Abstract
As a result of several anthropogenic factors, Cystoseira sensu lato forests have declined or become regionally extinct in many coastal regions of the Mediterranean. Given the low natural recovery of lost populations, research efforts have been encouraged to develop sustainable and efficient restoration of macroalgal forests on a large scale. By promoting growth and fertility of collected thallus branches under controlled laboratory conditions, the availability of seedlings for restoration could be ensured without jeopardizing natural populations. Here we investigated the effect of a commercial algal biostimulant (AlgatronCifo®) on the photophysiology, growth and fertility of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Phaeophyceae). In a factorial laboratory experiment, two different temperatures (10 ºC and 14 °C) and two culture media [i.e. seawater (SW) and Algatron (AT)] were tested. The photosynthetic performance of G. barbata doubled after three weeks of culture with AT, while it decreased by 25% when cultivated in SW. The highest photosynthetic performance and growth were achieved at 14ºC with AT, where fertile receptacles also developed, followed by seedling settlements. The thalli cultured in AT had similar or better photosynthetic performance than the initial control thalli. AT-cultured thalli had a greater ability to quench energy via photochemical pathways (qP) than those from the SW, which on the contrary, had higher levels of non-photochemical responses (qN, NPQmax). This limited photosynthetic performance was probably linked to the higher P-limitation experienced under that treatment. The algal biostimulant enhanced the physiological performance and induced fertility of G. barbata, demonstrating its valorization potential and setting a new path for improved restoration applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystoseira sensu lato (Fucales, Phaeophyceae) species are the main foundation species of Mediterranean algal forests (Fabbrizzi et al. 2020), listed in several international agreements for the conservation of marine species and habitats (e.g., Bern Convention, Barcelona Convention, Directive 92/43/EEC, European Red List of Habitats). However, as a result of multiple anthropogenic stressors (e.g., coastal urbanization, eutrophication, sediment input and overgrazing), these species and their habitats have been steadily declining in many coastal regions and are now critically endangered (Mangialajo et al. 2008; Falace et al. 2010; Vergés et al. 2014; Thibaut et al. 2015; Blanfuné et al. 2016; Valdazo et al. 2017; Mancuso et al. 2018). This trend of degradation is fueling intensive research efforts aimed at implementing effective measures to replenish declining populations of Cystoseira s.l. or restore lost forests (Falace et al. 2018; Verdura et al. 2018; De La Fuente et al. 2019; Orlando-Bonaca et al. 2021a, 2022; Savonitto et al. 2021).
Recruitment-enhancement, both ex situ and in situ, are both more sustainable methods than transplanting adult thalli (Falace et al. 2006) in order to restore macroalgal forests as they rely on harvesting only reproductive, fertile thallus branches which avoids the complete removal of adult plants from wild donor populations (Falace et al. 2018; Verdura et al. 2018). While the in-situ technique simulates the recruitment process by fixing fertile branches directly at the restoration site (Verdura et al. 2018), the ex-situ technique consists of cultivating seedlings in mesocosms to maximize recruitment and survival by setting the optimal culture conditions of temperature, light and nutrients (Falace et al. 2018; De La Fuente et al. 2019; Savonitto et al. 2021; Orlando-Bonaca et al. 2022).
However, scaling up restoration activities requires multiple and reiterative harvesting of fertile branches to obtain an adequate number of recruits. This could jeopardize the survival of donor populations and reduce their reproductive potential. On the other hand, the phenology of natural populations, their reproductive potential and consequently their restoration success are seriously threatened by increasingly frequent marine heat waves (Bevilacqua et al. 2019; Savonitto et al. 2019). Finally, the reproductive season in most species is limited to a few months and is increasingly unpredictable and altered due to climate change, as observed in the northern Adriatic after a heatwave (Bevilacqua et al. 2019; Savonitto et al. 2019). A possible solution to avoid burdening donor stocks and to decouple the availability of recruits from the natural reproductive cycle is to cultivate Cystoseira s.l. by inducing sexual maturity to ensure a seedling reservoir. However, seaweed farming is not an easy task as many problems may arise, such as growth loss, reduced plant quality, diseases, and competition from endo- or epiphytes, resulting in lower productivity and a shortage of seedlings (Hurtado and Critchley 2018; Jiksing et al. 2022).
Attempting to overcome these issues, seaweed-derived biostimulants have been used to increase the survival, growth and stress tolerance of a number of selected macroalgae, including brown (Hurtado and Critchley 2018, 2020; Umanzor et al. 2019, 2020a, b, 2022; Ali et al. 2021; Jiksing et al. 2022; Han et al. 2023). Macroalgal biostimulants are emerging as sustainable biological growth promoters and are increasingly used to improve agronomic production, plant growth and health (Crouch and Van Staden 1993; Battacharyya et al. 2015; Trivedi et al. 2018; Hurtado and Critchley 2020; Samuels et al. 2022). Their modes of action are not yet fully understood, but several studies have shown that this type of products has several beneficial effects on plants due to their broad spectrum of constituents (i.e. macro- and microelements, amino acids, hormones, phenolic compounds and saccharides) (Khan et al. 2009; Stirk et al. 2020; Ali et al. 2021; Sujeeth et al. 2022). The biostimulatory algal constituents promote natural processes for efficient nutrient uptake and utilization, chlorophyll content and photosynthesis, stress resistance, root development and also trigger early flowering and seed germination (Blunden and Wildgoose 1977; Crouch and Van Staden 1993; Arthur et al. 2003; Kumari et al. 2011; Spann and Little 2011; du Jardin 2015; Martynenko et al. 2016; Santaniello et al. 2017; Van Oosten et al. 2017; Zhang et al. 2019; Ali et al. 2021; Shukla et al. 2022; Sujeeth et al. 2022).
While the use of biostimulants from algae is currently gaining traction in marine agronomy (Hurtado and Critchley 2020; Jiksing et al. 2022), their application in the context of ecological restoration has not yet been explored. Therefore, the present study investigated the effects of a commercial algal biostimulant AlgatronCifo® derived from Macrocystis pyrifera (Linnaeus) C. Agardh (Laminariales, Phaeophyceae) on the photophysiology, growth and fertility of Gongolaria barbata (Stackhouse) Kuntze (= Cystoseira barbata) (Fucales, Phaeophyceae) to advance ex-situ restoration of macroalgal forests. We hypothesize that the algal biostimulant could lead to higher photosynthetic performance as compared to thalli grown in seawater, along with enhancement of photochemical pathways of photosynthesis in G. barbata and, thus, to promote its growth and fertility.
Materials and methods
Sampling site
The Gulf of Trieste is a shallow (max depth 25 m), semi-enclosed continental shelf area in the northeastern part of the Adriatic Sea with highly variable oceanographic features (Cozzi et al. 2020). The area is characterized by a marked seasonal cycle of seawater temperature (from winter minima of 8.0 °C to summer maxima of 28.4 °C) and strong salinity gradients (from 24.0 to 38.3‰) (Malačič et al. 2006; Kralj et al. 2019). The balance of nutrients and organic matter in the Gulf of Trieste are influenced by river discharges, especially by the Isonzo River, as well as by wastewater discharges and benthic fluxes (Cozzi et al. 2020). The trophic status of the Gulf also depends on the prevailing circulation patterns (Cibic et al. 2022).
The sampling site was located close to the Marano and Grado Lagoon, which is characterized by high seasonal and spatial variability of nitrogen, especially in the form of N-NO3−, and P limitation, as shown by the high DIN:SRP ratio (Acquavita et al. 2015). In situ nutrient concentrations from the area of study are summarized in Supplementary Information (Table S1).
Gongolaria barbata has declined in much of the Gulf of Trieste over the last three decades (Falace and Bressan 2003; Falace et al. 2005, 2010) and is now found only at a few sites with scattered populations (Orlando-Bonaca et al. 2021b; Savonitto et al. 2021).
In early November 2021, about 50 primary branches of G. barbata about 20 cm long (Fig. 1a) were collected on the beach near Grado (45°40′55.5 "N 13°26′04.2 "E) (Fig. 1b). Thalli collected were sterile and without receptacles as G. barbata normally reproduces from March to May in the northern Adriatic (Falace et al. 2006; Savonitto et al. 2021; Orlando-Bonaca et al. 2022). Fronds were transported in buckets of seawater in the dark and cold (4 °C) to the Phycological Laboratory of the University of Trieste within 1 h of collection.
Experimental set-up
In the laboratory only specimens without visible damage to the thallus or signs of degradation and depigmentation were selected for the experiment, then carefully wiped with paper towel and rinsed with filtered seawater (0.22 µm Durapore membrane filters, Merck Millipore Ltd) to remove visible epiphytes. For acclimation, thalli were maintained in filtered seawater (SW) for 48 h at 14 °C in tanks with a light intensity of 125 μmol photons m−2 s−1 (LED lamps, AM366 Sicce USA Inc., USA) and a photoperiod of 15:9 light:dark (Orlando-Bonaca et al. 2022). Light irradiance was measured using a LI-COR LI -190/R photometer (LICOR-Biosciences, USA).
After acclimatization (T0), a factorial laboratory experiment was performed in two environmentally controlled rooms to test two different temperatures and two culture media. Temperatures were set at 10 ºC (i.e., temperature measured in the field) and 14 °C (i.e. the mean temperature at which brown alga normally reproduces in the study area; Orlando-Bonaca et al. 2022) (Fig. 2). The culture media tested were filtered seawater (SW) and a solution of filtered SW enriched with the commercial biostimulant derived from Macrocystis pyrifera AlgatronCifo® (AT) (Cifo S.p.A., San Giorgio di Piano, Bologna, Italy. https://www.cifo.it/en/product/home-and-garden/hg-products/nutrition-and-beauty/specialities/pure-energy-for-all-plants/) at the concentration recommended by the manufacturer for foliar and fruiting treatments on land plants (i.e., 4.5 mL L−1). The nutrient concentrations of the culture media used in this study (ie., SW and AT) are given in Table 1, and the concentrations of nutrients and major components of pure Algatron are given in Supplementary Information (Table S1). Furthermore, M. pyrifera extracts contain alginate, phytohormones and a variety of mineral nutrients, such as magnesium, molybdenum, calcium, phosphorus, iron, zinc and boron (Iparraguirre et al. 2023).
For each treatment, nine replicate flasks were filled with 2 L of culture medium, and each flask contained a thallus frond that was cultured for three weeks (T3) (Fig. 2). The medium in each flask was renewed twice a week to prevent possible nutrient limitation. Aerators provided oxygenation of the medium.
Growth and fertility induction
At T0 and T3, five fronds from each treatment were carefully blotted-dry to remove excess water, and wet biomass (WB) was measured. Growth was calculated as relative growth rate (RGR) according to the following equation (Lüning 1990):
where WBT0 and WBT3 are the initial and final wet biomass and T is the experimental duration (21 days).
Throughout the experiment branch tips were observed for possible reproductive structures. When present, the receptacles were examined with a stereo microscope (Leica, MZ 6) and photographed with a Nikon Coolpix 4500 camera. At T3, fertile receptacles were detached from branches cultured at 14 °C in AT (i.e., the only condition under which they developed) to check whether they fertilized and produced viable zygotes. Receptacles were stored according to the protocol for ex-situ cultivation of G. barbata (Savonitto et al. 2021; Orlando-Bonaca et al. 2022) for 24 h at 4 °C in order to induce gamete release, and then randomly distributed to three Petri dishes, each containing nine fertile receptacles, and filled with AT. The receptacles were removed from the Petri dishes after 24 h. After fertilization (AF), the zygotes were cultured for 4 weeks (T5). The number of embryos per Petri dish was determined at 2 weeks AF (T4) and at T5. The development of the embryos was photographed with a Canon Powershot G9 on an inverted microscope (Leica, DM IL LED).
Photosynthetic performance
In vivo chlorophyll-a fluorescence (ChlaF) of photosystem II (PSII) allowed the assessment of the photosynthetic activity (Krause and Weis 1984; Murchie and Lawson 2013) of the specimens of G. barbata. ChlaF was measured on the apical part of fronds (10 cm) using a PAM-Imaging Fluorometer Open FluorCam (Photon Systems Instruments, Czech Republic). Photosynthetic efficiency was measured on three randomly selected fronds (i.e. Excel function RANDOM) at the end of acclimation (T0), after 7 days (T1), after 14 days (T2) and after 21 days (T3). To avoid signal overflow, the shutter time and sensitivity of the charge-coupled device (CCD) camera were set to 1 and 10 respectively. Each frond was placed at a constant distance of 17.5 cm below the camera lens, and the lamps were placed at an angle of 45° to the center of the measurement area. Prior to the measurements, each frond was dark-adapted for 20 min to allow complete oxidation of the PSII reaction centers.
The basal fluorescence (F0) was measured. Then a saturating pulse of actinic light (1990 μmol photons m−2 s−1, 0.8 s) was administered to induce maximum fluorescence (Fm). The maximum quantum yield was calculated after equation (Maxwell and Johnson 2000):
Rapid Light Curves (RLCs) consisted of 8 actinic light steps at the following intensities 29, 88, 147, 206, 324, 442, 795, 1149 μmol photons m−2·s−1 and lasting 60 s each. Irradiance period was 60 s to ensure the minimum (steady-state) fluorescence in actinic light (Ft) as in Nielsen and Nielsen (2008). A saturating light pulse of 4040 μmol photons m−2 s−1 was applied at the end of every step to determine the minimum and maximum fluorescence (F and F'm), and thus the effective quantum yield of PSII according to equation (Genty et al. 1989):
The relative electron transport rate (rETR) was calculated by multiplying Y(II) by the respective PAR values at each light step according to the equation (Genty et al. 1989):
RLCs with rETR as a function of PAR were fitted to the Platt et al. (1980) model using the phytotools package (Silsbe and Malkin 2015) in R (R Core Team 2022). The initial slope alpha (α), the light saturation coefficient (Ek) and the maximum relative electron transport rate (rETRmax) were calculated from each curve following equation:
For each light step, the non-photochemical quenching (NPQ) of ChlaF was also calculated using the following equation:
To obtain the values of the maximum non-photochemical quenching (NPQmax),which represents the maximum thermal energy dissipation (Joliot and Johnson 2011), the NPQ vs. PAR curves were fitted according to Serôdio and Lavaud (2011), using “phytotools” package version 1.0 (Silsbe and Malkin 2015) in R (R Core Team 2022).
Additionally, photochemical and non-photochemical quenching was estimated from RLC data. The proportion of opened reaction centers, and thus the efficiency of the photochemical pathway during the energy dissipation by activated Chlorophyll a was described by qP (Maxwell and Johnson 2000) and calculated after Schreiber et al. (1986). Non-photochemical quenching represents the energy dissipation different from the fluorescence and the photochemical pathway, being in relation with heat dissipation (Maxwell and Johnson 2000) and was calculated as qN (Schreiber et al. 1986) and NPQ (Bilger and Björkman 1991).
Additionally, the relationships between photochemical (qP) and non-photochemical quenching (qN) of thalli of G. barbata against E/Ek were compared among treatments. Parameters qP and qN were represented against the ratio E/Ek to analyze them at the limiting (E/Ek < 1), optimal (E/Ek = 1) and saturating (E/Ek > 1) range of irradiances for RLCs (Burdett et al. 2019). Response curves of quenching parameters to E/Ek ratio were fitted by non-linear regression to a standard dose–response curve (four-parameter logistic model), which is used when X values are logarithms of doses, following the equation:
where X is the log of the ratio E/Ek [the ratio between incident irradiance (PAR = E) and the saturating irradiance for photosynthesis derived from the rapid light curves (Ek)]; Y is the quenching response (photochemical-qP, or non-photochemical qN); Top and Bottom are the plateaus from the logistic model in the same units than quenching parameters; logE/Ek50 is the ratio at which 50% of maximum quenching response is reached (unitless); and HillSlope is the slope factor (unitless), which represent the highest rate of change of the quenching parameters in the model.
Statistical analyses
Analysis of Variance (ANOVA) was performed to test for the effect of medium, temperature and time of exposure on Fv/Fm, rETRmax, αETR and NPQmax. The design for the analysis consisted of three factors: Time (Ti, fixed, 3 levels), Medium (Me, fixed, 2 levels, crossed), and Temperature (Te, 2 levels, crossed), with n = 3 for each combination of factors. Significant interaction terms were examined by performing a post hoc pairwise t-test. The assumption of normality of response variables was tested with the Shapiro–Wilk test. Cochran’s C-test was used to test for the assumption of homogeneity of variances prior to analysis (Underwood 1997). For all response variables except Fv/Fm, the assumptions of normal distribution and variance homogeneity were verified. In this last case, non-normality and variance heterogeneity persisted after transformation. Therefore, data were analyzed through a permutational multivariate analysis of variance (PERMANOVA: Anderson 2001) based on Euclidean distance, with 5,000 permutations. This analysis returns the classical univariate F statistic, and it can be used to substitute univariate ANOVA, but it does not make any assumption on data distribution and is robust to variance heterogeneity for experiments with balanced designs (Anderson 2017).
Two-way ANOVA was used to test for the effects of culture medium (two levels: SW, AT) and temperature (two levels: 10 °C, 14 °C) on RGR (n = 5). The assumption of normality and homoscedasticity were tested as for previous analyses. Tukey’s HSD post-hoc test was used to examine pairwise significant differences between the factor combinations. All analyses were performed in R version 4.2.2 (R Development Core Team 2022) using the packages “GAD” (Sandrini-Neto and Camargo 2022), “vegan” (Oksanen et al. 2022).
Fits for the quenching parameters versus the E/Ek ratio were compared between treatments including the initial control (T0) by means of Extra-sum of squares F-test in GraphPad Prism (GraphPad Software Inc.). Since time and temperature had no significant effect on these parameters, the data were pooled among Ti × Te combinations.
Results
Growth and fertility
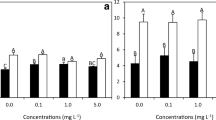
RGR was significantly affected by the culture medium (P = 0.019), although with slight differences, but not by temperature (P = 0.059) nor by their interaction (P = 0.209) (Table S2). The highest values were at 14 °C in AT (0.027 ± 0.004 day−1) and the lowest at 10 °C in SW (0.013 day−1 ± 0.001 SE) (Fig. 3).
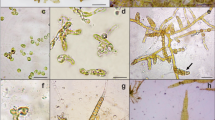
The receptacles began to develop at T2 (Fig. 4a, b). The fertile conceptacles released viable zygotes (Fig. 4b, c, d). After two weeks of cultivation (T4), the mean number was 444.6 ± 182.8 germlings Petri−1 and at T5 it was 45.3 ± 6.5 germlings Petri−1 (Table S3).
Development and maturation of Gongolaria barbata receptacles (14 °C in AT); a) apical receptacle (scale bar = 2 mm); b) development of G. barbata conceptacles (scale bar = 500 µm) c) fertilization and development of G. barbata embryos during 1 week (scale bar = 200 µm); d) two-week-old germlings (scale bar = 1 mm)
Photosynthetic performance
At T1, T2 and T3, Fv/Fm values showed a significant interaction between culture medium and temperature (Table S4, F(1,36) = 23.21, P < 0.001), i.e. slight but significant differences between media changed with temperature. At 10 °C, the values between the two media were comparable, while 14 °C AT (0.71 ± 0.01) had higher values than SW (0.65 ± 0.04) (Fig. 5a). The Fv/Fm values under the AT treatment were similar to those of the control at T0 (0.73 ± 0.02), they decreased by 11% in the SW treatment at 14 ºC after three weeks (Two tailed t-test, t = 3.82, df = 13, P = 0.002).
Fv/Fm, αETR, rETRmax, and NPQmax of Gongolaria barbata thalli at the three times (T1 = 7 days, T2 = 14 days, T3 = 21 days) and temperatures (10 °C and 14 °C) of exposure to the two media, namely Algatron (light green bars) and seawater (blue bars). Dotted lines indicate values at T0. Units for Fv/Fm and NPQmax: dimensionless; rETRmax, μmol electrons m–2 s–1; αETR, mol electrons (mol photons)−1. Data are mean (n = 3) ± SE
The interaction between temperature, time and medium had a significant effect on F0 (Table S5), therefore the differences changed over time depending on temperature and culture medium. Specifically, at AT, F0 was stable from T1 to T3 at both temperatures, while at SW, F0 decreased significantly by 17% at 10 °C, but increased by 23% at 14 °C (Table S5).
Analysis of the rETRmax values derived from the RLCs curves revealed a significant Ti × Me interaction, indicating that the temporal patterns of rETRmax values differed significantly between media (Table S6). Specifically, rETRmax values increased over time in the AT samples, from 95.12 ± 9.21 (T1) to 153.06 ± 32.75 (T3) at 10 °C and from 93.12 ± 13.58 (T1) to 128.83 ± 9.69 (T3) at 14 °C, while they remained stable in SW, with a mean pooled value of 57.4 ± 15.1 (Fig. 5b). Respect to initial control values (T0), rETRmax increased by twofold between T0 and T3, while thalli grown in SW had a significant effect of medium, regardless of temperature and time, for αETR values (Fig. 5c): AT samples showed higher values (0.69 ± 0.07 at 10 °C; 0.66 ± 0.06 at 14 °C) than SW (0.57 ± 0.10 at 10 °C; 0.52 ± 0.13 at 14 °C).
Maximum non-photochemical quenching (NPQmax) showed significant differences between the different culture media, independent of the temperature and time (Fig. 5d): samples grown in SW showed slightly higher values (4.09 ± 0.42 at 10 °C; 3.84 ± 0.33 at 14 °C) compared to samples grown in AT (3.58 ± 0.50 at 10 °C; 3.22 ± 0.64 at 14 °C).
Model comparison from pooled qN and qP data (Ti × Te) revealed significantly different responses curves among culture media and with control values (T0) (Fig. 6, Table S7). Differences in photochemical quenching (qP) were mostly attributed to changes in E/Ek50 (i.e. the E/Ek value at which half of the reaction centers were open, T0 < AT < SW) and the HillSlope (i.e. the rate at which qP declined, SW < T0 < AT). At the light-limiting region, photochemical quenching (qP) was close to 1 for all treatments (Fig. 6a). At E/Ek = 1, qP values from the control and AT treatment were around 0.7–0.9, indicating that around 80% of reaction centers were opened. In contrast, thalli of G. barbata cultured with SW had lower qP values with a greater variability (ranging from 0.5 to 0.75) and 50% of reaction centers were still open at higher E/Ek values than for the control or AT (Fig. 6a). Within the RLC light saturating region (E/Ek > 1), SW-cultured thalli showed the lowest values (Fig. 6a).
a) Photochemical (qP) and b) non-photochemical (qN) quenching of thalli of Gongolaria barbata exposed to two culture media, namely Algatron (green dots) and seawater (blue dots) and the initial control (T0, orange dots), represented against the ratio between incident irradiance (PAR = E) and the saturating irradiance for photosynthesis derived from the rapid light curves (Ek), as E/Ek. Data from different experimental times (T1, T2, T3) and temperatures (10, 14 °C) were pooled with a total of n = 144 per culture media and n = 95 for T0
Non-photochemical quenching (qN) values began rising exponentially at lower E/Ek values in AT than in SW or T0. Only T3 had a significant effect on qN of G. barbata, with higher values at 10 ºC than 14 ºC (Extra-sum of squares F-test, AT: F(4,40) = 3.57, P = 0.014; SW, F(4,40) = 14.2, P < 0.0001). For SW the greatest differences were detected around the theoretical optimal range for photosynthesis (E/Ek = 1, Table S4). The differences in the qN curves were mainly due to the E/Ek50 values. Although half of the maximum qN was reached at E/Ek ~ 0.6 for both culture media, the initial control samples required higher saturation to reach 50% of the maximum qN capacity than thalli cultured in SW or AT (Table S7, Fig. 6b). Nevertheless, SW and T0 had a similar rise in qN towards the RLC light saturating region, whereas thalli from AT experienced a steadier rise in qN, based on their lower HillSlope values (Fig. 6b, Table S6).
Discussion
The RGR of Gongolaria barbata in AlgatronCifo® (AT) was higher than in seawater, probably due to the presence of phytohormones and the polysaccharide alginate, which have been shown to promote growth (Briceño-Domínguez et al. 2014; Sujeeth et al. 2022; Umanzor et al. 2022). The highest growth was observed at 14 °C, which corresponds to the spring temperature in the Gulf of Trieste, when vegetative development of G. barbata is known to be maximal (Falace et al. 2006) and coincides with the temperature at which Orfanidis (1991) found the highest growth for the same species.
Fertile receptacles also developed exclusively at 14 °C-AT, followed by the release of viable zygotes. The number of zygotes released, and the survival of embryos was comparable to previous ex-situ cultures from receptacles harvested in the same geographical area (Savonitto et al. 2021). Therefore, this study indicates that the switch from vegetative to reproductive phase can be initiated by AT within two weeks. In contrast, thalli of G. barbata cultured for 16 months at different temperatures in von Stosch's enriched SW (i.e., from -1 to 33 °C) remained sterile under all conditions (Orfanidis 1991). In the case of Ericaria barbatula (Kützing) Molinari & Guiry, which was cultivated in SW, fertility was only achieved after six months (Papadimitriou et al. 2022). The biostimulant AT has been shown to significantly increase plant growth and flowering of Lobivia spp. (Prisa 2021). An increase in flowering has been documented in plant crops treated with macroalgal biostimulants (Ali et al. 2021), which has been linked to the phytohormones (or phyco-elicitors), especially cytokinins, contained in the extracts (Khan et al. 2009).
Our study also showed that G. barbata cultured with a commercial algal biostimulant AT increased its photosynthetic capacity (rETRmax) and efficiency (αETR) by 2 to 2.5-fold and enhanced photochemical quenching (qP) compared to its culture in seawater (SW). On the one hand, the higher photosynthetic efficiency (αETR) in AT could be related to an increase in the biosynthesis of light-harvesting centers due to N enrichment. A higher density of PSII centers was observed in the kelp Saccharina latissimi (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders grown under optimal N supply and temperature (Gerard 2008). Nitrogen has been shown to improve the physiological performance and growth rates of fucoid algae and mitigate the negative effects of stress (Fernández et al. 2020; Gerdol et al. 2020). Apart from the higher nutrient availability, the addition of the biostimulant may have induced changes in algal metabolism and signaling pathways specifically related to nutrient uptake and/or nutrient translocation, a mechanism that has also been observed in plants where biostimulants improved nutrient use efficiency (Jannin et al. 2013; Saa et al. 2015; Sujeeth et al. 2022).
Molybdenum was one of the micronutrients in AT (Table S1), naturally occurring in M. pyrifera (Iparraguirre et al. 2023), that plays a role in nitrate reduction and ion absorption in seaweeds (DeBoer 1981). This micronutrient is known to promote leaf production and fruiting in plants in synergy with other biostimulant components (La Bella et al. 2021). For G. barbata, Ak et al. (2020) reported that Mo concentrations were detected in field samples, but its concentration increased eightfold when cultured with F/2 medium (0.03 µM Na2MoO4.2H2O, Guillard 1975). The Mo concentrations of the biostimulant used in our experiment were 0.04–0.05 µM, which added to the average Mo concentration in seawater (0.10 µM, Abbott 1977) would result in a 1.5-fold enrichment that can be assimilated by G. barbata. In this context, it can be hypothesized that bio-enrichment with Mo may have promoted the photophysiology of G. barbata, as is the case with other micronutrients such as Cu (Celis-Plá et al. 2018).
Non-photochemical quenching parameters (NPQmax, qN) followed an opposite trend to the other photo-physiological parameters, with higher values in SW treatments. NPQ values represent the ability to efficiently dissipate excess energy through non-photochemical quenching, indicating a high photoprotective capacity (Celis-Plá et al. 2014). High NPQmax values may indicate environmental stress in response to high light intensity, desiccation, temperature, or nutrient deficiency (Ballottari et al. 2007; Gerotto et al. 2011; Cardol and Krieger-Liszkay 2017). In our experiment, culture media were frequently renewed (every 2–3 days) and provided at similar (SW) or higher concentrations than in their natural habitat (i.e., AT had a 58-fold higher DIN). However, a possible N-limitation cannot be ruled out, as the turnover rates in the field may provide a higher supply in the field than under laboratory conditions. This fact would explain why the physiological performance of SW fronds and the initial control were more similar (T0), while the photosynthetic responses in AT were promoted even above control values. On the other hand, nutrient availability and ratios can interact with their uptake and assimilation and affect photosynthesis and growth (Roleda and Hurd 2019). According to the DIN:SRP ratio at which nutrients were supplied in each treatment (121 and 382 for SW and AT, respectively), there would be an external P-limitation in both culture media. According to Dodds (2003), the DIN:SRP ratio should be interpreted altogether with the absolute amounts of each nutrient to avoid misuse. In this regard, although AT would indicate a greater P-limitation (due to 58 × N supply), PO43− values in AT were twice those in SW (Table 1), and therefore SW would be more P-limited. Under nutrient limiting situations, and particularly P, the photochemical pathway of ATP synthesis is limited leading to lower ETR values (Geider et al. 1993; Wykoff et al. 1998). Such disruptions in ATP synthase leads to marked proton gradients (ΔpH) across thylakoidal membranes (Muller et al. 2001), due to lower proton requirements and ATP consumption (Gauthier and Turpin 1997). These alterations have been linked to an enhanced energy dissipation via non-photochemical pathways (Krieger et al. 1992), detected in nutrient limited photoautotrophs by increased NPQ or qN (e.g. Dal Bosco et al. 2004; Rodríguez-Román and Iglesias-Prieto 2005; Weng et al. 2008; Zhang et al. 2019). This hypothesis would be supported by the lower qP values and enhanced non-photochemical response (qN, NPQmax) we found in G. barbata cultured with SW, a culture medium where it was particularly evident in the saturating region of the RLCs due to a greater need of dissipating excess heat.
Long-term series of PO43− and DIN concentrations in the Gulf of Trieste have shown that the availability of key nutrients in these coastal waters has changed, leading to almost permanent P deficiency over the last two decades (Cozzi et al. 2020). Increasingly dystrophic waters, together with climate change, are likely responsible for the major ecosystem shifts in the Gulf of Trieste, where habitat-forming macroalgae have almost completely disappeared and been replaced by ephemeral species (Orlando-Bonaca and Rotter 2018). Thus, higher nutrient concentrations in coastal areas bordering lagoons and freshwater discharges could be an explanation for the greater presence of G. barbata populations in these areas.
The enhancing effect of the Macrocystis extract occurred only during the first week of treatment, which probably contributed to the overall higher growth and enhanced physiological condition of the treated thalli and could be due to the presence of mannitol, which serves as an energy reserve in the short term (Briceño-Domínguez et al. 2014; Hurd et al. 2014). The nutritional strategy of G. barbata is largely unknown, but the higher photosynthetic and growth capacity when cultured with AT suggests that G. barbata may benefit from transient pulses of high nutrient concentrations, as previously observed in Cystoseira humilis Schousboe ex Kützing (Vaz-Pinto et al. 2014). The ability to surge uptake could be particularly beneficial for G. barbata populations growing in a coastal area such as Grado, where nutrient fluctuations and transient nutrient surpluses occur during the largest freshwater flows (Acquavita et al. 2015; Cozzi et al. 2020). Future studies should address the nutrient uptake, assimilation, and storage abilities of this species to fully understand its nutritional physiology.
Temperature had a secondary but significant role in the photo-physiological responses of G. barbata, interacting mainly with the culture media. This interaction could be due to higher nutrient demand through increased photosynthesis and growth rate at 14 ºC, as shown by the higher effective quantum yield of PSII (Fv/Fm), photosynthetic capacity (rETRmax) and greater RGR with algal biostimulant treatment (AT). At low temperature, photosynthetic electron transport may be limited by the increased stiffness of the thylakoid membrane and the resulting reduction in the movement of the proteins of the photosynthetic apparatus across the membranes. Moreover, the enzymatic reactions of the Calvin Benson cycle operate at reduced rates at low temperatures and consume less ATP and NADPH. These conditions generate an excess of light energy that can be dissipated by boosting non-photochemical pathways. However, this mechanism would only explain the higher non-photochemical quenching of thalli grown at 10 ºC in SW at T3, which is probably related to the greater P-limitation experienced after three weeks under that condition.
We cannot exclude that other unknown hormone-like compounds of the commercial biostimulant contributed to the improvement of the overall physiological state as well as the growth and reproduction of G. barbata. The components of the biostimulants are thought to act synergistically (Fornes et al. 2002; Vernieri et al. 2005; Yakhin et al. 2017; La Bella et al. 2021), as the uptake of the whole extracts is more beneficial than that of their individual components (Ali et al. 2021). Furthermore, the molecular mechanisms of action of these extracts are not yet fully understood (El Boukhari et al. 2020; Hurtado and Critchley 2020; Sujeeth et al. 2022) and require further functional studies to determine how they work and what effects they have on nutrient uptake and algal growth. Further investigations are required to test dose–response, in conjunction with nutrient-enriched media, to promote thalli and seedling growth. The mechanisms of action of these seaweed-derived biostimulants, using -omics techniques (e.g. metabolomics and/or transcriptomics) will help to unravel the effects of the various components.
Macroalgal-derived biostimulants offer an innovative strategy to improve macroalgal culture by boosting growth and promoting early fertility for sustainable restoration without harming the natural stands. Triggering reproduction under controlled laboratory conditions could ensure the availability of seedlings for a longer periods, thus promoting the restoration of Cystoseira s.l. forests on a larger scale, regardless of the availability of donor populations.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Abbott OJ (1977) The toxicity of ammonium molybdate to marine invertebrates. Mar Poll Bull 8:204–205
Acquavita A, Aleffi IF, Benci C, Bettoso N, Crevatin E, Milani L, Tamberlich F, Toniatti L, Barbieri P, Licen S, Mattassi G (2015) Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon (northern Adriatic Sea). Reg Stud Mar Sci 2:132–144
Ak I, Cankiriligil EC, Türker G, Sever O (2020) Assessment of light intensity and salinity regimes on the element levels of brown macroalgae, Treptacantha barbata: Application of response surface methodology (RSM). Food Sci Technol 41:944–952
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 10:531
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). In: Balakrishnan N, Colton T, Everitt B, Piegorsch W, Ruggeri F, Teugels JL (eds) Wiley statsref: statistics reference online, pp 1–15. https://doi.org/10.1002/9781118445112.stat07841
Arthur GD, Stirk WA, Van Staden J, Scott P (2003) Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S Afr J Bot 69:207–211
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic (Amsterdam) 196:39–48
Bevilacqua S, Savonitto G, Lipizer M, Mancuso P, Ciriaco S, Srijemsi M, Falace A (2019) Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 100:1–4
Bilger W, Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184:226–234
Blanfuné A, Boudouresque CF, Verlaque M, Beqiraj S, Kashta L, Nasto I, Ruci S, Thibaut T (2016) Response of rocky shore communities to anthropogenic pressures in Albania (Mediterranean Sea): Ecological status assessment through the CARLIT method. Mar Pollut Bull 109:409–418
Blunden G, Wildgoose PB (1977) The effects of aqueous seaweed extract and kinetin on potato yields. J Sci Food Agric 28:121–125
Briceño-Domínguez D, Hernández-Carmona G, Moyo M, Stirk W, van Staden J (2014) Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J Appl Phycol 26:2203–2210
Burdett HL, Wright H, Smale DA (2019) Photophysiological responses of canopy-forming kelp species to short-term acute warming. Front Mar Sci 6:516
Cardol P, Krieger-Liszkay A (2017) From light capture to metabolic needs, oxygenic photosynthesis is an ever-expanding field of study in plants, algae and cyanobacteria. Physiol Plant 161:2–5
Celis-Plá PSM, Korbee N, Gómez-Garreta A, Figueroa FL (2014) Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci Mar 78:377–388
Celis-Plá PSM, Brown MT, Santillán-Sarmiento A, Korbee N, Sáez CA, Figueroa FL (2018) Ecophysiological and metabolic responses to interactive exposure to nutrients and copper excess in the brown macroalga Cystoseira tamariscifolia. Mar Pollut Bull 128:214–222
Cibic T, Baldassarre L, Cerino F, Comici C, Fornasaro D, Kralj M, Giani M (2022) Benthic and pelagic contributions to primary production: Experimental insights from the Gulf of Trieste (Northern Adriatic Sea). Front Mar Sci 9:877935
Cozzi S, Cabrini M, Kralj M, De Vittor C, Celio M, Giani M (2020) Climatic and anthropogenic impacts on environmental conditions and phytoplankton community in the Gulf of Trieste (northern Adriatic Sea). Water 12:2652
Crouch IJ, Van Staden J (1993) Commercial seaweed products as biostimulants in horticulture. J Home Consum Hortic 1:19–76
Dal Bosco C, Lezhneva L, Biehl A, Leister D, Strotmann H, Wanner G, Meurer J (2004) Inactivation of the chloroplast ATP synthase γ subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem 279:1060–1069
De La Fuente G, Chiantore M, Asnaghi V, Kaleb S, Falace A (2019) First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 7:7290
DeBoer JA (1981) Nutrients. In: Lobban CS, Wynne MK (eds) The Biology of Seaweeds. Blackwell Scientific, Oxford, pp 356–91
Dodds WK (2003) Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J N Am Benthol Soc 22:171–181
du Jardin P (2015) Plant biostimulants: Definition, concept, main categories and regulation. Sci Hortic (Amsterdam) 196:3–14
El Boukhari MEM, Barakate M, Bouhia Y, Lyamlouli K (2020) Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 9:359
Fabbrizzi E, Scardi M, Ballesteros E, Benedetti-Cecchi L, Cebrian E, Ceccherelli G, De Leo F, Deidun A, Guarnieri G, Falace A, Fraissinet S, Giommi C, Mačić V, Mangialajo L, Mannino AM, Piazzi L, Ramdani M, Rilov G, Rindi L, Rizzo L, Sarà G, Souissi JB, Taskin E, Fraschetti S (2020) Modeling macroalgal forest distribution at Mediterranean scale: present status, drivers of changes and insights for conservation and management. Front Mar Sci 7:20
Falace A, Bressan G (2003) Changes of algal flora in the Gulf of Trieste (Northern Adriatic Sea). Bocconea 16:1033–1037
Falace A, Di Pascoli A, Bressan G (2005) Valutazione della biodiversità nella Riserva Marina di Miramare (Nord Adriatico): macroalghe marine bentoniche. Biol Mar Mediterr 12:88–98
Falace A, Zanelli E, Bressan G (2006) Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull Mar Sci 78:161–166
Falace A, Alongi G, Cormaci M, Furnari G, Curiel D, Cecere E, Petrocelli A (2010) Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem Ecol 26:77–90
Falace A, Kaleb S, De La Fuente G, Asnaghi V, Chiantore M (2018) Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS One 13: e0193011
Fernández PA, Gaitán-Espitia JD, Leal PP, Schmid M, Revill AT, Hurd CL (2020) Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: implications for acclimation under thermal stress. Sci Rep 10:3186
Fornes F, Sanchez-Perales M, Guardiola JL (2002) Effect of a seaweed extract on the productivity of ‘de Nules’ clementine mandarin and navelina orange. Bot Mar 45:486–489
Gauthier DA, Turpin DH (1997) Interactions between inorganic phosphate (Pi) assimilation, photosynthesis and respiration in the Pi-limited green alga Selenastrum minutum. Plant Cell Environ 20:12–24
Geider RJ, La Roche J, Greene RM, Olaizola M (1993) Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J Phycol 29:755–766
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subj 990:87–92
Gerard VA (2008) The role of nitrogen nutrition in high-temperature tolerance of the kelp, Laminaria saccharina (Chromophyta). J Phycol 33:800–810
Gerdol M, Visintin A, Kaleb S, Spazzali F, Pallavicini A, Falace A (2020) Gene expression response of the alga Fucus virsoides (Fucales, Ochrophyta) to glyphosate solution exposure. Environ Pollut 267:115483
Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T (2011) Role of PSBS and LHCSR in Physcomitrella patens acclimation to high light and low temperature. Plant Cell Environ 34:922–932
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of Marine Invertebrate Animals. Plenum Press, NY, pp 29–60
Han S, Song HI, Park JS, Kim YJ, Umanzor S, Yarish C, Kim JK (2023) Sargassum horneri and Ascophyllum nodosum extracts enhance thermal tolerance and antioxidant activity of Neopyropia yezoensis. J Appl Phycol 35:201–207
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed Ecology and Physiology. Cambridge University Press, Cambridge
Hurtado AQ, Critchley AT (2018) A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J Appl Phycol 30:2859–2873
Hurtado AQ, Critchley AT (2020) Time for applications of biostimulants in phyconomy: Seaweed Extracts for Enhanced Cultivation of Seaweeds (SEECS). In: Torres MD, Kraan S, Dominguez H (eds) Sustainable Seaweed Technologies. Elsevier, Amsterdam, pp 103–127
Iparraguirre J, Llanes A, Masciarelli O, Zocolo GJ, Villasuso AL, Luna V (2023) Formulation technology: Macrocystis pyrifera extract is a suitable support/medium for Azospirillum brasilense. Algal Res 69:102916
Jannin L, Arkoun M, Etienne P, Laîné P, Goux D, Garnica M, Fuentes M, Francisco SS, Baigorri R, Cruz F, Houdusse F (2013) Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J Plant Growth Regul 32:31–52
Jiksing C, Ongkudon MM, Thien VY, Rodrigues KF, Lym Yong WT (2022) Recent advances in seaweed seedling production: a review of eucheumatoids and other valuable seaweeds. Algae 37:105–121
Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci 108:13317–13322
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Kralj M, Lipizer M, Čermelj B, Celio M, Fabbro C, Brunetti F, Francé J, Mozetič P, Giani M (2019) Hypoxia and dissolved oxygen trends in the northeastern Adriatic Sea (Gulf of Trieste). Deep Sea Res II 164:74–88
Krause GH, Weis E (1984) Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. Photosynthesis Res 5:139–157
Krieger A, Moya I, Weis E (1992) Energy-dependent quenching of chlorophyll a fluorescence: effect of pH on stationary fluorescence and picosecond-relaxation kinetics in thylakoid membranes and photosystem II preparations. Biochim Biophys Acta Bioenerg 1102:167–176
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633
La Bella S, Consentino BB, Rouphael Y, Ntatsi G, De Pasquale C, Iapichino G, Sabatino L (2021) Impact of Ecklonia maxima Seaweed Extract and Mo Foliar Treatments on Biofortification, Spinach Yield, Quality and NUE. Plants 10:1139
Lüning K (1990) Seaweeds: Their Environment, Biogeography and Ecophysiology. John Wiley & Sons, Inc., New York
Malačič V, Celio M, Čermelj B, Bussani A, Comici C (2006) Interannual evolution of seasonal thermohaline properties in the Gulf of Trieste (northern Adriatic) 1991–2003. J Geophys Res Ocean 111:CO8009. https://doi.org/10.1029/2005JC003267
Mancuso FP, Strain EMA, Piccioni E, De Clerck O, Sarà G, Airoldi L (2018) Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Mar Pollut Bull 129:762–771
Mangialajo L, Chiantore M, Cattaneo-Vietti R (2008) Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar Ecol Prog Ser 358:63–74
Martynenko A, Shotton K, Astatkie T, Petrash G, Fowler C, Neily W, Critchley AT (2016) Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. Springer Plus 5:1393
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Nielsen HD, Nielsen SL (2008) Evaluation of imaging and conventional PAM as a measure of photosynthesis in thin- and thick-leaved marine macroalgae. Aquat Biol 3:121–131
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, R. O’Hara RB et al. (2022) « vegan: Community Ecology Package ». 450 https://CRAN.R-project.org/package=vegan
Orfanidis S (1991) Temperature responses and distribution of macroalgae belonging to the warm-temperate Mediterranean-Atlantic distribution group. Bot Mar 34:541–552
Orlando-Bonaca M, Rotter A (2018) Any signs of replacement of canopy-forming algae by turf-forming algae in the northern Adriatic Sea? Ecol Indic 87:272–284
Orlando-Bonaca M, Pitacco V, Slavinec P, Šiško M, Makovec T, Falace A (2021a) First restoration experiment for Gongolaria barbata in Slovenian coastal waters. What can go wrong? Plants 10:239
Orlando-Bonaca M, Pitacco V, Lipej L (2021b) Loss of canopy-forming algal richness and coverage in the northern Adriatic Sea. Ecol Indic 125:107501
Orlando-Bonaca M, Savonitto G, Asnaghi V, Trkov D, Pitacco V, Šiško M, Makovec T, Slavinec P, Lokovšek A, Ciriaco S, Chiantore M, Kaleb S, Descourvières EP, Srijemsi M and Falace A (2022) Where and how - new insight for brown algal forest restoration in the Adriatic. Front Mar Sci 9:988584
Papadimitriou A, Nakou K, Papathanasiou V, Orfanidis S (2022) Effects of photoperiod and temperature on ecophysiological responses of Ericaria barbatula (Phaeophyceae): a long-term study. Bot Mar 65:269–277
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Prisa D (2021) Biological mixture of brown algae extracts influences the microbial community of Lobivia arachnacantha, Lobivia aurea, Lobivia jojoiana and Lobivia grandiflora in pot cultivation. GSC Adv Res Rev 08(03):043-053
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rodríguez-Román A, Iglesias-Prieto R (2005) Regulation of photochemical activity in cultured symbiotic dinoflagellates under nitrate limitation and deprivation. Mar Biol 146:1063–1073
Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58:552–562
Saa S, Olivos-Del Rio A, Castro S, Brown PH (2015) Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] DA Webb). Front Plant Sci 6:87
Samuels LJ, Setati ME, Blancquaert EH (2022) Towards a better understanding of the potential benefits of seaweed based biostimulants in Vitis vinifera L. cultivars. Plants 11:348
Sandrini-Neto AL, Camargo MG (2022). GAD: General ANOVA Designs. Centro de Estudos do Mar da Universidade Federal do Parana (Brazil), Av. Beira-mar s/n. P.O. Box 50002, Pontal do Parana (PR), CEP:83255000, Brazil.
Santaniello A, Scartazza A, Gresta F, Loreti E, Biasone A, Di Tommaso D, Piaggesi A, Perata P (2017) Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front Plant Sci 8:1362
Savonitto G, Alongi G, Falace A (2019) Reproductive phenology, zygote embryology and germling development of the threatened Carpodesmia barbatula (= Cystoseira barbatula) (Fucales, Phaeophyta) towards its possible restoration. Webbia 74:317–323
Savonitto G, De La Fuente G, Tordoni E, Ciriaco S, Srijemsi M, Bacaro G, Chiantore M, Falace A (2021) Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat Conserv Mar Freshw Ecosyst 31:1611–1623
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Serôdio J, Lavaud J (2011) A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res 108:61–76
Shukla PS, Yadav NS, Critchley AT, Prithiviraj B (2022) Editorial: Biostimulants as an avenue of abiotic stress tolerance improvement in crops. Front Sustain Food Syst 6:908555
Silsbe GM, Malkin SY (2015) Package “phytotools”: Phytoplankton Production Tools. CRAN library repository. http://cran.nexr.com/web/packages/phytotools/phytotools.pdf
Spann TM, Little HA (2011) Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’sweet orange nursery trees. Hort Science 46:577–582
Stirk WA, Rengasam KR, Kulkarni MG, van Staden J (2020) Plant biostimulants from seaweed: an overview. In: Geelen D, Xu L (eds) The Chemical Biology of Plant Biostimulants. John Wiley & Sons Ltd, London pp 31–55
Sujeeth N, Petrov V, Guinan KJ, Rasul F, O’Sullivan JT, Gechev TS (2022) Current insights into the molecular mode of action of seaweed-based biostimulants and the sustainability of seaweeds as raw material resources. Int J Mol Sci 23(14):7654
Thibaut T, Blanfuné A, Boudouresque CF, Verlaque M (2015) Decline and local extinction of Fucales in French Riviera: the harbinger of future extinctions? Medit Mar Sci 16(1):206–224
Trivedi K, Vijay Anand KG, Kubavat D, Patidar R, Ghosh A (2018) Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J Appl Phycol 30:2001–2015
Umanzor S, Shin S, Marty-Rivera M, Augyte S, Yarish C, Kim JK (2019) Preliminary assessment on the effects of the commercial seaweed extract, AMPEP, on growth and thermal tolerance of the kelp Saccharina spp. from the Northwest Atlantic. J Appl Phycol 31:3823–3829
Umanzor S, Jang S, Antosca R, Critchley AT, Yarish C, Kim JK (2020a) Optimizing the application of selected biostimulants to enhance the growth of Eucheumatopsis isiformis, a carrageenophyte with commercial value, as grown in land-based nursery systems. J Appl Phycol 32:1917–1922
Umanzor S, Shin S, Yarish C, Augyte S, Kim JK (2020b) Exploratory evaluation of the effects of Kelpak® seaweed extract on cultivated kelp Saccharina spp. exposed to sublethal and lethal temperatures. J World Aquac Soc 51:960–969
Umanzor S, Han S, Song HI, Park JS, Critchley AT, Yarish C, Kim JK (2022) Ascertaining the interactions of brown seaweed-derived biostimulants and seawater temperature on spore release, germination, conchocelis, and newly formed blades of the commercially important red alga Neopyropia yezoensis. Algal Res 64:102692
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Valdazo J, Viera-Rodríguez MA, Espino F, Haroun R, Tuya F (2017) Massive decline of Cystoseira abies-marina forests in Gran Canaria island (Canary Islands, eastern Atlantic). Sci Mar 81:499–507
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4:5
Vaz-Pinto F, Martínez B, Olabarria C, Arenas F (2014) Neighbourhood competition in coexisting species: The native Cystoseira humilis vs the invasive Sargassum muticum. J Exp Mar Biol Ecol 454:32–41
Verdura J, Sales M, Ballesteros E, Cefalì ME, Cebrian E (2018) Restoration of a canopy-forming alga based on recruitment enhancement: Methods and long-term success assessment. Front Plant Sci 9:1832
Vergés A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, Heck KL, Booth DJ, Coleman MA, Feary DA, Figueira W, Langlois T, Marzinelli EM, Mizerek T, Mumby PJ, Nakamura Y, Roughan M, van Sebille E, Gupta AS, Smale DA, Tomas F, Wernberg T, Wilson SK (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B 281:20140846
Vernieri P, Borghesi E, Ferrante A, Magnani G (2005) Application of biostimulants in floating system for improving rocket quality. J Food Agric Environ 3:86
Weng XY, Xu HX, Yang Y, Peng HH (2008) Water-water cycle involved in dissipation of excess photon energy in phosphorus deficient rice leaves. Biol Plant 52:307–313
Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117:129–139
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049
Zhang Y, Wu H, Yuan C, Li T, Li A (2019) Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply. J Appl Phycol 31:2797–2809
Acknowledgements
The authors would like to thank Prof. Monia Renzi for Algatron nutrient analysis and the Agenzia Regionale per la Protezione dell’Ambiente del Friuli Venezia Giulia (ARPA-FVG) for providing seawater nutrient data. We would also like to thank Dr. Marina Srijemsi for help with the laboratory culture and Saul Ciriaco for field work. We thank the reviewers for their helpful and constructive comments which significantly improved the manuscript.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. This work was supported by grants from the LIFE financial instrument of the European Community, project REEForest-LIFE (101074309 LIFE21-NAT-IT-REEForest).
Author information
Authors and Affiliations
Contributions
SK, RSdP, EBE, AF conceived the study; SK, GS performed the lab measurements; SK, RSdP, EBE, GS, SB, SN, AA, AF performed data analysis; SK, RSdP, EBE, AF wrote the original draft of the manuscript. All authors contributed to the revision of the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaleb, S., Sánchez de Pedro, R., Bañares-España, E. et al. Cultivation of Gongolaria barbata (Fucales, Phaeophyceae) with a seaweed-derived biostimulant in order to improve photophysiological fitness and promote fertility to advance the restoration of marine macroalgal forests. J Appl Phycol 35, 2337–2350 (2023). https://doi.org/10.1007/s10811-023-02984-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02984-3