Abstract
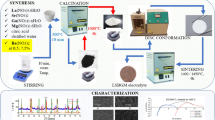
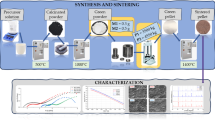
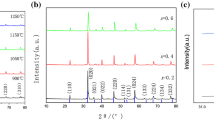
In this work, we prepared La1 − xSrxGa1−yMgyO3 (LSGM) by the fast combustion method and assessed the electrical properties with respect to the composition and sintering temperature (1200, 1300, and 1400 °C by 6 h) as an electrolyte material for the reversible solid oxide cells (ReSOCs). For the preparation of samples, two different fuels, such as tartaric acid (TA) and citric acid (CA), with corresponding nitrate salts as precursors, were adopted for the fast combustion method (at 500 °C for 10 min). From the X-ray diffractograms, two main phases corresponding to LSGM orthorhombic (space group Imma) and LSGM-cubic (space group Pm-3 m) were identified. From the literature, both structures are reported as high oxygen ion conductive species, but normally they are not reported to appear together. Additionally, in some cases, an isolating (secondary) phase of LaSrGaO4 in a low concentration < 1.98% was observed. The scanning electron microscopy (SEM) studies on samples sintered at 1200 and 1300 °C revealed the smaller grain size and irregular morphology. The SEM micrographs depicted a well-defined superficial morphology with less porosity for the samples sintered at 1400 °C. For comparative analysis, the conductivity (S.cm− 1) was measured at varying temperatures (300–800 °C) for the samples sintered at 1300 and 1400 °C. Because of the large number of insulating phases produced by the incomplete sintering process, the samples sintered at 1300 °C had lower conductivities. A higher conductivity of 0.125 S.cm− 1 was observed for La0.80Sr0.20Ga0.80Mg0.20O3 (LSGM), which was obtained using the citric acid (sintered at 1400 °C), which is in the range of earlier reported similar studies. The observed variation in the conductivity with respect to different phases of LSGM, the influence of the secondary phase, and the wt% of the constituents of LSGM are discussed.






Similar content being viewed by others
Data Availability statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
NOAA, Earth System Research Laboratory, (2021). https://www.Esrl.Noaa.Gov/Gmd/Ccgg/Trends/Weekly.Html
A. Dicks, D. Rand, Fuel Cell Systems Explained, 2018
L. Blum, R. Deja, R. Peters, D. Stolten, Comparison of efficiencies of low, mean and high temperature fuel cell Systems. Int. J. Hydrogen Energy 36, 11056–11067 (2011). https://doi.org/10.1016/j.ijhydene.2011.05.122
T. Ishihara, H. Matsuda, Y. Takita, Doped LaGaO3 Perovskite Type Oxide (as a New Oxide Ionic Conductor, 1994)
P.S. Cho, S.Y. Park, Y.H. Cho, S.J. Kim, Y.C. Kang, T. Mori, J.H. Lee, Preparation of LSGM powders for low temperature sintering. Solid State Ionics. 180, 788–791 (2009). https://doi.org/10.1016/j.ssi.2008.12.040
T. Ishihara, Perovskite oxide for solid oxide fuel cells (Springer, 2009)
T. Ishihara, Development of new fast oxide ion conductor and application for intermediate temperature Solid Oxide Fuel Cells. Bull. Chem. Soc. Jpn 79, 1155–1166 (2006). https://doi.org/10.1246/bcsj.79.1155
R. Singh, R.K. Singh, Electrical properties of Ba doped LSGM for electrolyte material of solid oxide fuel cells, in: AIP Conf. Proc., 2013: pp. 976–977. https://doi.org/10.1063/1.4791368
K. Huang, J.B. Goodenough, Solid Oxide Fuel Cell Technology: Principles, Performance and Operations (CRC Press, 2009), https://doi.org/10.1533/9781845696511
Ç Öncel, M. Gulgun, Chemical Synthesis of Mixed Oxide Powders for Solid Oxide Fuel Cell (SOFC) Electrolyte and Electrodes, Assess. Hydrog. Energy Sustain. Dev. (2007) 147–159
D. Kioupis, A. Gaki, G. Kakali, Wet chemical synthesis of La1-xSrxGa 0.8Mg0.2O3-σ (x = 0.1, 0.2, 0.3) powders, Mater. Sci. Forum Trans. Tech. Publications Ltd., 2010: 908–913. https://doi.org/10.4028/www.scientific.net/MSF.636-637.908
P. Huang, A. Petric, Superior Oxygen Ion Conductivity of Lanthanum Gallate Doped with Strontium and Magnesium, Electrochem. Soc. 143 (1996)
M. Pechini, Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor, 3330697A, 1967
M. Kakihana, Invited Review “Sol-Gel” Preparation of High Temperature Superconducting Oxides*. J. Sol-Gel Sci. Technol. 6, 7–55 (1996)
E. Djurado, M. Labeaub, Second Phases in Doped Lanthanum Gallate Perovskites. J. Eur. Ceram. Soc. 18, 1397–1404 (1998)
P. Majewski, M. Rozumek, C.A. Tas, F. Aldinger, Processing of (La,Sr)(Ga,Mg)O 3 Solid Electrolyte. J Electroceram. 8, 65–73 (2002)
M.M. Guenter, M. Lerch, H. Boysen, D. Toebbens, E. Suard, C. Baehtz, Combined neutron and synchrotron X-ray diffraction study of Sr/Mg-doped lanthanum gallates up to high temperatures. J. Phys. Chem. Solids 67, 1754–1768 (2006). https://doi.org/10.1016/j.jpcs.2006.04.001
Y. Wang, X. Liu, G.-D. Yao, R.C. Liebermann, M. Dudley, High temperature transmission electron microscopy and X-ray diffraction studies of twinning and the phase transition at 145°C in LaGaO3. Mater. Sci. Eng. A 132, 13–21 (1991)
K. Huang, R. Tichy, J. Goodenought, Superior Perovskite Oxide-Ion Conductor; Strontium-and Magnesium-Doped LaGaO 3: I, Phase Relationships and Electrical Properties. J. Ame Ceram. Soc. 81, 2565–2575 (1998)
R.C. Biswal, K. Biswas, Novel way of phase stability of LSGM and its conductivity enhancement. Int. J. Hydrogen Energy 40, 509–518 (2015). https://doi.org/10.1016/j.ijhydene.2014.10.099
R.K. Singh, P. Singh, Synthesis of La0.9Sr0.1Ga0.8Mg 0.2O3-δ electrolyte via ethylene glycol route and its characterizations for IT-SOFC. Ceram. Int. 40, 7177–7184 (2014). https://doi.org/10.1016/j.ceramint.2013.12.056
D. Marrero-López, J.C. Ruiz-Morales, J. Peña-Martínez, M.C. Martín-Sedeño, J.R. Ramos-Barrado, Influence of phase segregation on the bulk and grain boundary conductivity of LSGM electrolytes. Solid State Ionics. 186, 44–52 (2011). https://doi.org/10.1016/J.SSI.2011.01.015
S. Yu, H. Bi, J. Sun, L. Zhu, H. Yu, C. Lu, X. Liu, Effect of grain size on the electrical properties of strontium and magnesium doped lanthanum gallate electrolytes. J. Alloys Compd. 777, 244–251 (2019). https://doi.org/10.1016/J.JALLCOM.2018.10.257
E. Gomes, M.R. Soares, F.M. Figueiredo, F.M.B. Marques, Conductivity of La0.95Sr0.05Ga0.90Mg0.10O3 – δ obtained by mechanical activation. J. Eur. Ceram. Soc. 25, 2599–2602 (2005). https://doi.org/10.1016/J.JEURCERAMSOC.2005.03.109
Y.-M. Chen, T.-N. Lin, M.-W. Liao, H.-Y. Kuo, C.-Y. Yeh, W.-X. Kao, S.-F. Yang, K.-T. Wu, T. Ishihara, Applications of the Glycine Nitrate Combustion Method for Powder Synthesis on the LSGM-based Electrolyte-Supported Solid Oxide Fuel Cells. ECS Trans. 78, 773–781 (2017). https://doi.org/10.1149/07801.0773ecst
M. Morales, J.J. Roa, J. Tartaj, M. Segarra, A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: From materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 36, 1–16 (2016). https://doi.org/10.1016/j.jeurceramsoc.2015.09.025
S.L. Reis, E.N.S. Muccillo, Influence of small amounts of gallium oxide addition on ionic conductivity of La0.9Sr0.1Ga0.8Mg0.2O3-δ solid electrolyte. Ceram. Int. 44, 115–119 (2018). https://doi.org/10.1016/j.ceramint.2017.09.139
L. Cong, T. He, Y. Ji, P. Guan, Y. Huang, W. Su, S ynthesis and characterization of IT-electrolyte with perovskite structure La Sr Ga Mg O by glycine-nitrate combustion method, 2003
K. Huang, M. Feng, J. Goodenough, Sol-Gel Synthesis of a New Oxide-Ion Conductor Sr- and Mg-Doped LaGaO3 Perovakite. J. Am. Ceram. Soc. 79, 1100–1104 (1996)
L. Vasylechko, A. Senyshyn, Y. Pivak, M. Berkowske, V. Vashook, H. Ullmann, C. Bähtz, U. Bismayer, LSGM Single Crystals: Crystal Structure, Thermal Expansion, Phase Transitions and Conductivity, Mix. Ion. Electron. Conduct. Perovskites Adv. Energy Syst. (2004) 231–237
P. Datta, P. Majewski, F. Aldinger, Thermal expansion behaviour of Sr- and Mg-doped LaGaO3 solid electrolyte. J. Eur. Ceram. Soc. 29, 1463–1468 (2009). https://doi.org/10.1016/j.jeurceramsoc.2008.08.029
M.S. Khan, M.S. Islam, D.R. Bates, Dopant Substitution and Ion Migration in the LaGaO 3-Based Oxygen Ion Conductor, 1997
Acknowledgements
The authors acknowledge the financial support of FONDECYT (ANID) Projects No.:1181703, 11220335 and DOCTORADO NACIONAL: 21202168, Government of Chile. The authors thank Mónica Uribe from Instituto de Geología Aplicada, UDEC; the Centro de Microscopía Avanzada, CMA BIO-BIO, Proyecto PIA-ANID ECM-12 for their contribution to this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepúlveda, E., Mangalaraja, R.V., Udayabhaskar, R. et al. Preparation of LSGM electrolyte via fast combustion method and analysis of electrical properties for ReSOC.. J Electroceram 49, 85–93 (2022). https://doi.org/10.1007/s10832-022-00294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-022-00294-7