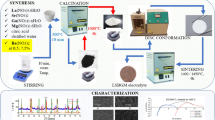
Abstract
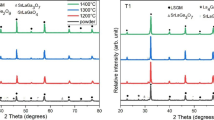
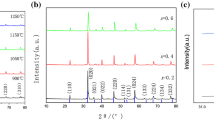
In this work, La0.85Sr0.15Ga0.85Mg0.15O3−δ (LSGM) was prepared as an electrolyte for solid oxide cell (SOC) applications. A fast combustion method was used, starting with nitrate salts and citric acid as fuel. Different parameters, such as mass and pressing load, in the pre-sintering step were used to obtain a highly ionic conductive material at intermediate temperatures. The aim is to find optimal processing conditions for energy savings. SEM analysis showed similar grain sizes and distributions for all samples. The XRD spectra showed two main phases corresponding to LSGM orthorhombic (space group Imma) and LSGM cubic (space group Pm-3m). LaSrGaO4 appeared in lighter samples. The EIS revealed that heavier samples present high conductivity, showing a clear relationship between conductivity, sample mass (during the pre-sintering step), and the LSGM phase amount. The effect of pressure was less evident. The highest conductivity was 0.013 and 0.063 S cm−1 at 600 and 800 °C, respectively.
Graphical abstract



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
T. Ishihara, T. Kanno, Steam electrolysis using LaGaO3 based perovskite electrolyte. ISIJ Int. 50(9), 1291–1295 (2010)
H. Maria van der, Technology roadmap hydrogen and fuel cells. (2015). [Online]. Available: https://www.iea.org/publications/freepublications/publication/TechnologyRoadmapHydrogenandFuelCells.pdf
J. Töpler, J. Lehmann, Hydrogen and fuel cell (Springer, Berlin, 2016)
Y. Ling et al., Review of experimental and modelling developments for ceria-based solid oxide fuel cells free from internal short circuits. J. Mater. Sci. 55(1), 1–23 (2020). https://doi.org/10.1007/s10853-019-03876-z
A. Dicks, D. Rand, Fuel cell systems explained (John Wiley & Sons, Hoboken, 2018)
T. Ishihara, H. Matsuda, Y. Takita, Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. (1994) [Online]. Available: https://pubs.acs.org/sharingguidelines.
T. Ishihara, Perovskite oxide for solid oxide fuel cells (Springer, New York, 2009)
T. Ishihara, Development of new fast oxide ion conductor and application for intermediate temperature solid oxide fuel cells. Bull. Chem. Soc. Jpn. 79(8), 1155–1166 (2006). https://doi.org/10.1246/bcsj.79.1155
K. Huang, J.B. Goodenough, Solid oxide fuel cell technology: principles, performance and operations (CRC Press, Cambridge, 2009)
P. Huang, A. Petric, Superior oxygen ion conductivity of lanthanum gallate doped with strontium and magnesium. Electrochem. Soc. 143(5), 1644 (1996)
M. Shi et al., Synthesis and characterization of Sr- and Mg-doped Lanthanum gallate electrolyte materials prepared via the Pechini method. Mater. Chem. Phys. 114(1), 43–46 (2009). https://doi.org/10.1016/j.matchemphys.2008.06.051
Y.-M. Chen et al., Applications of the glycine nitrate combustion method for powder synthesis on the LSGM-based electrolyte-supported solid oxide fuel cells. ECS Meet. Abstr. (2017). https://doi.org/10.1149/ma2017-03/1/58
P. Majewski, M. Rozumek, C.A. Tas, F. Aldinger, Processing of (La, Sr) (Ga, Mg)O3 solid electrolyte. J. Electroceram. 8, 65–73 (2002)
E. Djurado, M. Labeaub, Second phases in doped lanthanum gallate perovskites. J. Eur. Ceram. Soc. 18, 1397–1404 (1998)
C. Oncel, B. Ozkaya, M.A. Gulgun, X-ray single phase LSGM at 1350 °C. J. Eur. Ceram. Soc. 27(2–3), 599–604 (2007). https://doi.org/10.1016/j.jeurceramsoc.2006.04.115
M. Shi et al., Synthesis and characterization of La0.85Sr0.15Ga0.80Mg0.20O2.825 by glycine combustion method and EDTA combustion method. Powder Technol. 204(2–3), 188–193 (2010). https://doi.org/10.1016/j.powtec.2010.07.020
K. Huang, R. Tichy, J. Goodenought, Superior perovskite oxide-ion conductor; strontium-and magnesium-doped LaGaO3: I, phase relationships and electrical properties. J. Ame. Ceram. Soc. 81(10), 2565–2575 (1998)
V. Esteve, El método de rietveld, 2a edn. (Universitat Jaume, Castelló de la Plana, 2014)
E. Sepúlveda, R.V. Mangalaraja, L. Troncoso, J. Jiménez, C. Salvo, F. Sanhueza, Effect of barium on LSGM electrolyte prepared by fast combustion method for solid oxide fuel cells (SOFC). MRS Adv. 7(35), 1167–1174 (2022). https://doi.org/10.1557/s43580-022-00373-5
E. Sepúlveda et al., Preparation of LSGM electrolyte via fast combustion method and analysis of electrical properties for ReSOC. J. Electroceram. 49(2), 85–93 (2022). https://doi.org/10.1007/s10832-022-00294-7
M.M. Guenter, M. Lerch, H. Boysen, D. Toebbens, E. Suard, C. Baehtz, Combined neutron and synchrotron X-ray diffraction study of Sr/Mg-doped lanthanum gallates up to high temperatures. J. Phys. Chem. Solids 67(8), 1754–1768 (2006). https://doi.org/10.1016/j.jpcs.2006.04.001
Y. Wang, X. Liu, G.-D. Yao, R.C. Liebermann, M. Dudley, High temperature transmission electron microscopy and X-ray diffraction studies of twinning and the phase transition at 145 °C in LaGaO3. Mater. Sci. Eng. A 132, 13–21 (1991)
T.W. Li, S.Q. Yang, S. Li, Preparation and characterisation of perovskite La0.8Sr0.2Ga0.83Mg0.17O2.815 electrolyte using a poly(vinyl alcohol) polymeric method. J. Adv. Ceram. 5(2), 167–175 (2016). https://doi.org/10.1007/s40145-016-0186-0
D. Kioupis, A. Gaki, G. Kakali, Wet chemical synthesis of La1−xSrxGa0.8Mg0.2O3−σ (x = 0.1, 0.2, 0.3) powders. Mater. Sci. Forum 636–637, 908–913 (2010). https://doi.org/10.4028/www.scientific.net/MSF.636-637.908
R.C. Biswal, K. Biswas, Novel way of phase stability of LSGM and its conductivity enhancement. Int. J. Hydrogen Energy 40(1), 509–518 (2015). https://doi.org/10.1016/j.ijhydene.2014.10.099
X.P. Lin, H.T. Zhong, X. Cheng, B. Ge, D.S. Ai, Preparation and property of lsgm-carbonate composite electrolyte for low temperature solid oxide fuel cell. Solid State Phenomena 281, 754–760 (2018). https://doi.org/10.4028/www.scientific.net/SSP.281.754
K. Huang, R.S. Tichy, J.B. Goodenough, Superior perovskite oxide-ion conductor; Strontium- and magnesium-doped LaGaO3: II, ac impedance spectroscopy. J. Am. Ceram. Soc. 81(10), 2576–2580 (1998). https://doi.org/10.1111/j.1151-2916.1998.tb02663.x
S. Li, B. Bergman, Doping effect on secondary phases, microstructure and electrical conductivities of LaGaO3 based perovskites. J. Eur. Ceram. Soc. 29(6), 1139–1146 (2009). https://doi.org/10.1016/j.jeurceramsoc.2008.08.017
S. Yu et al., Effect of grain size on the electrical properties of strontium and magnesium doped lanthanum gallate electrolytes. J. Alloys Compd. 777, 244–251 (2019). https://doi.org/10.1016/j.jallcom.2018.10.257
G.M. Rupp, M. Glowacki, J. Fleig, Electronic and ionic conductivity of La0.95Sr0.05Ga0.95Mg0.05O3−δ (LSGM) single crystals. J. Electrochem. Soc. 163(10), F1189–F1197 (2016). https://doi.org/10.1149/2.0591610jes
Acknowledgments
The authors acknowledge the financial support of FONDEF VIU (ANID) Project No.:22P0087. Government of Chile. The authors thank Mónica Uribe from Instituto de Geología Aplicada. UDEC; the Centro de Microscopía Avanzada.
Funding
The authors thank the Project: FONDEF VIU 22P0087 from the Agencia Nacional de Investigación y Desarrollo (ANID), Chile.
Author information
Authors and Affiliations
Contributions
ES contributed toward Full redaction, EIS analysis, and compilation. FS contributed toward characterization XRD, SEM. RC contributed toward morphology, size distribution, and graphs. JJ contributed toward XRD and Rietveld refinement. MRV contributed toward translation and final revision.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepúlveda, E., Sanhueza, F., Cobo, R. et al. Relationship among the powder mass, press charge, and final properties of an LSGM electrolyte for solid oxide cells. MRS Advances (2024). https://doi.org/10.1557/s43580-024-00771-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43580-024-00771-x