Abstract
Climate change represents a threat to narrow-ranged mountain species living in low-altitude massifs. We studied the disjunct Apennine population of Lasiommata petropolitana (Lepidoptera, Nymphalidae) in the Gran Sasso and Monti della Laga National Park. We quantified the altitudinal shifts undergone in the last decades (1964–2021) in the Alps and Apennines and estimated the local extinction risk due to climate change. We also sequenced the COI mitochondrial marker of seven Apennine specimens, comparing them with those available across the Palearctic. We projected the probability of presence for the species under a future climatic scenario using an ensemble forecasting approach. We found that, despite geographical isolation, the Apennine population of L. petropolitana displays a single widespread COI haplotype also occurring in most European populations. In the Alps and Apennines, this species has shifted uphill an average of 6.3 m per year since 1964. Accordingly, our model predicted a likely extinction in the Apennines by about 2060, due to a reduction of the climatic suitability in this region.
Implications for insect conservation
Implications for insect conservation Despite its potential loss in the Apennines would not erode mitochondrial diversity, L. petropolitana characterises the butterfly community of the Gran Sasso massif as an alpine enclave. The loss of the Apennine population, together with those of other orophilous butterflies, could trigger a homogenization of alpha and beta diversity and induce a loss of functional diversity in the impoverished high-altitude biotas. As habitat heterogeneity is a key aspect for populations to endure climate change, the maintenance of varied microhabitats, mainly through grazing management, could address the decline of this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in climate have produced dramatic alterations in species distributions over geological times (Peterson and Lieberman 2012; Blois et al. 2013). The recent human-induced climate changes represent a growing threat to biodiversity (Halsch et al. 2021; Habibullah et al. 2022). Their impacts are now widely documented (Parmesan 2006; Renner and Zohner 2018; Shah et al. 2020) and are especially noticeable in European southern mountain ecosystems, where populations that were more widely distributed during the coldest phases of the late Quaternary, are now restricted to the highest altitudes (Grabherr et al. 2010; Ernakovich et al. 2014; Ohlemüller et al. 2008). In these areas, even a moderate increase in temperature could determine significant changes in community composition and species richness (Shah et al. 2020; Viterbi et al. 2020).
Among invertebrates, butterflies are considered good models to study climate change effects because their distribution has been generally well studied across time and because they often have a relatively small climatic niche (Parmesan et al. 1999; van Swaay et al. 2010; Habel et al. 2011; Schweiger et al. 2014). Moreover, butterflies tend to react quickly to environmental changes by virtue of biological features such as their complex demands during life cycle, their mobility and short lifespan (van Swaay et al. 2010; Bonelli et al. 2018). Different consequences of climate change have been documented for mountain butterflies, including distribution shifts towards higher altitudes (Forister et al. 2010; Rödder et al. 2021) and an overall expansion of thermophilous and generalist species, in parallel to a decrease of more specialised ones (Zografou et al. 2014; Cerrato et al. 2019; Macgregor et al. 2019; Bonelli et al. 2022).
Many butterfly species inhabiting relatively cold habitats were presumably more widely distributed during the last glacial maximum and in the current interglacial they shifted towards high latitudes and/or to scattered mountain areas. Examples among Holarctic butterflies are included in the genera Erebia (De Groot et al. 2009; Hinojosa et al. 2018; Minter et al. 2020; Sistri et al. 2022) and Parnassius (Ashton et al. 2009; Todisco et al. 2010).
Upward shifts are associated with the reduction and the fragmentation of suitable habitats for orophilous butterflies, increasing their extinction risk because of fitness lowering and genetic impoverishment (Schmitt and Hewitt 2004; Habel et al. 2011). In the short term, an elevational displacement might have a positive effect on a given butterfly population, as at high altitudes land use intensity is generally lower than in lowland areas, but in the long run climate change is expected to lead to a reduction in habitat area (Hülber et al. 2020).
Indeed, the possibility to shift towards higher elevations is clearly limited for populations inhabiting mountains, since the area of the habitats rapidly decreases with altitude (Habel et al. 2011). In addition to ecological consequences of population extinction, the loss of intra-specificgenetic diversity is an important aspect to consider, since many relict populations are markedly genetically distinct from the other lineages, as a result of their protracted isolation (Schmitt et al. 2005; Konvička et al. 2014; Minter et al. 2020; Sistri et al. 2022). A high extinction risk has been predicted for several species with disjunct populations in the Apennines, such as Erebia gorge (Hübner, [1804]) (Piazzini and Favilli 2020) and Erebia pandrose (Borkhausen, 1788) (Sistri et al. 2022), the latter represented by a genetically differentiated population on these mountains.
In this study, we focused on the Apennine population of the butterfly Lasiommata petropolitana (Fabricius, 1787) (Nymphalidae, Satyrinae). This species occurs from the Pyrenees to Scandinavia and as far as eastern Russia (Bozano 1999). While its distribution is relatively continuous in northern parts of its range, towards the south, particularly in Europe, it becomes fragmented into a series of increasingly isolated populations restricted to mountainous areas (Kudrna et al. 2011). In Italy, the distribution of L. petropolitana is similar to that of other butterfly species such as Polyommatus eros (Ochsenheimer, 1808), Boloria pales ([Denis & Schiffermüller], 1775), Erebia pandrose and E. pluto (Prunner, 1798), which are widely distributed in the Alps and restricted to a few mountain areas in the Apennines. These isolated populations often represent endemic lineages responsible for a strong genetic distinctiveness between the communities of the Apennines and the Alps (Ehl et al. 2020; Menchetti et al. 2021; Schmitt et al. 2021; Sistri et al. 2022). In the Apennines, L. petropolitana only occurs in a few sites in three neighbouring massifs: Monti della Laga, Gran Sasso and Monti Marsicani (Balletto et al. 2007). These areas are included within the National Park of Gran Sasso and Monti della Laga and that of Abruzzo, Lazio and Molise. The presence of the species in the Apennines was confirmed in the 1980s after the first report dating back to the end of the XIX century (Prola et al. 1978). Then, this population was described as a separate subspecies (Pararge petropolitana centralapennina Teobaldelli and Floriani 1983), mainly on the basis of wing pattern. In Italy, L. petropolitana inhabits grassy habitats in woodland (e.g., with Fagus sylvatica L. and Larix decidua Mill.) and shrub areas (e.g., with Pinus mugo Turra), mainly on rocky substrates and in cold and humid sites (Prola et al. 1978; D’Alessandro et al. 2008; Hellmann and Parenzan 2010; Bonato et al. 2014). In the Apennines the species is monovoltine, with a flight-period between late May and early July, occurring above 1200 m of altitude in areas close to the limit of beech forests (Prola et al. 1978; Teobaldelli and Floriani 1983; Balletto et al. 2007).
Our study has two main research goals: (i) to assess the future extinction risk of this population and ii) to evaluate the potential impoverishment of mitochondrial genetic diversity if the Apennine population of L. petropolitana would be lost. To achieve these goals, (a) we applied Species Distribution Modelling (SDM) to high-resolution occurrence data to predict the persistence of L. petropolitana in the Apennines during the next decades; (b) we evaluated the historical elevational shift of the species over the Alps and Apennines; and (c) we obtained cytochrome c oxidase subunit 1 (COI) sequences to evaluate the mitochondrial DNA (mtDNA) genetic identity of the Apennine population with respect to other populations from Europe and Asia.
Methods
Data collection
We surveyed the Central Apennines in 2021, recording the geographic coordinates and the elevation (10 m precision) of all the sites where we observed L. petropolitana (Online Resource 1). Occurrence data for the Alps and the Apennines, as defined by Menchetti et al. (2021) (Fig. 1), were retrieved from literature, museum and private collections with reference to the Italian CKmap project (Balletto et al. 2007) and from collection data stored in Roger Vila’s laboratory at the Institut de Biologia Evolutiva CSIC-UPF (Barcelona, Spain). Data deposited in the platform iNaturalist have also been included if they were associated with high-resolution coordinates (error lower than 1000 m) and if accompanied by a photograph in order to avoid the risk of confusion with the similar L. maera (Linnaeus, 1758). A subset of occurrences was obtained using only the specimens with year and altitude recorded. We found a total of 147 suitable occurrence data. Due to the different sources of these data, and to reduce clustered occurrences, we then filtered the dataset selecting a minimum distance between the occurrence points of 2 km using the ‘thin’ function of ‘spThin’ R package (Aiello-Lammens et al. 2015) in R v. 4.0.3 (R Core Team, 2020). This reduced the final dataset used for the Species Distribution Modelling to 109 records (Fig. 1).
Species distribution modelling
We modelled the distribution of L. petropolitana in the study areas as a single entity, although Alps and Apennine populations may be differently adapted to local conditions. Recent studies highlighted the importance of including adaptive genetic variation in climate change vulnerability assessment (Razgour et al. 2019). However, given the very limited distribution of L. petropolitana in the Apennines (4 raster cells) this was not possible. Modelling the entire species as a single entity is expected to generate more generous estimates of species tolerance to environmental conditions.
In order to reduce the risk of overfitting through the inclusion of spurious correlates (Araújo et al. 2019; Fourcade et al. 2017), which can be particularly problematic in climate change projections of species distributions (Santini et al. 2021), we restricted the selection of predictor variables to those for which we could hypothesize a causal relationship with species presence. There are few studies on physiological and life history traits of L. petropolitana and they do not clearly suggest which climatic variables are most influential on the species (Nylin et al. 1996; Gotthard 1998). However, previous research on butterfly physiology and distribution documented some major climatic constraints in Southern Europe. In general, the loss of snow cover strongly affects the survival of butterflies at high altitudes due to exposure to low winter temperatures (Vrba et al. 2012, 2017; Konvicka et al. 2021) while, in summer, the main influencing factor is water availability (Vodă et al. 2015), which also counteracts the strong seasonal increase in temperature. We obtained 19 climatic variables from the CHELSA database (Karger et al. 2017, 2018) (Online Resource 3) with a resolution similar to that of the occurrence data (30 s, ~ 1 km2), and clipped them for the study area. Then we carried out a PCA on the variables to obtain their correlation structure (Online Resource 4). Afterwards, based on the available biological knowledge and the correlation structure among available climatic variables, we selected four weakly correlated variables: 1) minimum temperature of the coldest month (‘bio6’ in CHELSA database); (2) precipitation of the coldest quarter (‘bio18’); (3) precipitation of the warmest quarter (‘bio19’); (4) temperature annual range (‘bio7’). Regarding the climatic variables in the study region, in addition to a generalised increase in temperature, there is evidence of a decrease in precipitation frequency and amounts in the last decades (Valt and Cianfarra 2010; Di Lena et al. 2012; Gobiet et al. 2014; Brugnara and Maugeri 2019).
We evaluated the Variance Inflation Factor (VIF) for each selected variable using the R package ‘usdm’ (Naimi et al. 2014) and verified that all values were < 3, which indicates very low multicollinearity (Alin 2010; Kock and Lynn 2012) (Online Resource 5). Then, we downloaded and clipped the same variables for future projections for the 2041–2060 period, as well as the Representative Concentration Pathway (RPC) 4.5, described by Moss et al. (2008) as the intermediate scenario. For future climatic variables we used the median of four Global Circulation Models (GCMs): NorESM1, CM5A-MR, MPI-ESM-MR, HadGEM2-AO.
Species distribution modelling was performed using the R package ‘biomod2’ (Thuiller 2014). In general, overall statistical models tend to be less prone to overfitting than machine learning models, but the latter usually achieve higher accuracy in present predictions (e.g. Merow et al. 2013). As such, it is recommended to use multiple and diverse algorithms (Araújo and New 2007; Norberg et al. 2019) and validate them using a robust blocking approach to estimate the uncertainty around predictions (Bahn and McGill, 2013; Roberts et al. 2017). Accordingly, we used four statistical models to forecast the current and future species distribution: generalised additive model (GAM), generalised linear model (GLM), maximum entropy (MAXENT) and random forests (RF). The first two are statistical linear models, while MAXENT and RF are machine learning algorithms, so the entire set covers a wide range of complexity in distribution models (Merow et al. 2013) allowing appropriate estimates of uncertainty in model projections (Buisson et al. 2010).
Thereafter, we generated 10 datasets of 1000 randomly selected background points, one dataset for each of 10 repetitions used for the model validation. To estimate the relative importance of the predictor variables, we correlated the predicted probabilities of presence, using the full dataset, with the predicted probabilities of presence obtained after permuting the variable of interest. The relative importance of each variable was quantified as one minus the Pearson rank correlation coefficient (Thuiller et al. 2009). We validated the models using spatial block validation with the R package ‘blockCV’ (Valavi et al. 2019). We split the study area into six spatial blocks and two folds (Online Resource6), iteratively fitted the model on all but one block, and tested against the left-out block. This procedure is known to provide a more objective assessment of model transferability for future projections (Bahn and McGill 2013; Roberts et al. 2017). We measured model performance using the True Skill Statistic (TSS) and the area under the curve (AUC). For both present and future projections, we obtained one occurrence probability raster for each statistical model by calculating the mean of all the projections of models with a TSS > 0.5 and an AUC > 0.7. In order to obtain a single projection for each scenario, we then averaged the four rasters and excluded cells with an occurrence probability < 10%. In the results, the term ‘cells’ will thus indicate the cells with an occurrence probability > 10%. Then we calculated the difference between the two scenarios by subtracting the current average predictions from the future ones; raster cells with positive delta values indicate a predicted improvement of climatic conditions, whereas raster cells with negative delta values indicate a deterioration of climatic conditions. To estimate the uncertainty in the predictions due to disagreements among the four different algorithms, we assigned − 1 to all cells with negative values, + 1 to all cells with positive values (and 0 otherwise) of the average single-model predictions, and assessed the consensus of model predictions by summing the four binarized maps. This resulted in a raster map with values ranging between − 4 and + 4, with extreme values indicating that all the four statistical models predicted a decrease (− 4) or increase (+ 4) in the probability of occurrence, and intermediate values indicating partial (± 2–3) or high disagreement (− 1 to 1) among the predictions of the algorithms.
To ensure transparency and full replicability of the methods we provide an Overview, Data, Model, Assessment and Prediction (ODMAP) protocol as part of the supplementary material, that details all methodological choices and parameters (Online Resource 7; Zurell et al. 2020). The occurrence data used for species distribution modelling are available in the Online Resource 8.
Changes in elevation over time
Lasiommata petropolitana shifts in elevation were evaluated from 1964 to 2021, assuming 1964 as one of the dates suggested to mark the beginning of the Anthropocene (Lewis and Maslin 2015). When explicitly reported, elevation values were obtained from literature and collections data. For the occurrences without a clear report of elevation, but with precise coordinates (error lower than 1000 m like most citizen science data), the elevations were extracted using a 1 km2 layer available at https://www.earthenv.org/topography (Amatulli et al. 2018). The final dataset was composed of 323 records over a period of 57 years. An ordinary least squares (OLS) regression between time and elevation was used to test for the existence of a temporal trend in elevational shifts.
COI analyses
To evaluate the genetic identity of the Apennine population of L. petropolitana we gathered COI sequences for this species from across its distribution range (Eurasia) by downloading data publicly available in GenBank and in BOLD (Barcode of Life Data System), and by adding a series of seven unpublished sequences specifically analysed for this study, representing specimens collected in 2021 in Monti della Laga and Gran Sasso massifs. COI sequences from the Apennine specimens were obtained using LepF1 and LepR1 primers, following the protocol by Platania et al. (2020) at the Institut de Biologia Evolutiva CSIC-UPF (Barcelona, Spain). We aligned all the sequences with MegaX. We removed potential contaminant sequences as well as sequences shorter than 600 bp. This resulted in a final alignment of 55 specimens (Online Resource 2). We constructed maximum parsimony haplotype networks using the COI alignments and TCS 1.21 (Clement et al. 2000) by setting a 95% connection limit (11 steps). The haplotype network was graphically edited with tcsBU (Múrias dos Santos et al. 2016).
Haplotype colours were assigned according to p-distances projected after Principal Coordinates Analysis (PCoA) on the RGB space as done by the ‘recluster.col’ function of ‘recluster’ R package https://cran.r-project.org/web/packages/recluster/index.html) (Dapporto et al. 2013). Subsequently, the colours were plotted on a map using the recluster.plot.pie function of the same package. The haplotypes in the network were coloured according to their geographical origin.
Results
We recorded L. petropolitana in four sites in the massifs of Gran Sasso and Monti della Laga within the borders of the homonymous National Park. The species was found in grassy areas on rocky substrate in beech forest clearings, as well as in adjacent pastures with sparse shrubs, in an altitudinal range between 1455 and 1775 m above sea level (Online Resource 1). Among literature data for the Apennines, we excluded the single pre-1980 record for the massif of Monti Marsicani in the Abruzzo, Lazio and Molise National Park (Prola et al. 1978), as it lacks both coordinates and altitude. In the latter area, which constitutes the southern range limit of L. petropolitana in the Apennines, we have not detected the species and therefore its current presence needs confirmation.
Species distribution modelling
After thinning a total of 147 occurrence data, a dataset of 109 records was selected for the species distribution modelling of Alpine and Apennine populations (Fig. 1).The spatial-block validation of the statistical models indicated good predictive performances, with TSS = 0.53–0.82 and AUC = 0.81–0.93 (Online Resource 9). The most important variable in predicting the occurrence of L. petropolitana, according to all four statistical models, was the minimum temperature of the coldest month (values of the mean importance of all variables are given in the Online Resource 10). Accordingly, the smoothed response curves showed a strict dependence of L. petropolitana occurrence probability on the minimum temperature of the coldest month, with a great concordance between different algorithms. Conversely, the three other variables showed weaker relationships and lower concordance between the algorithms (Online Resources 10 and 11).
The projections of each statistical model (Online Resource 12) pictured slightly different results that were averaged in the ensemble model. The ensemble model projections for the present and the future indicated a high probability of occurrence across the Alps, but not over the Apennines (Figs. 2a, b). The predicted change in the probability of occurrence raster highlighted a widespread decline in climatic suitability across the distribution range of the species in the Apennines (Fig. 2c). We also found a high consensus among model predictions, indicating a high probability of decline throughout the current distribution of the species (Fig. 2d). Similarly, many marginal areas of the pre-Alps are predicted to deteriorate their climatic suitability for L. petropolitana (Fig. 2c).
Species distribution modelling of Lasiommata petropolitana (cells with an occurrence probability < 10% were excluded). The panels (a–d) are accompanied by insets showing a detail of the Central Apennines area and four yellow arrows indicate the position of the four occurrence records for this area. Panels (a) and (b) show the current (1980–2021) and future (2041–2060) distribution of the species, respectively. They are based on the ensemble models, ensemble present (a) and ensemble future (b), and the colours represent the species occurrence probability in a range between 0 and 1. c Ensemble delta: this panel shows the occurrence probability difference between future projections and current projections (future probability minus present probability for each cell). Negative values (in red) indicate a predicted reduction of suitability in the future, whereas positive values (in blue) indicate a predicted increase of suitability in the future (the values between -0.1 and + 0.1 are excluded). d Consensus change. This panel shows the confidence in directional change of the four statistical models. The colours represent the number of statistical models which agree in the future occurrence predictions: green areas indicate full agreement among all the models (+ 4 + 3 − 3 − 4 values), while red areas indicate an intermediate agreement in which different models predict different results (+ 1 + 2 0 − 1 − 2 values). e Histogram showing the proportion of raster cells depending on the probability of the occurrence of L. petropolitana in present (light blue) and future (red with white stripes). The numbers (1, 2, 3, 4) indicate the occurrence probability values of the four sites in the Apennines both for the present (blue numbers) and for the future (red numbers). These numbers are associated with those in Online Resources 1 and 13. f Frequency of the confidence in directional changes among the four statistical models represented as the percentage of cells for which models agreed. The consensus categories are indicated on the x-axis and their percentages on the y-axis (the colours are associated with (d)
The occurrence suitability for both present and future of all the cells is reported in Fig. 2e. The four cells assessed for the Apennines are indicated by numbers from one to four (Fig. 2e). These cells will shift from a present mean value of 0.47 ± 0.12 mean ± s.d.) to 0.24 ± 0.15 (mean ± s.d.) in the future (Online Resource 13). Only 2,7% of the occupied cells have a probability of 0.24 in the present.This value is much lower than the 10% quantile threshold used in similar studies to predict future occurrence at a given site (e.g., Habel et al. 2011).Overall, 75.4% of the cells will decrease their suitability in the future (Online Resource 14). More than half of the cells (54.1% + 19.3%) have a high consensus value between the four algorithms (− 4, − 3; + 3, + 4 respectively; Fig. 2f) and belong to high elevation areas (Fig. 2d).
Regression between elevation and year of records indicated an average increase of 6.3 m (± 1.5 s.e.) in altitude per year since 1964 (t = 4.28, P < 0.001, Fig. 3).
COI structure
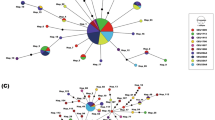
We retrieved a total of 48 COI sequences with a length between 600 and 658 bp and obtained seven original sequences (all with 658 bp) for the four Apennine sites. The haplotype network analysis identified few (8) haplotypes, with a star-like distribution (Fig. 4c). One haplotype appeared as highly frequent and widespread, while the other 7 haplotypes are rarer, separated by one or two mutations from the main haplotype.
The mitochondrial (COI) genetic structure of Lasiommata petropolitana. a Map of L. petropolitana haplotype distribution (2° resolution). The haplotype colours are attributed according to their genetic distance, based on a PCoA configuration on p-distances projected in RGB space. b Relative genetic distance plot among the different L. petropolitana haplotypes. Colours association corresponds to Fig. 2a. c Maximum parsimony COI haplotype network. The haplotypes (coloured circles) are separated by segments; each segment represents a single mutation between two different haplotypes. Haplotype colours have been assigned according to the geographical origin of the COI sequences. The circle area is proportional to the number of specimens showing each haplotype, as shown in the legend
While the most common haplotype is widely distributed across the range of L. petropolitana, including the isolated Apennine population, rare haplotypes are segregated in different regions. If we exclude the 5 singletons, the 2 remnant haplotypes are endemic to the Alps and the Pyrenees (Fig. 4c).
Discussion
According to species distribution modelling and the analysis of the altitudinal trend, the population of L. petropolitana in Central Italy is strongly threatened by climate change, as there is evidence that the climatic suitability of the areas currently occupied in the Apennines will lower critically in the next decades.
The analysis of the COI mitochondrial marker showed that the Apennine population belongs to a common haplotype occurring all over the distribution range of the species, therefore questioning the validity of its subspecific status attributed by Teobaldelli and Floriani (1983). This pattern is in agreement with recent findings on this genus (Platania et al. 2020), and reflects the tendency of boreo-alpine species to show a limited genetic differentiation after postglacial dynamics of populations (Hewitt 2000; Mutanen et al. 2012; Wallis and Arntzen 1989). Among the selected variables the minimum temperature of the coldest month (‘bio6’ in CHELSA database) explained most of the distribution of the species across the Alps and Apennines (Online Resources 10, 11), with an optimum value around − 10 °C. During the current interglacial these temperatures only occur on high mountain systems in Southern Europe. Presumably, L. petropolitana was more largely and quite homogeneously distributed in non-glaciated areas of Central and Southern Europe before the onset of the current interglacial period that started 11.600 years ago (Otto-Bliesner et al. 2021). During the interglacial period, L. petropolitana should have quite rapidlyexpanded far to the north in areas formerly covered by ice-sheets (Scandinavia) and retreated to scattered mountain areas in Central and Southern Europe (Pyrenees, Alps, Apennines, Balkans, Carpathians) (Mutanen et al. 2012). Likely, this process is currently exacerbated by human-induced climate changes and we found a significant elevational shift of about 6 m per year, in line with results obtained with Lepidoptera in other studies (Forister et al. 2010; Rödder et al. 2021; Sistri et al. 2022). A consistent shift in altitude may force the Apennine population to occupy smaller areas still characterised by locally suitable environmental conditions. Such areas may indeed act as microclimate refugia in the short term, but in the long run their suitability will be reduced due to climate change, probably leading to the local extinction of the species. On the Apennines, the minimum altitude recorded in recent years for the species is 1455 m a.s.l. (site 4, Online Resource 1). The relatively low altitude of the mountains in its range determines that suitable sites are isolated from each other by valleys that do not conform to the species’ demands in terms of climate and habitat, thus limiting the possibility of dispersal among the mountain groups.
In these areas L. petropolitana lives near the treeline, a limit around which there is increasing evidence for butterfly decline in the Apennines (Scalercio et al. 2014; Cini et al. 2020). The local morphology of the territory could be also a limiting factor for the dispersal of the species, such as in areas where rocky cliffs enclose the suitable habitats, without allowing connections with nearby ones (e.g., site 4, Online Resource 1).
Another important factor responsible for the range shift of many butterfly species is land-use change (van Swaay et al. 2010; Bonelli et al. 2011). We have no information on the historical occurrence of L. petropolitana in the Apennines, as its presence there has been confirmed in the 1980s (Prola et al. 1978). Furthermore, recent records are few and we don’t know if anthropic activities, e.g. linked to livestock or forestry activities, have contributed to its rarity. At present, however, the distribution range of the species is included in a National Park, where land use changes are limited thanks to current regulations.
Despite being clearly threatened at a local scale, L. petropolitana is assigned to the Least Concern category in the European Red List of Butterflies (van Swaay et al. 2010). Nevertheless, in some countries it is recognised as a species under extinction risk, being listed as endangered in Germany (Reinhardt and Bolz 2011) and Poland (Buszko and Nowatzki 2000). In the Czech Republic it is now extinct, following habitat disappearance due to land use changes, including cessation of grazing and afforestation (Spitzer et al. 2017). These examples refer to populations at the margins of their main distribution range, comprising massifs with lower altitudes than the Alps and the Pyrenees and/or areas located at the southern limit of the Northern Europe population. This tendency has been highlighted in other butterflies at their range limit (Wilson et al. 2015; Fourcade and Öckinger 2017). Lasiommata petropolitana is assigned to the Least Concern category also in the Italian IUCN Red List, due to the presence of large and relatively stable populations in the Alps (Bonelli et al. 2018), but the marginal population in the Apennines should be categorised as Endangered according to the B2ab (iii, v) IUCN criteria (http://www.iucn.it/scheda.php?id=-1662973159) and our projections for the next decades confirm this assessment.
Although L. petropolitana does not show a particularly conspicuous variability in the COI marker across its range, some of its biological features, probably having a genetic basis, are known to vary among different geographical areas, such as larval diapause in alpine populations in contrast to pupal diapause reported further north(Nylin et al. 1996; Gotthard 1998). However, mtDNA may show an evolutionary trajectory that may be different than that of nDNA. From this point of view, it would be worth checking in future studies whether the Apennine population represents an Evolutionary Significant Unit (Casacci et al. 2013) based on high-throughput sequencing on the nuclear genome.
Even if the potential loss of the Apennine population of L. petropolitana in the future would not erode the mitochondrial genetic diversity of the species, the prospect of its local extinction is a cause for concern. Indeed, L. petropolitana is a species that characterises the rich butterfly community of the Gran Sasso and Monti della Laga National Park. Together with communities from neighbouring high-altitudes mountains, these massifs are recognised as alpine enclaves in the Apennines, because of both species’ richness and composition (Balletto et al. 1982). Among the most relevant species recorded in the areas inhabited by L. petropolitana there are Parnassius mnemosyne (Linnaeus, 1758), Leptidea reali Reissinger, 1990, Lycaena hippothoe (Linnaeus, [1760]) and Erebia euryale (Esper, 1805), all fairly widespread in the Alps but more localised in the Apennines (Balletto et al. 2007; Dincă et al. 2011).
The loss of L. petropolitana in the Apennines, together with other orophilous butterflies (Bonelli et al. 2011) could trigger a homogenization of alpha and beta diversity in the region and possibly induce a loss of functional diversity in the impoverished high-altitude biota. In turn, these losses could erode the ecosystem services provided by a restricted number of butterflies species occurring on high altitude grassy habitats (Noriega et al. 2018). Highly charismatic butterfly species that characterise unique communities can also act as attractive components for tourism in protected areas, and their persistence could benefit the touristic offer of the Gran Sasso and Monti della Laga National Park.
It is difficult to hypothesise any action to avoid the climate change impacts on the Apennine population of L. petropolitana. Translocation to other Apennine massifs makes little sense since our predictions indicate a decrease of the climatic suitability for the entire mountain chain in the next decades, and no positive trend is expected anywhere in the region (Fig. 2c). However, the entire range of the species in the Apennines is included within protected areas, where it is possible to implement conservation measures. In general, the maintenance of habitat heterogeneity is a key aspect for the conservation of rich butterfly communities (van Swaay et al. 2012). In the study area, L. petropolitana partly occurs in grassy habitats maintained by cattle and sheep grazing, which prevents the expansion of shrubs and arboreal vegetation near the treeline. The maintenance of a moderate grazing, as long as it is correctly planned and adapted to local conditions (Metera et al. 2010; Jerrentrup et al. 2016; Johansson et al. 2017; Cutter et al. 2022), is thus desirable at the wood margins, considering that the treeline generally tends to advance rapidly both with land abandonment and climate warming (Gerhig-Fasel et al. 2007; Wieser et al. 2014).However, there are also large upland areas in Gran Sasso and Monti della Laga National Park, including some where L. petropolitana occurs (e.g. sites 1 and 2, Online Resource 1), which are subject to intense grazing both by cattle and sheep. A decrease of the treeline elevation is reported on the southern slopes of Gran Sasso massif, probably due to the combination between water shortage in summer and alteration of microclimate conditions when beech cover was reduced for pasturage in the past (Bonanomi et al. 2020). At these sites, some efforts should therefore be dedicated to allow the natural advance of the beech woods, in order to limit the mismatch between the elevational shift of L. petropolitana and the availability of suitable vegetation structure units for the species. Moreover, in such areas it could be possible to implement local strategies to limit the impact of grazing on herbaceous vegetation and on the biodiversity associated with it. Among the insects occurring in the Park, other localised species would also benefit from this measure, such as the endemic bumble bee Bombus konradini Reinig, 1965, for which intensive grazing is recognised as a major threat (Quaranta et al. 2018).
Continuous monitoring will also help in detecting negative population trends and prime for the need to implement changes in management. Italian National Parks, including Gran Sasso and Monti della Laga are called upon to conserve biodiversity, including threatened butterfly populations (Cini et al. 2020; Sistri et al. 2022). The same Parks have been recently committed by the Italian Environment Ministry to monitor pollinators using periodic transects following the EU Pollinators Initiative. The information obtained by transect counts will allow the monitoring of population trends and, in the case of species threatened by climate change such as L. petropolitana, they will enable to evaluate whether a reduction of estimated climatic suitability is associated with a reduction in population size.
References
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2018) Data from: Climatologies at high resolution for the earth’s land surface areas. Dryad. http://datadryad.org/stash/dataset/doi:10.5061/dryad.kd1d4
Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38(5):541–545. https://doi.org/10.1111/ecog.01132
Alin A (2010) Multicollinearity. WIREs. Comput Stat 2(3):370–374. https://doi.org/10.1002/wics.84
Amatulli G, Domisch S, Tuanmu M-N, Parmentier B, Ranipeta A, Malczyk J, Jetz W (2018) A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Scientific Data 5(1):180040. https://doi.org/10.1038/sdata.2018.40
Araújo MB, New M (2007) Ensemble forecasting of species distributions. Trends Ecol Evol 22(1):42–47. https://doi.org/10.1016/j.tree.2006.09.010
Araújo MB, Anderson RP, Márcia Barbosa A, Beale CM, Dormann CF, Early R et al (2019) Standards for distribution models in biodiversity assessments. Sci Adv 5(1):4858. https://doi.org/10.1126/sciadv.aat4858
Ashton S, Gutiérrez D, Wilson RJ (2009) Effects of temperature and elevation on habitat use by a rare mountain butterfly: implications for species responses to climate change. Ecol Entom 34:437–446. https://doi.org/10.1111/j.1365-2311.2008.01068.x
Bahn V, McGill BJ (2013) Testing the predictive performance of distribution models. Oikos 122(3):321–331. https://doi.org/10.1111/j.1600-0706.2012.00299.x
Balletto E, Toso GG, Barberis G (1982) Le comunità di Lepidotteri Ropaloceri nei consorzi erbacei dell’Appennino. In: La Greca M (ed.) Quaderni sulla Struttura delle zoocenosi terrestri. 2. La montagna. II. 1. I pascoli altomontani. Collana del progetto finalizzato “Promozione della qualità dell’ambiente”, Roma, pp 7–144
Balletto E, Bonelli S, Cassulo LA (2007) Insecta Lepidoptera Papilionoidea. In: Ruffo S, Stoch F (eds.) Checklist and distribution of the Italian Fauna. 10,000 terrestrial and freshwater species. Memorie del Museo Civico di Storia Naturale di Verona, 2° serie, Sez. Scienze della Vita, Vol. 17, Verona, pp. 257–261
Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S (2013) Climate change and the past, present, and future of biotic interactions. Science 341(6145):499–504. https://doi.org/10.1126/science.1237184
Bonanomi G, Zotti M, Mogavero V, Cesarano G, Saulino L, Rita A et al (2020) Climatic and anthropogenic factors explain the variability of Fagus sylvatica treeline elevation in fifteen mountain groups across the Apennines. Forest Ecosyst 7(1):1–13. https://doi.org/10.1186/s40663-020-0217-8
Bonato L, Uliana M, Beretta S (2014) Farfalle del Veneto: atlante distributivo Regione Veneto Musei Civici di Venezia. Marsilio Editori, Venezia
Bonelli S, Cerrato C, Loglisci N, Balletto E (2011) Population extinctions in the Italian diurnal Lepidoptera: an analysis of possible causes. J Insect Conserv 15(6):879–890. https://doi.org/10.1007/s10841-011-9387-6
Bonelli S, Casacci LP, Barbero F, Cerrato C, Dapporto L, Sbordoni V, Scalercio S, Zilli A, Battistoni A, Teofili C, Rondinini C, Balletto E (2018) The first red list of Italian butterflies. Insect Conserv Divers 11(5):506–521. https://doi.org/10.1111/icad.12293
Bonelli S, Cerrato C, Barbero F, Boiani MV, Buffa G, Casacci LP, Fracastoro L, Provenzale A, Rivella E, Zaccagno M, Balletto E (2022) Changes in alpine butterfly communities during the last 40 years. InSects 13(1):43. https://doi.org/10.3390/insects13010043
Bozano GC (1999) Guide to the butterflies of the Palearctic region Satyridae part I. Omnes Artes, Milano
Brugnara Y, Maugeri M (2019) Daily precipitation variability in the southern Alps since the late 19th century. Int J Climatol 39(8):3492–3504. https://doi.org/10.1002/joc.6034
Buisson L, Thuiller W, Casajus N, Lek S, Grenouillet G (2010) Uncertainty in ensemble forecasting of species distribution. Glob Change Biol 16(4):1145–1157. https://doi.org/10.1111/j.1365-2486.2009.02000.x
Buszko J, Nowacki J (eds) (2000) The Lepidoptera of Poland A distributional checklist. Polskie towarzystwo entomologiczne, Poznań
Casacci LP, Barbero G, Balletto E (2013) The “Evolutionarily Significant Unit” concept and its applicability in biological conservation. Ital J Zool 2013:1–12. https://doi.org/10.1080/11250003.2013.870240
Cerrato C, Rocchia E, Brunetti M, Bionda R, Bassano B, Provenzale A, Bonelli S, Viterbi R (2019) Butterfly distribution along altitudinal gradients: temporal changes over a short time period. Nat Conserv 34:91–118. https://doi.org/10.3897/natureconservation.34.30728
Cini A, Barbero F, Bonelli S, Bruschini C, Casacci LP, Piazzini S, Scalercio S, Dapporto L (2020) The decline of the charismatic Parnassius mnemosyne (L.) (Lepidoptera: Papilionidae) in a Central Italy national park: a call for urgent actions. J Insect Biodiv 16(2):47–54. https://doi.org/10.12976/jib/2020.16.2.2
Cutter J, Hovick T, McGranahan D, Harmon J, Limb R, Spiess J, Geaumont B (2022) Cattle grazing results in greater floral resources and pollinators than sheep grazing in low-diversity grasslands. Ecol Evol 12(1):e8396. https://doi.org/10.1002/ece3.8396
D’Alessandro C, Sala G, Zilli A (2008) Le farfalle diurne del Parco Nazionale d’Abruzzo. Bollettino Dell’associazione Romana Di Entomologia 63(1–4):91–154
Dapporto L, Ramazzotti M, Fattorini S, Talavera G, Vila R, Dennis RL (2013) Recluster: an unbiased clustering procedure for beta-diversity turnover. Ecography 36(10):1070–1075. https://doi.org/10.1111/j.1600-0587.2013.00444.x
De Groot M, Rebeušek F, Grobelnik V, Govedič M, Šalamun A, Verovnik R (2009) Distribution modelling as an approach to the conservation of a threatened alpine endemic butterfly (Lepidoptera: Satyridae). Eur J Entomol 106(1):77–84. https://doi.org/10.14411/eje.2009.012
Di Lena B, Antenucci F, Mariani L (2012) Space and time evolution of the Abruzzo precipitation. Ital J Agrometeorol 1:5–20
Dincă V, Lukhtanov VA, Talavera G, Vila R (2011) Unexpected layers of cryptic diversity in wood white Leptidea butterflies. Nat Commun 2(1):1–8. https://doi.org/10.1038/ncomms1329
Ehl S, Ehl S, Kramp K, Schmitt T (2020) Interglacials are driving speciation and intraspecific differentiation in the cold-adapted butterfly species group Boloria pales/napaea (Nymphalidae). J Biogeogr 48(1):134–146. https://doi.org/10.1111/jbi.13988
Ernakovich JG, Hopping KA, Berdanier AB, Simpson RT, Kachergis EJ, Steltzer H, Wallenstein MD (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20(10):3256–3269. https://doi.org/10.1111/gcb.12568
Forister ML, McCall AC, Sanders NJ, Fordyce JA, Thorne JH, O’Brien J, Waetjen DP, Shapiro AM (2010) Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc Natl Acad Sci 107(5):2088–2092. https://doi.org/10.1073/pnas.0909686107
Fourcade Y, Öckinger E (2017) Host plant density and patch isolation drive occupancy and abundance at a butterfly’s northern range margin. Ecol Evol 7(1):331–345. https://doi.org/10.1002/ece3.2597
Fourcade Y, Besnard AG, Secondi J (2017) Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob Ecol Biogeogr 27(2):245–256. https://doi.org/10.1111/geb.12684
Gehrig-Fasel J, Guisan A, Zimmermann NE (2007) Tree line shifts in the Swiss Alps: climate change or land abandonment? J Veg Sci 18(4):571–582. https://doi.org/10.1111/j.1654-1103.2007.tb02571.x
Gobiet A, Kotlarski S, Beniston M, Heinrich G, Rajczak J, Stoffel M (2014) 21st century climate change in the European alps - a review. Sci Total Environ 493:1138–1151. https://doi.org/10.1016/j.scitotenv.2013.07.050
Gotthard K (1998) Life history plasticity in the satyrine butterfly Lasiommata petropolitana: investigating an adaptive reaction norm. J Evol Biol 11:21–39
Grabherr G, Gottfried M, Pauli H (2010) Climate change impacts in alpine environments. Geogr Compass 4(8):1133–1153. https://doi.org/10.1111/j.1749-8198.2010.00356.x
Habel JC, Rödder D, Schmitt T, Nève G (2011) Global warming will affect the genetic diversity and uniqueness of Lycaena helle populations. Glob Change Biol 17(1):194–205. https://doi.org/10.1111/j.1365-2486.2010.02233.x
Habibullah MS, Din BH, Tan S-H, Zahid H (2022) Impact of climate change on biodiversity loss: global evidence. Environ Sci Pollut Res 29(1):1073–1086. https://doi.org/10.1007/s11356-021-15702-8
Halsch CA, Shapiro AM, Fordyce JA, Nice CC, Thorne JH, Waetjen DP, Forister ML (2021) Insects and recent climate change. Proc Natl Acad Sci 118(2):e2002543117. https://doi.org/10.1073/pnas.2002543117
Hellmann F, Parenzan P (2010) I Macrolepidotteri del Piemonte Museo Regionale di Scienze Naturali. Monografie XLVI, Torino
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405(6789):907–913. https://doi.org/10.1038/35016000
Hinojosa JC, Monasterio Y, Escobés R, Dincă V, Vila R (2018) Erebia epiphron and Erebia orientalis: sibling butterfly species with contrasting histories. Biol J Linn Soc 126(2):338–348. https://doi.org/10.1093/biolinnean/bly182
Hülber K, Kuttner M, Moser D, Rabitsch W, Schindler S, Wessely J, Gattringer A, Essl F, Dullinger S (2020) Habitat availability disproportionally amplifies climate change risks for lowland compared to alpine species. Glob Ecol Conserv 23:e01113. https://doi.org/10.1016/j.gecco.2020.e01113
Jerrentrup JS, Klimek S, Marchiori E, Bittante G, Ramanzin M, Sturaro E, Marini L (2016) Impact of dairy farming on butterfly diversity in Alpine summer pastures. Agric Ecosyst Environ 232:38–45. https://doi.org/10.1016/j.agee.2016.07.023
Johansson V, Knape J, Franzén M (2017) population dynamics and future persistence of the clouded apollo butterfly in southern Scandinavia: the importance of low intensity grazing and creation of habitat patches. Biol Conserv 206:120–131. https://doi.org/10.1016/j.biocon.2016.12.029
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017) Climatologies at high resolution for the earth’s land surface areas. Scientific Data 4(1):170122. https://doi.org/10.1038/sdata.2017.122
Kock N, Lynn G (2012) Lateral Collinearity and Misleading Results in Variance-Based SEM: An Illustration and Recommendations (SSRN Scholarly Paper ID 2152644). Social Science Research Network. https://papers.ssrn.com/abstract=2152644. Accessed 10 February 2022
Konvička M, Mihaly CV, Rákosy L, Beneš J, Schmitt T (2014) Survival of cold-adapted species in isolated mountains: the population genetics of the Sudeten ringlet, Erebia sudetica sudetica, in the Jeseník Mts., Czech Republic. J Insect Conserv 18(2):153–161. https://doi.org/10.1007/s10841-014-9621-0
Konvicka M, Kuras T, Liparova J, Slezak V, Horázná D, Klečka J, Kleckova I (2021) Low winter precipitation, but not warm autumns and springs, threatens mountain butterflies in middle-high mountains. PeerJ 9:e12021. https://doi.org/10.7717/peerj.12021
Kudrna O, Harpke A, Lux K, Pennerstorfer SO, Settele J (2011) Distribution Atlas of Butterflies in Europe. Gesellschaft für Schmetterlingsschutz, Halle
Lewis SL, Maslin MA (2015) Defining the Anthropocene. Nature 519(7542):171–180. https://doi.org/10.1038/nature14258
Macgregor CJ, Thomas CD, Roy DB, Beaumont MA, Bell JR, Brereton T et al (2019) Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat Commun 10(1):1–10. https://doi.org/10.1038/s41467-019-12479-w
Menchetti M, Talavera G, Cini A, Salvati V, Dincă V, Platania L, Bonelli S, Balletto E, Vila R, Dapporto L (2021) Two ways to be endemic. Alps and apennines are different functional refugia during climatic cycles. Mol Ecol 30(5):1297–1310. https://doi.org/10.1111/mec.15795
Merow C, Smith MJ, Silander JA Jr (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36(10):1058–1069. https://doi.org/10.1111/j.1600-0587.2013.07872.x
Metera E, Sakowski T, Słoniewski K, Romanowicz B (2010) Grazing as a tool to maintain biodiversity of grassland-a review. Animal Sci Papers Reports 28(4):315–334
Minter M, Dasmahapatra KK, Thomas CD, Morecroft MD, Tonhasca A, Schmitt T, Siozios S, Hill JK (2020) Past, current, and potential future distributions of unique genetic diversity in a cold-adapted mountain butterfly. Ecol Evol 10(20):11155–11168. https://doi.org/10.1002/ece3.6755
Moss R, Babiker M, Brinkman S, Calvo E, Carter T, Edmonds J, Elgizouli I et al (2008) Towards new scenarios for analysis of emissions, climate change, impacts, and response strategies Technical Summary. Intergovernmental Panel on Climate Change, Geneva
Múrias dos Santos A, Cabezas MP, Tavares AI, Xavier R, Branco M (2016) tcsBU: a tool to extend TCS network layout and visualization. Bioinformatics 32(4):627–628. https://doi.org/10.1093/bioinformatics/btv636
Mutanen M, Hausmann A, Hebert PDN, Landry J-F, Waard JR, Huemer P (2012) Allopatry as a gordian knot for taxonomists: patterns of DNA barcode divergence in arctic-alpine lepidoptera. PLoS ONE 7(10):e47214. https://doi.org/10.1371/journal.pone.0047214
Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37(2):191–203. https://doi.org/10.1111/j.1600-0587.2013.00205.x
Norberg A, Abrego N, Blanchet FG, Adler FR, Anderson BJ, Anttila J, Araújo MB, Dallas T, Dunson D, Elith J, Foster SD, Fox R, Franklin J, Godsoe W, Guisan A, O’Hara B, Hill NA, Holt RD, Hui FKC et al (2019) A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol Monogr 89(3):e01370. https://doi.org/10.1002/ecm.1370
Noriega JA, Hortal J, Azcárate FM, Berg MP, Bonada N, Briones MJI, Del Toro I, Goulson D, Ibanez S, Landis DA et al (2018) Research trends in ecosystem services provided by insects. Basic Appl Ecol 26:8–23. https://doi.org/10.1016/j.baae.2017.09.006
Nylin S, Gotthard K, Wiklund C (1996) Reaction norms for age and size at maturity in Lasiommata butterflies: predictions and tests. Evolution 50:1351–1358. https://doi.org/10.1111/j.1558-5646.1996.tb02377.x
Ohlemüller R, Anderson BJ, Araújo MB, Butchart SHM, Kudrna O, Ridgely RS, Thomas CD (2008) The coincidence of climatic and species rarity: high risk to small-range species from climate change. Biol Lett 4(5):568–572. https://doi.org/10.1098/rsbl.2008.0097
Otto-Bliesner BL, Brady EC, Zhao A, Brierley CM, Axford Y, Capron E et al (2021) Large-scale features of Last Interglacial climate: results from evaluating the lig127k simulations for the coupled model intercomparison project (CMIP6)–paleoclimate modeling intercomparison project (PMIP4). Climate of the past 17(1):63–94. https://doi.org/10.5194/cp-17-63-2021
Parmesan C (2006) Ecological and Evolutionary Responses to Recent Climate Change. Annu Rev Ecol Evol Syst 37(1):637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399(6736):579–583. https://doi.org/10.1038/21181
Peterson AT, Lieberman BS (2012) Species’geographic distributions through time: playing catch-up with changing climates. Evol Educ Outreach 5(4):569–581. https://doi.org/10.1007/s12052-012-0385-2
Piazzini S, Favilli L (2020) Rediscovery of a species considered to be extinct in the Pollino massif (Calabria, Italy): Erebia gorge (Hübner, [1804]) (Lepidoptera: Nymphalidae). Fragmenta Entomologica 52(1):43–46. https://doi.org/10.13133/2284-4880/404
Platania L, Vodă R, Dincă V, Talavera G, Vila R, Dapporto L (2020) Integrative analyses on Western Palearctic Lasiommata reveal a mosaic of nascent butterfly species. J Zool Syst Evol Res 58(4):809–822. https://doi.org/10.1111/jzs.12356
Prola C, Provera P, Racheli T, Sbordoni V (1978) I macrolepidotteri dell’Appennino centrale. Parte I. Diurna. Bombyces e Sphinges Fragmenta Entomologica 14:1–217
Quaranta M, Cornalba M, Biella P, Comba M, Battistoni A, Rondinini C, Teofili C (2018) Lista Rossa IUCN delle api italiane minacciate. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma.
Razgour O, Forester B, Taggart JB, Bekaert M, Juste J, Ibáñez C, Puechmaille SJ, Novella-Fernandez R, Alberdi A, Manel S (2019) Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc Natl Acad Sci 116(21):10418–10423. https://doi.org/10.1073/pnas.1820663116
Reinhardt R, Bolz R (2011) Rote Liste und Gesamtartenliste der Tagfalter (Rhopalocera) (Lepidoptera: Papilionoidea et Hesperioidea) Deutschlands. In: Binot-Hafke M, Balzer S, Becker N, Gruttke H, Haupt H, Hofbauer N, Ludwig G, Matzke-Hajek G, Strauch M (eds.) Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 3: Wirbellose Tiere (Teil 1). Landwirtschaftsverlag – Naturschutz und Biologische Vielfalt, Münster, pp 167–194
Renner SS, Zohner CM (2018) Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu Rev Ecol Evol Syst 49(1):165–182. https://doi.org/10.1146/annurev-ecolsys-110617-062535
Roberts DR, Bahn V, Ciuti S, Boyce MS, Elith J, Guillera-Arroita G, Hauenstein S, Lahoz-Monfort JJ, Schröder B, Thuiller W, Warton DI, Wintle BA, Hartig F, Dormann CF (2017) Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 40(8):913–929. https://doi.org/10.1111/ecog.02881
Rödder D, Schmitt T, Gros P, Ulrich W, Habel JC (2021) Climate change drives mountain butterflies towards the summits. Sci Rep 11(1):14382. https://doi.org/10.1038/s41598-021-93826-0
Santini L, Benítez-López A, Maiorano L, Čengić M, Huijbregts MA (2021) Assessing the reliability of species distribution projections in climate change research. Divers Distrib 27(6):1035–1050. https://doi.org/10.1111/ddi.13252
Scalercio S, Bonacci T, Mazzei A, Pizzolotto R, Brandmayr P (2014) Better up, worse down: bidirectional consequences of three decades of climate change on a relict population of Erebia cassioides. J Insect Conserv 18(4):643–650. https://doi.org/10.1007/s10841-014-9669-x
Schmitt T, Hewitt GM (2004) The genetic pattern of population threat and loss: a case study of butterflies. Mol Ecol 13(1):21–31. https://doi.org/10.1046/j.1365-294X.2004.02020.x
Schmitt T, Cizek O, Konvicka M (2005) Genetics of a butterfly relocation: large, small and introduced populations of the mountain endemic Erebia epiphron silesiana. Biol Conserv 123(1):11–18. https://doi.org/10.1016/j.biocon.2004.09.018
Schmitt T, Fritz U, Delfino M, Ulrich W, Habel JC (2021) Biogeography of Italy revisited: genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front Zool 18:1–13. https://doi.org/10.1186/s12983-021-00418-9
Schweiger O, Harpke A, Wiemers M, Settele J (2014) Climatic niche characteristics of the butterflies in Europe. ZooKeys 367:65. https://doi.org/10.3897/zookeys.367.6185
Shah AA, Dillon ME, Hotaling S, Woods HA (2020) High elevation insect communities face shifting ecological and evolutionary landscapes. Curr Opin Insect Sci 41:1–6. https://doi.org/10.1016/j.cois.2020.04.002
Sistri G, Menchetti M, Santini L, Pasquali L, Sapienti S, Cini A, Platania L, Balletto E, Barbero F, Bonelli S, Casacci LP, Dincă V, Vila R, Mantoni C, Fattorini S, Dapporto L (2022) The isolated Erebia pandrose Apennine population is genetically unique and endangered by climate change. Insect Conserv Divers 15(1):136–148. https://doi.org/10.1111/icad.12538
Spitzer L, Beneš J, Konvička M (2017) Extinction of Lasiommata petropolitana (Fabricius, 1787) (Lepidoptera: Nymphalidae) in the Czech Republic: a case of habitat loss at a range margin. Acta Musei Silesiae, Scientiae Naturales 66(3):271–279. https://doi.org/10.1515/cszma-2017-0030
Teobaldelli A, Floriani G (1983) Pararge petropolitana centralapennina n. spp. dei Monti della Laga (Appennini) (Lepidoptera Satyridae). Boll Soc Ent Ital 115(8–10):161–163
Thuiller W (2014) Editorial commentary on ‘BIOMOD - optimizing predictions of species distributions and projecting potential future shifts under global change.’ Glob Change Biol 20(12):3591–3592. https://doi.org/10.1111/gcb.12728
Thuiller W, Lafourcade B, Engler R, Araújo MB (2009) BIOMOD – a platform for ensemble forecasting of species distributions. Ecography 32(3):369–373. https://doi.org/10.1111/j.1600-0587.2008.05742.x
Todisco V, Gratton P, Cesaroni D, Sbordoni V (2010) Phylogeography of Parnassius apollo: hints on taxonomy and conservation of a vulnerable glacial butterfly invader. Biol J Linn Soc 101(1):169–183. https://doi.org/10.1111/j.1095-8312.2010.01476.x
Valavi R, Elith J, Lahoz-Monfort JJ, Guillera-Arroita G (2019) blockCV: an R package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Biorxiv. https://doi.org/10.1101/357798
Valt M, Cianfarra P (2010) Recent snow cover variability in the Italian Alps. Cold Reg Sci Technol 64(2):146–157. https://doi.org/10.1016/j.coldregions.2010.08.008
Van Swaay C, Cuttelod A, Collins S, Maes D, López Munguira M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhof I (2010) European Red List of Butterfies. Publications Office of the European Union, Luxembourg
Van Swaay C, Collins S, Dušej G, Maes D, Munguira ML, Rakosy L, Ryrholm N, Šašić M, Settele J, Thomas J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2012) Dos and Don’ts for butterflies of the habitats directive of the european union. Nat Conserv 1:73–153. https://doi.org/10.3897/natureconservation.1.2786
Viterbi R, Cerrato C, Bionda R, Provenzale A (2020) Effects of temperature rise on multi-taxa distributions in mountain ecosystems. Diversity 12(6):210. https://doi.org/10.3390/d12060210
Vodă R, Dapporto L, Dincă V, Vila R (2015) Why do cryptic species tend not to co-occur? A case study on two cryptic pairs of butterflies. PLoS ONE 10(2):e0117802. https://doi.org/10.1371/journal.pone.0117802
Vrba P, Konvička M, Nedvěd O (2012) Reverse altitudinal cline in cold hardiness among Erebia butterflies. CryoLetters 33(4):251–258
Vrba P, Nedvěd O, Zahradníčková H, Konvička M (2017) More complex than expected: cold hardiness and the concentration of cryoprotectants in overwintering larvae of five Erebia butterflies (Lepidoptera: Nymphalidae). European J Entomol 114:470–480. https://doi.org/10.14411/eje.2017.060
Wallis GP, Arntzen JW (1989) Mitochondrial-Dna variation in the crested newt superspecies: limited cytoplasmic gene flow among species. Evolution 43(1):88–104. https://doi.org/10.1111/j.1558-5646.1989.tb04209.x
Wieser G, Holtmeier FK, Smith WK (2014) Treelines in a changing global environment. In: Taus G (ed) Trees in a changing environment. Springer, Dordrecht, pp 221–263
Wilson RJ, Bennie J, Lawson CR, Pearson D, Ortúzar-Ugarte G, Gutiérrez D (2015) Population turnover, habitat use and microclimate at the contracting range margin of a butterfly. J Insect Conserv 19(2):205–216. https://doi.org/10.1007/s10841-014-9710-0
Zografou K, Kati V, Grill A, Wilson RJ, Tzirkalli E, Pamperis LN, Halley JM (2014) Signals of climate change in butterfly communities in a mediterranean protected area. PLoS ONE 9(1):e87245. https://doi.org/10.1371/journal.pone.0087245
Zurell D, Franklin J, König C, Bouchet PJ, Dormann CF, Elith J, Fandos G, Feng X, Guillera-Arroita G, Guisan A, Lahoz-Monfort JJ, Leitão PJ, Park DS, Peterson AT, Rapacciuolo G, Schmatz DR, Schröder B, Serra-Diaz JM, Thuiller W et al (2020) A standard protocol for reporting species distribution models. Ecography 43(9):1261–1277. https://doi.org/10.1111/ecog.04960
Acknowledgements
This study has been carried out in collaboration with the Gran Sasso e Monti della Laga National Park within the project “Ricerca e conservazione sui lepidotteri diurni di sei Parchi Nazionali dell’Appennino Centro-Settentrionale”. MB and LD thank Giorgio Davini (Gran Sasso and Monti della Laga National Park) for his contribution in the project. VD also acknowledges the Visiting Professor fellowship awarded by the Research Institute of the University of Bucharest.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. This study was funded by the Ministero Italiano della Transizione Ecologica within the project “Ricerca e conservazione sui lepidotteri diurni di sei Parchi Nazionali dell’Appennino Centro-Settentrionale”. Support was also provided by the Academy of Finland (Academy Research Fellow, decision no. 328895) to VD. RV is supported by Grant PID2019-107078 GB-I00 funded by Ministerio de Ciencia e Innovación and Agencia Estatal de Investigación (MCIN/AEI/https://doi.org/10.7554/eLife.76576).
Author information
Authors and Affiliations
Contributions
Conceptualization: MB, LD, LP. Methodology: LD, MM, LS. Data collection: MB, LD, LP, RV, EB. Data analysis: MB, LD, LP. Original draft preparation: MB, LD. Review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
N/A
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonifacino, M., Pasquali, L., Sistri, G. et al. Climate change may cause the extinction of the butterfly Lasiommata petropolitana in the Apennines. J Insect Conserv 26, 959–972 (2022). https://doi.org/10.1007/s10841-022-00441-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00441-z