Abstract
The aim of this study was to determine the combined effects of iron and sulfur on microalgal biomass concentration and removal efficiency of nitrogenous compounds using factorial design. Scenedesmus acuminatus (currently accepted name Tetradesmus lagerheimii) was separately cultivated in batch photobioreactors using modified N-8 media with two nitrogen sources, nitrate, and ammonium. To study the interaction effect between iron and sulfur and to reduce the total number of experimentally studied combinations, a factorial design was used. Three iron (0.1, 1, and 1.9 mg L−1) and three sulfur concentrations (3.7, 20, and 35.8 mg L−1) were employed to the modified N-8 media in this study. The results show that the final microalgal biomass concentration and nitrogen removal efficiency were more sensitive to the changes in iron and sulfur concentrations in the media with nitrate than with ammonium possibly because of the different assimilation mechanisms used by microalgae for these two nitrogen sources. The created models demonstrated that iron had a statistically significant effect on the microalgal biomass concentration and nitrate removal efficiency while sulfur did not. In addition, the interaction effect between iron and sulfur was not significant on microalgal biomass concentration and nitrogen removal. In synthetic medium with nitrate as nitrogen source, the highest microalgal biomass concentration was obtained with 1.0 mg L−1 iron and 35.8 mg L−1 sulfur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae have gained increasing attention in the past decades as a promising feedstock for, e.g., bioenergy and biofuels, as well as for wastewater treatment (for reviews, see Lakaniemi et al. 2013; Abinandan and Shanthakumar 2015). Despite the wide range of potential applications for microalgae-based technologies, maximizing growth of microalgae is often critical to ensure a profitable and efficient process. Many algal studies have focused on changing physicochemical parameters such as pH, temperature, light, and medium composition in order to optimize microalgal growth (Liu et al. 2008; Bartley et al. 2016; Schnurr et al. 2016). Of the studies that deal with medium composition, most have focused on optimizing the concentration of primary nutrients, nitrogen, and phosphorus to enhance microalgal biomass production (Rhee 1978; Hulatt et al. 2012; Lv et al. 2019). Micronutrients such as iron, magnesium, sulfur, zinc, and copper are typically supplied to growth media at low levels because they are essential for microalgal growth (Mandalam and Palsson 1998; Yeesang and Cheirsilp 2011). However, at too high concentration, many metals, such as iron, magnesium, and copper, are toxic to microalgae (Yeesang and Cheirsilp 2011; Gorain et al. 2013; Huang et al. 2014). This study focuses on the micronutrients iron and sulfur because they are constituents of enzymes required for nitrogen utilization by microalgae (Padmavathi et al. 2008; Shibagaki and Grossman 2008) and reported to have impacts on microalgal growth (Yeesang and Cheirsilp 2011; Mera et al. 2016).
In addition to nitrogen assimilation, iron is important for microalgae as a building block in the enzymes required for photosynthetic electron transfer and assimilation of sulfur (Raven 1990; Padmavathi et al. 2008). For example, when compared to a medium lacking iron, low levels of iron addition (0.00067–0.67 mg L−1) enhanced biomass production and lipid accumulation in Chlorella vulgaris, resulting in 3 to 7-fold higher lipid content (56.6%) when grown in the media containing 0.67 mg L−1 FeCl3 than in other media (Liu et al. 2008). Singh et al. (2015) confirmed that iron significantly affected lipid productivity of microalgae and that a high iron concentration (9 mg L−1) with 750 mg L−1 nitrogen and 0 mg L−1 phosphorus resulted in the highest lipid content of 59.6% and highest lipid productivity of 74 mg L−1 day−1 in Ankistrodesmus falcatus.
Sulfur is also essential for microalgal growth as it is required for the production of proteins, lipids, and polysaccharides (Shibagaki and Grossman 2008). The addition of sulfate as a source of sulfur has been shown to increase microalgal biomass production as well as nitrogen and phosphorus removal efficiencies (Mera et al. 2016; Lv et al. 2017). The highest biomass concentration of Chlamydomonas moewusii was obtained in a medium with ammonium as the nitrogen source when sulfur concentrations were between 3.2–96 mg L−1 (Mera et al. 2016), while the final Chlorococcum sp. biomass concentration and specific growth rates were similar in a synthetic wastewater with nitrate as nitrogen source when sulfur concentration was 6, 15, 25.7, 45.3, and 90.3 mg L−1 (Lv et al. 2017). The highest biomass concentration was obtained in similar sulfur concentration range with the two different microalgal species (Mera et al. 2016; Lv et al. 2017). However, Tao et al. (2017) observed different microalgal biomass concentration and ammonium removal efficiencies were obtained when Scenedesmus acuminatus was grown in two kinds of liquid digestates from pulp and paper industry and one potential reason for the difference was different initial sulfur concentration of the digestates.
Ammonium and nitrate are the form of nitrogen most often studied for microalgal growth as they are often present in natural waters and various wastewaters (Hulatt et al. 2012; Cai et al. 2013). Ammonium seems more widely utilized by algae compared to nitrate (Raven and Giordano 2016). However, in some cases, ammonium may not be an appropriate nitrogen source for microalgae due to the growth inhibition caused by, e.g., low pH in un-buffered solutions (Hulatt et al. 2012; Raven and Giordano 2016; Lv et al. 2019) and ammonium loss via volatilization from the medium at high pH (> 8.0) and temperature (Emerson et al. 1975; Zimmo et al. 2003). However, ammonium is considered as an energetically preferred nitrogen source for microalgae (Flynn and Hipkin 1999) because ammonium can be directly incorporated into amino acids using enzyme glutamine synthetase, while nitrate assimilation by microalgae requires two additional reduction reactions via nitrate reductase and nitrite reductase after which it is utilized as ammonium by the cells (Rowell et al. 1977; Hellebust and Ahmad 1989; Solomonson and Barber 1989). In the first reaction, nitrate is reduced into nitrite using nicotinamide adenine dinucleotide phosphate as reducing agent and ferredoxins are used to catalyze nitrite to ammonium in the second reaction (Hellebust and Ahmad 1989; Barsanti and Gualtieri 2014). The ferredoxins (iron-sulfur clusters) are the catalysts used during nitrate reduction, which indicates that the micronutrients iron and sulfur may have a combined influence on how nitrogen is utilized by microalgae. In addition, the combined effects of iron and sulfur on microalgal growth and nitrogen removal efficiency might vary between nitrate and ammonium supplied cultures. However, most studies looking at the impact of iron and sulfur on microalgal growth have only focused on one form of nitrogen (e.g., ammonium or nitrate) (Liu et al. 2008; Yeesang and Cheirsilp 2011; Mera et al. 2016; Lv et al. 2017). In addition, the combined effects of iron and sulfur on microalgal growth and nitrogen removal have not been previously investigated.
The aim of this study was to assess the combined effect of iron and sulfur on microalgal growth and nitrogen removal efficiency using two different nitrogen sources: ammonium and nitrate. The specific research questions addressed were the following: (1) What is the effect of iron and sulfur on microalgal growth and nitrogen removal efficiency? (2) Is there any combined effect of iron and sulfur on microalgal growth and nitrogen removal efficiency? (3) Do the effects of iron and sulfur vary with different nitrogen sources? The factorial experimental design used in this study allows for greater insight into potential relationships between the factors and reveals the optimal levels of both elements, while also limiting the number of experiments required (Bezerra et al. 2008). Scenedesmus acuminatus was used in this study due to its high growth rates and yields observed with pulp and paper mill biosludge digestates (Tao et al. 2017).
Materials and methods
Microalgal strain and medium
Scenedesmus acuminatus (SAG 38.81) (note that the currently accepted name is Tetradesmus lagerheimii (Wynne and Guiry 2016)), was obtained from the SAG Culture Collection of Algae at the University of Göttingen, Germany. The stock culture was maintained in 100 mL modified N-8 medium in 250-mL Erlenmeyer flasks on an orbital shaker (150 rpm) at room temperature and at a light intensity of 40 μmol photons m−2 s−1. The modified N-8 medium consisted of KNO3, 0.5055 g L−1; KH2PO4, 0.7400 g L−1; Na2HPO4, 0.2598 g L−1; MgSO4·7H2O, 0.0500 g L−1; CaCl2·2H2O, 0.0175 g L−1; FeNaEDTA·3H2O, 0.0115 g L−1, ZnSO4·7H2O, 0.0032 g L−1; MnCl2·4H2O, 0.0130 g L−1; CuSO4·5H2O, 0.0183 g L−1; and Al2(SO4)3·18H2O, 0.0070 g L−1 (Praveenkumar et al. 2014). As S. acuminatus did not grow in the modified N-8 medium with a natural pH of 6.5, the pH was adjusted to an optimal value of 8.0 (Xu et al. 2015) by adding 5 M NaOH.
Photobioreactors
Photobioreactors used consisted of a 1-L glass bottle (Pyrex) and a plastic cap with two holes serving as the gas inlet and outlet. Air at a flow rate of 0.2 L min−1 was sparged from the bottom of the bottle by a glass distribution tube (porosity 0, ⌀ 22 mm, Duran Group, Germany) that was inserted into the photobioreactor through one of the holes in the cap. White fluorescent lamps (Osram L 18 W/965 De Luxe Cool Daylight, Germany) were used to continuously illuminate the photobioreactors from two sides of the glass bottles with a light intensity of 240 μmol photons m−2 s−1, which is the optimal light intensity for Scenedesmus dimorphus based on Xu et al. (2015). Scenedesmus acuminatus was inoculated from the stock cultures to the photobioreactors to provide an initial optical density of 0.27–0.29 at a wavelength of 680 nm (OD680). The initial total culture volume in the reactors was 700 mL. The temperature of the reactors was maintained at 22 ± 2 °C.
Experimental design and data analysis
To reveal potential interactions between the effects of iron and sulfur, a 22 full-factorial experimental design was used. This method allows for a minimum number of total experiments while still differentiating between the effects of individual variables and revealing any possible interaction between the variables. In a 22 factorial design, two independent variables are evaluated at two different levels.
The study started with a set of experiments (the first experimental phase) that evaluated the response surface of microalgal growth and nitrogen removal efficiency based on low (coded as − 1) and medium (coded as + 1) levels of iron and sulfur (added as FeNaEDTA·3H2O and Na2SO4, respectively) in the media with nitrate or ammonium as the nitrogen source. KNO3 and NH4Cl were used as the nitrogen sources in the NO3− assay and the NH4+ assay, respectively. MgSO4·7H2O in the modified N-8 medium was replaced by equimolar concentrations of MgCl2·6H2O to keep same amount of magnesium as in the modified N-8 medium used for stock cultures while the sulfur concentration was varied according to the experimental design. As iron and sulfur did not show a significant effect on microalgal growth or nitrogen recovery when using ammonium as the nitrogen source, the second phase of experiments was conducted using only the medium with nitrate. During the second experimental phase, wider ranges of iron and sulfur including the medium (+ 1) and high (+ 3) levels were tested with the aim to optimize biomass growth and nutrient removal efficiency. When taken together, the experiments with nitrate as nitrogen source consisted of iron and sulfur factors at low (− 1), medium (+ 1), and high concentrations (+ 3) (e.g., FeL&SL represents: low concentration of iron and low concentration of sulfur) as represented in Table 1.
The low and medium levels of iron (0.1 and 1 mg L−1) were selected based on previous studies where the highest growth rates were observed at iron concentrations between 0.67 to 1.2 mg L−1 and followed by those with iron concentrations from 0.067 to 0.12 mg L−1 (Liu et al. 2008; Ren et al. 2014). The high level of iron was set to 1.9 mg L−1 in this study because iron concentrations between 2.07 and 41.4 mg L−1 were shown to negatively affect microalgal growth (Ren et al. 2014; Islami and Assareh 2019). To minimize chemical changes from inoculum medium to modified medium, the low level of sulfur (3.7 mg L−1) was chosen to be the same as the sulfur concentration used in the modified N-8 medium used for stock cultures. The medium level of sulfur (20 mg L−1) was close to the sulfur concentrations (15–18 mg L−1) used in previous studies in which high microalgal concentrations have been obtained (Mera et al. 2016; Tao et al. 2017). The results of the first batch of low and medium sulfur levels were used to determine the high level of sulfur (35.8 mg L−1) using the surface response plot and the linear models indicating a local optimal growth point in response to a higher sulfur concentration (NIST/SEMATECH 2012).
The duration of the batch cultivations was 7 days in the first experimental phase, and 14 days in the second experimental phase, as stationary phase of microalgal growth was not reached and nitrogen was not completely consumed within the 7 day incubations of the first experimental phase. In the first experimental phase, water evaporated at a rate of 15 mL per day during the cultivation. To ensure proper biomass and nutrient concentration calculations, distilled water was added during the second experimental phase to compensate for the evaporated water volume (marked with lines on the photobioreactors) each time before taking final samples.
The results of the microalgal biomass concentrations and nitrogen removal efficiencies were evaluated using R Statistical Software (version 3.5.1). The effects of independent factors on the dependent factors were analyzed by linear (first-order models) or quadratic equations (second-order models):
where Y is the response variable (microalgal biomass concentration or nitrogen removal efficiency), a0 is Y-intercept, a1 and a2 are linear coefficients, a11 and a22 are the squared term coefficients, and a12 is the interaction coefficient. X1 and X2 are iron and sulfur concentrations, respectively. In the obtained models, coefficient of determination (R2) or adjusted R2 were used to evaluate the model fit to the experimental data (Carley et al. 2004; González-Fernández et al. 2011). p values less than 0.05 indicated that a model term was significant for the response variable and the overall model p values were used to choose the best model fit (González-Fernández et al. 2011).
Analytical methods
The culture pH was measured using a WTW 330 pH meter (WTW, Germany) with a Slimtrode electrode (Hamilton, Germany). The light intensity was calculated by measuring the average value of six sites on two sides of the photobioreactor outer surface by a MQ-200 quantum meter (Apogee, USA).
Determination of microalgal growth
The microalgal biomass concentration was measured using both OD680 and ash-free dry weight (AFDW). OD680 was measured using a Shimadzu UV-1700 Pharmaspec spectrophotometer from non-filtrated samples. The non-filtrated samples were diluted with distilled water to give absorbance values between 0.2–0.7. The AFDW was measured by filtering 10–15 mL of culture solution through a glass fiber filter (Whatman GF/A). Each filter containing the suspended solids was dried at 105 °C overnight, then weighed and combusted in a 550 °C muffle furnace for 2 h before being weighed again. The AFDW was determined gravimetrically as the difference between the filters after treatment at these two temperatures.
The growth rates were calculated using the following equation:
where X1 is the concentration of biomass measured as AFDW (g L−1) at time t1 and X2 is the concentration of biomass at a time t2.
Determination of carbon and nutrient removal efficiency
The supernatant after the AFDW filtration was used for the analysis of dissolved organic carbon (DOC), dissolved inorganic carbon (DIC) and nutrient (N, P, S) concentrations. DOC and DIC were measured with a total organic carbon analyzer (Shimadzu Model TOC-5000) with an ASI-5000 autosampler. Ammonium-nitrogen (NH4+-N) was measured with an ion-selective electrode (Thermo Scientific Orion ISE meter). Nitrate (NO3−), nitrite (NO2−), phosphate (PO43−) and sulfate (SO42−) were measured using an ICS-1600 ion chromatograph (Dionex, USA) with an AS-DV autosampler, Ion-Pac AS4A-SC anion exchange column, and ASRS-300 suppressor (2 mm). The system was operated in isocratic mode using an eluent containing 1.9 mM Na2CO3 and 1.7 mM NaHCO3 and an eluent flow rate of 1 mL min−1.
Results
The effects of iron and sulfur at low and medium levels on the microalgal biomass concentration and nitrogen removal
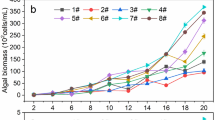
During the first experimental phase, the effects of iron and sulfur at low and medium levels (FeL&SL, FeL&SM, FeM&SL and FeM&SM) on the microalgal biomass concentration and nitrogen removal efficiency were studied separately with NO3− and NH4+ as the nitrogen source. The different iron and sulfur concentrations resulted in varied AFDW with both NO3− and NH4+. The OD680 and ADFW values showed linear correlation (R2 > 0.96) in all of the cultures. The final AFDW in the NH4+ assay (0.83 ± 0.15–0.99 ± 0.02 g L−1) was higher than that obtained in the NO3− assay (0.57 ± 0.11–0.80 ± 0.13 g L−1) (Fig. 1a, b). The microalgal growth reached the stationary phase during the 7-day cultivation only in FeM&SM with NO3−.
Ash-free dry weight (as g L−1) during the cultivation of Scenedesmus acuminatus in the modified N-8 media with different iron and sulfur concentrations (L refers to low and M refers to medium concentration of iron/sulfur) with a NO3− and b NH4+ as the nitrogen source. The results of ash-free dry weight are presented as the means of n = 4 (2 cultivations, 2 measurements from each); error bars represent standard deviation
The final NH4+-N removal efficiency was higher than the final NO3−-N removal efficiency after the 7-day cultivations (Fig. 2a, b). The final NO3−-N removal efficiencies in the media with higher iron concentration (FeM&SL: 71.4%, FeM&SM: 75.1%) were higher than those in the media with lower iron concentration (FeL&SL: 43.5%, FeL&SM: 46.4%). However, the studied iron and sulfur concentrations did not significantly affect the final NH4+-N removal efficiency, which was in the similar range (82.9–93.2%) in all cultures. Also, the ammonium removal rate was very similar in all of the cultures with the different Fe and S concentrations (Fig. 2b).
a NO3−-N and b NH4+-N concentrations during the cultivation of Scenedesmus acuminatus in the modified N-8 media (FeL&SL: low conc. of iron and low conc. of sulfur, FeL&SM: low conc. of iron and medium conc. of sulfur, FeM&SL: medium conc. of iron and low conc. of sulfur, and FeM&SM: medium conc. of iron and medium conc. of sulfur) with NO3− and NH4+ as nitrogen source, respectively. The results of NO3−-N are presented as the means of n = 4 (2 cultivations, 2 measurements from each); error bars represent standard deviation. The results of NH4+-N are presented as the means of n = 2 (2 cultivations, 1 measurement from each); error bars represent standard error
The effects of iron and sulfur on the microalgal biomass concentration and nitrogen removal with nitrate as nitrogen source
The second experimental phase focused on the effect of high levels of iron and sulfur with nitrate as the nitrogen source on microalgal growth and nitrate removal. The AFDW on day 7 ranged from 1.07 ± 0.14 to 1.15 ± 0.04 g L−1, which was slightly higher than obtained in the first experimental phase (Fig. 3b) and more than 99% nitrate was consumed by day 9 in all cultures (Fig. 3c). Compared to microalgal growth in the second experimental phase, in the first experimental phase, a lag phase of 1 day in microalgal biomass concentration was observed (Fig. 3b). Therefore, the highest specific growth rates were observed during the second experimental phase a few days earlier than during the first experimental phase (Table 2). However, the highest specific growth rates of FeM&SM during both experimental phases were similar.
a pH, b ash-free dry weight (as g L−1), and c NO3−-N during the cultivation of Scenedesmus acuminatus in the modified N-8 media. The results of pH are presented as the means of n = 2 (2 cultivations, 1 measurement from each); error bars represent standard error. The results of ash-free dry weight and nitrate are presented as the means of n = 4 (2 cultivations, 2 measurements from each); error bars represent standard deviation
Microalgal biomass concentration and nitrate removal efficiency models with nitrate as nitrogen source
First-order model in NO3− assay
The equation with iron and sulfur at low and medium concentrations representing as coded unit for the nitrate removal efficiency was:
The p value for the model in the NO3− assay was 0.00017 (< 0.001), which shows the regression analysis is statistically significant. The adjusted R2 = 95.68% indicates that the model explaining effects of iron and sulfur on nitrate removal efficiency fits well to the experimental data. In this model, the most significant factor affecting nitrate removal efficiency was iron (p value = 0.00006) followed by sulfur (p value = 0.282). However, the interaction effect between iron and sulfur was not a significant variable and was not therefore included in the model.
Second-order models in NO3− assays
Results of microalgal biomass concentration and nitrate removal efficiency from the first and second experimental phases were used to generate quadratic models for the NO3− assays. To consider lag phase in the NO3− assay and reduce the influences of nitrate deficiency on microalgal growth after day 7, the measured microalgal biomass concentrations from days 6 and 5 were used for statistical analysis for the first and second experimental phase, respectively (Table 2). Meanwhile, the statistical analysis of nitrate removal efficiency was conducted using experimental results from days 5 and 4 for the first and second experimental phases, respectively. The dates for biomass concentration and nitrate removal efficiency were different due to the different sampling dates.
The regression results of the AFDW are listed in Online Resource 1. The model with the best fit is presented as contour plot (Fig. 4). The quadratic polynomial equation (iron and sulfur concentrations representing as coded units) was derived from the regression results based on the p value of overall model and adjusted determination coefficient and was the following:
Contour plot showing the ash-free dry weight (g L−1) as a function of iron and sulfur. Independent variables are represented by their coded values and concentration values (mg L−1) inside brackets. The different colors represent the varied levels of microalgal biomass concentration. For example, the level increases from 0.5 to 0.8 with color changing from green to orange
The p value for the overall model was 0.0004 (< 0.05), thus the statistical relation was at 95% confidence level, which indicates that the regression analysis is statistically significant. The value of the adjusted determination coefficient R2 = 71.1% indicates that the model can explain more than 70% of the total variation, and thus the model fits the experimental data. In this study, iron (p = 0.0006) and iron2 (p = 0.007) affected the AFDW more significantly than sulfur (p = 0.074).
The regression results for the models of nitrate removal efficiency are listed in Online Resource 2, while the model with the best fit is presented as contour plot (Fig. 5). The quadratic polynomial equation (iron and sulfur concentration representing as united codes) which had the best fit was the following:
Contour plot showing the nitrate removal efficiency (%) as a function of iron and sulfur. Independent variables are represented by their coded values and concentration values (mg L−1) inside brackets. The different colors represent the varied levels of nitrate removal efficiency. For example, the level increases from 30 to 50 with color changing from green to orange
Iron (p< 0.0001) and iron2 (p < 0.001) significantly contributed to predicting about 84.3% of the variation in nitrate removal efficiency, while the p value for the overall model was less than 0.0001. The order of influencing factors for nitrate removal efficiency was iron > sulfur.
Discussion
This study was carried out to understand the combined effect of iron and sulfur on microalgal growth and nitrogen removal efficiency using factorial experiment design. The whole study was divided into two experimental phases. The results during the first experimental phase showed that the obtained microalgal biomass concentration was slightly more sensitive to the changes in iron and sulfur concentration in the media with NO3− than with NH4+ because the variation of final biomass concentration in the NO3− assay (0.23 g L−1) was higher than that in the NH4+ assay (0.16 g L−1). Regardless of nitrogen source and sulfur concentration, the final microalgal biomass concentration increased when the iron concentration was increased. At the same iron concentration, higher microalgal biomass concentration was obtained in the medium with NH4+ than with NO3−. In addition, the lag phase was observed in NO3− assay (Fig. 1) likely due to the additional enzymatic steps required for nitrate assimilation by the microalgae (Rowell et al. 1977; Hellebust and Ahmad 1989; Solomonson and Barber 1989). The similar phenomenon was also observed in the study by Lv et al. (2019). Flynn and Hipkin (1999) also indicated via theoretical calculations that higher iron concentrations were required in algal growth simulations for nitrate assimilation to achieve the same growth rate as for ammonium assimilation. However, Kim et al. (2013) obtained higher microalgal biomass concentration in a medium with nitrate than with ammonium when batch-cultivating Chlorella sorokiniana, while in a study by Hulatt et al. (2012), Chlorella vulgaris did not grow at all in a medium with ammonium due to poor buffering capacity of the medium (Hulatt et al. 2012). Thus, it seems to depend on the growth conditions and microalgal species whether the microalgae grow more efficiently with NO3− or NH4+ as their nitrogen source.
In the NO3− assay, the highest AFDW by cultivation day 5 was obtained in FeM&SM but the biomass concentration started to decline after that. This demise was likely due to carbon limitation caused by pH increase on day 5. Culture pH in FeM&SM of the NO3− assay increased likely due to fast nitrate uptake by the microalgae (Goldman and Brewer 1980) and at high pH (e.g., > 8.7) specific carbon uptake rate by microalgae decreases with increasing pH (Azov 1982). Compared to air, air supplemented with CO2 could promote the microalgal growth by providing sufficient inorganic carbon source and adjusting pH in un-buffered solution.
During the first experimental phase, the results also show that the nitrogen removal efficiency was more sensitive to the changes of iron and sulfur in the media with NO3− than with NH4+ as the nitrogen source. The results are consistent to the theoretical knowledge that ferredoxins (iron-sulfur proteins) contribute more to nitrate assimilation than ammonium assimilation due to additional reduction steps of nitrate into amino acids (Rowell et al. 1977; Hellebust and Ahmad 1989; Solomonson and Barber 1989). In addition, Jin et al. (1998) reported that the algal nitrite reductase is a ferredoxin-dependent enzyme and the concentration of ferredoxins may be the limiting factor for nitrite reduction rate.
In the NO3− assay, the initial iron concentration was shown to have more significant effects on both the microalgal biomass concentration and nitrate removal efficiency when compared to sulfur. The reason could be related to the different roles and uptake mechanisms of iron and sulfur in microalgal cells. Iron is an important trace element for microalgae because it is used in cells as a building block for many proteins (i.e., iron-sulfur proteins) required for photosynthetic electron transfer, as well as nitrogen and sulfur assimilation (Raven 1990; Padmavathi et al. 2008). Sulfur is involved in the production of protein, lipids and polysaccharides, and sulfate as the most stable sulfur form in nature can be taken up by microalgae (Shibagaki and Grossman 2008).
Iron uptake by microalgae likely includes two pathways: a passive adsorption process on the microalgal cell surface and an active absorption process through the membrane (Li and Zheng 2011; Sutak et al. 2012). The active process of iron uptake is further divided into two systems: a low-affinity transport and a high-affinity transport according to the availability of inorganic Fe species and iron sufficiency in cells (Marchetti and Maldonado 2016). In algal cells, sulfate is first activated with adenosine triphosphate to 5′-adenylsulfate (APS), which is then reduced to sulfite by APS reductase (Schiff and Hodson 1970). After sulfite is reduced to sulfide by sulfite reductase (SiR), the generated sulfide is incorporated into cysteine (Schiff and Hodson 1970). The iron-sulfur proteins consist of iron and sulfur, the latter of which is desulfurized from cysteine (Lill and Mühlenhoff 2008). Before the reduced sulfur is incorporated into cysteine, however, iron-sulfur proteins are also involved in sulfate assimilation to provide electrons for SiR when reducing sulfite to sulfide (Padmavathi et al. 2008). This indicates that iron as the iron-sulfur protein is involved in sulfate assimilation, thus, the availability of iron can affect sulfate assimilation in cells while sulfate seemed to not affect iron transport in cells according to current reported studies (Giordano et al. 2008).
The measured data during the first experimental phase were expected to create four first-order models: final microalgal biomass concentration in the NO3− assay, nitrate removal efficiency, final microalgal biomass concentration in the NH4+ assay, and ammonium removal efficiency. However, the combined effects of iron and sulfur on ammonium removal efficiency and microalgal biomass concentration in the media with NH4+ or NO3− could not be described with the first-order models as the overall p values were > 0.05. This indicates that the linear functions did not accurately describe the relationship between iron-sulfur concentrations and ammonium removal efficiency/microalgal biomass concentration. The first-order model could not describe the effects of iron and sulfur on ammonium removal efficiency and microalgal biomass concentration due to the insignificant regression analyses obtained in this study likely because the data have curvature or no model is suitable in the studied factors’ range.
Based on the obtained first-order model, the sulfur concentration was not a significant factor influencing nitrate removal efficiency and the combined effects of iron and sulfur on the microalgal growth in the media with nitrate were not clear. Therefore, the second experiment phase included wider iron and sulfur concentration range and the fitted second-order models using the NO3− assay data from the first and second experimental phases proved that the data set presented curvature.
The highest specific growth rate was obtained at high iron concentration likely due to the sufficient iron availability in the initial medium (Table 2). However, the bioavailable iron and sulfur may be deficient in the late-exponential growth (Hasegawa et al. 2018), thus, did not result in the highest final biomass concentration in this batch study. It is reasonable to optimize the medium composition for microalgal growth as the provided iron and sulfur may not be completely utilized for microalgal growth and nitrogen assimilation. In large-scale systems, optimal iron content should be applied for microalgal cultivation as iron is a significant factor for both growth and nitrate removal. The presence of sulfur can promote microalgal growth and no inhibition on microalgal growth was observed at high concentration of sulfur in this study. Further work should be carried out to study the effects of sulfur at higher concentrations (more than 1 g L−1) on microalgal growth, as microalgae may be used to treat wastewater containing high amounts of sulfur, e.g., from pulp and paper mill (Thompson et al. 2001).
Conclusions
The varied iron and sulfur concentrations in the medium with nitrate affected microalgal biomass concentration and nitrogen removal efficiency more than in the medium with ammonium. This was likely due to the different assimilation mechanisms used by microalgae for these two nitrogen species. Iron and sulfur concentrations affected the microalgal biomass concentration and nitrate removal efficiency, while iron was the only statistically significant factor. The models describing combined effects of iron and sulfur on microalgal biomass concentration and nitrate removal efficiency, which fitted best the experimental data, did not indicate any interaction effect between iron and sulfur. In the medium with nitrate as the nitrogen source, the highest final microalgal biomass concentration was obtained with initial iron and sulfur concentrations of 1.0 mg L−1 and 35.8 mg L−1, respectively.
References
Abinandan S, Shanthakumar S (2015) Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: a review. Renew Sust Energ Rev 52:123–132
Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemistry, and biotechnology. CRC Press, Boca Raton
Bartley ML, Boeing WJ, Daniel D, Dungan BN, Schaub T (2016) Optimization of environmental parameters for Nannochloropsis salina growth and lipid content using the response surface method and invading organisms. J Appl Phycol 28:15–24
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369
Carley KM, Kamneva NY, Reminga J (2004) Technical report of response surfaces methodology CASOS. School of Computer Science, USA http://www.casos.cs.cmu.edu/publications/. Accessed 23 April 2019
Emerson K, Russo RC, Lund RE, Thurston RV (1975) Aqueous ammonia equilibrium calculations: effect of pH and temperature. J Fish Board Can 32:2379–2383
Flynn KJ, Hipkin CR (1999) Interactions between iron, light, ammonium, and nitrate: insights from the construction of a dynamic model of algal physiology. J Phycol 35:1171–1190
Giordano M, Norici A, Ratti S, Raven JA (2008) Role of sulfur for algae: acquisition, metabolism, ecology and evolution. In: Hell R, Dahl C, Knaff DB, Leustek T (eds) Sulfur metabolism in phototrophic organisms Springer, Dordrecht, pp 397–415
Goldman JC, Brewer PG (1980) Effect of nitrogen source and growth rate on phytoplankton-mediated changes in alkalinity. Limnol Oceanogr 25:352–357
González-Fernández C, Molinuevo-Salces B, García-González MC (2011) Evaluation of anaerobic codigestion of microalgal biomass and swine manure via response surface methodology. Appl Energy 88:3448–3453
Gorain PC, Bagchi SK, Mallick N (2013) Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environ Technol 34:1887–1894
Hasegawa H, Nozawa A, Papry RI, Maki T, Miki O, Rahman MA (2018) Effect of biodegradable chelating ligands on Fe uptake in and growth of marine microalgae. J Appl Phycol 30:2215–2225
Hellebust JA, Ahmad I (1989) Regulation of nitrogen assimilation in green microalgae. Biol Oceanogr 6:241–255
Huang X, Wei L, Huang Z, Yan J (2014) Effect of high ferric ion concentrations on total lipids and lipid characteristics of Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis. J Appl Phycol 26:105–114
Hulatt CJ, Lakaniemi AM, Puhakka JA, Thomas DN (2012) Energy demands of nitrogen supply in mass cultivation of two commercially important microalgal species, Chlorella vulgaris and Dunaliella tertiolecta. Bioenergy Res 5:669–684
Islami HR, Assareh R (2019) Effect of different iron concentrations on growth, lipid accumulation, and fatty acid profile for biodiesel production from Tetradesmus obliquus. J Appl Phycol. https://doi.org/10.1007/s10811-019-01843-4
Jin T, Huppe HC, Turpin DH (1998) In vitro reconstitution of electron transport from glucose-6-phosphate and NADPH to nitrite. Plant Physiol 117:303–309
Kim S, Lee Y, Hwang SJ (2013) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegrad 85:511–516
Lakaniemi AM, Tuovinen OH, Puhakka JA (2013) Anaerobic conversion of microalgal biomass to sustainable energy carriers–a review. Bioresour Technol 135:222–231
Li SX, Zheng FY (2011) Effect of macronutrient enrichment on the size distribution, sorption, and bioconcentration factor of iron by coastal phytoplanktonic diatoms. Mar Environ Res 72:89–95
Lill R, Mühlenhoff U (2008) Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem 77:669–700
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Lv J, Guo J, Feng J, Liu Q, Xie S (2017) Effect of sulfate ions on growth and pollutants removal of self-flocculating microalga Chlorococcum sp. GD in synthetic municipal wastewater. Bioresour Technol 234:289–296
Lv J, Wang X, Feng J, Liu Q, Nan F, Jiao X, Xie S (2019) Comparison of growth characteristics and nitrogen removal capacity of five species of green algae. J Appl Phycol 31:409–421
Mandalam RK, Palsson B (1998) Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol Bioeng 59:605–611
Marchetti A, Maldonado MT (2016) Iron. In: Borowitzka MA, Beardall J, Raven J (eds) The physiology of microalgae. Springer, Dordrecht, pp 233–279
Mera R, Torres E, Abalde J (2016) Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: implications for the optimization of algal culture media. J Phycol 52:75–88
NIST/SEMATECH (2012) e-Handbook of statistical methods. https://www.itl.nist.gov/div898/handbook/pri/section7/pri7.htm. Accessed 26 April 2019
Padmavathi L, Ye H, Pilon-Smits EA, Pilon M (2008) Cysteine desulfurase-mediated sulfur donation pathways in plants and phototrophic bacteria. In: Hell R, Dahl C, Knaff DB, Leustek T (eds) Sulfur metabolism in phototrophic organisms. Springer, Dordrecht, pp 131–147
Praveenkumar R, Kim B, Choi E, Lee K, Cho S, Hyun JS, Park JY, Lee YC, Lee HU, Lee JS, Oh YK (2014) Mixotrophic cultivation of oleaginous Chlorella sp. KR-1 mediated by actual coal-fired flue gas for biodiesel production. Bioprocess Biosyst Eng 37:2083–2094
Raven JA (1990) Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol 116:1–8
Raven JA, Giordano M (2016) Combined nitrogen. In: Borowitzka MA, Beardall J, Raven J (eds) The physiology of microalgae. Springer, Cham, pp 143–154
Ren HY, Liu BF, Kong F, Zhao L, Xie GJ, Ren NQ (2014) Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour Technol 169:763–767
Rhee GY (1978) Effects of N: P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake 1. Limnol Oceanogr 23:10–25
Rowell P, Enticott S, Stewart WD (1977) Glutamine synthetase and nitrogenase activity in the blue-green alga Anabaena cylindrica. New Phytol 79:41–54
Schiff JA, Hodson RC (1970) Pathways of sulfate reduction in algae. Ann N Y Acad Sci 175:555–576
Schnurr PJ, Espie GS, Allen GD (2016) The effect of photon flux density on algal biofilm growth and internal fatty acid concentrations. Algal Res 16:349–356
Shibagaki N, Grossman A (2008) The state of sulfur metabolism in algae: from ecology to genomics. In: Hell R, Dahl C, Knaff DB, Leustek T (eds) Sulfur metabolism in phototrophic organisms. Springer, Dordrecht, pp 231–267
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29
Solomonson LP, Barber MJ (1989) Structure-function relationships of algal nitrate reductases. In: Wray JL, Kinghorn JR (eds) Molecular and genetic aspects of nitrate assimilation. Oxford Science Publishers, Oxford, pp 88–100
Sutak R, Botebol H, Blaiseau PL, Léger T, Bouget FY, Camadro JM, Lesuisse E (2012) A comparative study of iron uptake mechanisms in marine microalgae: iron binding at the cell surface is a critical step. Plant Physiol 160:2271–2284
Tao R, Lakaniemi AM, Rintala JA (2017) Cultivation of Scenedesmus acuminatus in different liquid digestates from anaerobic digestion of pulp and paper industry biosludge. Bioresour Technol 245:706–713
Thompson G, Swain J, Kay M, Forster CF (2001) The treatment of pulp and paper mill effluent: a review. Bioresour Technol 77:275–286
Wynne MJ, Guiry MD (2016) Tetradesmus lagerheimii M.J.Wynne & Guiry, nomen novum, a replacement name for Tetradesmus acuminatus (Lagerheim) M.J.Wynne, nom. illeg. (Sphaeropleales, Chlorophyta). Notulae Algarum 12:1
Xu X, Shen Y, Chen J (2015) Cultivation of Scenedesmus dimorphus for C/N/P removal and lipid production. Electron J Biotechnol 18:46–50
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040
Zimmo OR, Van der Steen NP, Gijzen HJ (2003) Comparison of ammonia volatilisation rates in algae and duckweed-based waste stabilisation ponds treating domestic wastewater. Water Res 37:4587–4594
Acknowledgments
This work was supported by the Marie Skłodowska-Curie European Joint Doctorate (EJD) in Advanced Biological Waste-To-Energy Technologies (ABWET) funded from Horizon 2020 (grant number 643071). We would also like to thank Tarja Ylijoki-Kaiste and Peter Heerings for their help in the laboratory.
Funding
Open access funding provided by Tampere University including Tampere University Hospital, Tampere University of Applied Sciences (TUNI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tao, R., Bair, R., Lakaniemi, AM. et al. Use of factorial experimental design to study the effects of iron and sulfur on growth of Scenedesmus acuminatus with different nitrogen sources. J Appl Phycol 32, 221–231 (2020). https://doi.org/10.1007/s10811-019-01915-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01915-5