Abstract
In the coming years biostimulants will play a key role in the sustainable intensification of agriculture due to their capacity to improve crops quality, nutrient use efficiency and tolerance to abiotic stresses. Cyanobacteria are nowadays considered one of the most promising sources of new biostimulants; however, in vivo studies using cyanobacteria are still scarce and often limited to a few genera. In this work the biostimulant activity of five cyanobacterial hydrolysates was evaluated on Ocimum basilicum L. grown in hydroponics. Plants were treated weekly with foliar applications of the cyanobacterial hydrolysates and of two commercial products. Three of the tested cyanobacterial hydrolysates, administered at the concentration of 1 g L-1, were effective in increasing plant growth (up to +32%), and number (up to +24%) and fresh weight (up to +26%) of the leaves compared to controls. Moreover, the cyanobacterial hydrolysates performed better than the commercial biostimulants. The biochemical characterization of the hydrolysates suggests that the observed bioactivity can be related to a high carbohydrate content. Our results indicate that cyanobacteria-based biostimulants can be an effective tool for sustainably enhancing plant growth and yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The greatest agricultural challenge of our century will be to increase global food production in a sustainable way, reducing the massive use of chemical inputs typical of conventional agriculture (Petersen and Snapp 2015). Fertilizer use in modern agriculture is highly inefficient and over the years has contributed to environmental pollution through greenhouse gases production, ocean acidification and eutrophication, and soil salinization (Halpern et al. 2015). Therefore, in recent years interest in biostimulants to improve the efficiency of fertilization by enhancing crop nutrients uptake and resilience, has increased exponentially and efforts have been made to define and categorize these emerging products (Du Jardin 2015).
According to the European Union (EU) fertilizer regulation 2019/1009 biostimulants are defined as “a product containing any substance or microorganism stimulating plant nutrition processes independently of its nutrient content, with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency; (b) tolerance to abiotic stress; (c) quality traits; (d) availability of confined nutrients in the soil or rhizosphere”. This definition encompasses a wide range of raw materials including algal biomass. Seaweeds have been largely exploited for the production of plant growth biostimulants (Mutale-joan et al. 2019); to date seaweed extracts represent 37% of the global biostimulant market and are expected to register the fastest growth rate by 2025 (Grand View Research 2018).
Interest in the use of microalgae (including cyanobacteria) has increased in many different sectors, such as food, feed, biofuels, fertilizers production and wastewater treatment (Muller-Feuga 2004; Bhatia et al. 2020; Ganesan et al. 2020; Chai et al. 2021; Swain et al. 2021), while studies on the biostimulant activity of these microorganisms are scarce and often limited to a few genera. In particular, with regard to cyanobacteria, over half of the research articles published in this field concerns Arthrospira, which is the most widely cultivated cyanobacterium in the world, with about 15,000 t of biomass produced per year (Hu 2019). Since cyanobacterial biodiversity is very high, this microbial group represents a resource worth to be explored for plant growth promoting applications (Norton et al. 1996; Suganya et al. 2016). The biostimulant potential of these microorganisms is becoming evident, as many strains have been proved to produce a wide range of bioactive molecules capable of positively affecting plant growth and resistance to abiotic stresses, such as phytohormones (auxins, cytokinins, gibberellins, abscisic acid), vitamins, aminoacids, betaines, antioxidants, polyamines and polysaccharides (Ördög and Pulz 1996; Sergeeva et al. 2002; Hajimahmoodi et al. 2010; Chaiklahan et al. 2013; de Morais et al. 2015; Borowitzka 2016; Mógor et al. 2018; Mutale-joan et al. 2019). Moreover, compared to seaweeds, that are typically harvested from natural environments, cyanobacteria are cultivated in artificial systems (open ponds and photobioreactors) which improve standardization of the raw material quality and offer the possibility to optimize the conditions for the production of large amounts of the bioactive molecules (Santini et al. 2021). In fact, it has been demonstrated that the chemical composition of seaweeds varies with age, environmental conditions, nutrient availability and time of harvesting (Shukla et al. 2019; Stirk et al. 2020; Ali et al. 2021), thus risking modification of the biostimulant activity and the active doses of the final product (Garcia-Vaquero et al. 2017).
This work aimed to broaden the knowledge on the biostimulant properties of cyanobacteria through a screening of five hydrolysates obtained from five different cyanobacterial strains on basil (Ocimum basilicum L.) grown in hydroponics, coupled with a first characterization of the hydrolysates to search for the molecules potentially responsible for the bioactivity on plants. Basil, a popular fresh culinary herb that constitutes a component of the Mediterranean diet (Simon et al. 1999), was chosen as a model species to test biostimulant activity due to its rapid life cycle and easiness of cultivation in hydroponics (Sgherri et al. 2010; Bulgari et al. 2017). In particular, the cultivation of basil in hydroponic systems in controlled environments allows year-round production and higher yields and quality (Sgherri et al. 2010). Two widely used commercial biostimulants were also tested: a hydrolysate from the brown seaweed Ascophyllum nodosum and a protein hydrolysate of animal origin. All the hydrolysates have been applied to plants by foliar spraying as this method allows the use of lower doses of product compared to basal application, thus improving the economic sustainability of treatments (Santini et al. 2021).
Materials and Methods
Cyanobacterial hydrolysates and plant material
The hydrolysates, obtained from five different cyanobacteria: Nostoc sp., Anabaena sp. and Tolypothrix sp., belonging to the Nostocales; Leptolyngbya sp., belonging to the Synechococcales; Arthrospira sp., belonging to the Oscillatoriales, were provided by Fotosintetica & Microbiologica S.r.l. (Florence, Italy). Cyanobacterial biomasses were produced in bubble column reactors with a surface-to-volume ratio of about 50 m-1. The hydrolysates were obtained by heating at 70 °C overnight the freeze-dried biomasses resuspended in water. Aliquots of the hydrolysates were dried in an oven at 100 °C until constant weight to determine the hydrolysate dry weight and then calculate the appropriate dilutions to be applied in the trials.
Rooted basil (Ocimum basilicum L. cv. Genovese) plants were used for the hydroponic growth assays. Basil seeds were sown in Petri dishes on inert rockwool substrate, irrigated with distilled water, and placed in a growth chamber under a 16:8 h light:dark photoperiod at an average light intensity of 280 μmol photons m-2 s-1. Once germinated (5 days after sowing) uniform seedlings were transferred to pots with universal soil (COMPO SANA, Compo Italia srl) inside a growth chamber under constant temperature of 22 °C, 70% relative humidity and the lighting conditions described above. After 18 days from germination, uniform seedlings were transferred to the hydroponic system after gently washing away soil residues from the roots. At the time of transplanting the plants had 5-6 leaves, corresponding to two nodes, were about 6 cm high and presented an average fresh weight ranging from 1.08 to 2.06 g in the different trials (Table 1).
Overall, three different trials were performed as shown in Table 1. The first and second trials lasted 21 days each. The commercial biostimulants were tested in the first trial, and the Arthrospira and Nostoc sp. hydrolysates in the second trial. In the third trial, lasting 28 days due to the lower fresh weight of the transplanted seedlings, the Tolypothrix, Anabaena and Leptolyngbya sp. hydrolysates were tested. In each trial, a control sprayed with deionized water was also set up.
Hydrolysates analysis
The cyanobacterial hydrolysates and the commercial biostimulants were analyzed for their macro- and microelement content. The elemental composition was determined using a CHNSO Analyzer (Flash EA, 1112 Series, Thermo Electron Corporation, USA) (Gnaiger and Bitterlich 1984) for nitrogen (N) and carbon (C) content and by inductively coupled plasma-optical emission spectroscopy (ICP-OES; iCAP 7400 DUO, Thermo Fisher Scientific, USA) for K, P, Ca, Na, S, Mg, Fe and for heavy metals (Pb, Hg, As, Cd, Ni, Cr).
Furthermore, all the hydrolysates were analyzed for protein, carbohydrate and lipid content. Total carbohydrate was determined according to Dubois et al. (1956) and lipid following Marsh and Weinstein (1966). Crude protein was calculated from the nitrogen content by multiplying for a nitrogen-to-protein conversion factor of 4.44 (González López et al. 2010).
Hydroponic cultivation trials
Plants were cultivated in a deep flow hydroponic system placed in a growth chamber under the environmental conditions described above and under uniform conditions of mineral fertilization.
The system consisted of polystyrene sheets supporting five plants each and floating in plastic tanks filled with 15 L of half-strength Hoagland nutrient solution (Hoagland and Arnon 1950) containing 3 mM KNO3, 2 mM Ca(NO3)2·H2O, 1 mM NH4H2PO4, 0.5 mM MgSO4·7H2O, 20 μM Fe-NaEDTA, 1 μM KCl, 25 μM H3BO3, 2 μM MnSO4·H2O, 2 μM ZnSO4·7H2O, 0.1 μM CuSO4·5H2O and 0.1 μM (NH4)6Mo7O24·4H2O. The nutrient solution was continuously air-bubbled so as to maintain the oxygen concentration above 6 mg L-1. The experimental set-up consisted of three randomly distributed tanks per treatment for a total of 15 plants per treatment.
Every week the water lost by evapotranspiration was replenished with distilled water and the pH and the electrical conductivity (EC) of the culture medium were monitored. The pH fluctuated between 5.5 and 6.3 and the EC at the beginning of the trials was on average 883 ± 6.7 μS cm-1, while at the end it was on average 459 ± 16.3 μS cm-1, with no significant differences recorded between treatments and control within each trial.
Plant treatments
Plants were cultivated for 21 (1st and 2nd trial) or 28 (3rd trial) days, depending on the fresh weight of the seedlings at the time of transplanting (Table 1), and were treated every week with foliar applications of the hydrolysates. The cyanobacterial hydrolysates were applied at the concentration of 1 g (dry weight) L-1 and volumes were progressively increased from 3 to 8 mL per plant to follow the increase in plant size. Treatment volumes were determined as the minimum volume needed to obtain a uniform wetting of the leaves for each application.
For treatments with commercial biostimulants the same protocols were adopted, except for the concentrations applied, that were those recommended on the label for horticultural crops, equal to 1 g (dry weight) L-1 for the A. nodosum hydrolysate and 1.77 g (dry weight) L-1 for the animal protein hydrolysate. In each trial 15 plants were sprayed with deionized water (controls).
Biometric parameters
On a weekly basis, plants were temporarily removed from hydroponics, excess water from roots was gently wiped, then plants were weighed and measured to determine fresh weight and height, and number of leaves and nodes. The measurements made during the trials were non-destructive. At the end of the trial plants were harvested and roots, stem and leaves were weighed separately. All samples were then oven-dried at 80 °C until constant weight, and dry weight (DW) was measured. Plant growth was calculated as the difference between the fresh weight (FW) at the time of harvesting and the fresh weight at the time of transplanting to hydroponics. FW and DW of the whole plant recorded at the time of harvesting were used to calculate plant water content (WC) as (FW-DW)/FW.
Due to the differences in the initial fresh weights of the plants and the different length of the trials (Table 1), the biometric parameters recorded in each trial were compared to the respective controls. All measured parameters are presented as means ± standard error.
Statistical analysis
Statistical analyses were conducted using Statgraphics. One-way ANOVA coupled with Tukey’s test was used to assess significant differences compared to the control within each trial. Significance level was P ≤ 0.05. ANOVA tables and results of Tukey’s multiple comparisons test are reported in the supplementary material (files S1, S2 and S3).
Results
Effect of cyanobacterial hydrolysates on plant biometric parameters
Three of the tested cyanobacterial hydrolysates, those from Nostoc sp., Tolypothrix sp. and Leptolyngbya sp., significantly increased (P < 0.05) the fresh weight of the plants starting from the 14th day after transplanting (Fig. 1), when the second foliar treatment was applied. At the end of the trials, the highest increase in plant growth compared to control was recorded in plants treated with the hydrolysate from Leptolyngbya sp. (+34.4%). Hydrolysates from Tolypothrix sp. and Nostoc sp. led to an increase of 31.8 and 28.7%, respectively (Fig. 1d). The Anabaena sp. and Arthrospira sp. hydrolysates increased plant growth by 21.3 and 8.0%, respectively, but the increase was not significant (Fig. 1d).
Average fresh weight of basil plants (n=15 per treatment) measured weekly during the first (a), second (b), and third (c) trial. Growth increase (n=15 per treatment) of treated plants at the end of the trials compared to their respective control (d). Bars represent standard error. * indicates significant differences between treatments and controls at P ≤ 0.05. For ANOVA results see tables in S1.
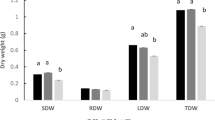
Treatments with Tolypothrix sp. and Leptolyngbya sp. hydrolysates had similar effects on shoot and root fresh weight. In particular, at the end of the trial, an average increase of 30% for shoot and 36% for root fresh weight with respect to control was observed (Fig. 2). The treatment with Nostoc sp. hydrolysate exhibited a predominant effect on root development, producing a 53% increase in root fresh weight and a 27% increase in shoot fresh weight (Fig. 2) leading to a significant higher value of the root/shoot ratio (0.34 ± 0.02) compared to that of the control (0.28 ± 0.01).
Increase in shoot (a) and root (b) fresh weight (n=15 per treatment) with respect to the controls recorded at the end of the trials. Bars represent standard error. * indicates significant differences at P ≤ 0.05; ** indicates significant differences at P ≤ 0.01. For ANOVA results see tables in S2.
Except for Arthrospira sp., all the treatments with cyanobacterial hydrolysates displayed significant effects on the number of leaves (Fig. 3). Plants treated with Nostoc sp., Tolypothrix sp. and Leptolyngbya sp. hydrolysates presented at the end of the trials one node more than the control and the number of leaves increased by about 24% (Fig. 3). In the same treatments, the fresh weight of the leaves at harvest increased by an average of 26% compared to the control (Fig. 3). Comparable effects on the number of leaves and nodes were observed with the application of Anabaena sp. hydrolysate, which however did not significantly affect the fresh weight of the leaves (Fig. 3) nor the overall fresh weight of the plants (Fig. 1d). The application of the Anabaena sp. hydrolysate produced a lower average leaf weight (220 mg per leaf) and root fresh weight (14.5 g) compared to the effective treatments in the same trial (on average 240 mg per leaf and 16.4 g of root fresh weight, respectively), which was reflected in a lower and not significant overall biostimulant activity on the plant.
Increase in number and fresh weight of leaves (n=15 per treatment) at harvest time compared to controls. Bars represent standard error. * indicates significant differences at P ≤ 0.05; ** indicates significant differences at P ≤ 0.01. For ANOVA results see tables in S3.
Only two of the tested hydrolysates (from Tolypothrix sp. and Anabaena sp.) affected plant height, increasing it by 20% compared to the control. No significant differences were found between controls and treatments with respect to plant water content, which ranged from 92 to 95% in all the trials.
None of the commercial biostimulants tested produced significant effects on basil growth. The application of the A. nodosum hydrolysate produced a slight and not significant increase in plant fresh weight (Fig. 1d) and number and fresh weight of leaves (Fig. 3) compared to the control. In plants treated with the animal protein hydrolysate a non significant decrease in plant growth (Fig. 1d) and no effect on the number of leaves (Fig. 3) were observed. Moreover, the two commercial biostimulants did not affect the height of the plants.
Hydrolysates characterization
Figure 4a shows the biochemical composition of the five cyanobacterial hydrolysates and the two commercial biostimulants used in the trials. Arthrospira sp. and Anabaena sp. hydrolysates showed a high protein (>65%) and a low carbohydrate (about 17%) content. Nostoc sp., Tolypothrix sp. and Leptolyngbya sp. hydrolysates had lower protein (from 25 to 48%) and higher carbohydrate (from 36 to 60%) content. The animal protein hydrolysate showed 70% protein with an extremely low carbohydrate (1%) content. Lipids were very low in all the cyanobacterial hydrolysates as well as in the two commercial products (Fig. 4a). The largest fractions of unidentified compounds, which comprises ashes, were found in the hydrolysates from marine species (Leptolyngbya sp. and A. nodosum) and in that obtained from animal residues (Fig. 4a).
The cyanobacterial hydrolysates contained all the macro- and microelements required for plant nutrition; nitrogen content was higher in Arthrospira sp. and Anabaena sp. hydrolysates, corresponding on average to 15% of the hydrolysate dry weight (Fig. 4b). In the A. nodosum hydrolysate nitrogen and phosphorus content (1.6 and 0.1%, respectively) was lower than in the cyanobacterial hydrolysates, while potassium content (11.2%) was higher (Fig. 4b). Leptolyngbya sp. and A. nodosum hydrolysates had the highest sodium content, on average 3%, among the tested hydrolysates. Iron was not detectable in the A. nodosum hydrolysate. The animal protein hydrolysate showed a high nitrogen content, similar to that of Arthrospira sp. and Anabaena sp. hydrolysates, while phosphorus, potassium, magnesium and iron were extremely low or not detectable. The unidentified fraction, comprising oxygen and hydrogen that were not analyzed, ranged between 33 and 55% in all the tested hydrolysates, with the highest values being recorded in Leptolyngbya sp. and A. nodosum hydrolysates (50 and 55%, respectively) (Fig. 4b).
In all the hydrolysates, the maximum amounts of heavy metals were below the limit values set by the European regulation 2019/1009 (Table 2). Mercury was not detected in any of the tested hydrolysates. Arsenic was not detected in the cyanobacterial hydrolysates and in the animal protein hydrolysate, whereas a high content (36 ppm on dry weight) was found in the A. nodosum hydrolysate, although below the limit allowed by the EU regulation 2019/1009 (40 ppm) (Table 2).
Discussion
The results of this study show that foliar applications of cyanobacterial hydrolysates can have high and significant stimulation effects on basil growth. In particular, three hydrolysates, obtained from Nostoc sp., Tolypothrix sp. and Leptolyngbya sp., have been able to enhance vegetative growth of basil by more than 30% compared to the controls.
The Nostocales are among the most well-known photosynthetic microorganisms used in agriculture, due to their ability to fix atmospheric nitrogen and improve soil structure and water retention capacity (Hegazi et al. 2010; Sahu et al. 2012). Recently, their ability to produce biologically active molecules able to stimulate growth and defence responses in plants has also been demonstrated (Singh et al. 2011; Singh 2014; Priya et al. 2015; Shariatmadari et al. 2015; Sharma et al. 2020). Nostoc piscinale biomasses subjected to freeze-drying and sonication and administered at concentrations of 0.3 and 1 g DW L-1by foliar application on winter wheat, significantly increased grain yield by 27 and 38%, respectively, compared to control sprayed with tap water (Takács et al. 2019). In the present study two Nostocales strains out of the three tested significantly increased the commercial yield (i.e., leaf weight) of basil by an average of 26% compared to the controls.
Few studies are currently available on the in vivo biostimulant activity of Synechococcales. According to Toribio et al. (2020), basal irrigation with an aqueous extract of a Leptolyngbya strain did not significantly affect growth of cucumber seedlings while in our work, treatment with the hydrolysate from Leptolyngbya sp. was among the most effective. However, it is to consider that in our experiments foliar spraying was used instead of basal application.
Application of Nostoc sp., Tolypothrix sp. and Leptolyngbya sp. hydrolysates enhanced root fresh weight by more than 35% over the controls. A larger root system increases drought tolerance and improves mineral uptake (Mutale-joan et al. 2020), which in turn influences photosynthetic efficiency and plant growth (Makino et al. 2000). This could suggest a potential role of hydrolysates in improving plant tolerance to abiotic stresses.
The maximum root fresh weight increase was obtained with Nostoc sp. hydrolysate, which also led to a significant increase in the root/shoot ratio (+21%) compared to the control. These results are in agreement with those found by Toribio et al. (2020), in whose work a Nostoc strain was able to increase the root/shoot ratio in cucumber seedlings by 20% compared to control plants.
Arthrospira is one of the most studied cyanobacteria for biostimulant applications and many works report positive effects of Arthrospira on various plant species (Hegazi et al. 2010; Singh et al. 2011; Aghofack-Nguemezi et al. 2015; Tuhy et al. 2015; Godlewska et al. 2019; Mutale-joan et al. 2020), while in other species such as chili pepper and strawberry the stimulatory effect on vegetative growth was weak and not significant (Jufri and Sulistyono 2016; Soppelsa et al. 2019). According to our knowledge, to date there are no studies on the effect of extracts or hydrolysates from cyanobacteria, including Arthrospira, on basil. The Arthrospira sp. hydrolysate tested in the present study did not produce effects on basil growth. Since the effectiveness of a cyanobacterial species can vary among different plant species and even different cultivars of the same species (Santini et al. 2021) we cannot exclude that the Arthrospira sp. hydrolysate we have tested would be effective if applied on a different plant.
In our work, the animal protein hydrolysate caused a slight depression of plant growth and, in some cases, leaf yellowing. Repeated foliar treatments with protein hydrolysates obtained by chemical hydrolysis, one of the most common method for animal waste (Colla et al. 2015), are reported to be phytotoxic due to the abundance of free amino acids with high levels of racemization (Lisiecka et al. 2011).
The weekly foliar and basal application of Ascophyllum nodosum extracts were found to increase leaf number, weight and plant height in mint and basil (Elansary et al. 2016), while in the present study, at the tested doses, no significant differences with respect to the control were detected in these parameters. Although the beneficial effects of seaweed extracts are known since the early 1940s (Craigie 2011), several researches have highlighted the variable nature of these products, which frequently do not have reproducible effects on plants (Chojnacka et al. 2012; Sharma et al. 2012; Goñi et al. 2016; Yakhin et al. 2017; Boukhari et al. 2020). According to a recent transcriptomic study, two extracts obtained from A. nodosum resulted in dysregulation of 4.47 and 0.87% of the transcriptome of Arabidopsis thaliana, which implies an important variability in the responses elicited (Goñi et al. 2016). Moreover, in nature seaweeds can accumulate heavy metals (Besada et al. 2009), as observed in this work for the A. nodosum hydrolysate, that contained arsenic in quantities just below the maximum value set by the European regulation.
The A. nodosum hydrolysate used in the present work showed a considerably lower carbohydrate content than that reported in literature for brown seaweeds (Yuan and Macquarrie 2015). This may be explained by seasonal variations in A. nodosum biomass composition (Tabassum et al. 2016), and by the fact that the phenol-sulfuric acid method may not detect all types of fibers without sample pretreatment. In our study, protein and lipid content in A. nodosum was in line with literature data (Craigie 2011; Blanco-Pascual et al. 2014). Furthermore, the A. nodosum hydrolysate had a larger fraction of unidentified compounds compared to the other hydrolysates tested. This may be due, besides to the high content of fiber (MacArtain et al. 2007), to mineral residues present in the collected biomass and derived from alkaline hydrolysis.
According to the results of the present study, the raw composition of a hydrolysate might influence its efficacy. In the most active cyanobacterial hydrolysates, carbohydrates were the major component (on average 47%), followed by proteins (35%) and lipids (5%), while the hydrolysates that did not produce significant effects on plant growth presented the highest values of proteins (on average 66%). Many cyanobacteria are known to contain high amounts of carbohydrates in the form of intracellular monosaccharides, polymeric reserve α-glucans and structurally complex extracellular polysaccharides (Rossi and De Philippis 2016). The potential use of cyanobacterial polysaccharides as foliar biostimulants was recently reported (Elarroussi et al. 2016, 2018; Rachidi et al. 2020). The polysaccharides extracted or released from cyanobacteria (A. platensis) and microalgae (Dunaliella salina and Porphyridium sp.) showed growth promoting effects in tomato and pepper (Elarroussi et al. 2016, 2018; Rachidi et al. 2020).
Despite carbohydrates are one of the major components of the cyanobacterial hydrolysates and may contribute to the observed effects, other bioactive molecules may also work as plant growth promoters (Rouphael and Colla 2018). Cyanobacteria are able to synthesize phytohormones, mainly auxins (Sergeeva et al. 2002; Hashtroudi et al. 2013), but also cytokinins (Hussain et al. 2010), abscisic acid (Zahradníčková et al. 1991; Maršálek et al. 1992; Esch et al. 1994) and polyamines (Mógor et al. 2018) which are known to play crucial roles in plant development. Macro- and microelements of the hydrolysates can also take part in the biostimulation process. For instance, it has been reported that potassium in seaweed extracts has a positive effect in regulating water status and enhancing plant photosynthesis and meristematic growth (Hernández-Herrera et al. 2014). In the present study we have observed that cyanobacterial hydrolysates contain macro- and microelements that can be readily absorbed by leaves through stomata and cuticle hydrophilic pores. However, taking into consideration their low concentrations in the hydrolysate, the amount of hydrolysate and the number of doses applied, as well as the nutrient sufficient growth condition of the basil plants, we could rule out the contribution of macro- and microelements in the cyanobacterial hydrolysates to plant growth through fertilization. In fact, according to the doses applied (on average 20 mg of hydrolysate dry weight per plant for the whole cycle), treatments with the nitrogen richest hydrolysates, namely Arthrospira sp. and Anabaena sp. hydrolysates, provided on average 3 mg of nitrogen per plant, treatments with Nostoc sp., Tolypothrix sp. and Leptolyngbya sp. hydrolysates provided on average 1.6 mg per plant, while half-strength Hoagland medium supplied 252 mg per plant. Therefore, the nitrogen contribution of the cyanobacterial hydrolysates amounted to 0.6-1.2% of the total nitrogen provided to plants. In addition, the cyanobacterial hydrolysates provided on average 1.2% of Fe, 1.1% of S, 1% of Mg, 0.21% of P, 0.02% of K and 0.08% of Ca with respect to the total amount of nutrients supplied with the half-strength Hoagland medium.
Conclusions
The present study reveals the remarkable biostimulant properties on basil plants of three cyanobacterial hydrolysates, which performed much better than two commercial products in the given experimental conditions. These results, together with those already available in the literature, candidate cyanobacteria to become a sustainable raw material for the development of a new category of plant biostimulants. However, not all the cyanobacterial hydrolysates tested had a significant effect on plant growth, which suggests that the biostimulant properties of cyanobacteria might be species-specific and might depend on the metabolites produced under the experimental conditions adopted. In particular, the most efficient hydrolysates exhibited a high carbohydrate content, suggesting the importance of this class of molecules in biostimulation. The results obtained are a first step and further studies are needed to determine the contribution of carbohydrates in biostimulation and to characterize components of this fraction as well as other bioactive molecules (e.g., phytohormones) in the hydrolysates.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Aghofack-Nguemezi J, Schinzoumka PA, Tatchago V (2015) Effets des extraits ou de la poudre de Spirulina platensis et Jatropha curcas sur la croissance et le développement de la tomate. J Appl Biosci 90:8413–8420
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plants 10:531
Besada V, Andrade J, Schultze F, Gonzalez JJ (2009) Heavy metals in edible seaweeds commercialised for human consumption. J Mar Syst 75:305–313
Bhatia SK, Mehariya S, Bhatia RH, Kumar M, Pugazhendhi A, Awasthi MK, Atabani AE, Kumar G, Kim W, Seo S-O, Yang Y-H (2020) Wastewater based microalgal biorefinery for bioenergy production: progress and challenges. Sci Total Environ 751:141599
Blanco-Pascual N, Montero MP, Gómez-Guillén MC (2014) Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll 37:100–110
Borowitzka MA (2016) Chemically-mediated interactions in microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The Physiology of Microalgae. Developments in Applied Phycology. Springer, Dordrecht, pp 321–357
Boukhari MEME, Barakate M, Bouhia Y, Lyamlouli K (2020) Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 9:359
Bulgari R, Baldi A, Ferrante A, Lenzi A (2017) Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. NZ J Crop Hortic Sci 45:119–129
Chai WS, Tan WG, Halimatul Munawaroh HS, Gupta VK, Ho S-H, Show PL (2021) Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ Pollut 269:116236
Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B (2013) Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int J Biol Macromol 58:73–78
Chojnacka K, Saeid A, Witkowska Z, Tuhy L (2012) Biologically active compounds in seaweed extracts - the prospects for the application. Open Conf Proc J 3:20–28
Colla G, Nardi S, Cardarelli M, Ertani A, Lucini L, Canaguier G, Rouphael Y (2015) Protein hydrolysates as biostimulants in horticulture. Sci Hortic 196:28–38
Craigie J (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
de Morais MG, da Silva VB, de Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Bio Med Res Inter 4:835761
Du Jardin P (2015) Plant biostimulants: Definition, concept, main categories and regulation. Sci Hortic 196:3–14
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Elansary HO, Yessoufou K, Shokralla S, Mahmoud EA, Skalicka-Woźniak K (2016) Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind Crop Prod 92:50–56
Elarroussi H, Elmernissi N, Benhim AR, El Kadmir IIM, Bendaou N, Smouni A, Wahbya I (2016) Microalgae polysaccharides a promising plant growth biostimulant. J Algal Biomass Utln 7:55–63
Elarroussi H, Benhima R, Elbaouchi A, Sijilmassi B, Elmernissi N, Aafsar A, Meftah-Kadmiri I, Bendaou N, Smouni A (2018) Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J Appl Phycol 30:2929–2941
Esch H, Hundeshagen B, Schneider-Poetsch HJ, Bothe H (1994) Demonstration of abscisic acid in spores and hyphae of the arbuscular-mycorrhizal fungus Glomus and in the N2-fixing cyanobacterium Anabaena variabilis. Plant Sci 99:9–16
Ganesan R, Manigandan S, Samuel MS, Shanmuganathan R, Brindhadevi K, Chi NTL, Duc PA, Pugazhendhi A (2020) A review on prospective production of biofuel from microalgae. Biotech Rep 27:e00509
Garcia-Vaquero M, Rajauria G, O’Doherty JV, Sweeney T (2017) Polysaccharides from macroalgae: recent advances, innovative technologies and challenges in extraction and purification. Int Food Res J 99:1011–1020
Gnaiger E, Bitterlich G (1984) Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62:289–298
Godlewska K, Michalak I, Pacyga P, Baśladyńska S, Chojnacka K (2019) Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J Microbiol Biotechnol 35:80
Goñi O, Fort A, Quille P, McKeown PC, Spillane C, O’Connell S (2016) Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: same seaweed but different. J Agric Food Chem 64:2980–2989
González López CV, Cerón García MC, Acién Fernández FG, Segovia Bustos C, Chisti Y, Fernández Sevilla JM (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Grand View Research (2018) Biostimulants market size, share & trends analysis report by active ingredient (acid based, seaweed extract, microbial), by crop type (row crops & cereals), by application (foliar, soil) and segment forecasts, 2018-2025. https://www.grandviewresearch.com/industry-analysis/biostimulants-market; searched on 14 February 2022
Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22:43–50
Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U (2015) The use of biostimulants for enhancing nutrient uptake. Adv Agron 130:141–174
Hashtroudi MS, Ghassempour A, Riahi H, Shariatmadari Z, Khanjir M (2013) Endogenous auxins in plant growth-promoting cyanobacteria—Anabaena vaginicola and Nostoc calcicola. J Appl Phycol 25:379–386
Hegazi AZ, Mostafa SSM, Ahmed HMI (2010) Influence of different cyanobacterial application methods on growth and seed production of common bean under various levels of mineral nitrogen fertilization. Nat Sci 8:183–194
Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, Norrie J, Hernández-Carmona G (2014) Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J Appl Phycol 26:619–628
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn 347:1–32
Hu Q (2019) Current status, emerging technologies, and future perspectives of the world microalgal industry. In: Book of Abstracts AlgaEurope Conference. European Algae Biomass Association-EABA, Florence, Italy, p 139
Hussain A, Krischke M, Roitsch T, Hasnain S (2010) Rapid determination of cytokinins and auxin in cyanobacteria. Curr Microbiol 61:361–369
Jufri AFS, Sulistyono E (2016) Effects of dry Spirulina platensis and antitranspirant on growth and yield of chili pepper (Capsicum annuum L.). J Agron Indones 44:170–175
Lisiecka J, Knaflewski M, Spiżewski T, Frąszczak B, Kałużewicz A, Krzesiński W (2011) The effect of animal protein hydrolysate on quantity and quality of strawberry daughter plants cv. “Elsanta”. Acta Sci Pol 10:31–40
MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR (2007) Nutritional value of edible seaweeds. Nutr Rev 65:535–543
Makino A, Nakano H, Mae T, Shimada T, Yamamoto N (2000) Photosynthesis, plant growth and N allocation in transgenic rice plants with decreased Rubisco under CO2 enrichment. J Exp Bot 51:383–389
Maršálek B, Zahradníčková H, Hronková M (1992) Extracellular abscisic acid produced by cyanobacteria under salt stress. J Plant Physiol 139:506–508
Marsh JB, Weinstein DB (1966) Simple charring method for determination of lipids. J Lipid Res 7:574–576
Mógor AF, Ördög V, Pereira Lima GP, Molnár Z, Mógor G (2018) Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J Appl Phycol 30:453–460
Muller-Feuga A (2004) Microalgae for aquaculture: the current global situation and future trends. In: Richmond A (ed) Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Blackwell Science, Oxford, pp 352–364
Mutale-joan C, Merghoub N, Arroussi ELH (2019) Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J Microbiol Biotechnol 35:177
Mutale-joan C, Redouane B, Najib E, Yassine K, Lyamlouli K, Laila S, Zeroual Y, EL Arroussi H (2020) Screening of microalgae liquid extracts for their biostimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L.. Sci Rep 10:2820.
Norton TA, Melkonian M, Andersen RA (1996) Algal biodiversity. Phycologia 35:308–326
Ördög VL, Pulz O (1996) Diurnal changes of cytokinin-like activity in a strain of Arthronema africanum (Cyanobacteria) determined by bioassay. Algol Stud 82:57–67
Petersen B, Snapp S (2015) What is sustainable intensification? Views from experts. Land Use Policy 46:1–10
Priya H, Prasanna B, Ramakrishnan N, Bidyarani N, Babu S, Thapa S, Renuka N (2015) Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol Res 171:78–89
Rachidi F, Benhima R, Sbabou L, Elarroussi H (2020) Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotech Rep 25:e00426
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 565–590
Rouphael Y, Colla G (2018) Synergistic biostimulatory action: designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci 9:1655
Sahu D, Priyadarshani I, Rath B (2012) Cyanobacteria as potential biofertilizer. CIBTech J Microbiol 1:20–26
Santini G, Biondi N, Rodolfi L, Tredici MR (2021) Plant biostimulants from cyanobacteria: an emerging strategy to improve yields and sustainability in agriculture. Plants 10:643
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Sgherri C, Cecconami S, Pinzino C, Navari-Izzo F, Izzo R (2010) Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem 123:416–422
Shariatmadari Z, Riahi H, Abdi M, Hashtroudi MS, Ghassempour AR (2015) Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. J Appl Phycol 27:2279–2287
Sharma SHS, Lyons G, McRoberts C, McCall D, Carmichael E, Andrews F, Swan R, McCormack R, Mellon R (2012) Biostimulant activity of brown seaweed species from Strangford Lough: compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigna mungo L.) and pak choi (Brassica rapa chinensis L.). J Appl Phycol 24:1081–1091
Sharma V, Prasanna R, Hossain F, Muthusamy V, Nain L, Das S (2020) Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. Biotech 10:1–15
Shukla PS, Mantin EG, Adil M, Bajpai S, Critchley AT, Prithiviraj B (2019) Aschophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:655
Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z (1999) Basil: A source of aroma compounds and a popular culinary and ornamental herb. In: Janick J (ed) Perspectives on new crops and new uses. ASHS Press, Arlington, pp 449–505
Singh S (2014) A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J Appl Microbiol 117:1221–1244
Singh DP, Prabha R, Yandigeri MS, Arora DK (2011) Cyanobacteria-mediated phenylpropanoids and phytohormones in rice (Oryza sativa) enhance plant growth and stress tolerance. Antonie Van Leeuwenhoek 100:557–568
Soppelsa S, Kelderer M, Casera C, Bassi M, Robatscher P, Matteazzi A, Andreotti C (2019) Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agron 9:483
Stirk W, Rengasamy K, Kulkarni M, van Staden J (2020) Plant biostimulants from seaweed: an overview. In: Geelen D, Xu L (eds) The chemical biology of plant biostimulants. John Wiley & Sons, London, pp 33–55
Suganya T, Varman M, Masjuki HH, Renganathan S (2016) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sust Energ Rev 55:909–941
Swain PK, Biswal T, Panda RB (2021) Role of microalgae as biofertilizer for sustainable environment. In: Acharya SK, Mishra DP (eds) Current advances in mechanical engineering, lecture notes in mechanical engineering. Springer, Singapore, pp 371–382
Tabassum MR, Xia A, Murphy JD (2016) Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour Technol 216:219–226
Takács G, Stirk WA, Gergely I, Molnár Z, van Staden J, Ördög V (2019) Biostimulating effects of the cyanobacterium Nostoc piscinale on winter wheat in field experiments. S Afr J Bot 126:99–106
Toribio AJ, Suárez-Estrella F, Jurado MM, López MJ, López-González JA, Moreno J (2020) Prospection of cyanobacteria producing bioactive substances and their application as potential phytostimulating agents. Biotechnol Rep 26:e00449
Tuhy Ł, Samoraj M, Witkowska Z, Chojnacka K (2015) Biofortification of maize with micronutrients by Spirulina. Open Chem 13:1119–1126
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049
Yuan Y, Macquarrie DJ (2015) Microwave assisted acid hydrolysis of brown seaweed Ascophyllum nodosum for bioethanol production and characterization of alga residue. ACS Sustain Chem Eng 3:1359–1365
Zahradníčková H, Maršálek B, Polišenská M (1991) High-performance thin-layer chromatographic and high-performance liquid chromatographic determination of abscisic acid produced by cyanobacteria. J Chromatogr 555:239–245
Acknowledgments
Authors acknowledge Massimo D’Ottavio for the hydrolysates biochemical characterization. Authors also thanks Centro di Competenza VALORE, Florence, Italy (Regione Toscana, Par-FAS 2007-2013 Projects) where part of the analyses were carried out.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: Gaia Santini, Liliana Rodolfi, Natascia Biondi, Mario R. Tredici; Formal analysis: Gaia Santini; Investigation: Gaia Santini, Giacomo Sampietro; Writing-Original Draft: Gaia Santini; Writing-Review & Editing: Gaia Santini, Liliana Rodolfi, Natascia Biondi, Giacomo Sampietro, Mario R. Tredici.
Corresponding author
Ethics declarations
Competing Interests
The cyanobacterial hydrolysates were provided by Fotosintetica & Microbiologica S.r.l., in which Giacomo Sampietro, Mario R. Tredici and Liliana Rodolfi have a financial interest. All the other authors have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santini, G., Rodolfi, L., Biondi, N. et al. Effects of cyanobacterial-based biostimulants on plant growth and development: a case study on basil (Ocimum basilicum L.). J Appl Phycol 34, 2063–2073 (2022). https://doi.org/10.1007/s10811-022-02781-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02781-4