Abstract
Nearly four years after its first appearance, and having gone from pandemic to endemic, the SARS-CoV-2 remains out of control globally. The purpose of this study was to evaluate the clinical efficacy of vitamin D (VD) in COVID-19 and long COVID-19, explain the discrepancy in clinical outcomes and highlight the potential impact of metformin on VD efficacy in recent articles. Articles from January 2022 to August 2023 were selected for this review. The objective of this study was achieved by reviewing, analyzing, and discussing articles demonstrating (1) the mechanism of action of VD (2) observational or randomized clinical trials (RCTs) that support or not the beneficial clinical effects of VD in COVID-19 or long COVID. (3) genetic and non-genetic reasons for the variation in the effects of VD. Articles were collected from electronic databases such as PubMed, Scopus, MEDLINE, Google Scholar, Egyptian Knowledge Bank, Science Direct, and Cochrane Database of Systematic Reviews. Twenty three studies conducted in vitro or in animal models indicated that VD may act in COVID-19 through protecting the respiratory system by antimicrobial peptide cathelicidins, reducing lung inflammation, regulating innate and adaptive immune functions and up regulation of autophagy gene activity. Our review identified 58 clinical studies that met the criteria. The number of publications supporting a beneficial clinical activity of VD in treating COVID-19 was 49 (86%), including 12 meta-analyses. Although the total patients included in all articles was 14,071,273, patients included in publications supporting a beneficial role of VD in COVID-19 were 14,029,411 (99.7%). Collectively, extensive observational studies indicated a decisive relationship between low VD levels and the severity of COVID-19 and mortality outcomes. Importantly, evidence from intervention studies has demonstrated the effectiveness of VD supplements in treating COVID-19. Furthermore, the results of 4 observational studies supported the beneficial role of VD in alleviating symptoms of long COVID-19 disease. However, eight RCTs and one meta-analysis of RCTs may contain low-grade evidence against a beneficial role of VD in COVID-19. Twenty-five articles have addressed the association between VDR and DBP genetic polymorphisms and treatment failure of VD in COVID-19. Impaired VDR signaling may underlie the variability of VD effects as non-genetic mechanisms. Interestingly, in recent studies, metformin has a beneficial therapeutic role in COVID-19 and long COVID-19, possibly by improving AMPK signaling of the VDR and enhancing the efficacy of the VD. In conclusion, evidence has been significantly strengthened over the past 18 months, with several meta-analyses and RCTs reporting conclusive beneficial effects of VD supplementation against COVID-19 and highlighting metformin to improve VDR sensitivity and efficacy in treating COVID-19 and long COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly four years after its first appearance, and after transition from a pandemic to an endemic phase, SARS-CoV-2 remains among the most troublesome respiratory viruses and is still out of control globally (Mahajan et al. 2023; WHO 2023). Furthermore, many studies published on long COVID indicate that in 50% to 70% of COVID-19 survivors may experience several post-COVID symptoms for up to 6 months which include a wide range of persistent health problems (Davis et al. 2023). The search for effective drugs for the treatment and prevention of coronavirus (COVID-19) is still underway. Numerous studies have shown potential for current therapies for prevention and treatment including antivirals, but the only clarity so far is that there is no effective drug driving clinical management in the WHO health emergencies programmer (Looi 2023). Furthermore, few studies attempt to investigate treatments for long post-COVID-19 syndrome for which there is no evidence of efficacy and little biological plausibility.
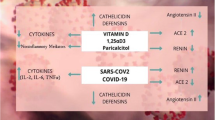
Extra-Skeletal functions of VD including differentiation and proliferation of cells, antioxidant, antibacterial, anti-inflammatory and immunomodulatory properties have been investigated in various tissues or cells by many investigators (Holick et al. 2023). Several epidemiologic studies have observed that low VD levels are found in a large percentage of COVID-19 patients with acute respiratory failure. Furthermore, it has been investigated that low levels of 25 hydroxyvitamin D are associated also with long COVID syndrome in survivors of COVID-19 (Filippo et al. 2023a). Vitamin D deficiency is widespread throughout the world, especially in southern European countries, and the Covid-19 virus has had a significant impact in these countries (Chiodini et al. 2021). Therefore, the use of VD supplements to prevent the spread of COVID-19 is a potential therapeutic strategy that is easy to implement (Cicero et al. 2022; Argano et al. 2023). VD works in more ways than one against COVID-19. Vitamin D interacting with its receptor (VRD)—Triggers the regulation of several genes involved in the immune system and enhances the innate and adaptive immune response against respiratory infections. In macrophages, it promotes the production of antiviral and antimicrobial proteins such as cathelicidins and beta-defensin-2 proteins that inhibit viral particle replication and promote removal of virus from cells by autophagy (Sartika and Gayatri. 2022). Analog calcitriol of vitamin D increased the expression of angiotensin-converting enzyme II (ACE2) in the lungs and alleviated acute lung injury (Xu et al. 2017). It also prevents cytokine storm and inflammatory processes in COVID-19 (Gilani et al. 2022; Bae et al. 2022).
Despite promising initial results, neither government agencies nor the World Health Organization has recommended incorporation of vitamin D into COVID-19 preventive or treatment guidelines. This could be possibly due to other studies found no such effects of VD (Brunvoll et al. 2022; Jolliffe et al. 2022).In addition many investigators attribute the reason for the contradiction in the results of the VD in the treatment or prevention of COVID-19 to the inaccuracy and heterogeneity with respect to design, drug dosage and population characteristics of the articles published in the first period after the outbreak of the pandemic (Jolliffe et al. 2022). Fortunately, the accuracy and heterogeneity regarding design, drug dose, and demographic characteristics of articles on COVID-19 treatment published in 2022 have been improved so far. In addition, several studies have observed that VDR gene polymorphisms may modulate response to VD therapy. The purpose of this study was to provide an up-to-date assessment of the evidence, evaluating the clinical efficacy of vitamin D in COVID-19, and long post COVID-19 and explaining discrepancy of clinical outcomes in articles published from January 2022 until now.
Methods
Articles from January 2022 to August 2023 were selected for this review. Original research articles, whether experimental, observational, clinical trials or meta-analyses were included in this study. Articles published in languages other than English or published in a journal not indexed by Scopus were excluded from the review. The objective of this study was achieved by reviewing articles that elucidate (1) Mechanism of action of VD in treating COVID-19 in experimental studies (2) observational or randomized clinical trials that support the beneficial clinical effects of VD in COVID-19 (3) observational or RCTs have found no effect of VD in COVID-19 (4) Clinical studies support the beneficial effect of VD on long-COVID-19 (5) Genetic and non-genetic reasons for the differences in the effects of VD (6) potential impact of metformin on VD efficacy.
Qualitative and quantitative data were extracted from each study. Publication dates and number of included studies, patient characteristics, clinical status, sample size, VD supplementation and its effect on infection incidence, hospital stay, ICU admission rate, ventilation requirements, and mortality in COVID patients were extracted from the selected articles. Reports of published articles were collected from electronic databases such as PubMed, Scopus, MEDLINE, Google Scholar, Egyptian Knowledge Bank, Science Direct, and Cochrane Database of Systematic Reviews.
Results and discussion
Mechanism of action of vitamin D in treating COVID-19 in experimental studies
Twenty three studies conducted in vitro or in animal models on the mechanism of effect of vitamin D in COVID-19 infection were published from January 2022 to Aug. 2033.These studies indicate that vitamin D plays an important role in protecting the respiratory system through antimicrobial peptide cathelicidins, which have direct antimicrobial effects on bacteria, viruses, and fungi. In an in vitro study, calcitriol showed significant efficacy against SARS-CoV-2 in cell-based assays (Mok et al. 2023). They suggested that calcitriol acts by modulating the vitamin D receptor pathway to increase the expression of cathelicidin. It has effects on several innate immune mechanisms in the airway (Stapleton et al. 2022). The conflicting effects of calcitriol may be due to differences in vitamin D metabolism and the dose of calcitriol given to mice may have been too low to maintain adequate levels.
Meanwhile, Arora et al. (2022) investigated that high-dose VD reduced lung inflammation in mice but not hamsters. They observed faster recovery in VD-treated mice that survived SARS-CoV-2 infection. However, there was no action on gene expression of SARS-CoV-2 in the lungs of mice or hamsters. They observed that VD deficiency increased disease severity, while VD sufficiency or supplementation reduced inflammation after H1N1 and SARS-CoV-2 infection. Several investigators have also pointed to the reduction of inflammation by VD in COVID-19 infection as the main mechanism of VD action. In addition, other researchers observed that new biomarkers for inflammation such as the systemic inflammatory index and response were negatively associated with VD concentrations (Dziedzic et al. 2022).
The potential effect of VD in regulating the innate and adaptive immune function in SARS-CoV2 infection has been reviewed by several investigators (Bikle 2022). Regarding innate immune systems, it modulates constitutive expression of recognition receptors such as TLRs to identify SARS-CoV2. It promotes the production of antimicrobial peptides such as cathelicidins and b-defensins from neutrophils, macrophages and from epithelial respiratory cells which stimulate clearance of these viruses. Importantly, the attenuating effect of VD on chronic activation of innate immunity that results in a cytokine/bradykinin storm. It works by down regulating TLRs and increasing IL-10 production by regulatory T cells, while inhibiting Th17 cells and the TNF/NFκB and IFNγ signaling pathways. The active metabolite, 1, 25(OH) 2D, regulates adaptive immunity by increasing the production of virus-specific IgG1 antibodies. However, it reduces DC maturation and regulation of key transcription factors such as STAT3 and STAT3 activator of IL-6 transforming T cell. Therefore, it inhibits inflammatory processes (Bikle 2022). Briceno Noriega and Savelkoul (2022) confirmed that VD plays an important role against the endemic phase of COVID-19 respiratory tract infection by acting as an immune modulator. It would play a protective role in the endemic phase of COVID-19 by stimulating the cellular receptor angiotensin converting enzyme 2 (ACE2)/Ang (1–7)/Mas G and inhibiting the expression of renin and the angiotensin II receptor type I (AT1R) axis. Moreover, cathelicidin LL-37 and human-defensin 2 induced by 1, 25(OH) 2D interact with the SARS-CoV-2 spike protein and inhibit viral binding to ACE2 (Pouremamali et al. 2022). Laboratory evidence of clinical studies has also shown that VD plays an immunomodulatory role during COVID-19 infection in preventing hyperinflammatory conditions associated with COVID-19(Sharif-Askari et al. 2022).
Cimmino et al. (2022) hypothesized that VD prevents IL-6 deleterious effects in COVID-19 infection. IL-6 induces COVID-related thrombosis via endothelial dysfunction with tissue Factor and adhesion molecules expression up regulation and ACE2r. Moreover, it was observed that the expression of VDR was statistically lower in patients with COVID-19 than healthy subjects and that the level of IL-6 was statistically higher in the COVID-19 group. Therefore, patients with severe COVID-19 may benefit from vitamin D supplementation, which would help reduce the production of IL-6 that causes a cytokine storm and thus reduce the severity of the disease. (Azmi et al. 2023; Chileshe et al. 2022; Holick et al. 2023). Furthermore, calcitriol has been shown to improve the barrier function of 16HBE cell layers based on two independent measures by inhibiting TNF-α-induced barrier leakage of epithelial cells in a human lung culture model (Rybakovsky et al. 2023).
The mechanism of action of VD in COVID-19 diseases may also be through regulation of autophagy gene activity by VD/VDR. VD promotes autophagy via genomic or nongenomic signaling pathway to regulate a wide range of functions of many organs (Sartika and Gayatri 2022). By activating autophagy, VD protects various organs from oxidative stress and apoptosis and regulates immune modulation, cell proliferation and differentiation, and control of inflammation. Furthermore, VD supplementation can enhance autophagy to prevent many human diseases as a part of human homeostasis mechanism (Bhutia et al. 2022). In vivo and in vitro studies, VD3 supplementation was able to activate autophagy and in vitro significantly enhances gene expression of VDRs and autophagy (Chen et al. 2022) (Fig. 1).
Clinical studies of Vitamin D in COVID-19
For this study, 58 publications published from January 2022 to August 2023 that met the eligible criteria were selected. Number of publications supporting the beneficial clinical effect of VD in treating COVID-19 is 49 (86%) including 12 meta-analyses. Overall 14,071273 patients were included in these publications while 14,029,411 (99.7%) patients were included in publications supporting the beneficial clinical effect of VD in COVID-19. Publications are discussed under three headings, observational studies, RCTs (intervention) that support a beneficial clinical effect of VD, and clinical studies that disapprove of the use of VD in the treatment of COVID-19.
Observational studies
Although there is a small number of conflicting evidence from interventional studies on the benefits of VD supplementation in COVID-19 patients, these epidemiological observational studies collectively confirm the association of low levels of vitamin D with COVID-19 susceptibility, severity, and mortality outcomes. In this study, 29 observational studies were published from January 2022 to August 2023, confirming the previous conclusion. In a very large population-based meta-analyses study by Petrelli et al. (2023), including 74 articles, 27 meta-analyses noted that vitamin D3 deficiency or insufficiency is associated with increased risk of SARS-CoV-2 infection, COVID-19 severity, and mortality risk, with highly suggestive evidence. Equally important, the results of a large number of observational groups, a retrospective case–control study published by Israel et al. in 2022, showed that there is an inverse relationship between the level of VD and the risk of SARS-CoV-2 infection and disease severity in infected patients. Moreover, in a prognostic association study, vitamin D deficiency contributes to an increased risk of severe outcomes of COVID-19, which was evident in a retrospective observational study conducted on 2342 patients with COVID-19 in a clinical hospital of infectious disease (Toban et al. 2023) and also in a retrospective observational cohort study including 2908 patients(Ramirez-Sandoval et al. 2022). These and 18 other studies suggest that low VD may be a risk factor for poor prognosis among patients admitted for COVID-19, therefore, serum levels of 25(OH)D for COVID-19 patients can be used as an independent indicator of prognosis (Table1).
A large pharmacoepidemiological study (220,265 patients) indicated that VD3 and VD2 supplements reduced the risks associated with COVID-19 infection by 20% and 28%, and mortality by 33% and 25%, respectively. Furthermore, patients with initially low VD levels benefited more from VD supplementation than did patients with a higher serum level. This study also suggested that patients receiving higher bolus doses and higher daily doses had significantly reduced rates of COVID-19 infection compared to patients receiving lower doses with similar levels of VD (Gibbons et al. 2022). Another canter-based observational prospective study (93,685 patients) demonstrated that higher VD intake was associated with decreased risk of ischemic stroke and pneumonia mortality (Nanri et al. 2023). Interestingly, in an observational cohort study, two doses of calcifediol 450 mcg each for two consecutive days reduced the percentage of mortality with the best prognosis in COVID-19 patients significantly higher than those who did not receive calcifediol. (Mingiano et al. 2023). Furthermore, VD levels were inversely correlated with inflammatory markers as TNFa, TNFa mRNA, IL-6, and D-dimer levels with lower mortality and severity of COVID-19 at higher VD levels (Beheshti et al. 2023). In general, extensive observational studies indicate a decisive relationship between low serum VD levels and mortality outcomes. However, the results of cohort studies generally suffer from insufficient follow-up time, completeness of follow-up, and the number of influencing factors cannot be well controlled. Therefore, correlation in observational studies cannot be equated with causation in actual RCTs.
Randomized controlled trials
Due to the characteristics of RCT, strict patient exclusion and inclusion criteria lead to limitations in the representativeness bias and external validity of the research results. RCTs of VD supplementation in COVID-19 are necessary to conclusively demonstrate benefit. As shown in Table 2, in this study, the scale of patients enrolled in 11 RCTs was 297,494 and in 11 meta-analyses of RCTs was 296,087 which were published from January 2022 to August 2023. Although different doses of VD have been used to test the efficacy of VD against COVID-19, all studies confirm the clinical safety of high-dose VD supplementation and the clinical benefit of VD in COVID-19. However, high-dose supplementation regimens had a significantly better clinical outcome compared to lower doses ( Sarhan et al. 2022; Cervero et al. 2022; Cicero et al. 2022; Shah et al. 2022; Annweiler et al. 2022; Tentolouris et al. 2022; Torres et al. 2022; Menger et al. 2022; Asla et al. 2023). Moreover, parenteral high dose was associated with a greater impact on reduction of mortality (Menger et al. 2022; Sarhan et al. 2022; Zaazouee et al. 2023; Asla et al. 2023). Additionally, VD may have greater benefit if given early in mild to moderate COVID-19 cases. In a randomized, double-blind, parallel trial enrolling highly exposed workers from four hospitals in Mexico City, a small daily dose (4000 IU) of VD supplementation prevented SARS-CoV-2 infection without serious complications and regardless of serum VD level. (Villasis-Keever et al. 2022).
Several meta-analyses compare the effectiveness of low-dose or high-dose on the clinical severity of COVID-19 (Shah et al. 2022; Cicero et al. 2022; Cervero et al. 2022; D'Ecclesiis et al. 2022; Hariyanto et al. 2022; Zaazouee et al. 2023). Anweiler et al. (2022) suggested that early administration of high dose (400,000 IU) versus standard dose (50,000 IU) of cholecalciferol to at-risk patients with COVID-19 reduced overall mortality. Likewise, Hosseini et al. (2022) meta-analysis found no change in the incidence of COVID-19 infection after VD supplementation, while showing protective effects against mortality and ICU admission in COVID-19 patients.The only meta-analysis with different results was published by Tentolouris et al. (2022). They suggested that daily oral doses as small as 1,000 IU of cholecalciferol to high doses as 400,000 IU of cholecalciferol have a beneficial role on ICU admission, but not on mortality. In cohort intervention clinical trial, vitamin D reduced markers of inflammation and shortened the length of stay in the intensive care unit. Vitamin D also led to decreased levels of STAT3, JNK, and AKT pathways, and decreased levels of proinflammatory cytokines such as IL-6, IL-17, and IL-1β (Sharif-Askari et al. 2022; Hafezi et al. 2022). Collectively, the evidence from these intervention studies has demonstrated clear efficacy of VD supplementation in treating COVID-19. This evidence has been significantly strengthened over the past 18 months, with several meta-analyses reporting conclusive, specific, and indisputable protective effects of VD supplementation against admission of COVID-19 patients to the ICU(D’Ecclesiis et al. 2022; Hariyanto et al. 2022; Cicero et al. 2022; Argano et al. 2023; Petrelli et al. 2023; Asla et al. 2023).
Clinical studies have not found a beneficial clinical effect for vitamin D in treating COVID-19
Eight randomized control trials (RCT) and one met a-analysis of RCTs published from January 2022 to Aug. 2023 did not approve the use of vitamin D in the treatment and prevention of COVID-19., The scale of patients enrolled in these studies was 41,862. All randomized controlled trials confirm the clinical safety of high doses of vitamin D supplements, however, benefit has not been observed in any of the COVID-19 outcomes including length of hospital stay, disease incidence, number of days on respiratory support, mortality, admission to Intensive care unit, and prognosis. Four randomized controlled trials(44% of studies) evaluated the effect of a single oral bolus dose of cholecalciferol (100,000–500,000 IU) on length of hospital stay and respiratory deterioration, and found no effect in 649 patients with insufficient vitamin D level (Cannata-Andía et al. 2022; Mariani et al. 2022; Jaun et al. 2023; Abroug et al. 2023). Eight cohort studies and eight randomized controlled studies involving 3359 patients with COVID-19 were included in the only meta- analysis study (Zhang et al. 2023). This study showed that the results of the pooled analysis of cohort studies indicated that VD supplementation had a significant effect on reducing mortality in COVID-19 patients, while the results of the pooled analysis of RCTs showed that VD supplementation did not significantly change the mortality rate (Table 3).
In a large-scale, quadruple-blind, randomized controlled trial (34,601 patients), 10 mcg of vitamin D daily for six months in the winter did not reduce the incidence of SARS-CoV-2 infection and severe COVID-19 outcomes compared with placebo(Brunvoll et al. 2022). Similar conclusions were achieved in three arm, parallel, randomised controlled trial observed that 800 IU/day or3200 IU/day vitamin D for six months had no effect on incidence of covid-19(Jolliffe et al. 2022). However, the overall number of randomized controlled trials that failed to find a beneficial role for vitamin D was small with a small sample size of the enrolled population and heterogeneous with respect to study design, dosing and intervention strategies. In addition to the conflicting results in the meta-analysis as described with Zhang et al. (2023). Therefore, these studies are considered to have low-grade evidence against the beneficial role of vitamin D in COVID-19.
Impact of vitamin D on long COVID-19 syndrome
Two years after the SARS-CoV-2 virus began spreading globally, reports from most parts of the world indicate that a significant proportion of people who have recovered from COVID-19 have various health problems referred to as “long COVID-19.”. Several published studies on long COVID suggest that in 50% to 70% of COVID-19 survivors suffer from post-COVID symptoms for more than 3 months after acute disease (Fernández et al. 2021). Furthermore, other studies suggest that individuals may remain symptomatic months after initial recovery and an estimated 65 million or more people are living with the effects of long COVID-19 (Davis et al. 2023). long COVID-19 is an emerging chronic disease that has the potential to impact overall health and patients may experience mild to moderate symptoms including fatigue, chest pain, muscle pain, shortness of breath, cough, headache and “brain fog.” These symptoms last longer after infection with the Omicron variant of the SARS-CoV 2 virus (Thaweethai et al. 2023). However, there is a cumulative risk of post-acute sequelae, which may include various acute cardiac, pulmonary, or neurological and psychiatric symptoms. The incidence of long COVID-19 is increasing proportionally with the number of SARS-CoV-2 infections, especially in older people. Therefore, the number of cases of post-acute sequelae is expected to increase in the future (Boufidou et al. 2023).
Unfortunately, no effective approved treatment against Long COVID-19 has been discovered yet. The primary management of long-term COVID-19 currently relies on supportive treatment, symptomatic treatment and rehabilitation. A key part of the multidisciplinary approach to treatment involves the patient taking an active role in their recovery and self-monitoring (Schrimpf et al. 2022; Banerjee et al. 2022; Chee et al. 2023). Interestingly, the US National Institutes of Health (NIH) recently announced a series of clinical trials for potential treatments for long-term Covid-19 disease (Tanne 2023). There is now reasonable evidence that vaccination reduces the risk of long COVID-19. In a meta-analysis conducted in March 2023, people who received two doses of the vaccine were significantly less likely to develop long Covid-19 than unvaccinated people (Marshall 2023). However, reliable comparative studies, including randomized controlled trials, are needed to provide strong evidence of the efficacy of vaccination in preventing or relieving long COVID-19. A recent study observed a promising effect of metformin in preventing long-term COVID-19 disease compared to ivermectin or fluvoxamine. Outpatient treatment with metformin has been shown to reduce the incidence of long Covid by about 41%, compared to placebo. However, there was no significant effect of ivermectin or fluvoxamine on the cumulative incidence of long Covid compared to placebo (Bramante et al. 2023).
There are promising reasons to enhance research on the effects of vitamin D supplements in long COVID-19 patients. Several narrative reviews have indicated vitamin D as a mitigation agent for long COVID-19 and highlighted the potential role of hypovitaminosis D as a risk factor for long COVID-19 (Men´endez et al. 2022; Barrea et al. 2022; Moukayed 2023; Marks 2023). These publications are supported by the results of 4 observational studies published from January 2022 to Aug. 2023. However, no randomized controlled trial has been published to date that has found that vitamin D affects long COVID-19 syndrome. In one study, 681 post-COVID outpatient participants with long- COVID-19 were re-evaluated 6 months after hospital discharge. It was noted that vitamin D deficiency was detected in 35.6% of participants, and therefore vitamin D was an independent risk factor for long-COVID-19. Furthermore, vitamin D deficiency was associated with decreased performance with older participants (Galluzzo et al. 2022). In line with these findings, a previous study showed that COVID-19 survivors with vitamin D deficiency had lower exercise tolerance (Townsend et al. 2021).
In a retrospective case study, conducted from June 20 to July 31, 2022, blood concentrations of vitamin D, zinc, and fibrinogen were determined in patients infected with Omicron, a variant of the COVID-19 virus, who developed post-COVID-19 symptoms. This study showed that low serum VD level is associated with delayed recovery from long COVID-19 syndrome (Chen et al. 2023). In another retrospective cross-sectional study, fifty subjects with long COVID-19 and 50 subjects without long COVID-19 were enrolled on a 1:1 basis from the post-COVID-19 outpatient clinic. It was apparent that COVID-19 survivors with long COVID-19 had lower levels of VD 25(OH) than matched patients without long COVID-19 (di Filippo et al. 2023b). There is accumulating evidence to support the use of VD supplements, before and after infection with SARS-CoV-2, as a preventive strategy to reduce the risk of COVID-19, however, few other studies have reported a non-significant effect of VD in COVID-19. Metformin use may improve AMPK signaling of VDR and enhance VD efficacy in COVID-19 and long COVID-19. Randomized trials are needed to provide conclusive evidence of the effectiveness of VD or VD with metformin in preventing or ameliorating long COVID-19 disease in all patients.
Genetic polymorphisms of the vitamin D could explain the controversy surrounding the clinical outcomes of VD supplementation
VD deficiency is highly prevalent worldwide and appears to be on the rise, and is common in critically ill patients (Xie et al. 2022; Cui et al. 2023). VD deficiency has been associated with a higher risk of severe COVID-19 infection (Dissanayake et al. 2022; Topan et al. 2023). However, the importance of VD supplementation in COVID-19 remains controversial. While some articles have observed a significant positive effect of VD on COVID-19 severity (ASLA et al. 2023; Petrelli et al. 2023), other studies have failed to find any benefit (Zhang et al. 2023). This controversy can be explained by differences in study design, samples studied, race, age, and genetic variations. Changes in serum 25(OH) D relative to VD supplementation vary widely among individuals. Recent genome studies have revealed associations of 25(OH)D concentration with proteins involved in vitamin D metabolism and transport. Variation in the expression and activities of these proteins could result in genetic differences in the production, transport and degradation of 25(OH) D that lead to differences in the level of 25(OH) D after vitamin D supplementation. Changes in the 25 (OH) D levels alter the dynamics and kinetics of VD. For example, increased endogenous production of vitamin D3 could be due to decreased enzymatic activity of 7-dehydrocholesterol reductase, encoded by the DHCR7 gene (Charoenngam et al. 2023).
Currently, several publications have addressed the relationship between genetic variation for vitamin D and treatment failure for COVID-19. Twenty-five articles on the association of genetic polymorphisms in the VDR or vitamin D binding protein (DBP) gene and individual responses to VD supplementation were published from January 2022 to August 2023. Several studies have indicated that there is significant variability in response to VD supplementation between people (Kelishadi et al. 2020; Ammar et al. 2023). This variability may result from a DBP polymorphism that contributes to the variability in the total plasma 25(OH) D concentrations of the VD supplementation. DBP gene encodes a DBP protein of 52 to 59 kDa, regulates absorption and plays important role in the transport of VD and its metabolites (Mehramiz et al. 2019; Slow et al. 2020; Ammar et al. 2023). This DBP polymorphism may explain the meta-analysis conclusion of Menger et al. (2022) about the superior effect on reducing COVID-19 mortality of parenteral VD supplementation. Other studies have indicated that gene polymorphisms in the VD metabolism pathway may alter susceptibility and severity of COVID-19 infection. Saria Santamira et al. (2023) observed an association of the CYP24A1 rs6127099 (A > T) polymorphism with a lower risk of COVID-19 infection. Furthermore, Foruhari et al. (2023) explain the variation of vitamin D levels across populations by epigenetic polymorphisms of CYP24A1 methylation.
Meanwhile, several studies noted that levels of 25(OH) D were not associated with the severity and mortality of COVID-19. These articles showed that response to VD supplementation can be altered by genetic variants of the VDR gene. The VDR genes are important for VD signaling and are modulated by genetic and non-genetic factors. Some VDR gene polymorphisms are independently linked with COVID-19 severity and patient survival (Ghiasvand et al. 2022; Apaydin et al. 2022; Jafarpoor et al. 2022). The TaqI variant allele and the FF variant FokI genotype can alter response to vitamin D supplementation and are associated with a better response (Usategui-Martín et al. 2022). Other researchers indicated that the mortality rate or severity of SARS-CoV-2 with different variants was associated with a low level of 25-OHD and that VDR SNPs regulate susceptibility to infection with COVID-19 (Camporesi et al. 2022; Al-Gharawi et al. 2023; Albu-Mohammed et al. 2022; Protas et al. 2023). Furthermore, it has been shown that patients with FokI and TaqI gene polymorphisms may be at higher risk of COVID-19 pandemic infection, and VD supplementation is recommended for individuals in the period surrounding or after the COVID-19 pandemic (Mamurova et al. 2023; Zeidan et al. 2023; Shawi et al. 2023). It is clear from previous studies, VD deficiency and VDR polymorphisms are risk factors for COVID-19 and could explain the controversy surrounding the clinical outcomes of VD supplementation in the treatment of COVID-19. Large parenteral dose of VD supplementation may be recommended to reduce risk of DBP gene polymorphisms.
Non-genetic reasons for differences in VD effects in COVID-19 and the use of metformin to improve VDR sensitivity
Unresponsiveness or VD resistance is not only caused by genetic disorders in vitamin D receptor expression, but impairment of vitamin D signaling (receptor resistance) may also be the cause as non-genetic mechanisms for variability of VD effects (Hampl and Vondra 2017; Macova et al. 2018). Metformin is the most frequently used first-line drug for the treatment of T2D. It attenuates insulin resistance or improves insulin receptor sensitivity through activation of AMPK or AMPK signaling and other mechanisms including several AMPK-independent mechanisms, such as affecting mitochondrial function, restoring redox homeostasis, and regulating several other signals, such as mTOR, SIRT1 and FBP1(Du et al. 2022). Several observations indicate that metformin is a unique multi-acting drug that targets multiple pathological pathways of COVID-19 in a diabetes-independent manner (Wiernsperger et al. 2022). Therefore, we hypothesize that metformin can improve VDR sensitivity through activation of AMPK or AMPK-independent mechanisms. This hypothesis is supported by preclinical, observational and clinical evidence that metformin may be beneficial in patients with acute, severe SARS-CoV-2 infection, long-COVID-19 and moderate evidence of benefit of metformin in preventing health care outcomes in COVID-19. In three clinical trials, metformin showed some effectiveness in protecting against severe cases of COVID-19 (Erickson et al. 2023).
In a comprehensive meta-analysis by Ganesh and Randall (2022), metformin improved outcomes in COVID-19 patients with DM and reduced mortality. In another meta-analysis including 22 retrospective observational studies, there was a significant association between reduced in-hospital mortality and outpatient metformin treatment for T2D in patients hospitalized for COVID-19 (Ma, Krishnamurth, 2023). Consistent with these studies, Pedrosa et al. (2023) examined the association between risk of death among COVID-19 patients and metformin use in 26 retrospective studies. They found that metformin use was significantly associated with a 13 to 90% reduction in death rate in patients with COVID-19. Importantly, a recent study showed that early use of metformin in treating COVID-19 outpatients reduced healthcare utilization for severe COVID-19 by 42.3% and the risk of long-term COVID by 41.3% over 10 months of follow-up( Bramante et al. 2023). Therefore, our study indicates a theoretical and practical basis for the use of metformin as a promising drug for improving VDR sensitivity, and its combination with vitamin D supplementation would be preferable in combating SARS-CoV-2 infection.
Conclusion
There is accumulating evidence to support the use of VD supplements, before and after infection with SARS-CoV-2 (Long COVID-19), as a preventive strategy to reduce the risk of COVID-19 infection and mortality as well as prevent and treat post-COVID-19 syndrome. Several studies have indicated that early intramuscular administration of high-dose long-acting cholecalciferol had significantly better clinical outcomes in patients infected with COVID-19. However, few other studies with low-grade evidence have reported a non-significant effect of VD in COVID-19.This controversy surrounding the clinical outcomes of VD supplementation in the treatment of COVID-19 can be explained by VDR gene polymorphisms, DBP gene polymorphisms and impaired VDR signaling as non-genetic causes. Our study indicates a theoretical and clinical basis for the use of metformin as a promising drug to improve VDR sensitivity, and its combination with VD supplementation would be better in combating SARS-CoV-2 infection.
Availability of data and materials
Data on relevant human studies in the current review. https://docs.google.com/document/d/1d1BtamJEQzGDzQPtAR4O93-4Mx15hkQ7/edit?usp=sharing&ouid=113303836144481485524&rtpof=true&sd=true
Abbreviations
- AMPK:
-
AMP activated protein kinase
- ARDS:
-
Acute respiratory distress Syndrome
- ACE2:
-
Angiotensin-converting enzyme 2
- DBP:
-
Vitamin D binding protein
- COVID-19:
-
Coronavirus disease 2019
- 25(OH) D2:
-
Ergocalciferol; 1,25(OH)2 D3: cholecalciferol
- CRP:
-
C-reactive protein
- DM:
-
Diabetes mellitus
- HMGB1:
-
High-mobility group box 1 protein
- iNOS:
-
Inducible nitric oxide synthase
- IL-1β:
-
Interleukin one beta
- IL-6:
-
Interleukin-6
- JAK:
-
Janus kinase
- JNK:
-
C-Jun N-terminal kinase
- LTB4:
-
Leukotriene B4
- mTOR:
-
Mammalian target of rapamycin
- PRKA:
-
Protein kinase AMP-activated
- NF-κB:
-
Nuclear factor kappa B
- RCT:
-
Clinical controlled trial
- SIRT1:
-
Sirtuin
- SARS-CoV2:
-
Severe acute respiratory syndrome coronavirus2
- TLR:
-
Toll-like receptor 2
- TNF-α:
-
Tumor necrosis factor alpha
- T2D:
-
Diabetes type 2
- VDR:
-
Vitamin D receptor
References
Abroug H, Maatouk A, Bennasrallah C, Dhouib W, Ben Fredj M, Zemni I, Kacem M, Mhalla S et al (2023) Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial. Trials 24(1):123. https://doi.org/10.1186/s13063-023-07114-5. (PMID: 36803273; PMCID: PMC9940050)
Albu-Mohammed WHM, Anvari E, Fateh A (2022) Evaluating the role of BglI rs739837 and TaqI rs731236 polymorphisms in Vitamin D receptor with SARS-CoV-2 variants mortality rate. Genes 13(12):2346. https://doi.org/10.3390/genes13122346. (PMID: 36553614; PMCID: PMC9777972)
Al-Gharrawi ANR, Anvari E, Fateh A (2023) Association of ApaI rs7975232 and BsmI rs1544410 in clinical outcomes of COVID-19 patients according to different SARS-CoV-2 variants. Sci Rep 13(1):3612. https://doi.org/10.1038/s41598-023-30859-7. (PMID: 36869206; PMCID: PMC9983525)
AlYafei N, Fathima Jaleel BN, Abdel-Salam AG, Ali Al-Saadi H, Al Abdulla SA (2022) Association of serum Vitamin D level and COVID-19 infection: a case-control Study. Qatar Med J 5(4):48. https://doi.org/10.5339/qmj.2022.48. (PMID:36504923;PMCID:PMC9720159)
Ammar M, Heni S, Tira MS, Khalij Y, Hamdouni H, Amor D, Ksibi S, Omezzine A, Bouslama A (2023) Variability in response to vitamin D supplementation according to vitamin D metabolism related gene polymorphisms in healthy adults. Eur J Clin Nutr 77(2):189–194. https://doi.org/10.1038/s41430-022-01218-y. (PMID: 36167979; PMCID: PMC9514197)
Annweiler C, Beaudenon M, Gautier J, Gonsard J, Boucher S, Chapelet G, Darsonval A, Fougère B, Guérin O, Houvet M, Ménager P, Roubaud-Baudron C, Tchalla A, Souberbielle JC, Riou J, Parot-Schinkel E, Célarier T, COVIT-TRIAL study group, (2022) High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med 19(5):e1003999. https://doi.org/10.1371/journal.pmed.1003999. (PMID: 35639792; PMCID: PMC9154122)
Apaydin T, Polat H, Dincer Yazan C, Ilgin C, Elbasan O, Dashdamirova S, Bayram F, Tukenmez Tigen E, Unlu O, Tekin AF, Arslan E, Yilmaz I, Haklar G, Ata P, Gozu H (2022) Effects of vitamin D receptor gene polymorphisms on the prognosis of COVID-19. Clin Endocrinol (oxf) 96(6):819–830. https://doi.org/10.1111/cen.14664. (Epub 2021 Dec 25 PMID: 34919268)
Argano C, Mallaci Bocchio R, Natoli G, Scibetta S, Lo Monaco M, Corrao S (2023) Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals (basel) 16(1):130. https://doi.org/10.3390/ph16010130. (PMID:36678627;PMCID:PMC9864223)
Arora J, Patel DR, Nicol MJ, Field CJ, Restori KH, Wang J, Froelich NE, Katkere B, Terwilliger JA, Weaver V, Luley E, Kelly K, Kirimanjeswara GS, Sutton TC, Cantorna MT (2022) Vitamin D and the ability to produce 1,25(OH)2D are critical for protection from viral infection of the lungs. Nutrients 14(15):3061. https://doi.org/10.3390/nu14153061.PMID:35893921;PMCID:PMC9332570
Asla MM, Nawar AA, Elsayed A, Farahat RA, Abdel Gadir A, Vitamin D on COVID-19 Patients During the Pandemic et al (2022) A systematic review and meta-analysis curr. Res Nutr Food Sci Jour 11(1):37–60. https://doi.org/10.12944/CRNFSJ.11.1.3
Azmi A, Rismani M, Pourmontaseri H, Mirzaii E, Niknia S, Miladpour B (2023) The role of vitamin D receptor and IL-6 in COVID-19. Mol Genet Genomic Med 11:e2172. https://doi.org/10.1002/mgg3.2172
Bae JH, Choe HJ, Holick MF, Lim S (2022) Association of Vitamin D status with COVID-19 and its severity : Vitamin D and COVID-19: a narrative review. Rev Endocr Metab Disord 23(3):579–599. https://doi.org/10.1007/s11154-021-09705-6.PMID:34982377;PMCID:PMC8724612
Banerjee I, Robinson J, Sathian B (2022) Treatment of long COVID or post COVID syndrome: a pharmacological approach. Nepal J Epidemiol. 12(3):1220–1223. https://doi.org/10.3126/nje.v12i3.48532. (PMID: 36407052; PMCID: PMC9659683)
Barrea L, Verde L, Grant WB, Frias-Toral E, Sarno G, Vetrani C, Ceriani F, Garcia-Velasquez E et al (2022) (2022) Vitamin D: a role also in long COVID-19? Nutrients 14(8):1625. https://doi.org/10.3390/nu14081625. (PMID:35458189;PMCID:PMC9028162)
Barrett R, Youssef M, Shah I, Ioana J, Lawati AA, Bukhari A et al (2022) Vitamin D status and mortality from SARS CoV-2: a prospective study of unvaccinated caucasian adults. Nutrients 14(16):3252. https://doi.org/10.3390/nu14163252. (PMID:36014757;PMCID:PMC9413855)
Basińska-Lewandowska M, Lewandowski K, Horzelski W, Lewiński A, Skowrońska-Jóźwiak E (2023) Frequency of COVID-19 infection as a function of Vitamin D levels. Nutrients 15(7):1581. https://doi.org/10.3390/nu15071581. (PMID: 37049423; PMCID: PMC10097275)
Baxter BA, Ryan MG, LaVergne SM, Stromberg S, Berry K, Tipton M, Natter N, Nudell N, McFann K, Dunn J et al (2022) Correlation between 25-hydroxyvitamin D/D3 deficiency and COVID-19 disease severity in adults from northern colorado. Nutrients 14:5204. https://doi.org/10.3390/nu14245204
Beheshti M, Neisi N, Parsanahad M, Rasti M, Nashibi R, Cheraghian B (2023) Correlation of Vitamin D levels with serum parameters in Covid-19 patients. Clin Nutr ESPEN 55:325–331. https://doi.org/10.1016/j.clnesp.2023.04.012.PMID:37202065;PMCID:PMC10114307
Bhutia SK (2022) Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J Nutr Biochem 99:108841. https://doi.org/10.1016/j.jnutbio.2021.108841. (Epub 2021 Aug 14 PMID: 34403722)
Bikle DD (2022) Vitamin D regulation of immune function. Curr Osteoporos Rep 20:186–193. https://doi.org/10.1007/s11914-022-00732-z
Boufidou F, Medić S, Lampropoulou V, Siafakas N, Tsakris A, Anastassopoulou C (2023) SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int J Mol Sci 24(16):12962. https://doi.org/10.3390/ijms241612962. (PMID:37629143;PMCID:PMC10454552)
Bramante CT, Buse JB, Liebovitz DM, Nicklas JM, Puskarich MA, Cohen K, Belani HK et al (2023) Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(23)00299-2
Briceno Noriega D, Savelkoul HFJ (2022) Vitamin D: a potential mitigation tool for the endemic stage of the COVID-19 pandemic? Front Public Health 10:888168. https://doi.org/10.3389/fpubh.2022.888168
Brunvoll SH, Nygaard AB, Ellingjord-Dale M, Holland P, Istre MS, Kalleberg KT et al (2022) Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial. BMJ 378:e071245. https://doi.org/10.1136/bmj-2022-071245
Camporesi G, Hernández Payró R, Levy Esses T, Peláez Samperio MJ, Macho González A, Sánchez-Muniz FJ (2022) Vitamina D y polimorfismos de los genes VDR y GC en la severidad y mortalidad por COVID-19 Una revisión sistemática [Vitamin D and polymorphisms of VDR and GC genes in the severity and mortality from COVID-19 A systematic review]. Nutr Hosp 39(6):1397–1407. https://doi.org/10.20960/nh.04299. (PMID: 36327123)
Cannata-Andía JB, Díaz-Sottolano A, Fernández P, Palomo-Antequera C, Herrero-Puente P (2022) COVID-VIT-D trial collaborators et al a single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med 20(1):83. https://doi.org/10.1186/s12916-022-02290-8.PMID:35177066;PMCID:PMC8853840
Cao M, He C, Gong M, Wu S, He J (2023) The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials. Front Nutr 22(10):1132528. https://doi.org/10.3389/fnut.2023.1132528.PMID:37426183;PMCID:PMC10325578
Cervero M, López-Wolf D, Casado G, Novella-Mena M, Ryan-Murua P, Taboada-Martínez ML et al (2022) Beneficial effect of short-term supplementation of high dose of Vitamin D3 in hospitalized patients with COVID-19: a multicenter, single-blinded, prospective randomized pilot clinical trial. Front Pharmacol 13:863587. https://doi.org/10.3389/fphar.2022.863587
Charoenngam N, Jaroenlapnopparat A, Mettler SK, Grover A (2023) Genetic variations of the Vitamin D metabolic pathway and COVID-19 susceptibility and severity: current understanding and existing evidence. Biomedicines 11(2):400. https://doi.org/10.3390/biomedicines11020400. (PMID:36830936;PMCID:PMC9953304)
Chee YJ, Fan BE, Young BE, Dalan R, Lye DC (2023) Clinical trials on the pharmacological treatment of long COVID: A systematic review. J Med Virol 95(1):e28289. https://doi.org/10.1002/jmv.28289. (PMID: 36349400; PMCID: PMC9878018)
Chen Z, Huang D, Yongyut P, Li G, Esteban MA, Jintasataporn O, Deng J, Zhang W, Ai Q, Mai K, Zhang Y (2022) Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-kB/ inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy. Front Immunol 13:986593. https://doi.org/10.3389/fimmu.2022.986593
Chen KY, Lin CK, Chen NH (2023) Effects of vitamin D and zinc deficiency in acute and long COVID syndrome. J Trace Elem Med Biol 80:127278. https://doi.org/10.1016/j.jtemb.2023.127278
Chileshe M, Mulamba M, Julius V, Kwangu M, Mubita SM (2022) Analysis of Vitamin D and its correlation with interleukin-6 in COVID-19 patients at mary begg health services, Zambia: implication for patient management. J Biomed Res Environ Sci 3(4):335–343. https://doi.org/10.37871/jbres1445
Chiodini I, Gatti D, Soranna D et al (2021) Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes. Front Public Health 9:736665
Cicero AFG, Fogacci F, Borghi C (2022) Vitamin D supplementation and COVID-19 outcomes: mounting evidence and fewer doubts. Nutrients 14:3584. https://doi.org/10.3390/nu14173584
Cimmino G, Conte S, Morello M, Pellegrino G, Marra L, Morello A, Nicoletti G, De Rosa G, Golino P, Cirillo P (2022) Vitamin D inhibits IL-6 Pro-Atherothrombotic effects in human endothelial cells: a potential mechanism for protection against COVID-19 infection? J Cardiovasc Dev Dis 9:27. https://doi.org/10.3390/jcdd9010027
Cui A, Zhang T, Xiao P, Fan Z, Wang H, Zhuang Y (2023) Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7 9 million participants. Front Nutr 10:1070808. https://doi.org/10.3389/fnut.2023.1070808. (PMID: 37006940; PMCID: PMC10064807)
Davis HE, McCorkell L, Vogel JM, Topol EJ (2023) Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 21(3):133–146. https://doi.org/10.1038/s41579-022-00846-2. (PMID: 36639608; PMCID: PMC9839201)
De Niet S, Trémège M, Coffiner M, Rousseau AF, Calmes D, Frix AN et al (2022) Positive effects of Vitamin D supplementation in patients hospitalized for COVID-19: a randomized, double-blind placebo-controlled trial. Nutrients 14(15):3048. https://doi.org/10.3390/nu14153048. (PMID:35893907;PMCID:PMC9330587)
D’Ecclesiis O, Gavioli C, Martinoli C, Raimondi S, Chiocca S, Miccolo C et al (2022) Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS ONE 17(7):e0268396. https://doi.org/10.1371/journal.pone.0268396. (PMID:35793346;PMCID:PMC9258852)
di Filippo L, Frara S, Nannipieri F, Cotellessa A, Locatelli M, Rovere Querini P, Giustina A (2023a) Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgad207. (PMID: 37051747)
di Filippo L, Uygur M, Locatelli M, Nannipieri F, Frara S, Giustina A (2023b) Low vitamin D levels predict outcomes of COVID-19 in patients with both severe and non-severe disease at hospitalization. Endocrine 80(3):669–683. https://doi.org/10.1007/s12020-023-03331-9. (PMID: 36854858; PMCID: PMC9974397)
Dissanayake HA, de Silva NL, Sumanatilleke M, de Silva SDN, Gamage KKK et al (2022) Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J Clin Endocrinol Metab 107:1484–1502
Domazet Bugarin J, Dosenovic S, Ilic D, Delic N, Saric I, Ugrina I, Stojanovic Stipic S, Duplancic B, Saric L (2023) Vitamin D supplementation and clinical outcomes in severe COVID-19 patients-randomized controlled trial. Nutrients 15(5):1234. https://doi.org/10.3390/nu15051234
Dror AA, Morozov N, Daoud A, Namir Y, Yakir O, Shachar Y et al (2022) Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 17(2):e0263069. https://doi.org/10.1371/journal.pone.0263069. (PMID:35113901;PMCID:PMC8812897)
Du Y, Zhu YJ, Zhou YX, Ding J, Liu JY (2022) Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study. Mol Biomed 3(1):41. https://doi.org/10.1186/s43556-022-00108-w. (PMID: 36484892; PMCID: PMC9733765)
Dziedzic EA, Ga˛siorJS, Tuzimek A, Da˛browski M, Jankowski P, (2022) The Association between Serum Vitamin D concentration and new inflammatory biomarkers—systemic inflammatory index (SII) and systemic inflammatory response (SIRI)—in patients with ischemic heart disease. Nutrients 14:4212. https://doi.org/10.3390/nu14194212
Elmi ZAI, Sighakoli S, Tetteh J et al (2023) Case–control study of serum vitamin D concentrations in hospitalised patients with COVID-19 and hospitalised controls suffering with respiratory tract infections of differing aetiology. BMJ Nutr Prev Health. https://doi.org/10.1136/bmjnph-2022-000428
Erickson SM, Fenno SL, Barzilai N, Kuchel G, Bartley JM, Justice JN, Buse JB, Bramante CT (2023) Metformin for treatment of acute COVID-19: systematic review of clinical trial data against SARS-CoV-2. Diabetes Care 46(7):1432–1442. https://doi.org/10.2337/dc22-2539. (PMID: 37339345; PMCID: PMC10300519)
Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL (2021) Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 18(5):2621
Forouhari A, Heidari-Beni M, Veisi S, Poursafa P, Kelishadi R (2023) Effect of epigenetics on vitamin D levels: a systematic review until December 2020. Arch Public Health 81(1):106. https://doi.org/10.1186/s13690-023-01122-2. (PMID: 37322552; PMCID: PMC10268530)
Galluzzo V, Ciciarello F, Tosato M, Zazzara MB, Pais C, Savera G, Calvani R, Picca A, Marzetti E, Landi F, Gemelli Against COVID-19 Post-Acute Care Team (2022) Association between vitamin D status and physical performance in COVID-19 survivors: Results from the Gemelli against COVID-19 post-acute care project. Mech Ageing Dev 205:111684. https://doi.org/10.1016/j.mad.2022.111684. (PMID: 35568146; PMCID: PMC9093083)
Ganesh A, Randall MD (2022) Does metformin affect outcomes in COVID-19 patients with new or pre-existing diabetes mellitus? A systematic review and meta analysis. Br J Clin Pharmacol 88(6):2642–2656. https://doi.org/10.1111/bcp.15258
Ghiasvand R, Rashidian A, Abaj F, Rafiee M (2022) Genetic variations of vitamin D receptor and vitamin D supplementation interaction in relation to serum vitamin D and metabolic traits: a systematic review and meta-analysis. Int J Vitam Nutr Res. https://doi.org/10.1024/0300-9831/a000762. (PMID: 3599720)
Gholamalizadeh M, Rabbani F, Ahmadzadeh M, Hajipour A, Musavi H, Mobarakeh KA et al (2023) The association between vitamin D intake with inflammatory and biochemical indices and mortality in critically ill patients with COVID-19: A case-control study. Immun Inflamm Dis 11(4):e844. https://doi.org/10.1002/iid3.844. (PMID:37102656;PMCID:PMC10132183)
Gholi Z, Yadegarynia D, Eini-Zinab H, Vahdat Shariatpanahi Z (2022) Vitamin D deficiency is associated with increased risk of delirium and mortality among critically Ill, elderly covid-19 patients. Compl Ther Med 70:102855. https://doi.org/10.1016/j.ctim.2022.102855. (PMID: 35868492; PMCID: PMC9293788)
Gibbons JB, Norton EC, McCullough JS, Meltzer DO, Lavigne J, Fiedler VC et al (2022) Association between vitamin D supplementation and COVID-19 infection and mortality. Sci Rep 12(1):19397. https://doi.org/10.1038/s41598-022-24053-4. (PMID:36371591;PMCID:PMC9653496)
Gilani SJ, Bin-Jumah MN, Nadeem MS, Kazmi I (2022) Vitamin D attenuates COVID-19 complications via modulation of proinflammatory cytokines, antiviral proteins, and autophagy. Expert Rev Anti Infect Ther 20(2):231–241. https://doi.org/10.1080/14787210.2021.1941871. (PMID: 34112047; PMCID: PMC8477590)
Hafezi S, Saheb Sharif-Askari F, Saheb S-A et al (2022) Vitamin D enhances type I IFN signaling in COVID-19 patients. Sci Rep 12:17778. https://doi.org/10.1038/s41598-022-22307-9
Hampl R, Vondra K (2017) Peripheral sensitivity to steroids revisited. Physiol Res 66(Suppl 3):S295–S303
Hariyanto TI, Intan D, Hananto JE, Harapan H, Kurniawan A (2022) Vitamin D supplementation and Covid-19 outcomes: A systematic review, meta-analysis and meta-regression. Rev Med Virol 32(2):e2269. https://doi.org/10.1002/rmv.2269.PMCID:PMC8420388
Holick MF, Mazzei L, García Menéndez S, Martín Giménez VM, Al Anouti F, Manucha W (2023) Genomic or non-genomic? a question about the pleiotropic roles of Vitamin D in inflammatory-based diseases. Nutrients 15:767. https://doi.org/10.3390/nu15030767
Hosseini B, El Abd A, Ducharme FM (2022) Effects of Vitamin D supplementation on COVID-19 related outcomes: a systematic review and meta-analysis. Nutrients 14(10):2134. https://doi.org/10.3390/nu14102134. (PMID:35631275; PMCID: PMC9147949)
Huang H, Zheng J, Liu Y, Zhou Q, Peng D (2023) Effect of vitamin D status on adult COVID-19 pneumonia induced by Delta variant: A longitudinal, real-world cohort study. Front Med (lausanne) 10:1121256. https://doi.org/10.3389/fmed.2023.1121256. (PMID:37035323;PMCID:PMC10080157)
Israel A, Cicurel A, Feldhamer I, Stern F, Dror Y, Giveon SM, Gillis D, Strich D, Lavie G et al (2022) Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: a retrospective case-control study. Intern Emerg Med 17(4):1053–1063. https://doi.org/10.1007/s11739-021-02902-w.PMID:35000118;PMCID:PMC8742718
Jafarpoor A, Jazayeri SM, Bokharaei-Salim F, Ataei-Pirkooh A, Ghaziasadi A, Soltani S et al (2022) VDR gene polymorphisms are associated with the increased susceptibility to COVID-19 among iranian population: A case-control study. Int J Immunogenet 49(4):243–253. https://doi.org/10.1111/iji.12585. (PMID: 35861117; PMCID: PMC9350092)
Jaun F, Boesing M, Luethi-Corridori G, Abig K, Bloch N, Giezendanner S, Grillmayr V et al (2023) Effect of single high dose Vitamin D substitution in hospitalized COVID-19 patients with Vitamin D deficiency on length of hospital stay. Biomedicines 11(5):1277. https://doi.org/10.3390/biomedicines11051277. (PMID:37238948;PMCID:PMC10215464)
Jolliffe DA, Holt H, Greenig M, Talaei M, Perdek N, Pfeffer P, Vivaldi G, Maltby S, Symons J et al (2022) Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ 378:e071230. https://doi.org/10.1136/bmj-2022-071230
Karonova TL, Golovatyuk KA, Kudryavtsev IV, Chernikova AT, Mikhaylova AA, Aquino AD et al (2022) Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label. Single-Center Study Nutrients 14(13):2602. https://doi.org/10.3390/nu14132602. (PMID:35807783;PMCID:PMC9268385)
Kelishadi R, Heidari-Beni M, Akbarian SA, Hasan Tajadini M, Haghjooy Javanmard S (2020) Genetic variation in cytochrome P450 2R1 and Vitamin D binding protein genes are associated with Vitamin D deficiency in adolescents. Int J Vitam Nutr Res 90:339–345. https://doi.org/10.1024/0300-9831/a000632
Looi MK (2023) What is the future for covid drugs and treatments? BMJ 382:1797
Ma Z, Krishnamurthy M (2023) Is metformin use associated with low mortality in patients with type 2 diabetes mellitus hospitalized for COVID-19? a multivariable and propensity score-adjusted meta-analysis. PLoS ONE 18(2):e0282210. https://doi.org/10.1371/journal.pone.0282210
Máčová L, Bičíková M, Hampl R (2018) Impaired vitamin D sensitivity. Physiol Res 67(Suppl 3):S391–S400. https://doi.org/10.33549/physiolres.934006. (PMID: 30484666)
Mahajan R, Lim WE, Kumar S, Sareen M (2023) COVID-19 and management education: From pandemic to endemic, The International Journal of Management Education, 2: 21. ISSN 100801:1472–8117. https://doi.org/10.1016/j.ijme.2023.100801
Mamurova B, Akan G, Mogol E, Turgay A, Tuncel G, Evren EU, Evren H, Suer K, Sanlidag T, Ergoren MC (2023) Strong Association Between Vitamin D receptor gene and severe acute respiratory syndrome coronavirus 2 infectious variants. Glob Med Genet 10(1):27–33. https://doi.org/10.1055/s-0043-1761924.PMID:36819669;PMCID:PMC9935054
Mariani J, Antonietti L, Tajer C, Ferder L, Inserra F, Sanchez Cunto M, Brosio D, Ross F et al (2022) High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 17(5):e0267918. https://doi.org/10.1371/journal.pone.0267918. (PMID:35622854;PMCID:PMC9140264)
Marks R (2023) Long COVID in the older adult and Vitamin D. J Gerontol Geriatr Med 9:155
Marshall M (2023) Long COVID: answers emerge on how many people get better. Nature 619(7968):20. https://doi.org/10.1038/d41586-023-02121-7. (PMID: 3736979)
Mehramiz M, Khayyatzadeh SS, Esmaily H, Ghasemi F, Sadeghi-Ardekani K, Tayefi M et al (2019) Associations of vitamin D binding protein variants with the vitamin D-induced increase in serum 25-hydroxyvitamin D. Clin Nutr ESPEN 29:59–64
Menéndez SG, Martín Giménez VM, Holick MF, Barrantes FJ, Manucha W (2022) COVID-19 and neurological sequelae: Vitamin D as a possible neuroprotective and/or neuroreparative agent. Life Sci 297:120464. https://doi.org/10.1016/j.lfs.2022.120464. (PMID: 35271880; PMCID: PMC8898786)
Menger J, Lee ZY, Notz Q, Wallqvist J, Hasan MS, Elke G, Dworschak M, Meybohm P, Heyland DK, Stoppe C (2022) Administration of vitamin D and its metabolites in critically ill adult patients: an updated systematic review with meta-analysis of randomized controlled trials. Crit Care 26(1):268. https://doi.org/10.1186/s13054-022-04139-1. (PMID:36068584;PMCID:PMC9446655)
Mingiano C, Picchioni T, Cavati G, Pirrotta F, Calabrese M, Nuti R, Gonnelli S, Fortini A et al (2023) Vitamin D Deficiency in COVID-19 Patients and Role of Calcifediol Supplementation. Nutrients 15(15):3392. https://doi.org/10.3390/nu15153392.PMID:37571329;PMCID:PMC10421093
Mok CK, Ng YL, Ahidjo BA, Aw ZQ, Chen H, Wong YH, Lee RCH, Loe MWC et al (2023) Evaluation of in vitro and in vivo antiviral activities of Vitamin D for SARS-CoV-2 and variants. Pharmaceutics 15(3):925. https://doi.org/10.3390/pharmaceutics15030925.PMID:36986786;PMCID:PMC10058714
Montini F, Nozzolillo A, Rancoita PMV, Zanetta C, Moiola L, Cugnata F, Esposito F, Rocca MA, Martinelli V, Filippi M (2023) Modifiable risk factors of COVID-19 in patients with multiple sclerosis: a single-centre case-control study. J Neurol 270(4):1835–1842. https://doi.org/10.1007/s00415-023-11618-0. (PMID: 36795147; PMCID: PMC9933018)
Mostafa S, Mohammed SA, Elshennawy SI, Zakaria DM, Mahmoud SAK, Alsadek AM, Ahmad IH, Mohammed DS, Mohammed MA, Eltrawy HH (2022) Clinical and prognostic significance of baseline serum vitamin d levels in hospitalized Egyptian COVID-19 patients. Int J Gen Med 15:8063–8070. https://doi.org/10.2147/IJGM.S386815. (PMID:36389016;PMCID:PMC9651079)
Moukayed M (2023) A narrative review on the potential role of Vitamin D3 in the prevention, protection, and disease mitigation of acute and long COVID-19. Curr Nutr Rep 12(2):215–223. https://doi.org/10.1007/s13668-023-00471-2.PMID:37145350;PMCID:PMC10161182
Nanri A, Mizoue T, Goto A, Noda M, Sawada N, Tsugane S (2023) Vitamin D intake and all-cause and cause-specific mortality in Japanese men and women: the Japan Public Health Center-based prospective study. Eur J Epidemiol 38(3):291–300. https://doi.org/10.1007/s10654-023-00968-8. (PMID: 36719520; PMCID: PMC9887248)
Nielsen NM, Junker TG, Boelt SG, Cohen AS, Munger KL, Stenager E, Ascherio A, Boding L, Hviid A (2022) Vitamin D status and severity of COVID-19. Sci Rep 12(1):19823. https://doi.org/10.1038/s41598-022-21513-9. (PMID: 36396686; PMCID: PMC9672358)
Pedrosa AR, Martins DC, Rizzo M, Silva-Nunes J (2023) Metformin in SARS-CoV-2 infection: a hidden path-from altered inflammation to reduced mortality a review from the literature. J Diabetes Complicat 37(2):108391. https://doi.org/10.1016/j.jdiacomp.2022.108391. (PMID: 36621213; PMCID: PMC9807268)
Petrelli F, Oldani S, Borgonovo K, Cabiddu M, Dognini G, Ghilardi M, Parati MC (2023) Vitamin D3 and COVID-19 outcomes: an umbrella review of systematic reviews and meta-analyses. Antioxidants 12:247. https://doi.org/10.3390/antiox12020247
Pouremamali A, Babaei A, Malekshahi SS, Abbasi A, Rafiee N (2022) Understanding the pivotal roles of ACE2 in SARS-CoV-2 infection: from structure/function to therapeutic implication. Egypt J Med Hum Genet 23(1):103. https://doi.org/10.1186/s43042-022-00314-9. (PMID: 37521846; PMCID: PMC9206724)
Protas VV, Pogossyan GP, Li KG, Zhumina A, Bisseneva AK, Shaikina DN (2023) Plasma 25-Hydroxyvitamin D Level and VDR Gene Single Nucleotide Polymorphism rs2228570 Influence on COVID-19 Susceptibility among the Kazakh Ethnic Group—A Pilot Study. Nutrients 15:1781. https://doi.org/10.3390/nu15071781
Rachman A, Rahmaniyah R, Khomeini A, Iriani A (2023) Impact of vitamin D deficiency in relation to the clinical outcomes of hospitalized COVID-19 patients. Research 12:394. https://doi.org/10.12688/f1000research.132214.3)
Ramirez-Sandoval JC, Castillos-Ávalos VJ, Paz-Cortés A, Santillan-Ceron A, Hernandez-Jimenez S, Mehta R, Correa-Rotter R (2022) Very Low Vitamin D Levels are a Strong Independent Predictor of Mortality in Hospitalized Patients with Severe COVID-19. Arch Med Res 53(2):215–222. https://doi.org/10.1016/j.arcmed.2021.09.006. (PMID: 34711432; PMCID: PMC8516726)
Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD, Malhotra P (2022) Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J 98(1156):87–90. https://doi.org/10.1136/postgradmedj-2020-139065. (Epub 2020 Nov 12 PMID: 33184146)
Rybakovsky E, DiGuilio KM, Valenzano MC, Geagan S, Pham K, Harty RN, Mullin JM (2023) Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lung-derived epithelial cell culture model, 16HBE 14o. Physiol Rep 11(7):e15592 (10.14814/phy2.15592.PMID:37038908;PMCID:PMC10086678)
Sarhan N, AbouWarda AE, Sarhan RM, Boshra MS, Mostafa-Hedeab G, ALruwaili BF, et al (2022) Evidence for the Efficacy of a high dose of Vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: a randomized clinical trial. Medicina 58:1358. https://doi.org/10.3390/medicina58101358
Sarría-Santamera A, Mukhtarova K, Baizhaxynova A, Kanatova K, Zhumambayeva S, Akilzhanova A, Azizan A (2023) Association of CYP24A1 Gene rs6127099 (A > T) polymorphism with lower risk to COVID-19 Infection in Kazakhstan. Genes 14:307. https://doi.org/10.3390/genes14020307
Sartika KD, Gayatri AAAY (2022) Signaling pathway of vitamin D, vitamin D receptor and autophagy in infection: a systematic review. Int J Adv Med 9:1046–1052
Schrimpf A, Braesigk A, Lippmann S, Bleckwenn M (2022) Management and treatment of long COVID symptoms in general practices: an online-based survey. Front Public Health 10:937100. https://doi.org/10.3389/fpubh.2022.937100
Shah K, Varna VP, Sharma U, Mavalankar D (2022) Does vitamin D supplementation reduce COVID-19 severity?: a systematic review. QJM 115(10):665–672. https://doi.org/10.1093/qjmed/hcac040. (PMID:35166850;PMCID:PMC9383458)
Sharif-Askari FS, Hafezi S, Sharif-Askari NS, Alsayed HAH, Mdkhana B, Selvakumar B et al (2022) Vitamin D modulates systemic inflammation in patients with severe COVID-19. Life Sci 307:120909. https://doi.org/10.1016/j.lfs.2022.120909. (PMID: 36028169; PMCID: PMC9398944)
Shawi HRS, Anvari E, Fateh A (2023) Role of FokI rs2228570 and Tru9I rs757343 polymorphisms in the mortality of patients infected with different variants of SARS-CoV-2. Arch Med Res 54(4):310–318. https://doi.org/10.1016/j.arcmed.2023.03.006. (PMID: 37032232; PMCID: PMC10063569)
Slow S, Pearson JP, Florkowski CM, Elder PA, Lewis JG, Kennedy MA et al (2020) Effect of genetic factors on the response to vitamin D(3) supplementation in the VIDARIS randomized controlled trial. Nutrition 75–76:110761. https://doi.org/10.1016/j.nut.2020.110761
Stapleton EM, Keck K, Windisch R, Stroik MR, Thurman AL, Zabner J, Thornell IM, Pezzulo AA, Klesney-Tait J, Comellas AP (2022) Vitamin D-mediated effects on airway innate immunity in vitro. PLoS ONE 17(6):e0269647. https://doi.org/10.1371/journal.pone.0269647. (PMID:35666753;PMCID:PMC9170100)
Tan MKA, Lim Alba R, Li K (2023) Association of Vitamin D levels on the Clinical Outcomes of Patients Hospitalized for COVID-19 in a Tertiary Hospital. J ASEAN Fed Endocr Soc 38(1):81–89. https://doi.org/10.15605/jafes.038.01.07. (PMID: 37252418; PMCID: PMC10213170)
Tanne JH (2023) Covid-19: US agency launches raft of clinical trials of treatments for long covid. BMJ 382:1797. https://doi.org/10.1136/bmj.p1797Published
Tentolouris N, Samakidou G, Eleftheriadou I, Tentolouris A, Jude EB (2022) The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. a systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev 38(4):e3517. https://doi.org/10.1002/dmrr.3517. (PMID: 34965318; PMCID: PMC9015406)
Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA et al (2023) Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 329(22):1934–1946. https://doi.org/10.1001/jama.2023.8823. (PMID: 37278994; PMCID: PMC10214179)
Topan A, Lupse M, Calin M, Jianu C, Leucuta D, Briciu V (2023) 25 Hydroxyvitamin D serum concentration and COVID-19 severity and outcome—a retrospective survey in a Romanian hospital. Nutrients 15:1227. https://doi.org/10.3390/nu15051227
Torres M, Casado G, Vigón L, Rodríguez-Mora S, Mateos E, Ramos-Martín F et al (2022) Multidisciplinary Group of Study of COVID-19 (MGS-COVID); contributing members of the Multidisciplinary Group of Study of COVID-19 (in alphabetical order). changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomed Pharmacother 150:112965. https://doi.org/10.1016/j.biopha.2022.112965. (PMID: 35468580; PMCID: PMC9008199)
Townsend L, Dyer AH, McCluskey P, O’Brien K, Dowds J, Laird E, Bannan C et al (2021) Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients 13(7):2430. https://doi.org/10.3390/nu13072430
Usategui-Martín R, De Luis-Román DA, Fernández-Gómez JM, Ruiz-Mambrilla M, Pérez-Castrillón JL (2022) Vitamin D receptor (VDR) gene polymorphisms modify the response to Vitamin D supplementation: a systematic review and meta-analysis. Nutrients 14:360. https://doi.org/10.3390/nu14020360
Vásquez-Procopio J, Torres-Torres J, Borboa-Olivares H, Sosa SEY, Martínez-Portilla RJ et al (2022) Association between 25-OH Vitamin D deficiency and COVID-19 severity in pregnant women. Int J Mol Sci 23(23):15188. https://doi.org/10.3390/ijms232315188. (PMID:36499537;PMCID:PMC9735729)
Villasis-Keever MA, López-Alarcón MG, Miranda-Novales G, Zurita-Cruz JN, Barrada-Vázquez AS, González- Ibarra J et al (2022) Efficacy and safety of Vitamin D supplementation to prevent COVID-19 in frontline healthcare workers a randomized clinical trial. Arch Med Res 53(4):423–430. https://doi.org/10.1016/j.arcmed.2022.04.003. (PMID: 35487792; PMCID: PMC9013626)
WHO. WHO COVID-19 dashboard [Internet]. [cited 2023 Jan 14]. Available from: https://covid19.who.int
Wiernsperger N, Al-Salameh A, Cariou B, Lalau JD (2022) Protection by metformin against severe Covid-19: an in-depth mechanistic analysis. Diabetes Metab 48(4):101359. https://doi.org/10.1016/j.diabet.2022.101359. (PMID: 35662580; PMCID: PMC9154087)
Xie J, Chen Q, He D (2022) Abnormal blood 25-hydroxyvitamin D in critically ill patients: prevalence, predictors, and its association with in-hospital mortality. Eur J Med Res 27(1):111. https://doi.org/10.1186/s40001-022-00736-6. (PMID:35794582;PMCID:PMC9257555)
Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H (2017) Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep 16:7432–7438
Zaazouee MS, Eleisawy M, Abdalalaziz AM, Elhady MM, Ali OA, Abdelbari TM, Hasan SM et al (2023) Hospital and laboratory outcomes of patients with COVID-19 who received vitamin D supplementation: a systematic review and meta-analysis of randomized controlled trials. Naunyn Schmiedebergs Arch Pharmacol 396(4):607–620. https://doi.org/10.1007/s00210-022-02360-x. (PMID: 36508011; PMCID: PMC9743115)
Zeidan NMS, Lateef AE, Selim DM et al (2023) Vitamin D deficiency and vitamin D receptor FokI polymorphism as risk factors for COVID-19. Pediatr Res 93:1383–1390. https://doi.org/10.1038/s41390-022-02275-6
Zhang Y, Li J, Yang M, Wang Q (2023) Effect of vitamin D supplementation on COVID-19 patients: a systematic review and meta-analysis. Front Nutr 10:1131103. https://doi.org/10.3389/fnut.2023.1131103
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was not funded by any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
Author information
Authors and Affiliations
Contributions
AAG: resources, writing—original draft, writing—review and editing, supervision. YAA-W: resources, review and editing. RHT: review and editing; GAG: review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomaa, A.A., Abdel-Wadood, Y.A., Thabet, R.H. et al. Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy. Inflammopharmacol 32, 249–271 (2024). https://doi.org/10.1007/s10787-023-01383-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01383-x