Abstract
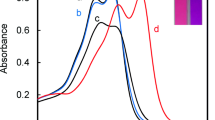
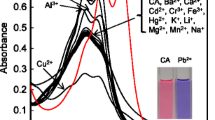
A new colorimetric chemosensor based on a simple ternary mixture of an anionic dye, pyrogallol red (PR), a cationic polyelectrolyte, poly(diallyldimethylammonium chloride) (PDADMAC), and a metal chelator, N-(2-hydroxyethyl) ethylenediaminetriacetic acid (HEDTA) for the colorimetric detection of Fe2+ and Fe3+ has been developed in an aqueous solution buffered at pH 5. Upon addition of Fe2+ or Fe3+ to the mixture, the absorption spectra showed a bathochromic shift; correspondingly, the solution color changed from red to blue, whereas other metal ions basically resulted in insignificant spectral and color changes. From the competitive experiments, no obvious interferences for the colorimetric detection of Fe2+ and Fe3+ were observed in the presence other metal ions. The results indicated that the mixture could be used as a potential Fe2+ and Fe3+ colorimetric and naked eye chemosensor in aqueous media. This research demonstrates that the ternary ensemble consisted of an organic dye, an oppositely charged polyelectrolyte, and a metal chelator is a versatile and convenient tool for the facile preparation of a novel chemosensor system.





Similar content being viewed by others
References
Quang, D.T., Kim, J.S.: Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 110, 6280–6301 (2010)
Sharma, H., Kaur, N., Singh, A., Kuwar, A., Singh, N.: Optical chemosensors for water sample analysis. J. Mater. Chem. C 4, 5154–5194 (2016)
Jeong, Y., Yoon, J.: Recent progress on fluorescent chemosensors for metal ions. Inorg. Chim. Acta 381, 2–14 (2014)
Beutler, E., Felitti, V., Gelbart, T., Ho, N.: Genetics of iron storage and hemochromatosis. Drug. Metab. Dispos. 29, 495–499 (2001)
Cairo, G., Pietrangelo, A.: Iron regulatory proteins in pathobiology. Biochem. J. 352, 241–250 (2000)
Touati, D.: Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373, 1–6 (2000)
D’Autreaux, B., Tucker, N.P., Dixon, R., Spiro, S.: A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 437, 769–772 (2005)
Lieu, P.T., Heiskala, M., Peterson, P.A., Yang, Y.: The roles of iron in health and disease. Mol. Aspects Med. 22, 1–87 (2001)
Burdo, J.R., Connor, J.R.: Brain iron uptake and homeostatic mechanisms: an overview. Biometals 16, 63–75 (2003)
Connor, J.R., Menzies, S.L., Martin, S.M.S., Mufson, E.J.: Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 27, 595–611 (1990)
Wójciak, R.W., Mojs, E., Stanislawska-Kubiak, M.: The occurrence of iron-deficiency anemia in children with type 1 diabetes. J. Investig. Med. 62, 865–867 (2014)
Simcox, J.A., McClain, D.A.: Iron and diabetes risk. Cell Metab. 17, 329–341 (2013)
Weinreb, O., Mandel, S., Youdim, M.B.H., Amit, T.: Targeting dysregulation of brain iron homeostasis in Parkinson’s disease by iron chelators. Free Radic. Biol. Med. 62, 52–64 (2013)
Torti, S.V., Torti, F.M.: Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13, 342–355 (2013)
Que, E.L., Domaille, D.W., Chang, C.J.: Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008)
Vanloot, P., Coulomb, B., Brach-Papa, C., Sergent, M., Boudenne, J.L.: Multivariate optimization of solid phase extraction applied to iron determination in finished waters. Chemosphere 69, 1351–1360 (2007)
Canfranc, E., Abarca, A., Sierra, I., Marina, M.L.: Determination of iron and molybdenum in a dietetic preparation by flame AAS after dry ashing. J. Pharm. Biomed. Anal. 25, 103–108 (2001)
Ling, I., Hashima, R., Sabah, K.J.: Sugar thiacrown-ether appended calix[4]arene as a selective chemosensor for Fe2+ and Fe3+ ions. RSC Adv. 5, 88038–88044 (2015)
Singh, G., Singh, J., Singh, J., Mangat, S.S.: Design of selective 8-methylquinolinol based ratiometric Fe2+ and Fe3+/H2PO4 – fluorescent chemosensor mimicking NOR and IMPLICATION logic gates. J. Lumin. 165, 123–129 (2015)
Oh, J.W., Kim, T.H., Yoo, S.W., Lee, Y.O., Lee, Y., Kim, H., Kim, J., Kim, J.S.: Multisignaling metal sensor: optical, electrochemical, and electrochemiluminescent responses of cruciform-shaped alkynylpyrene for selective recognition of Fe3+. Sens. Actuators B 177, 813–817 (2013)
Kaur, N., Kuma, S.: Colorimetric metal ion sensors. Tetrahedron 67, 9233–9264 (2011)
Kim, Y.S., Park, G.J., Lee, J.J., Lee, S.Y., Lee, S.Y., Kim, C.: Multiple target chemosensor: a fluorescent sensor for Zn(II) and Al(III) and a chromogenic sensor for Fe(II) and Fe(III). RSC Adv. 5, 11229–11239 (2015)
Lashgari, N., Badiei, A., Ziarani, G.M.: A fluorescent sensor for Al(III) and colorimetric sensor for Fe(III) and Fe(II) based on a novel 8-hydroxyquinoline derivative. J. Fluoresc. 26, 1885–1894 (2016)
Wang, S., Gwon, S.Y., Kim., S.H.: A highly selective and sensitive colorimetric chemosensor for Fe2+ based on fluoran dye. Spectrochim. Acta A 76, 293–296 (2010)
You, G.R., Park, G.J., Lee, S.A., Ryu, K.Ym, Kim, C.: Chelate-type schiff base acting as a colorimetric sensor for iron in aqueous solution. Sens. Actuators B 215, 188–195 (2015)
Kim, Y.S., Lee, J.J., Lee, S.Y., Jo, T.G., Kim, C.: A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(II) and Fe(III) and the fluorometric detection of Zn(II) in aqueous media. RSC Adv. 6, 61505–61515 (2016)
Higby, K., Suiter, C.R., Silerkhodr, T.: A comparison between two screening methods for detection of microproteinuria. Am. J. Obstet. Gynecol. 173, 1111–1114 (1995)
Behr, S., Trumel, C., Palanche, F., Braun, J.P.: Assessment of a pyrogallol red technique for total protein measurement in the cerebrospinal fluid of dogs. J. Small Anim. Pract. 44, 530–533 (2003)
Ensafi, A.A., Khayamian, T., Khaloo, S.S.: Application of adsorptive cathodic differential pulse stripping method for simultaneous determination of copper and molybdenum using pyrogallol red. Anal. Chim. Acta 505, 201–207 (2004)
Ivanov, V.M., Mamedov, A.M.: 3,4,5-Trihydroxyfluorones as analytical reagents. J. Anal. Chem. 61, 1040–1062 (2006)
Pelit, L., Koçak, S., Pelit, F.O., Turkmena, H., Ertas, F.N.: A spectrophotometric method for determination of molybdenum in water samples by using pyrogallol red and a water soluble ionic liquid. Anal. Methods 5, 5792–5798 (2013)
Atala, E., Velásquez, G., Vergara, C., Mardones, C., Reyes, J., Tapia, R.A., Quina, F., Mendes, M.A., Speisky, H., Lissi, E., Ureta-Zañartu, M.S., Aspée, A., López-Alarcón, C.: Mechanism of pyrogallol red oxidation induced by free radicals and reactive oxidant species. A kinetic and spectroelectrochemistry study. J. Phys. Chem. B 117, 4870–4879 (2013)
Butler, G.B., Angelo, R.J.: Preparation and polymerization of unsaturated quaternary ammonium compounds VIII A proposed alternating intramolecular-intermolecular chain propagation. J. Am. Chem. Soc. 79, 3128–3131 (1957)
Assem, Y., Chaffey-Millar, H., Barner-Kowollik, C., Wegner, G., Agarwal, S.: Controlled/living ring-closing cyclopolymerization of diallyldimethylammonium chloride via the reversible addition fragmentation chain transfer process. Macromolecules 40, 3907–3913 (2007)
Wang, Y., Chen, J., Jiao, H., Chen, Y., Li, W., Zhang, Q., Yu, C.: Polymer-templated perylene-probe noncovalent self-assembly: a new strategy for label-free ultrasensitive fluorescence turn-on biosensing. Chem. Eur. J. 19, 12846–12852 (2013)
Dubas, S.T., Limsavarn, L., Iamsamai, C., Potiyaraj, P.: Assembly of polyelectrolyte multilayers on nylon fibers. J. Appl. Polym. Sci. 101, 3286–3290 (2006)
Cheng, K.L.: EDTA as masking agent in selective spectrophotometric determination of copper with triethenetetramine: an interpretation of masking. Anal. Chem. 34, 1392–1396 (1962)
Zhou, X., Lu, Y., Zhu, J.F., Chan, W.H., Lee, A.W.M., Chan, P.S., Wong, R.N.S., Mak, N.K.: Ratiometric fluorescent Zn2+ chemosensor constructed by appending a pair of carboxamidoquinoline on 1,2-diaminocyclohexane scaffold. Tetrahedron 67, 3412–3419 (2011)
Repo, E., Warchoł, J.K., Bhatnagar, A., Sillanpää, M.: Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J. Colloid Interface Sci. 358, 261–267 (2011)
Chen, H., Cutright, T.: EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere 45, 21–28 (2001)
Okemgbo, A.A., Hill, H.H., Metcalf, S.G., Bachelor, M.: Metal ion interferences in reverse polarity capillary zone electrophoretic analysis of Hanford Defense Waste for ethylenediaminetetraacetic acid (EDTA) and n-hydroxyethylethylenediaminetriacetic acid (HEDTA). Anal. Chim. Acta 396, 105–116 (1999)
Gonzalez, D., Obrador, A., Alvarez, J.M.: Behavior of zinc from six organic fertilizers applied to a navy bean crop grown in a calcareous soil. J. Agric. Food Chem. 55, 7084–7092 (2007)
Sakamaki, M., Aikawa, S., Fukushima, Y.: Colorimetric chemosensor for Zn2+ based on pyrogallol red and poly(diallyldimethylammonium chloride) in aqueous solution. Polym. Bull. 75, 1667–1680 (2018)
Irving, H.M.N.H., Freiser, H., West, T.S.: IUPAC Compendium of Analytical Nomenclature, Definitive Rules. Pergamon Press, Oxford (1978)
Wang, M., Wang, J., Xue, W., Wu, A.: A benzimidazole-based ratiometric fluorescent sensor for Cr3+ and Fe3+ in aqueous solution. Dyes Pigments 97, 475–480 (2013)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Grynkiewicz, G., Poenie, M., Tsien, R.Y.: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985)
Kim, H., Na, Y.J., Song, E.J., Kim, K.B., Bae, J.M., Kim, C.: A single colorimetric sensor for multiple target ions: the simultaneous detection of Fe2+ and Cu2+ in aqueous media. RSC Adv. 4, 22463–22469 (2014)
Sen, S., Sarkar, S., Chattopadhyay, B., Moirangthem, A., Basu, A., Dharad, K., Chattopadhyay, P.: A ratiometric fluorescent chemosensor for iron: discrimination of Fe2+ and Fe3+ and living cell application. Analyst 137, 3335–3342 (2012)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inoue, K., Aikawa, S., Masaru, S. et al. Colorimetric chemosensor for Fe2+ and Fe3+ based on a ternary mixture of an anionic dye, a cationic polyelectrolyte, and a metal chelator in aqueous solution. J Incl Phenom Macrocycl Chem 91, 171–177 (2018). https://doi.org/10.1007/s10847-018-0812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0812-2