Abstract
In-water remediation strategies, implemented in conjunction with traditional watershed management, could help minimize the impact of excess nitrogen (N) on marine ecosystems. Seaweed farming and harvesting may have potential as in-water N remediation tools in the Western Gulf of Maine (WGoM), but more understanding of the associated spatial and temporal variability is needed. In this study, Saccharina latissima was grown and collected from four WGoM sites in 2016–2019 and analyzed for tissue N content and stable isotopes. The source of N taken by the kelp was not obvious from monthly nor interannual mean δ15N measured in the kelp tissue, and the interannual means were significantly different between sites in the same bay. Mean kelp biomass across all sites and years was 9.84 (± 2.53)–14.84 kg (wet weight) per meter of longline at time of harvest (late May–early June). Nitrogen content of the S. latissima tissue was 1.04–3.82% (± 0.22) (dry weight) throughout the growing season and generally decreased through the spring. Using these results, we estimated that harvesting a hypothetical hectare of S. latissima after 6–7 months of cultivation in the WGoM would have the potential to remove 19.2 (± 4.8)–176.0 (± 7.7) kg N ha−1, depending on the density of longlines. The wide ranges of both biomass at time of harvest, and δ15N and percent N content in the kelp tissue, highlight the need for site-specific pilot studies, even within a specific bay, prior to implementing kelp aquaculture as an in-water tool for N bioextraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrient pollution is one of the principal causes of poor coastal water quality and habitat degradation (Nixon 1995, 1998; Diaz and Rosenberg 2008; Paerl et al. 2014). Globally, an estimated 245,000 km of coastline are considered “dead zones” triggered by excessive input of reactive nitrogen (N) and phosphorus (P) (Diaz and Rosenberg 2008). In the United States of America (USA), a nationwide excess of reactive N from anthropogenic sources has caused impairment to an estimated two-thirds of the country’s coastal waters (Bricker et al. 1999; Howarth et al. 2002). Moreover, the degree of coastal nutrient loading to the Northeastern USA coastline is considered one of the highest on Earth (Boesch 2002; Howarth 2008). Nutrient pollution, in combination with other trace elements supporting primary production, results in areas of hypoxia and anoxia, habitat degradation, altered food webs, loss of biodiversity, increased instances of green or harmful algal blooms, and greater susceptibility to localized ocean acidification (Nixon 1987, 1995; Paerl 1997; Paerl and Whitall 1999; Breitburg et al. 2009, 2018; Wallace et al. 2014).
In this study we focus on nutrient concerns in the Western Gulf of Maine (WGoM) bordering Massachusetts (MA), New Hampshire (NH), and Maine (ME), USA. Bays and estuaries adjacent to these states are waterbodies of emerging concern due to both point and nonpoint sources of reactive N (Castro et al. 2003; Liebman et al. 2012). Effluent from wastewater treatment facilities (WWTFs) is the most common point source of N to the WGoM; however, substantial N contributions from nonpoint N sources like stormwater runoff, agricultural runoff, and atmospheric deposition also occur in the region (Castro et al. 2003; Liebman et al. 2012; Trowbridge et al. 2014). Atmospheric N deposition is estimated to be 30–40% of the total N load in many locations and stormwater runoff has been estimated to contribute another 30–35% of the nonpoint source N loading (Castro et al. 2003; Liebman et al. 2012; Trowbridge et al. 2014). New Hampshire and Massachusetts have implemented N discharge limits and strategies targeting both point and nonpoint source N to address and minimize the deleterious effects of excess nutrients on the WGoM (Reitsma et al. 2017). Maine, the state with the most coastline bordering the WGoM, has yet to establish nutrient criteria.
In addition to improving point source discharges, resource managers in Maine are interested in nutrient bioextraction as part of a system-wide approach integrating watershed load reductions and enhanced nutrient assimilation (Liebman et al. 2012). Nutrient bioextraction strategies, also referred to as bioremediation, aim to remove nutrients that exceed the flushing and assimilation capacity of the system, regardless of their source (Krom 1986; Chopin et al. 2001; Neori et al. 2004). Bioextraction efforts in coastal water bodies typically target dissolved inorganic nitrogen (DIN) because it often limits primary production in temperate marine ecosystems (Ryther and Dunstan 1971; Lobban and Harrison 1994). Excess dissolved inorganic P and dissolved carbon (C), and small amounts of dissolved organic N and P when inorganic nutrient levels are low (Li et al. 2016), are also removed from the environment during bioextraction (Bianchi 2007).
Many primary producers are suitable for use in bioextraction, but recently more attention has been given to the use of macroalgae in this role. Macroalgae naturally extract N from the marine environment because N is one of the key macronutrients required for protein and nucleic acid synthesis; and kelps are highly productive (Gao and McKinley 1994; Valiela et al. 1997; Neori et al. 2004). Previous studies have evaluated a range of macroalgal species and cultivation systems, including temperate and tropical macroalgae, land-based systems, integrated multi-trophic aquaculture (IMTA) systems, and nearshore marine installations. Many of these studies strategically cultivated a desirable alga to remove DIN from the surrounding water (Goldman et al. 1974; Ryther et al. 1975; Neori et al. 1996, 2004; Chopin et al. 1999, 2001, 2012; Buschmann et al. 2001; Troell et al. 2003; Abreu et al. 2011; Sanderson et al. 2012; Wang et al. 2012, 2014; Broch et al. 2013; Handå et al. 2013; Kim et al. 2014, 2015; Yarish et al. 2017; Fossberg et al. 2018). To determine the origin of the removed DIN, the N isotope ratio (δ15N) in the algal tissue can be compared to the isotopically distinct δ15N of nitrogen originating from oceanic, atmospheric, treated wastewater, or fertilizer (Heaton 1986; Owens 1987; Peterson and Fry 1987).
Seaweed aquaculture and harvesting activities are expanding in the WGoM (Grebe et al. 2019; Maine Department of Marine Resources [MEDMR] 2019), which raises the question: can this growing industry potentially contribute to the maintenance or enhancement of the WGoM’s assimilative capacity for nutrients? Aquaculture leaseholders in Maine reported harvesting approximately 127 t wet weight (WW) of cultivated macroalgae in 2019 (MEDMR 2019), the majority of which was processed or sold as edible (Piconi et al. 2020). Maine’s seaweed production is projected to grow at 12–15% annually to reach a total annual yield of 1360–2720 t (WW) by 2035 and new market opportunities in livestock feed, fertilizer, pharmaceuticals, and carbon or nutrient offsets are expected (Piconi et al. 2020). Most of the current seaweed aquaculture expansion is focused on kelp (order Laminariales). The most commonly grown species in Maine are the following: Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders (sugar kelp), Saccharina angustissima (Collins) Augyte, Yarish, & Neefus (skinny kelp), and Alaria esculenta (Linnaeus) Greville (winged kelp or horsetail kelp) (Grebe et al. 2019; Bricknell et al. 2020). Of the three, S. latissima is also the most frequently grown species in the USA (Kim et al. 2015; Yarish et al. 2017). In this study, we focus only on the bioextraction potential of S. latissima.
Previous studies have estimated N bioextraction by S. latissima grown in other regions by multiplying the percent N content in the kelp tissue by biomass harvested and extrapolating to a larger area (Neori et al. 2004; He et al. 2008; Chopin et al. 2012; Kim et al. 2015; Wu et al. 2015; Xiao et al. 2017; Yarish et al. 2017). Findings from these studies suggest that S. latissima aquaculture can be a useful nutrient extraction strategy in specific regions or seasons, but there is a need for more long-term estimates from a wide range of locations. Along the Eastern USA coastline, the need for improved understanding of the temporal and spatial variability of N dynamics and the related bioextraction efficiencies of specific macroalgal species is especially strong (Kim et al. 2007, 2015; Liebman et al. 2012). Kim et al. (2015) and Yarish et al. (2017) provided N bioextraction estimates by S. latissima grown in New York, Connecticut, and southern Massachusetts, but the temperature gradient along the Eastern USA coastline is one of the steepest in the world, and temperature has a strong influence on S. latissima growth (Fortes and Lüning 1980; Bolton and Lüning 1982). A better understanding of the expected macroalgal N bioremediation ranges is essential from a management perspective because bioextraction can be expensive (Neori et al. 2004). Some commonly used nutrient management practices are not adequately assessed and later found to be moderately ineffective (Boesch et al. 2001). Overestimating efficiencies of management measures is costly from a financial perspective, but it also damages social capital that had to be built by resource managers prior to initiating the treatment strategy. Thus, identifying local-regional patterns or commonalities across local studies can help build a better understanding of the range of results expected from bioextraction efforts.
In this study, we aimed to expand on previous work evaluating bioextraction by macroalgae along the Eastern USA coast to gain a more comprehensive understanding of the N extraction potential in the region. First, we estimated the N extraction of kelp harvested from the WGoM in late spring and throughout the growing season to determine the effect of harvest timing, biomass, and percent tissue N content on the total N removed from the surrounding water. Then, we sought to characterize the source of DIN taken up by the kelp by measuring the δ15N in the collected tissue. Lastly, we provided regional context for the potential N removed through harvesting cultivated S. latissima from the WGoM by estimating the amount of harvested kelp needed to extract N equivalent to the N loading from atmospheric deposition, activities in the watershed upland of the coast, and treated wastewater effluent.
Materials and methods
Study site descriptions
The Gulf of Maine (GoM) is a temperate, biologically productive, waterbody extending from Nova Scotia, Canada, to Cape Cod, Massachusetts, USA (Fig. 1). Offshore, much of the GoM’s productivity is from the upwelling of nutrient-rich water from deep on the continental slope (Townsend 1998; Bricknell et al. 2020). In the coastal zone, nutrient delivery and cycling are influenced by vertical mixing by tides, wind-driven transport, small- and large-scale buoyancy forcing, large freshwater sources, atmospheric deposition, wastewater treatment facilities (WWTFs), and stormwater runoff (Garret et al. 1978; Townsend 1991; Pettigrew et al. 1998, 2005; Castro et al. 2003; Trowbridge et al. 2014).
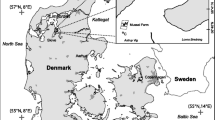
Map of the four study sites: Wood Island, Ram Island, Brothers Island, and Cow Ledge (gray diamonds). a Casco and Saco Bay, Maine, b Western Gulf of Maine (WGoM), and c the Northwestern Atlantic Ocean. Freshwater sources of interest are the Saco River, Scarborough River, Capisic Brook, Presumpscot River, and Royal River (black lines). White-circled black dots are the cities of Portland and South Portland, white dots are combined sewer overflows, and gray dots designate pollutant discharge elimination system outfalls administered by the Maine Department of Environmental Protection (https://www.maine.gov/dep/gis/datamaps/). Basemaps: QGIS Open Street Map and GADM (https://gadm.org/)
Cultivation and sampling occurred at four sites in the Western Gulf of Maine. Two of the sites were in Casco Bay, Maine. We refer to these sites as Brothers (Bros.) Island and Cow Ledge because they were near these geographical features. The Brothers Island and Cow Ledge sites were < 3 km apart and the longlines were oriented in a similar cardinal direction (north-south) which was parallel to the prevailing current. The other two cultivation and sampling sites, Ram Island and Wood Island, were in Saco Bay, Maine (Fig. 1). The Ram Island and Wood Island sites were < 4 km apart and the longlines were oriented in a similar cardinal direction (east-west) and parallel to the prevailing current.
Casco Bay has a relatively complex, indented shoreline, whereas Saco Bay is a relatively uniform, crescent bay (Tanner et al. 2006). Previous studies have concluded that land-based N sources dominate nearshore N concentrations in Casco Bay (Castro et al. 2003; Gray 2019). Less information is available for Saco Bay, but it is presumably also heavily impacted by land-based N. Both bays receive substantial freshwater and nutrient contributions from rivers draining upland watersheds (Wade et al. 2008; Tilburg et al. 2011, 2015; Gray 2019), WWTFs employing secondary treatment, and combined sewer overflows (Maine Department of Environmental Protection [MEDEP] 2019). Combined sewer overflows (CSOs) contribute land-based nutrients to the bays after heavy rainstorms when stormwater runoff is channeled into the combined sewer collection system at a volume that exceeds the capacity of the treatment facility (MEDEP 2019). In 2019, these CSOs collectively discharged 768,000 m3 of untreated stormwater runoff and wastewater into Casco Bay and 273 m3 into Saco Bay (Riley 2020).
Field measurements and laboratory procedures
Kelp cultivation and collection
Saccharina latissima sporelings were produced using the methodology described in Redmond et al. (2014). Briefly, we collected wild S. latissima reproductive tissue from nearby bays and stressed it in the laboratory to release spores. Thin line was inoculated in water containing the released spores (6000–8000 spores mL−1) over night and then transferred to aquaria. The sporelings grew in light and temperature-controlled aquaria for approximately 6–8 weeks. Outplanting occurred between October and December each year. Kelp installations at each site consisted of 1 (Wood Island and Ram Island), 2 (Brothers Island and Cow Ledge in 2018), and 5 (Cow Ledge in 2019) longlines suspended 2 m below the water’s surface. Each longline was 60–120 m long, and the spacing between each line was ≥ 6 m. Each site was less than 1 km from shore. Water depths on site were 7–17 m mean lower low water (MLLW).
Kelp cultivation occurred during four growing seasons: October to June 2016–2019. Sample collection typically began in January or February when the individual sporophytes were 30–50 cm long and 7–8 g (WW). The sporophytes were too small to obtain density estimates at that time. However, in mid-March, mean sporophyte density was typically 200–500 sporophytes m−1. At maturity in late May, mean sporophyte density was approximately 200 sporophytes m−1.
During sampling events, we maintained the sample integrity by removing the entire organism (holdfast, stipe, and blade) using nitrile gloves. Access to the sampling sites was weather dependent and thus, sampling frequency varied throughout the season and from year to year. During the most rigorous sampling season (2019), we completed approximately 10 sampling events at each site: roughly once per month, December through February, and 2–4 times per month from March to June. The timing of sampling was also variable across tides and time of day. At Cow Ledge in 2019, where there were 5 longlines, we collected kelp from the outermost line. All collected kelp was stored in plastic bags, transported in a covered cooler, and refrigerated at 8 °C until further processing. Transportation between the field and the laboratory was 1–2 h.
Biomass analysis
We removed and weighed all sporophytes from three, 10-cm sections of the longline to generate a mean biomass estimate for each sampling date. The location of the sections along the longline was haphazardly determined. (During a few sampling events and seasons, only one biomass measurement was possible. We do not report standard deviations for these cases). Then, we multiplied the mean biomass (WW) per 10 cm by 10 to obtain an estimate of kelp biomass (WW) per longline meter. We also established a wet to dry ratio for the samples by weighing the collected kelp upon removal from the plastic bag in the laboratory and again immediately after it had been lyophilized. The difference between the two weights was attributed to water loss and used to establish a wet to dry ratio.
Elemental and stable isotope analysis
On each sampling event, we haphazardly collected five individual sporophytes for elemental and stable isotope analysis. Within 12 h of collection, we excised a 4 cm2 cutout from the basal tissue near the meristem, where metabolic activity is concentrated (Nielsen et al. 2014; Boderskov et al. 2016). The tissue was rinsed with deionized water and lightly rubbed between gloved hands for 30 s. No epiphytic algae were visibly present on the sporophytes. A small percentage (< 5%) of the sporophytes had snails (Lacuna vincta) or egg rings attached to them, which we manually removed. The tissue samples were stored in a − 40 °C freezer. The frozen tissue was lyophilized at − 50 °C using a Labconco FreeZone Legacy 2.5 Liter Benchtop Freeze Dryer. After 24 h of drying, the lyophilized samples were homogenized into a fine powder using a mortar and pestle. The powder (2.5–5 mg) was encapsulated in tin capsules and shipped to the University of California Davis Stable Isotope Facility [UC Davis SIF] (https://stableisotopefacility.ucdavis.edu). The SIF analyzed each sample for total N, total C, 15N, and 14C using a PDZ Europa ANCA-GSL elemental analyzer interfaced with a PDZ Europa 20-20 isotope ratio mass spectrometer. The instruments have an analytical precision of 0.3‰ for 15N, and the instruments were calibrated before analysis with certified standards (UC Davis SIF 2017). Overall, 364 samples were analyzed for elemental content and stable isotope ratios. We calculated elemental ratios for samples using the measurements obtained from the UC Davis SIF. We obtained percent tissue N or C content by dividing the total weight of N or C measured in each sample by the encapsulated dry sample weight. Then, we calculated the C:N ratio (M:M) for each sample from these percentages.
A common approach for estimating N removed from the marine ecosystem is to multiply the percent tissue N content in the kelp at harvest by the biomass harvested (Neori et al. 2004; He et al. 2008; Kim et al. 2013, 2014, 2015). This methodology stems from the understanding that some N is immediately used to fuel macroalgal growth, and the surplus is stored as pigments, amino acids, and proteins (Martínez et al. 2012). Therefore, we also opted for this approach and estimated total N removed by S. latissima at the time of harvest using the mean percent tissue N calculated for the dry weight (DW) of the kelp during each sampling event (Eq. 1):
We adjusted the percent tissue N content by the WW:DW ratio and then multiplied by the estimated mean of kelp biomass (WW g m−1) for that site on the same sampling date. No assumptions regarding forms of N were included in these calculations.
The UC Davis SIF calculated the stable isotope ratios for each sample by comparing the difference in the 15N measured in the sample against the 15N in at least four different laboratory reference materials (Eq. 2) (Peterson and Fry 1987):
where R is 15N/14N. The δ15N of primary producers reflects the inorganic N sources used, plus a variable amount of fractionation (differential use of 15N vs. 14N during N uptake) (Fogel and Cifuentes 1993; Fry 2006). Thus, we compared the calculated ratios to known δ15N ranges for N from specific sources. The δ15N ranges most attributed to each N source are the following: − 2–0‰ for atmospheric N, − 3–3‰ for N from commercial fertilizers, 4–8‰ marine N from natural sources, and > 10‰ for N discharged from wastewater treatment processes (Heaton 1986; Macko and Ostrom 1994; McClelland et al. 1997; McClelland and Valiela 1998; Costanzo et al. 2001; Gartner et al. 2002; Cole et al. 2004; Kendall et al. 2007).
Environmental measurements
Water temperature was continuously measured using Hobo Pendant Temperature/Light 8K Data Loggers (Part #: UA-002-08). If a temperature logger was lost or compromised, we used temperature readings from the nearby University of Maine Land Ocean Biogeochemical Observatory buoys (2016 only) and a buoy maintained by the U.S. National Oceanic and Atmospheric Administration (NOAA) (Station CASM 1). A Tilt Current Meter (TCM-1; Lowell Instruments LLC) hanging inverted from the middle spacer-buoy on each longline continuously measured current velocity and direction. Photosynthetically active radiation (PAR) was measured at 2 m underwater using an LI-193 Spherical Quantum Sensor (LI-COR) during each sampling event May 2018–June 2019. Before these dates, we estimated photosynthetically active radiation (PAR) for each bay by transforming daily Global Horizontal Irradiance obtained from the National Solar Radiation Database (https://maps.nrel.gov/nsrdb-viewer) Physical Solar Model V.3 for the Portland International Jetport station (location ID 1364086). We considered all rainfall up to 60 h before sampling as potential runoff affecting the collected kelp but excluded snowfall. Rainfall data for Casco Bay sites came from the Portland International Airport weather station maintained by NOAA. The University of New England Marine Science Center Weather Station in Biddeford, ME, provided rainfall data for the Saco Bay sites.
We collected triplicate water samples from 2 m underwater during the 2018 and 2019 growing seasons using a horizontal Niskin bottle. All water samples were stored in sealed Whirl-pak bags, transported in a covered cooler to the laboratory, refrigerated at 8 °C, and processed within 4 h of collection. Each water sample was analyzed to estimate salinity, pH, and NO3−–N concentration at the time of sampling. We measured salinity using a Cole-Parmer RSA-BR90A Refractometer (0–90%) and a HACH benchtop meter (model #: PW172KB0703F01) calibrated to certified standards to measure pH. We determined the concentrations of NO3−–N in each sample spectrophotometrically using HACH Nitrate TNTplus Low Range Vial Tests and a HACH DR3900 Laboratory VIS Spectrophotometer calibrated before analysis with certified standards. We chose to enumerate NO3−–N because it is a common form of problematic reactive N in waterways impacted by anthropogenic activities (Galloway et al. 2004) and more easily measured through grab sampling than nitrite or ammonium.
Statistical analysis
We examined all data for assumptions related to normality and homogeneity of variance. We identified and removed outliers using quantile ranges, robust fit, a k-nearest neighbor analysis. Then, we examined data from each study area separately (i.e., individual sites) and collectively (all sites). We used multivariate analyses of variance (MANOVA) to compare the effect of 13 environmental factors on percent tissue N, percent tissue C, and δ13C, δ15N measured in the kelp tissue. The 13 environmental factors were as follows: site, bay, temperature, current, light, pH, salinity, ambient NO3−–N at the surface, ambient NO3−–N at 2 m deep, total rainfall received 60 h before sampling, grow-out week, distance from shore, and distance from nearest WWTF. When significant effects (p ≤ 0.05) were detected, each dependent variable was analyzed separately using one-way analyses of variance (ANOVA). We performed post hoc comparisons using Tukey’s honest significant difference HSD tests and measured Pearson R correlations between δ15N, percent tissue N, and the environmental factors. We used JMP Pro 14 for all statistical analyses.
Results
Environmental conditions
Monthly mean PAR, pH, salinity, NO3−–N, and water temperatures measured during the study varied by bay (Fig. 2). The ambient water conditions at the Saco Bay sites were generally colder, higher in nutrients, and lower in pH and salinity than the Casco Bay sites. The highest salinity (S) measured was in February (S = 35) and the lowest in early May (S = 8). The pH of water collected from the sites was 7.5–8.2. The highest pH values occurred in November, and the lowest values occurred in May. Mean monthly nitrate in the water samples was 0.29–11.8 μM NO3−–N. Peak nitrate values occurred in early March to mid-April and then declined mid-April through May. The water temperature at the kelp farms was 1–12 °C from November to June. We observed three distinct temperature intervals. The water temperature steadily declined from 10 °C in November to approximately 2–4 °C in mid-February. Then it oscillated between 2 and 4 °C from mid-February until mid-March. Then, water temperature began to increase before reaching 10–12 °C in late May. The current velocities measured at the Cow Ledge and Wood Island in 2018 and 2019 were 3–54 cm s−1. Specific current velocities are not available for Ram Island or Brothers Island, but they are probably like those at Cow Ledge and Wood Island, because tidal cycles drive most of the variability in currents within the nearshore WGoM.
Biomass and elemental analysis
Mean kelp biomass across all sites and years was 9.84 (± 2.53) to 14.84 kg WW m −1 longline at the time of harvest (Table 1). Wet to dry ratios of the kelp were 7.4:1 in March to 8.7:1 in May. The highest sampling frequency, and thus insight into biomass increase, occurred in Spring 2018 and Spring 2019 at the Wood Island site (Fig. 3). In 2018, biomass measurements at this site show that peak growth occurred in late May. Interestingly, measurements from 2019 at this site show peak growth from April until mid-May, followed by a decline in biomass in late May.
Kelp characteristics from the Wood Island site in 2018 and 2019. a Mean biomass (kg m−1) in 2018, b percent tissue nitrogen (DW) in 2018, c mean biomass (kg m−1) in 2019, and d percent tissue nitrogen (DW) in 2019. Error bars are standard deviation of ≥ 3 samples, and replicate biomass measurements were not collected in 2018
Mean N content of the S. latissima tissue, calculated for each sampling event, was 1.04–3.82% (± 0.22) DW throughout the growing season and generally decreased through the spring. Tissue N content at Wood Island in 2018 and 2019 illustrated the general trend: at this site, percent tissue N decreased 0.08–0.17% week−1 from mid-April to late-May (Fig. 3). In late May, percent tissue N contents were 1.04–2.29% (± 0.09) DW. Increasing water temperature was negatively associated with percent tissue N (MANOVA; F(9,155) = 37.49, p < 0.0001). Photosynthetically active radiation (PAR) was positively associated with percent tissue N (MANOVA; F(9,155) = 4.65, p = 0.032). Site also had significant effects on percent tissue N (MANOVA; F(9,155) = 4.10, p = 0.0078). There were no significant direct correlations (Pearson’s r) between the percent tissue N and environmental data. Across sites and all years, the mean C:N ratio (M:M) measured in the S. latissima tissue was 9.4 (± 0.7) to 23.4 (± 10.8). The lowest C:N ratios were in March; the highest ratios were in May and June (Fig. 4).
Stable isotopes
The interannual mean δ15N measured in the kelp tissue grown at the two Casco Bay sites was significantly different from the interannual mean δ15N measured in the kelp tissue grown at the two Saco Bay sites (Tukey’s HSD, df = 258, p < 0.0001). The widest range in distribution of δ15N was at the Wood Island site (0.79–10.09‰), and the narrowest range in distribution was at the Ram Island site (3.95–8.96‰) (Fig. 5). Cultivation site significantly affected δ15N values (MANOVA; F(9,155) = 19.33, p < 0.0001). Grow-out week had a positive effect on δ15N values (MANOVA; F(9,155) = 5.88, p < 0.0160), and ambient NO3−–N had a negative effect on δ15N values (MANOVA; F(9,155) = 5.12, p < 0.0240). There were no significant correlations (Pearson’s r) between δ15N, % tissue N, or environmental conditions at the sites. When combined by bay, the δ15N values mirror each other with the lowest values observed in February and then rising throughout the spring (Fig. 6).
Nitrogen isotope ratios (δ15N) in Saccharina latissima cultivated near Brothers Island, Cow Ledge, Ram Island, and Wood Island from 2016 to 2019. Shaded areas represent the range of δ15N commonly associated with nitrogen (N) from treated wastewater (δ15N = > 10‰), oceanic N (δ15N = 4–8‰), and fertilizers (δ15N= − 3–3‰). Unshaded ranges represent overlap between N sources. Ranges for δ15N from different sources were obtained from: Heaton 1986; Macko and Ostrom 1994; McClelland et al. 1997; McClelland and Valiela 1998; Costanzo et al. 2001; Gartner et al. 2002; Cole et al. 2004; Kendall et al. 2007
Monthly nitrogen isotope ratios (‰) measured in Saccharina latissima from Casco (gray fill) and Saco Bay (white fill) from 2016 to 2019. The range of δ15N commonly associated with nitrogen (N) from treated wastewater is (δ15N = > 10‰), oceanic N (δ15N = 4–8‰), and fertilizers (δ15N= − 3–3‰). Unshaded ranges represent overlap between N sources. Ranges for δ15N from different sources were obtained from: Heaton 1986; Macko and Ostrom 1994; McClelland et al. 1997; McClelland and Valiela 1998; Costanzo et al. 2001; Gartner et al. 2002; Cole et al. 2004; Kendall et al. 2007
Discussion
Biomass and bioextraction estimates
Saccharina latissima grew well in both Casco and Saco Bay. Biomass per longline meter at harvest (10–15 kg m−1) (WW) was on the higher end of the ranges previously reported in the literature (Table 3). The mean wet weight to dry weight ratio (WW:DW) ratio of the sporophytes at harvest was also slightly higher than the 7:1 reported by Sanderson et al. (2012) for cultivated S. latissima from an IMTA system in Scotland. In our data, the highest and lowest biomass measurements have almost 1% difference in the tissue N content (Cow 2018 vs. Wood 2019). The observed 1–4% N content is comparable to the range reported by other studies where S. latissima was grown in water with high DIN from anthropogenic or fish waste (Handå et al. 2013; Kim et al. 2015; Marinho et al. 2015; Yarish et al. 2017), but the higher 4% N tissue content also exceeded the upper value reported by several studies where S. latissima was grown in IMTA or relatively unimpacted water (Sanderson et al. 2012; Bruhn et al. 2016; Freitas et al. 2016; Fossberg et al. 2018). Maximum potential N removal did not coincide with peak percent tissue N and percent tissue N observed in the kelp at the time of May harvest was lower than previously observed for S. latissima in the Northwest Atlantic (Kim et al. 2015; Yarish et al. 2017). Almost all percent N and C:N ratios measured in the kelp tissue at harvest indicate that nitrogen was limiting. Like Kim et al. (2015) and Yarish et al. (2017), we observed a high degree of temporal and spatial variability of tissue N content in S. latissima.
As previously demonstrated, our results can be extrapolated to generate rough, hectare-scale estimates of potential bioextraction by kelp harvesting in the region. With a moderate, 6 m of spacing between longlines, a total of 1767 m of longline fit in one hectare of ocean surface. Multiplying this by the calculated kg kelp m−1 would result in a kelp harvest of 17.3 (± 4.4)–26.1 t WW ha−1. The intensive cultivation scenario, with 1.5-m spacing between longlines, has 6768 longline meters per hectare, which would produce 70.3–100.1 t WW ha−1. Converting by the WW:DW ratio (8.7:1), and then multiplying by the mean % tissue N measured in kelp from each site at harvest, results in an estimated 19.2 (± 4.8)–46.0 (± 2.0) kg N ha−1 that could be removed by harvesting a hectare of S. latissima with 6-m spacing between longlines and 73.5 (± 18.4)–176.0 (± 7.7) kg N ha−1 with 1.5-m spacing between longlines (Table 1).
Previously published estimates of N loading to Casco Bay (i.e., atmospheric N deposition, N loading from upland activities in the watersheds, and effluents from large WWTFs) help to put the potential bioextraction from kelp aquaculture in context (Table 2). We can calculate the approximate area of S. latissima harvest needed to remove a quantity of N equivalent to the N that is delivered to Casco Bay from these sources. In all examples considered, the quantity of N removed from Casco Bay by harvesting one hectare of S. latissima would be greater than the amount of N contributed to the Bay from one hectare of any loading sources. For example, even with 6-m spacing between longlines, the N extraction by 1 ha of S. latissima harvest is equivalent to the annual atmospheric deposition of N across 2.7 (± 0.7)–10.7 (±0.5) ha of Casco Bay or 5.1 (± 1.3)–12.1 (± 0.5) ha of activities in a nearby urban subwatershed. Insufficient data on N inputs prevents a direct comparison for Saco Bay, but we expect that the pattern would be similar.
These estimates of total N removed per hectare of kelp harvested from the WGoM (19.2–46 kg ha−1 with 6-m longline spacing) are higher than many of the ranges reported by other studies evaluating S. latissima for bioextraction at nearshore and IMTA sites (Table 3). Of particular interest, again, is the comparison between this study and those in closest proximity. In the Long Island Sound, CT and the Bronx River Estuary, NY, Kim et al. (2014, 2015) and Yarish et al. (2017) calculated 10–35 kg N ha−1 removed with 6-m longline spacing and 29–139 kg N ha−1 with 1.5-m longline spacing (Kim et al. 2014, 2015; Yarish et al. 2017). Additionally, Augyte et al. (2017) estimated 88.7 k g ha−1 N removal by closely related species, Saccharina angustissima (formerly Saccharina latissima forma angustissima), cultivated near Bristol, ME, and Sorrento, ME, using a 2.5-m spacing between longlines. We recalculated this to be 124 kg ha−1 N removal by S. angustissima with 1.5-m spacing between longlines and note that this estimate lies in the middle of the range reported by this study for S. latissima grown with the same longline spacing in Saco and Casco Bay.
This emphasizes the importance of considering cultivation density and harvest timing when evaluating bioextraction applications. Unsurprisingly, increasing the density of longlines on a hectare of ocean surface produced a much higher estimate of N extraction per hectare. However, we must consider these estimates with caution. The risk of overestimating bioextraction increases when extrapolating from dispersed longlines to higher densities because intensive cultivation reduces the water flow delivering nutrients, and thus the tissue N content, but values from low-density field studies do not reflect this (Kerrison et al. 2015; Marinho et al. 2015). Additionally, higher density cultivation could exceed the environmental or social carrying capacityFootnote 1 for kelp aquaculture in the region, which is why we have both evaluated a range of longline densities and underline the need for integrated management of N pollution.
The timing of kelp farm deployment and harvesting also influences bioextraction services of kelp grown in the WGoM. For example, from February through early May 2019, even as percent tissue N decreased throughout the spring, the biomass increased, and thus, so did the potential N removed through the harvest of all cultivated kelp. However, biomass did not increase in the same way during the last couple weeks of May 2019 due to reduced growth rates and sloughing, possibly associated with ambient water temperatures exceeding 10 °C. Therefore, to maximize the N extracted in 2019, the sugar kelp should have been harvested in early May rather than late May. The most dramatic example is from the Wood Island site in 2019, where harvesting one month earlier would have doubled N removal (27.4 kg ha−1 vs. 51 kg ha−1). However, these gains also appear to vary by site. At the other sites that same spring, harvesting 3–4 weeks earlier would have resulted in 3–22 kg ha−1 more N removed. Additionally, in 2018, the highest estimates of N removal were obtained in late May, possibly because the ambient water temperature did not reach 10 °C until that time. This highlights an opportunity for active monitoring of the ambient DIN and dissolved inorganic carbon at kelp aquaculture installations and N and C content in the kelp tissue. Using real-time estimates of N removal and ambient environmental conditions to schedule harvesting could maximize bioextraction effects.
Even with optimizations to harvest timing and density of longline arrays, kelp bioextraction must be part of a comprehensive N management strategy. Human activity has added reactive N to the landscape and changed nearshore habitats in ways that enhance N delivery to coastal ecosystems (Cleveland et al. 1999; Galloway et al. 2004). Comparing the maximum N potentially removed by harvesting a hectare of S. latissima to sources of nitrogen loading in Casco Bay reinforces the magnitude of anthropogenic disturbance in the N cycle. Encouragingly, the hectare-level comparisons generated for Casco Bay suggest that kelp bioextraction may be an efficient in-water tool to intercept nonpoint source pollution like atmospheric N deposition which, again, can be 30–40% of total N load to Casco Bay (Castro et al. 2003; Sonoma Technology Inc. 2003). However, the application of N bioextraction technologies must only be an additional measure for mitigating anthropogenic impacts on the environment. It should not be an alternative to improved management of point source and nonpoint source N by reducing combustion of fossil fuels, decreasing the application of N-based fertilizes, and tertiary treatment of wastewater. Using kelp aquaculture to remediate any substantial quantities of N will require a considerable shift in social acceptance of marine development and would have to be carefully evaluated against other commercial and ecological needs for this bay.
Environmental conditions
Careful consideration of environmental variables’ potential effect is important when anticipating how potential yields and nutrient concentrations reported by this study might vary. Many of the measured environmental conditions exhibited patterns like those reported by Kim et al. (2014, 2015) and Yarish et al. (2017); however, ambient salinity at our sites exhibited more dramatic swings than those observed in Long Island Sound. Mean ambient salinity measured at each sampling event declined from 30 to 23 in Saco Bay and 32 to 29 in Casco Bay in March and April, and salinity dropped as low as 16–17 at Cow Ledge and Wood Island in mid-April. This decline in salinity is earlier and steeper than the lowest salinities of 22–26 that Kim et al. observed at their sites in May. This discrepancy is notable regarding the timing of stress on the kelp crop. Saccharina latissima is semi-euryhaline; it can withstand 23–35 with no reduction in growth (Druehl 1967; Bartsch et al. 2008), but stress responses often develop at salinities below this range. A sharp decline in growth occurred in S. latissima in salinities consistently below 16 (Bartsch et al. 2008; Nielsen et al. 2014), and Gordillo et al. (2002) found that the closely related, Laminaria digitata, exhibited reduced nitrate uptake rates in low salinity conditions. Thus, the spring flush timing leading to freshening events in the WGoM could have affected the growth and tissue composition of the sampled kelp and may ultimately impact the potential N removed by kelp in this region.
The potential impact of combined stressors should also be considered when interpreting results from this study. No statistically significant relationships were observed between percent tissue N, δ15N, and the measured environmental conditions. One explanation for this may be that an alga’s tolerance range for one environmental factor may be influenced by other environmental factors (Hurd et al. 2014). For example, when Mortensen (2017) grew S. latissima and L. digitata in water enriched with nitrate and phosphate, the algae survived almost 2 weeks in brackish water (salinity = 18). In our study, one or several of the environmental conditions measured were less than optimal for S. latissima growth at some point during the growing seasons. For instance, temperatures at the sampling sites did not reach the 5–15 °C optimal growth range for S. latissima (Fortes and Lüning 1980; Bolton and Lüning 1982; Kim et al. 2015; Yarish et al. 2017) until mid-March. Photosynthetically active radiation measured during some sampling events was lower than the light-saturating level of 150–215 μmol photons m−2 s−1 reported for adult S. latissima sporophytes (Lüning 1979; Bartsch et al. 2008). Similarly, the range of current speeds (3–54 cm s−1) during periods of 2018 and 2019 seasons is broader than the optimal 10–25 cm s−1 flow rate for S. latissima (Kerrison et al. 2015). Lastly, initial sporophyte density (200–500 m−1) may have resulted in clumping and shading preventing adequate light and nutrients from reaching all sporophytes. The statistically significant effect of site on both the δ15N and percent tissue N observed in our results may be the result a combined stressor effect involving any of these ambient conditions and perhaps even other stressors that were not detected. Or inversely, the absence of a clear relationship between percent tissue N, δ15N, and may be because the algae were able to tolerate passing colder temperatures, low light, lower or higher current, or higher cultivation densities because the other environmental conditions were more than adequate.
Sources of N-stable isotopes
Mean δ15N measured in the kelp tissue did not show a clear indication that kelp grown and collected from Saco and Casco Bay took up N from anthropogenic sources. This finding contrasts with the general picture of coastal WGoM dynamics presented by Castro et al. (2003), Liebman et al. (2012), and Trowbridge et al. (2014). The absence of a clear N source relationship is also dissimilar to conclusions presented by Kim et al. (2015), who described clear indications that anthropogenic N sources were taken up by S. latissima in the Bronx River Estuary (− 2–6‰) and Long Island Sounds (9–19‰). The interannual, site-specific means δ15N for samples from Brothers Island (7.6 ‰), Cow Ledge (6.6‰), Ram Island (6.3‰), and Wood Island (5.7‰) sites fell within the δ15N ranges commonly attributed to N of marine origin (4–8‰) (Fig. 5). However, the high end of the δ15N range measured in kelp grown at Brothers Island (4.7–11.51‰) spans into the δ15N values commonly attributed to N from treated wastewater (10–12‰). In Saco Bay, the low end of the δ15N range measured in kelp grown at Wood Island reached into the δ15N values commonly attributed to N from fertilizers (−3–3‰). Looking at δ15N by bay (Fig. 6), the range of tissue δ15N in Saco Bay S. latissima reached its lowest values, indicative of N originating from fertilizer, in February. Also, during February, some measurements of tissue δ15N in Casco Bay S. latissima had values indicative of N originating from treated wastewater (> 10‰) but the sample mean was much lower (5.78‰). The monthly mean tissue δ15N in Casco Bay S. latissima continued to rise through May. Nutrient bioavailability, S. latissima ecophysiology, or unmeasured environmental changes may have influenced these results obtained in the present study.
Nutrient bioavailability at the study sites, affected by flushing rates and uptake by wild species, may have also limited exposure of the sampled kelp to anthropogenic N. Slow N supply rates and low amounts of N substrate are key considerations for N isotope distributions in primary producers because they limit reactions important for growth (Peterson and Fry 1987). In N-limited systems, macroalgae do little fractionation of their source material during N uptake (Peterson and Fry 1987; Savage and Elmgren 2004; Thornber et al. 2008); all available N will be consumed regardless of isotope content so long as redox conditions remain relatively stable. Given the stable redox conditions in this well-mixed, highly oxygenated environment, we assume the observed δ15N values in the tissue were representative of the N source (Wada and Hattori 1978; Mariotti et al. 1982; Pennock et al. 1996). However, fractionation by some macroalgae has occurred in water with high DIN concentrations, which resulted in tissue-δ15N values lower than that of the δ15N measured in the source N (Wada and Hattori 1978; Mariotti et al. 1982; Peterson and Fry 1987; Pennock et al. 1996; Wang et al. 2014). Examining the mean percent tissue N and the tissue C:N ratio in the S. latissima each month and at harvest indicates that there were periods during many of the growing seasons when the kelp was N limited. In S. latissima, > 3% DW tissue N content suggests N sufficiency, 1.9% is the minimum required for maximal growth, and < 1.3% DW tissue N indicates N limitation (Chapman et al. 1978; Wheeler and Weidner 1983; Kim et al. 2015). Therefore, δ15N values measured during or after a period of N limitation may not be comparable to when N was replete in the kelp tissue (Aberle and Malzahn 2007). The natural assimilatory capacity and high flushing rates of the WGoM may also explain the absence of a clear anthropogenic isotopic signature in the cultivated S. latissima despite the known contributions of anthropogenic N. Additionally, the WGoM has large, naturally occurring, Fucus spp. and Ascophyllum nodosum beds in the intertidal and subtidal zones. These wild algae may have also intercepted some anthropogenic N before it reached the study sites.
The nutrient ecophysiology of the sampled kelp (i.e., starving or N saturated) may have affected fractionation rates that are crucial assumptions for the application of stable isotope ratio assessments of primary producers. Fernandes et al. (2012) found that large N reserves in algal tissue can mask the isotopic signal of newly acquired N, and kelp cells have large vacuoles enabling N storage. When ambient N is abundant, kelp cells can store N as nitrate in cellular vacuoles and cytoplasm (Fong et al. 1994). Then, they draw on these reserves when ambient N is low (Chapman and Craigie 1977; Egan and Yarish 1990). It is plausible that this nutrient ecophysiology resulted in a muddled δ15N that is not representative of recent N use. For example, if S. latissima took up and stored N from the marine environment in December–February, the stored N would have a δ15N reflecting that source. When this stored N was assimilated into algal tissue later in the spring, because ambient N was insufficient for the sporophytes’ accelerated growth rates, the tissue sampled at that time would still exhibit a δ15N that was influenced by a marine N source despite the possibility that the algae could be using N from another source. Cellular N reserves in the S. latissima could also explain why there were no statistically significant relationships between percent tissue N, tissue δ15N, and ambient nitrate at each site.
Lastly, undetected environmental changes in the N sources or at the study sites may have affected the δ15N results. The isotopic composition of N species within aquatic environments is affected by many environmental processes including assimilation, denitrification, nitrification, and mineralization (Wada et al. 1975; Wada and Hattori 1978; McCready et al. 1983). Substantial changes in ambient environmental conditions can result in a shifted δ15N ratio for N sources, making it challenging to use stable isotope techniques to identify nutrient sources in field studies (Fry 2006; Wayland and Hobson 2001). For example, the presence, or pulses, of ammonium at the sites may help to explain why there was no correlation between ambient nitrate concentrations and δ15N. Saccharina latissima exhibits a preference for ammonium. Harrison et al. (1986) found that nitrate uptake in S. latissima was completely suppressed for 30 min following a pulse of ammonium. We assumed that any ammonium delivered to the sites would be immediately taken up, so we did not attempt to quantify ammonium in this study. However, frequent ammonium supplies or an ammonium pulse shortly prior to a sampling event may have also influenced N uptake rates or provided a contrasting δ15N signal.
Undocumented phytoplankton blooms are another example of an undetected environmental event that may be a source of variability influencing our dataset. Yarish et al. (2017) attributed low tissue N in kelp to a prolonged spring phytoplankton bloom, which may have been supported by mild winter conditions (i.e., harsh winter and spring results in more DIN available for the macroalgae). Anderson et al. (2005) also found correlations between spring snowmelt and spring phytoplankton blooms in southern New England. Releases of N from 15N-depleted sediments would have also affected the δ15N measured in the kelp tissue (Altabet 2006; Bianchi 2007; Sigman et al. 2009). Without knowledge or measurement of a release, it would be hard to correct for it when interpreting the data presented here. It is also possible that the natural variation between sites, or between published δ15N values for N sources and those in the WGoM, is so considerable that it exceeds the capacity of stable isotope analysis to differentiate between the N sources (Ostrom et al. 1997; Fry 2006). Due to logistical constraints, characterization of the δ15N in NO3− from specific N sources in Casco and Saco Bay was not possible. However, if future work can do this, it will reduce uncertainty regarding unmeasured environmental conditions and support the development of a stable isotope-specific mixing model for these locations.
Importantly, the isotope values reported in this study can help us to understand the current WGoM biogeochemistry and the existing degree of human perturbation in Casco and Saco Bay. If used in future studies, they will also help to better describe the direction and magnitude of nutrient cycling in the WGoM (Peterson and Fry 1987; Ostrom et al. 1997; Dethier et al. 2013). Establishing baseline stable isotope values for S. latissima in this region will help with the detection of potentially incipient eutrophication, which is preferable to restoration (McClelland et al. 1997). Additionally, if future studies can demonstrate a closer relationship between anthropogenic N pollution and bioextraction provided by kelp in the WGoM, it will garner stronger public support for cap and trade programs to include bioextraction as an eligible activity.
Conclusion
Identifying and implementing effective nutrient management technologies is critical to mitigating the impact of human activities on coastal ecosystems. This study measured biomass, δ15N, and tissue N content of Saccharina latissima grown from 2016 to 2019 at four sites in Casco and Saco Bay, Maine, to better understand how the N bioextraction achieved by harvesting cultivated kelp varies across space and time. Although the patterns in elemental content of the S. latissima tissue from the WGoM are like those reported from further south, total biomass at time of harvest was higher. Significant variation in biomass and tissue N content was observed between sites between the two bays, potentially due to combined environmental stressors, or the timing of seasonal temperature and salinity changes between the bays. High variation in δ15N also occurred between sites, and the monthly and interannual mean δ15N did not show explicit use of anthropogenic N sources like wastewater or fertilizer. The absence of clear source N relationships may be the result of physiological traits of S. latissima, biogeochemical characteristics of the WGoM, or unmeasured environmental changes. Our results further highlight the need for site-level pilot studies, even within the same bay, to characterize the seasonal and spatial variation of N assimilation before any kelp aquaculture is developed solely for bioextraction purposes in the WGoM. Finally, we extrapolated our results to estimate that harvesting cultivated kelp from the WGoM has the potential to extract 19.2 (± 4.8)–176.0 (± 7.7) kg N ha−1 depending on the cultivation density used, which emphasizes the importance of cultivation density and harvest time on theoretical kelp aquaculture bioextraction efficiencies. We conclude that kelp farming and harvesting could be a component within a broader, integrated approach to N mitigation in the region, but a substantial increase in kelp production and social acceptance of aquaculture will be required.
Notes
Carrying capacity is a system’s ability to tolerate activity without unacceptable impact to ecosystem characteristics like wildlife movement and habitat, recreational and commercial uses, water circulation, viewsheds, and other cultural uses (Group of Experts on the Scientific Aspects of Marine Environmental Protection 1986).
References
Aberle N, Malzahn AM (2007) Interspecific and nutrient-dependent variations in stable isotope fractionation: experimental studies simulating pelagic multitrophic systems. Oecologia 154:291–303
Abreu MH, Pereira R, Yarish C, Buschmann AH, Sousa-Pinto I (2011) IMTA with Gracilaria vermiculophylla: productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
Altabet MA (2006) Isotopic tracers of the marine nitrogen cycle: present and past. In: Volkman JK (ed) Marine organic matter: biomarkers, isotopes and DNA. The handbook of environmental chemistry. Springer, Berlin, pp 251–293
Anderson DM, Keafer BA, McGillicuddy DJ Jr, Mickelson MJ, Keay KE, Libby PS, Manning JP, Mayo CA, Whittaker DK, Hickey JM, He R (2005) Initial observations of the 2005 Alexandrium fundyense bloom in southern New England: general patterns and mechanisms. Deep-Sea Res II 52:2856–2876
Augyte S, Yarish C, Redmond S, Kim JK (2017) Cultivation of a morphologically distinct strain of the sugar kelp, Saccharina latissima forma angustissima, from coastal Maine, USA, with implications for ecosystem services. J Appl Phycol 29:1967–1976
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Bianchi (2007) Biogeochemistry of estuaries. Oxford University Press, New York
Boderskov T, Schmedes PS, Bruhn A, Rasmussen MB, Nielsen MM, Pedersen MF (2016) The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J Appl Phycol 28:1153–1165
Boesch DF (2002) Challenges and opportunities for science in reducing nutrient over-enrichment of coastal ecosystems. Estuaries 25:886–900
Boesch DF, Brinsfield RB, Magnien RE (2001) Chesapeake Bay eutrophication. J Environ Qual 30:303–320
Bolton JJ, Lüning K (1982) Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Mar Biol 66:89–94
Breitburg DL, Craig JK, Fulford RS, Rose KA, Boynton WR, Brady DC, Ciotti BJ, Diaz RJ, Friedland KD, Hagy JD, Hart DR (2009) Nutrient enrichment and fisheries exploitation: interactive effects on estuarine living resources and their management. Hydrobiologia 629:31–47
Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto GS (2018) Declining oxygen in the global ocean and coastal waters. Science 359:eaam7240
Bricker SB, Clement CG, Pirhalla DE, Orlando SP, Farrow DRG (1999) National estuarine eutrophication assessment: effects of nutrient enrichment in the nation’s estuaries. NOAA , National Ocean Service, Special Projects Office and the National Centers for Coastal Ocean Science. Silver Spring, MD. https://repository.library.noaa.gov/view/noaa/1693/noaa_1693_DS1.pdf. Accessed 3 March 2019
Bricknell IR, Birkel SD, Brawley SH, Van Kirk T, Hamlin H, Capistrant-Fossa K, Huguenard K, Van Walsum PG, Liu ZL, Zhu LH, Grebe G, Taccardi E, Miller M, Preziosi BM, Duffy K, Byron CJ, Quigley CTC, Bowden TJ, Brady D, Beal BF, Sappati PK, Johnson TR, Moeykens S (2020) Resilience of cold water aquaculture: a review of likely scenarios as climate changes in the Gulf of Maine. Rev Aquacult 13:460–503
Broch OJ, Ellingsen IH, Forbord S, Wang X, Volent Z, Alver MO, Handå A, Andresen K, Slagstad D, Reitan KI, Olsen Y (2013) Modelling the cultivation and bioremediation potential of the kelp Saccharina latissima in close proximity to an exposed salmon farm in Norway. Aquacult Env Interac 13:187–206
Bruhn A, Tørring DB, Thomsen M, Canal-Vergés P, Nielsen MM, Rasmussen MB, Eybye KL, Larsen MM, Balsby TJS, Petersen JK (2016) Impact of environmental conditions on biomass yield, quality, and bio-mitigation capacity of Saccharina latissima. Aquacult Env Interact 8:619–636
Buschmann AH, Correa JA, Westermeier R, del Carmen H-GM, Norambuena R (2001) Red algal farming in Chile: a review. Aquaculture 194:203–220
Castro MS, Driscoll CT, Jordan TE, Reay WG, Boynton WR (2003) Sources of nitrogen to estuaries in the United States. Estuaries 26:803–814
Chapman ARO, Craigie JS (1977) Seasonal growth in Laminaria longicruris: relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar Biol 40:197–205
Chapman ARO, Markham JW, Lüning K (1978) Effects of nitrate concentration on the growth and physiology of Laminaria saccharina (Phaeophyta) in culture. J Phycol 14:195–198
Chopin T, Yarish C, Wilkes R, Belyea E, Lu S, Mathieson A (1999) Developing Porphyra/salmon integrated aquaculture for bioremediation and diversification of the aquaculture industry. J Appl Phycol 11:463–472
Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-González JA, Yarish C, Neefus C (2001) Integrating seaweeds into marine aquaculture systems: a key toward sustainability. J Phycol 37:975–986
Chopin T, Robinson S, Sawhney M, Bastarache S, Belyea E, Shea R, ArmstrongW SI, Fitzgerald P (2004) The AquaNet integrated multi-trophic aquaculture project: rationale of the project and development of kelp cultivation as the inorganic extractive component of the system. Bull Aquac Assoc Can 104:11–18
Chopin T, Cooper JA, Reid G, Cross S, Moore C (2012) Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev Aquacult 4:209–220
Cleveland CC, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, Von Fischer JC, Elseroad A, Wasson MF (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem Cycl 13:623–645
Cole ML, Valiela I, Kroeger KD, Tomasky GL, Cebrian J, Wigand C, McKinney RA, Grady SP, Carvalho da Silva MH (2004) Assessment of a δ15N isotopic method to indicate anthropogenic eutrophication in aquatic ecosystems. J Environ Qual 33:124–132
Costanzo SD, O’donohue MJ, Dennison WC, Loneragan NR, Thomas M (2001) A new approach for detecting and mapping sewage impacts. Mar Pollut Bull 42:149–156
Dethier MN, Sosik E, Galloway AW, Duggins DO, Simenstad CA (2013) Addressing assumptions: variation in stable isotopes and fatty acids of marine macrophytes can confound conclusions of food web studies. Mar Ecol Prog Ser 478:1–4
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Druehl LD (1967) Distribution of two species of Laminaria as related to some environmental factors. J Phycol 3:103–108
Druehl LD, Baird R, Lindwall A, Lloyd KE, Pakula S (1988) Longline cultivation of some Laminariaceae in British Columbia, Canada. Aquac Res 19:253–263
Egan B, Yarish C (1990) Productivity and life history of Laminaria longicruris at its southern limit in the Western Atlantic Ocean. Mar Ecol Prog Ser 67:263–273
Fernandes M, Benger S, Sharma SK, Gaylard S, Kildea T, Hoare S, Braley M, Irving AD (2012) The use of δ15N signatures of translocated macroalgae to map coastal nutrient plumes: improving species selection and spatial analysis of metropolitan datasets. J Environ Monit 14:2399–2410
Fogel ML, Cifuentes LA (1993) Isotope fractionation during primary production. In: Engel MH, Macko SA (eds) Organic geochemistry. Topics in Geobiology. Springer, Boston, pp 73–98
Fong P, Donohoe RM, Zedler JB (1994) Nutrient concentration in tissue of the macroalga Enteromorpha as a function of nutrient history: an experimental evaluation using field microcosms. Mar Ecol Prog Ser 7:273–281
Fortes MD, Lüning K (1980) Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgol Meeresunters 34:15–29
Fossberg J, Forbord S, Broch OJ, Malzahn AM, Jansen H, Handå A, Førde H, Bergvik M, Fleddum AL, Skjermo J, Olsen Y (2018) The potential for upscaling kelp (Saccharina latissima) cultivation in salmon-driven integrated multi-trophic aquaculture (IMTA). Front Mar Sci 5:1–12
Freitas JR, Morrondo JMS, Ugarte JC (2016) Saccharina latissima (Laminariales, Ochrophyta) farming in an industrial IMTA system in Galicia (Spain). J Appl Phycol 28:377–385
Fry B (2006) Stable isotope ecology. Springer, New York
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Gartner A, Lavery P, Smit AJ (2002) Use of δ15N signatures of different functional forms of macroalgae and filter-feeders to reveal temporal and spatial patterns in sewage dispersal. Mar Ecol Prog Ser 235:63–73
Goldman JC, Tenore KR, Ryther JH, Corwin N (1974) Inorganic nitrogen removal in a combined tertiary treatment–marine aquaculture system–I. Removal efficiencies. Water Res 8:45–54
Gordillo FJ, Dring MJ, Savidge G (2002) Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity. Mar Ecol Prog Ser 234:111–118
Gray WJ (2019) Improved estimates of tributary nitrogen load to Casco Bay, Maine. University of Maine, MSc Thesis https://digitalcommons.library.umaine.edu/etd/3087. Accessed 5 Dec 2019
Grebe GS, Byron CJ, Gelais AS, Kotowicz DM, Olson TK (2019) An ecosystem approach to kelp aquaculture in the Americas and Europe. Aquacult Rep 15:100215
Group of Experts on the Scientific Aspects of Marine Environmental Protection (1986) Environmental capacity: an approach to marine pollution prevention. Reports and Studies GESAMP No. 30, pp 49. https://www.unenvironment.org/resources/report/gesamp-environmental-capacity-approach-marine-pollution-prevention. Accessed 3 March 2019
Handå A, Forbord S, Wang X, Broch OJ, Dahle SW, Størseth TR, Reitan KI, Olsen Y, Skjermo J (2013) Seasonal-and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414–415:191–201
Harrison PJ, Druehl LD, Lloyd KE, Thompson PA (1986) Nitrogen uptake kinetics in three year-classes of Laminaria groenlandica (Laminariales: Phaeophyta). Mar Biol 93:29–35
He P, Xu S, Zhang H, Wen S, Dai Y, Lin S, Yarish C (2008) Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res 42:1281–1289
Heaton TH (1986) Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review. Chem Geol 59:87–102
Howarth RW (2008) Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8:14–20
Howarth RW, Sharpley A, Walker D (2002) Sources of nutrient pollution to coastal waters in the United States: Implications for achieving coastal water quality goals. Estuaries 25:656–676
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, Cambridge
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Pub, Malden, pp 375–449
Kerrison PD, Stanley MS, Edwards MD, Black KD, Hughes AD (2015) The cultivation of European kelp for bioenergy: site and species selection. Biomass Bioenerg 80:229–242
Kim JK, Kraemer GP, Neefus CD, Chung IK, Yarish C (2007) Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J Appl Phycol 19:431–440
Kim JK, Kraemer GP, Yarish C (2014) Field scale evaluation of seaweed aquaculture as a nutrient bioextraction strategy in Long Island Sound and the Bronx River Estuary. Aquaculture 433:148–156
Kim JK, Kraemer GP, Yarish C (2015) Use of sugar kelp aquaculture in Long Island Sound and Bronx River Estuary for nutrient extraction. Mar Ecol Prog Ser 531:155–166
Krom MD (1986) An evaluation of the concept of assimilative capacity as applied to marine waters. Ambio 15:208–214
Li H, Zhang Y, Han X, Shi X, Rivkin RB, Legendre L (2016) Growth responses of Ulva prolifera to inorganic and organic nutrients: implications for macroalgal blooms in the southern Yellow Sea, China. Sci Rep 6:26498
Liebman M, Benoy G, Latimer J, Bricker S (2012) Eutrophication in the Gulf of Maine. State of the Gulf of Maine Report. https://www.researchgate.net/publication/281118458_Eutrophication_in_the_Gulf_of_Maine_State_of_the_Gulf_of_Maine_Report. Accessed 11 November 2019
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lüning K (1979) Growth strategies of three Laminaria species (Phaeophyceae) inhabiting different depth zones in the sublittoral region of Helgoland (North Sea). Mar Ecol Prog Ser 1:195–207
Macko SA, Ostrom NE (1994) Pollution studies using stable isotopes. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental science. Blackwell, Oxford, pp 45–62
Marinho GS, Holdt SL, Birkeland MJ, Angelidaki I (2015) Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. J Appl Phycol 27:1963–1973
Mariotti A, Mariotti F, Champigny ML, Amarger N, Moyse A (1982) Nitrogen isotope fractionation associated with nitrate reductase activity and uptake of NO3− by pearl millet. Plant Physiol 69:880–884
Martínez B, Pato LS, Rico JM (2012) Nutrient uptake and growth responses of three intertidal macroalgae with perennial, opportunistic and summer-annual strategies. Aquat Bot 96:14–22
McClelland JW, Valiela I (1998) Linking nitrogen in estuarine producers to land-derived sources. Limnol Oceanogr 43:577–585
McClelland JW, Valiela I, Michener RH (1997) Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnol Oceanogr 42:930–937
McCready RG, Gould WD, Barendregt RW (1983) Nitrogen isotope fractionation during the reduction of NO3− to NH4+ by Desulfovibrio sp. Can J Microbiol 29:231–234
MEDEP [Maine Department of Environmental Protection] (2008) Development of nutrient criteria for Maine’s coastal waters. https://www.maine.gov/dep/water/nutrient-criteria/nutrient_criteria_report_2008.pdf. Accessed 2 Feb 2020
MEDEP [Maine Department of Environmental Protection] (2019) Combined sewer overflow program. https://www.maine.gov/dep/water/cso/. Accessed 15 July 2020
MEDMR [Maine Department of Marine Resources] (2019) Maine aquaculture harvest data: Marine algae (2015 – 2019). https://www.maine.gov/dmr/aquaculture/harvestdata/index.html. Accessed 1 May 2020
Mortensen LM (2017) Remediation of nutrient-rich, brackish fjord water through production of protein-rich kelp S. latissima and L. digitata. J Appl Phycol 29:3089–3096
Neori A, Krom MD, Ellner SP, Boyd CE, Popper D, Rabinovitch R, Davison PJ, Dvir O, Zuber D, Ucko M, Angel D (1996) Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 141:183–199
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Nielsen MM, Krause-Jensen D, Olesen B, Thinggaard R, Christensen PB, Bruhn A (2014) Growth dynamics of Saccharina latissima (Laminariales, Phaeophyceae) in Aarhus Bay, Denmark, and along the species’ distribution range. Mar Biol 161:2011–2022
Nixon SW (1987) Chesapeake Bay nutrient budgets – a reassessment. Biogeochem 4:77–90
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–219
Nixon SW (1998) Enriching the sea to death. Sci Am 9:48–53
Ostrom NE, Macko SA, Deibel D, Thompson RJ (1997) Seasonal variation in the stable carbon and nitrogen isotope biogeochemistry. Geochim Cosmochim Acta 61:2929–2942
Owens NJP (1987) Natural variations in 15N in the marine environment. In: Blaxter JHS, Southward AJ (eds) Advances in marine biology, vol 24. Academic Press, London, pp 389–451
Paerl HW (1997) Coastal eutrophication and harmful algal blooms: importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol Oceanogr 42:1154–1165
Paerl HW, Whitall D (1999) Anthropogenically-derived atmospheric nitrogen deposition, marine eutrophication and harmful algal bloom expansion: Is there a link? Ambio 28:307–311
Paerl HW, Hall NS, Peierls BL, Rossignol KL (2014) Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuar Coast 37:243–258
Pennock JR, Velinsky DJ, Ludlam JM, Sharp JH, Fogel ML (1996) Isotopic fractionation of ammonium and nitrate during uptake by Skeletonema costatum: implications for δ15N dynamics under bloom conditions. Limnol Oceanogr 41:451–459
Peteiro C, Freire Ó (2013) Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J Appl Phycol 25:205–213
Peteiro C, Salinas JM, Freire Ó, Fuertes C (2006) Cultivation of the autoctonous seaweed Laminaria saccharina off the Galician coast (NW Spain): Production and features of the sporophytes for an annual and biennial harvest. Thalassas 22:45–53
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview. Algal Res 15:9–23
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Pettigrew NR, Townsend DW, Xue H, Wallinga JP, Brickley PJ, Hetland RD (1998) Observations of the Eastern Maine Coastal Current and its offshore extensions in 1994. J Geophys Res-Oceans 103:30623–30639
Pettigrew NR, Churchill JH, Janzen CD, Mangum LJ, Signell RP, Thomas AC, Townsend DW, Wallinga JP, Xue H (2005) The kinematic and hydrographic structure of the Gulf of Maine Coastal Current. Deep-Sea Res II 52:2369–2391
Piconi P, Veidenheimer R, Chase B (2020) Edible seaweed market analysis. Island Institute. http://www.islandinstitute.org/edible-seaweed-market-analysis-2020. Accessed 1 May 2020
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England seaweed culture handbook. https://opencommons.uconn.edu/seagrant_weedcult/1/. Accessed 20 September 2019
Reitsma J, Murphy DC, Archer AF, York RH (2017) Nitrogen extraction potential of wild and cultured bivalves harvested from nearshore waters of Cape Cod, USA. Mar Pollut Bull 116:175–181
Riley MS (2020) Maine Combined Sewer Overflow 2019 status report: Document No.: DEPLQ0972L-2020 Maine Department of Environmental Protection. https://www.maine.gov/dep/water/cso/. Accessed 23 November 2020
Ryther J, Dunstan WM (1971) Nitrogen, phosphorus and eutrophication in the coastal marine environment. Science 171:l0
Ryther JH, Goldman JC, Gifford CE, Huguenin JE, Wing AS, Clarner JP, Williams LD, Lapointe BE (1975) Physical models of integrated waste recycling-marine polyculture systems. Aquaculture 5:163–177
Sanderson JC, Dring MJ, Davidson K, Kelly MS (2012) Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) CE Lane, C. Mayes, Druehl & GW Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354:128–135
Savage C, Elmgren R (2004) Macroalgal (Fucus vesiculosus) δ15N values trace decrease in sewage influence. Ecol Appl 14:517–526
Sigman DM, Karsh KL, Casciotti KL (2009) Ocean process tracers: nitrogen isotopes in the ocean. In: Steele JH, Turekian KK, Thorpe SA (eds) Encyclopedia of ocean sciences. Academic Press, London, pp 4138–4152
Sonoma Technology Inc. (2003) Deposition of air pollutants to Casco Bay. https://www.cascobayestuary.org/wp-content/uploads/2014/07/2003_sonoma_deposition_air_pollutants4.pdf. Accessed 9 September 2019
Tanner BR, Perfect E, Kelley JT (2006) Fractal analysis of Maine’s glaciated shoreline tests established coastal classification scheme. J Coastal Res 22:1300–1304
Thornber CS, DiMilla P, Nixon SW, McKinney RA (2008) Natural and anthropogenic nitrogen uptake by bloom-forming macroalgae. Mar Pollut Bull 56:261–269
Tilburg CE, Gill SM, Zeeman SI, Carlson AE, Arienti TW, Eickhorst JA, Yund PO (2011) Characteristics of a shallow river plume: observations from the Saco River Coastal Observing System. Estuar Coast 34:785–799
Tilburg CE, Jordan LM, Carlson AE, Zeeman SI, Yund PO (2015) The effects of precipitation, river discharge, land use and coastal circulation on water quality in coastal Maine. Roy Soc Open Sci 2:140429
Townsend DW (1991) Influences of oceanographic processes on the biological productivity of the Gulf of Maine. Rev Aquat Sci 5:211–230
Townsend DW (1998) Sources and cycling of nitrogen in the Gulf of Maine. J Marine Syst 16:283–295
Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yarish C (2003) Integrated mariculture: asking the right questions. Aquaculture 226:69–90
Trowbridge P, Wood MA, Burack TS, Quiram VV, Forbes EJ (2014) Great Bay nitrogen non-point source study. PREP Reports and Publications 831. http://scholars.unh.edu/cgi/viewcontent.cgi?article=1384&context=prep. Accessed 4 Jan 2020
UC Davis SIF [University of California, Davis Stable Isotope Facility] (2017) Carbon (13C) and nitrogen (15N) analysis of solids by EA-IRMS. https://stableisotopefacility.ucdavis.edu/13cand15n.html. Accessed 29 Sept 2019
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
Wada E, Hattori A (1978) Nitrogen isotope effects in the assimilation of inorganic nitrogenous compounds by marine diatoms. Geomicrobiol J 1:85–101
Wada E, Kadonaga T, Matsuo S (1975) 15N abundance in nitrogen of naturally occurring substances and global assessment of denitrification from isotopic viewpoint. Geochem J 9:139–148
Wade TL, Sweet ST, Klein AG (2008) Assessment of sediment contamination in Casco Bay, Maine, USA. Environ Pollut 152:505–521
Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ (2014) Coastal ocean acidification: the other eutrophication problem. Estuar Coast Shelf Sci 148:1–13
Wang X, Olsen LM, Reitan KI, Olsen Y (2012) Discharge of nutrient wastes from salmon farms: environmental effects, and potential for integrated multi-trophic aquaculture. Aquacult Env Interac 2:267–283
Wang X, Broch OJ, Forbord S, Handå A, Skjermo J, Reitan KI, Vadstein O, Olsen Y (2014) Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: implications for integrated multi-trophic aquaculture. J Appl Phycol 26:1869–1878
Wayland M, Hobson KA (2001) Stable carbon, nitrogen, and sulfur isotope ratios in riparian food webs on rivers receiving sewage and pulp-mill effluents. Can J Zool 79:5–15
Wheeler WN, Weidner M (1983) Effects of external inorganic nitrogen concentration on metabolism, growth and activities of key carbon and nitrogen assimilatory enzymes of Laminaria saccharina (Phaeophyceae) in culture. J Phycol 19:92–96
Wu H, Huo Y, Zhang J, Liu Y, Zhao Y, He P (2015) Bioremediation efficiency of the largest scale artificial Porphyra yezoensis cultivation in the open sea in China. Mar Pollut Bull 95:289–296
Xiao X, Agusti S, Lin F, Li K, Pan Y, Yu Y, Zheng Y, Wu J, Duarte CM (2017) Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci Rep 7:46613
Yarish C, Kim JK, Lindell S, Kite-Powell H (2017) Developing an environmentally and economically sustainable sugar kelp aquaculture industry in southern New England: from seed to market. https://opencommons.uconn.edu/eeb_articles/38. Accessed 3 January 2020
Acknowledgments
We thank the following individuals for their innovative ideas and never-ending help in the laboratory and on the water: Arenti T, Beard K, Brawley S, Costa-Pierce B, Hollandbeck M, Jagoutz T, Johndrow K, Jones E, Olson T, Pierce E, St. Gelais A, and Waters A. Much appreciation to Liberti K, and Cleaver C, Koons B, and the anonymous reviewers for their constructive comments.
Funding
This activity was supported by National Science Foundation award #IIA-1355457 to Maine EPSCoR at the University of Maine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grebe, G.S., Byron, C.J., Brady, D.C. et al. The nitrogen bioextraction potential of nearshore Saccharina latissima cultivation and harvest in the Western Gulf of Maine. J Appl Phycol 33, 1741–1757 (2021). https://doi.org/10.1007/s10811-021-02367-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02367-6