Abstract
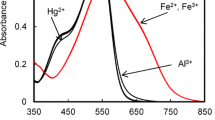
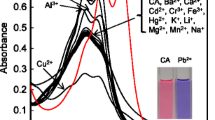
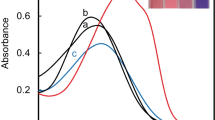
We have developed a colorimetric chemosensor based on an anionic organic dye, commercially available nuclear fast red (NFR) and a cationic polyelectrolyte, poly(diallyldimethylammonium chloride) (PDADMAC) for detection of Hg2+ in aqueous solution at physiological conditions. Upon addition of Hg2+, a bathchromic shift in the absorption was observed concomitantly with color change from pink to reddish violet which was easily detectable by the naked eye, while such a change was not observed for NFR alone, indicating that PDADMAC played an important role in detecting Hg2+. This investigation can propose the simple and valuable construction method for a novel chemosensor by mixture of a water-soluble organic dye and an oppositely charged polyelectrolyte.










Similar content being viewed by others
References
Saleem M, Lee KH (2015) Optical sensor: a promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv 5:72150–72287
Quang DT, Kim JS (2010) Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev 110:6280–6301
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
Sakamaki M, Aikawa S, Fukushima Y (2017) Colorimetric determination of Pb2+ in perfect aqueous solution using carminic acid as a selective chemosensor. J Fluoresc 27:1929–1935
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107:641–662
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108:3443–3480
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury-current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Gaedi M, Fathi MR, Shokrollahi A, Shajarat F (2006) Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Anal Lett 39:1171–1185
Hong YS, Rifkin E, Bouwer EJ (2011) Combination of diffusive gradient in a thin film probe and IC-ICP-MS for the simultaneous determination of CH3Hg+ and Hg2+ in oxic water. Environ Sci Technol 45:6429–6436
Park H, Hwang SJ, Kim K (2012) An electrochemical detection of Hg2+ ion using graphene oxide as an electrochemically active indicator. Electrochem Commun 24:100–103
Yoon S, Miller EW, He Q, Do PH, Chang CJ (2007) A bright and specific fluorescent sensor for mercury in water, cells, and tissue. Angew Chem Int Edit 46:6658–6661
Cheng X, Li Q, Qin J, Li Z (2010) A new approach to design ratiometric fluorescent probe for mercury(II) based on the Hg2+-promoted deprotection of thioacetals. ACS Appl Mater Interfaces 2:1066–1072
Ding H, Zheng C, Li B, Liu G, Pu S, Jia D, Zhou Y (2016) A rhodamine-based sensor for Hg2+ and resultant complex as a fluorescence sensor for I-. RSC Adv 6:80723–80728
Venkatesan P, Thirumalivasan N, Wu S-P (2017) A rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+ in vitro and in vivo. RSC Adv 7:21733–21739
Inoue K, Aikawa S, Fukushima Y (2018) Colorimetric detection of Hg2+ using a mixture of an anionic azo dye and a cationic polyelectrolyte in aqueous solution. Polym Int 67:755–760
Baar S (1957) A micromethod for the estimation of serum calcium. Clin Chim Acta 2:567–575
Sams A, Davies FMR (1967) Commercial varieties of nuclear fast red; their behaviour in staining after autoradiography. Stain Technol 42:269–276
Cui Y, Yu J, Feng S (2014) Nuclear fast red as highly sensitive“off/on”fluorescent probefor detecting guanine. Talanta 130:536–541
Li M, Fu Y, Jin L (2017) A dual-signal sensing system based on organic dyes-LDHs film for fluorescence detection of cysteine. Dalton Trans 46:7284–7290
Butler GB, Angelo RJ (1957) Preparation and polymerization of unsaturated quaternary ammonium compounds VIII a proposed alternating intramolecular-intermolecular chain propagation. J Am Chem Soc 79:3128–3131
Assem Y, Chaffey-Millar H, Barner-Kowollik C, Wegner G, Agarwal S (2007) Controlled/living ring-closing cyclopolymerization of diallyldimethylammonium chloride via the reversible addition fragmentation chain transfer process. Macromolecules 40:3907–3913
Wang Y, Chen J, Jiao H, Chen Y, Li W, Zhang Q, Yu C (2013) Polymer-templated perylene-probe noncovalent self-assembly: a new strategy for label-free ultrasensitive fluorescence turn-on biosensing. Chem Eur J 19:12846–12852
Dubas ST, Limsavarn L, Iamsamai C, Potiyaraj PJ (2006) Assembly of polyelectrolyte multilayers on nylon fibers. J Appl Polym Sci 101:3286–3290
Sakamaki M, Aikawa S, Fukushima Y (2018) Colorimetric chemosensor for Zn2+ based on pyrogallol red and poly(diallyldimethylammonium chloride) in aqueous solution. Polym Bull 75:1667–1680
Inoue K, Aikawa S, Sakamaki M, Fukushima Y (2018) Colorimetric chemosensor for Fe2+ and Fe3+ based on a ternary mixture of an anionic dye, a cationic polyelectrolyte, and a metal chelator in aqueous solution. J Incl Phenom Macrocycl Chem 91:171–177
Inoue K, Aikawa S, Sakamaki M, Fukushima Y (2018) Colorimetric Co2+ sensor based on an anionic pyridylazo dye and a cationic polyelectrolyte in aqueous solution. Polym Int 67:1589–1594
Inoue K, Aikawa S, Fukushima Y (2019) Colorimetric chemosensor for Ni2+ based on alizarin complexone and a cationic polyelectrolyte in aqueous solution. J Appl Polym Sci 136:47496
Fukushima Y, Aikawa S (2019) Colorimetric detection of Ni2+ based on an anionic triphenylmethane dye and a cationic polyelectrolyte in aqueous solution. Tetrahedron Lett 60:675–680
Lee SY, Lee JJ, Bok KH, Kim JA, Kim SY, Kim C (2016) A colorimetric chemosensor for the sequential recognition of mercury (II) and iodide in aqueous media. Inorg Chem Comm 70:147–152
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, Yang M (2015) A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron 63:506–512
Job P (1928) Formation and stability of inorganic complexes in solution. Ann Chim 9:113–203
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Fukushima Y (2001) Interaction of porphyrin derivatives with a β-sheet structure of a zwitterionic polypeptide in aqueous solution. Polym Bull 45:479–485
Liang W, He S, Fang J (2014) Self-assembly of J-aggregate nanotubes and their applications for sensing dopamine. Langmuir 30:805–811
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 123 kb)
Rights and permissions
About this article
Cite this article
Fukushima, Y., Aikawa, S. Colorimetric Chemosensor for Hg2+ Based on Nuclear Fast Red and a Cationic Polyelectrolyte in Aqueous Solution. J Fluoresc 30, 175–180 (2020). https://doi.org/10.1007/s10895-019-02482-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02482-1