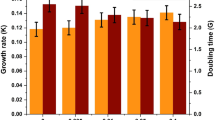
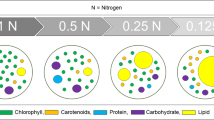
Abstract
Microalgae as sources for biodiesel production have been widely investigated. Microalgae biomass, lipid content and fatty acid profiles of microalgae are limiting factors for the cost-effective production of biodiesel. In this paper, the effects of high ferric ion concentrations on three species of microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis) were studied. The microalgae were cultured in different concentrations (1.2 × 10−2, 1.2 × 10−1, 1.2 and 12 mmol L−1) of ferric ion. The growth, lipid content and fatty acid profiles of the three microalgae were analysed. When algae were cultured in 1.2 mmol L−1 ferric ion for 10 days, the final cell density and specific growth rates of T. subcordiformis, N. oculata and P. viridis decreased significantly (p < 0.05), and the total lipid contents of the microalgae, 33.72, 37.34 and 29.48 % (dry mass) in T. subcordiformis, N. oculata and P. viridis, respectively, were higher than those at other concentrations. The neutral lipid/total lipid ratios of the three microalgae species increased with increasing ferric ion concentration. Neutral lipids accounted for 50.75, 48.37 and 46.59 % of the total lipid in T. subcordiformis, N. oculata and P. viridis, respectively, when cultured in 12 mmol L−1 ferric ion. The proportions of saturated fatty acids in all three species cultured in 12 mmol L−1 ferric ion were significantly higher than those cultured in lower ferric ion concentrations. An optimum ferric ion concentration can improve the properties of T. subcordiformis, N. oculata and P. viridis as sources for biodiesel.




Similar content being viewed by others
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sust Energ Rev 15:584–593
Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energy 88:3402–3410
Baky HHAI, El-Baroty GS, Bouaid A, Martinez M, Aracil J (2012) Enhancement of lipid accumulation in Scenedesmus obliquus by optimizing CO2 and Fe3+ levels for biodiesel production. Bioresour Technol 119:429–432
Behrens PW, Kyle DJ (1996) Microalgae as a source of fatty acids. J Food Lipids 3:259–272
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharm 37:911–917
Briat JF, Fobis-Loisy I, Grignon N, Lobréaux S, Pascal N, Savino G, Thoiron S, Wirén N, Wuytswinkel OV (1995) Cellular and molecular aspects of iron metabolism in plants. Biol Cell 84:69–81
Bruton T, Lyons H, Lerat Y, Stanley M, BoRasmussen M (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sust Energy Ireland 30–31
Cai ZP, Huang WW, Duan SS (2008) Iron concentration—induced changes in growth and biochemical compositions of marine diatom Phaeodactylum tricornutum (Bacillariophyceae). Ecol Environ 174:1327–1333
Chen CM, Zheng AR, Zhou CY, Chen YZ (1997) The effects of iron on growth, pigments and nitrogen assimilation of a marine algae Skeletonema costatum. Acta Oceanol Sin 19:50–56
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Cheng YX, Jiang XM, Chen XH, Huang XH, Huang XX, Zhou ZG, Zhang DM, Hou ZE, Chen KJ (2005) Live food cultivation. China Agriculture, Beijing
Chen SJ, Chen M, Lu HK, Chen BN, Xie KE (1977) On the foods of abalones—a preliminary report on the culture of benthic diatoms. Acta Zool Sinica 23:47–52
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Prog Process Intensif 48:1146–1151
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Doubnerová V, Ryšlavá (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Science 180:575–583
Francisco E, Neves D, Lopes E, Franco T (2009) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Gledhill M, Buck KN (2012) The organic complexation of iron in the marine environment: a review. Front Microbiol 3:1–17
Gong YM, Jiang ML (2011) Biodiesel production with microalgae as feedstock: from strains to biodiesel. Biotechnol Lett 33:1269–1284
Gouveia L, Olivera AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Guschina IA, Harwood JL (2009) Algae lipids and effect of the environment on their biochemistry. In: Arts MT, Brett MT, Kainz KJ (eds) Lipids in aquatic ecosystems. Springer, Dordrecht, pp 1–24
Hsieh C-H, Wu W-T (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Huang XX, Huang ZZ, Wen W, Yan JQ (2012) Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J Appl Phycol 25:129–137
Izui K, Matsumura H, Furumoto T, Kai Y (2004) Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol 55:69–84
Jiang XM (2002) Effects of temperatures, light intensity and nitrogen concentrations on the growth and fatty acid composition of Nannochloropsis oculata. Mar Science 26:9–12
Kadar E, Rooks P, Lakey C, White DA (2012) The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci Total Environ 439:8–17
Kadar E, Simmance F, Martin O, Voulvoulis N, Widdicombe S, Mitov S, Lead JR, Readman JW (2010) The influence of engineered Fe2O3 nanoparticles and soluble (FeCl3) iron on the developmental toxicity caused by CO2-induced seawater acidification. Environ Pollut 158:3490–3497
Kadar E, Tarran GE, Jha AW, Al-Subiai SN (2011) Stabilisation of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ Sci Technol 45:3245–3251
Keenan C, Goth-Goldstein R, Lucas D, Sedlak LD (2009) Oxidative stress induced by zero-valent iron nanoparticles and Fe (II) in human bronchial epithelial cells. Environ Sci Technol 43:4555–4560
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86: 1059–1070
LeBel C, Ischiropoulos H, Bondy S (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lee DH (2011) Algal biodiesel economy and competition among bio-fuels. Bioresour Technol 102:43–49
Lin Q, Gu N, Lin JD (2012) Effect of ferric ion on nitrogen consumption, biomass and oil accumulation of a Scenedesmus rubescens-like microalga. Bioresour Technol 112:242–247
Lin S, Gobler CJ, Carpenter EJ (2001) Cytological and biochemical responses of Dunaliella tertiolecta (Volvocales, Chlorophyta) to iron stress. Phycologia 40:403–410
Liu SC, Li DT, Gao JL, Huang BY, Zhang CH, Hao JM, Zhang L (2009) Lipid components of Ostrea rivularis, Paphia undulata and Pinctada martensii. J Fish China 33:666–671
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Meher LC, Vidya SD, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sustain Energy Rev 10:248–268
Muller-Feuga A, Moal J, Kaas R (2003) The microalgae of aquaculture. In: Stottrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell, Oxford, pp 206–252
Ou MM, Zhang MP, Feng YY (2002) Effects of various iron forms on the growth of Chlorella vulgaris in seawater. J Ocean Univ Qingdao 32:627–633
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy's aquatic species program-biodiesel from algae. Prepared for U.S. Department of Energy's Office of Fuels Development, by National Renewable Energy Laboratory, Golden, Colorado
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Storch TA, Dunham VL (1986) Iron mediated changes in the growth of lake Erie phytoplankton and axenic algae culture. J Appl Phycol 22:109–117
Sunda WG, Huntsman SA (1997) Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390:389–392
Sunda WG, Price NM, Morel FMM (2005) Trace metal ion buffers and their use in culture studies. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, pp 35–63
Vraspir J, Butler A (2009) Chemistry of marine ligands and siderofores. Annu Rev Mar Sci 1:43–63
Thakur A, Kumar HD (1999) Nitrate and phosphate uptake by the cells of Dunaliella salina. Bull Environ Contam Toxicol 62:70–78
Wynn JP, Hamid ABA, Ratledge C (1999) The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiol 145:1911–1917
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040
Zhang Y, Adams IP, Ratledge C (2007) Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiol 153:2013–2025
Zhu MY, Mu XY, Li RX, Rui H (2000) The effects of iron on growth, photosynthesis and biochemical composition of a marine algae Phaeodactylum tricornutum. Acata Oceanol Sinica 22/1:110–116
Zuo DM, Han ZG, Wu BG (2002) Effects of iron on the growth and photosythesis of red tide diatom Pseudonitzschia pungens Grunow. J Jinan Univ (China) 23:81–87
Acknowledgments
This study was financially supported by a project of the National High Technology Research and Development Program, China (2009AA064401), a project of the State Oceanic Administration of China (SHME2011SW02) and the Innovation Research Group Developing Project in the Universities of Shanghai (nutrition, feed and environment of animal aquaculture, the second).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, X., Wei, L., Huang, Z. et al. Effect of high ferric ion concentrations on total lipids and lipid characteristics of Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis . J Appl Phycol 26, 105–114 (2014). https://doi.org/10.1007/s10811-013-0056-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0056-x