Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the cytokine release syndrome (CRS) and leads to multiorgan dysfunction. Mitochondrial dynamics are fundamental to protect against environmental insults, but they are highly susceptible to viral infections. Defective mitochondria are potential sources of reactive oxygen species (ROS). Infection with SARS-CoV-2 damages mitochondria, alters autophagy, reduces nitric oxide (NO), and increases both nicotinamide adenine dinucleotide phosphate oxidases (NOX) and ROS. Patients with coronavirus disease 2019 (COVID-19) exhibited activated toll-like receptors (TLRs) and the Nucleotide-binding and oligomerization domain (NOD-), leucine-rich repeat (LRR-), pyrin domain-containing protein 3 (NLRP3) inflammasome. The activation of TLRs and NLRP3 by SARS‐CoV‐2 induces interleukin 6 (IL-6), IL-1β, IL-18, and lactate dehydrogenase (LDH). Herein, we outline the inflammatory circuit of COVID-19 and what occurs behind the scene, the interplay of NOX/ROS and their role in hypoxia and thrombosis, and the important role of ROS scavengers to reduce COVID-19-related inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
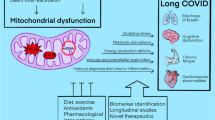
Coronavirus disease-19 (COVID-19) poses a menace to public health with almost half a billion cases and approximately six million deaths worldwide [1, 2]. Invading the human lungs, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interacts with the mucous membranes across different organs, such as the eyes, nose, and mouth. Older people with comorbidities such as the metabolic syndrome and diabetes experience severe COVID-19 symptoms. Moreover, increased mortality due COVID-19 was attributed to other risk factors such as older age, diabetes, hypertension, and renal disease. For instance, more than 65% of COVID-19 patients had diabetes and cardiovascular diseases, of which 63% were above 60 years [3]. In addition, SARS-CoV-2 damages mitochondria, alters autophagy, reduces nitric oxide (NO), increasing nicotinamide adenine dinucleotide phosphate oxidases (NOX) as well as reactive oxygen species (ROS). In COVID-19, SARS-CoV-2 also activates both toll-like receptors (TLRs) and the NOD−, LRR−, and pyrin domain-containing protein 3 (NLRP3) inflammasome [4,5,6,7,8,9,10,11,12]. The SARS-CoV open reading frame 9b (ORF-9b) manipulates the human mitochondrial antiviral signalling molecule (MAVS) to evade the innate host immunity, limit the antiinflammatory response, and overproduce ROS [10, 13]. The NOX protein family produces ROS that enhance viral pathogenicity in inflammatory cells [10, 11]. Mammalian NOX enzymes and subunits include NOX1-5, p22phox, p67phox, NOXO1 that are elevated in response to angiotensin II (ATII) in the kidneys, heart, and endothelial cells. Such enzymes and subunits are also involved in COVID-19 [14, 15]. Infection with SARS-CoV-2 mediates inflammatory cytokines and chemokines, where ATII-induced interleukin-6 (IL-6) synthesis usually requires NOX-derived ROS [7]. Patients and mice who are NOX2-deficient had enhanced immune response with a tendency to develop autoantibodies with low ROS levels [8, 9]. The activation of TLRs and NLRP3 by SARS‐CoV‐2 induces IL‐6, IL-1β, IL-18, and lactate dehydrogenase (LDH) [4, 5, 16,17,18,19,20,21,22,23]. Currently, research has discussed a higher number of involved systems in COVID-19, but from an individual perspective. Herein, the present review article combines the simultaneous detrimental effects of mitochondrial dysfunction, autophagy, NOX, NO, ROS, NLRP3, and TLRs during COVID-19 (Fig. 1). Moreover, we referred to the potential role of ROS scavengers in COVID-19.
BEHIND-THE-SCENE IN COVID-19
-
1.
The NOX-Mediated ROS Pathway of Inflammation
The dysregulation of NOX signalling is evident in COVID-19 patients with comorbidities, including obesity, diabetes, coronary artery disease, and heart failure [24]. In COVID-19 patients with acute respiratory distress syndrome (ARDS), ATII increases NOX and causes vasoconstriction and thrombosis via ROS, IL-6, tumour necrosis factor-Alpha (TNF-α), and other cytokines (Fig. 2) [25, 26]. The generation of NOX-dependent ROS elevates TNF-α, transforming growth factor-beta 1 (TGF-β1), ATII, and plasminogen activator inhibitor-1 (PAI-1), all of which are increased in COVID-19 patients [24, 27,28,29,30]. Numerous endogenous and exogenous processes produce ROS, such as NOX, the electron transport chain, xanthine oxidase, smoking, heavy metals, drugs, processed meat, and radiation (Fig. 2) [31]. Interferon-Gamma (IFN-γ) and ATII in vascular smooth muscle trigger NOX1 expression, while hypoxia/ischaemia and TNF-α stimulate NOX4 [32, 33]. Endosomal NOX2 produces the proinflammatory leukotriene B4 (LTB4) and increases the levels of IL-6 and ROS in virus-mediated pathogenicity [10, 34,35,36,37,38]. For example, influenzae A virus causes significantly less lung injury in the absence of NOX2, highlighting that NOX2-mediated ROS stimulates viral infection [35, 39]. In COVID-19, SARS-CoV-2 upregulates both ACE and ATII and therefore activates the phagocytes, metabolises haemoglobin, and causes hyperferritinaemia to produce hydroxyl radical (•OH), increasing the likelihood of inflammation and thrombosis (Fig. 3) [40,41,42,43,44,45,46,47,48,49,50,51].
The formation of •OH correlates with oxidative stress products such as 4-hydroxynonenal and malondialdehyde guanine adducts of DNA, which also are the products of the radical oxidation of phospholipids, related to COVID-19 dyslipidaemia [52,53,54,55]. Reactive oxygen species interact with lipids, carbohydrates, proteins, and nucleic acids, causing permanent destruction or alterations in their functions [56]. Hydroxyl radical is the most reactive and most toxic ROS that causes severe cellular damage by strongly interacting with DNA, carbohydrates, proteins, and lipids [57,58,59,60]. Haemochromatosis in different diseases (e.g., ageing and Parkinson’s disease) has gained attention because iron catalyses the formation of •OH [61,62,63,64]. Hydroxyl radical directly reacts with all DNA components, such as purine and pyrimidine bases, deoxyribose sugar backbone and causes single and double stranded breaks in DNA strand breaks and chemical modifications of nucleobases or nucleotides [60, 65, 66]. The uncontrolled production of ROS significantly contributes to infectious, inflammatory, and numerous chronic disorders. This evidence underpins the current hypothesis that NOX is an essential regulator in COVID-19 pathogenesis, and that blocking the expression of NOX might hinder the production of ATII-induced ROS and IL-6, minimising inflammation and tissue injury (Fig. 2).
-
2.
The Inflammatory Role of NLRP3
The tissues of postmortem COVID-19 patients show the active NLRP3 inflammasome and its products, including IL-1β, IL-18, and LDH [16,17,18,19,20,21,22,23]. Acute and chronic respiratory diseases, traumatic brain injury, acute kidney injury (AKI), and chronic kidney disease (CKD) also reported the involvement of the NLRP3 inflammasome [67]. Viral infections, metabolic abnormalities, tissue damage, and dysfunctional mitochondria generate ROS (e.g., •OH) that activate the NLRP3 inflammasome, triggering the production of proinflammatory cytokines [68,69,70,71,72,73]. Fortunately, mitochondria-targeted antioxidants such as molecular hydrogen (H2) can suppress the production of mitochondrial •OH, and therefore inhibit the expression of NLRP3 inflammasome, caspase-1, and IL-1β [74]. Molecular hydrogen is a potent scavenger that selectively scavenges •OH without adverse effects on the human body [75]. A recent multicentre trial revealed that the inhalation of hydrogen–oxygen gas mixture reduced COVID-19-related acute and chronic inflammation [76]. The intraperitoneal H2-rich saline suppressed the activation of the NLRP3 inflammasome, the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and the production of TNF-α and IL-1β in a mouse model with acute pancreatitis. Moreover, H2-rich saline improved the survival rate and ameliorated intestinal damage and inflammatory response, oedema, and apoptosis ameliorated intestinal ischaemia/reperfusion-mediated coagulopathy in rats. Molecular hydrogen-rich saline inhibited the activation of NF-κB and NLRP3 inflammasomes in peripheral blood mononuclear cells (PBMCs) [77]. Given this, H2 may reduce the SARS-CoV-2-induced inflammation by inhibiting the NLRP3 cascade and the release of proinflammatory cytokines.
-
3.
The Nitric Oxide (NO)/ROS Imbalance
Persistent inflammation due to COVID-19 disturbs the nitric oxide (NO)/ROS balance and causes multiorgan failure [78]. Patients with COVID-19 and common comorbidities (e.g., hypertension and diabetes) displayed significantly reduced endothelial NO, suggesting a strong relationship with acute lung injury (ALI) and NO/ROS imbalance [79,80,81,82,83,84,85]. Severe acute respiratory syndrome coronavirus 2 downregulates the expression of angiotensin-converting enzyme 2 (ACE2), producing proinflammatory cytokines and ROS that cause excessive inflammatory responses and lower the levels of NO by causing endothelial cell apoptosis (Fig. 4) [86,87,88,89]. Viral SARS-CoV-2 particles easily bind their protein spikes and enter into the cells due to the higher expression of ACE-2 receptors. Hence, people with impaired metabolic health are more prone to COVID-19 and comorbidities [3]. Severely ill COVID-19 patients exhibit excessive mitochondrial ROS that lead to mitochondrial dysfunction, reducing the production and bioavailability of NO by the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), AP-1 as well as the overexpression of cytokines and adhesion molecules (Fig. 2) [90,91,92]. The NO donor S-nitroso-N-acetylpenicillamine (SNAP) significantly inhibited cysteine proteases encoded by SARS-CoV-1 ORF1a and the membrane fusion of offspring virus S protein, decreasing viral replication by > 80% in VeroE6 cells [93,94,95,96,97]. Both SARS-CoV-2 and SARS-CoV exhibit a high degree of similarity in the receptor-binding domains of the spike proteins [98, 99]. Consequently, inhaled NO may prevent SARS-CoV-2 infection or treat mild, moderate, or severe COVID-19 patients, and could be used as an adjuvant therapy in mechanically ventilated patients (Fig. 4) [83, 100, 101].
-
4.
Mitochondrial Dysfunction and Autophagy
Hypoxia and other inflammatory mediators impair the function of mitochondria during COVID-19 [102, 103]. Mitochondrial dysfunction is a potential source of ROS that affect healthy mitochondria and promote cell death [104]. Mitochondria have emerged as critical dynamic organelles to maintain cellular homeostasis, metabolism, innate immune response, and determine the severity of viral infections [105]. Mitochondrial dynamics such as fusion, fission, and mitophagy protect against environmental insults; although, they are susceptible to viral infections, due to viral proteins or physiological alterations (e.g., disruption of Ca2+ homeostasis, endoplasmic reticulum stress, oxidative stress, and hypoxia) [106,107,108]. By interfering with mitochondria, viruses distort mitochondrial functions to create a favorable stressful environment for viral proliferation (i.e., low and higher amounts of mitochondrial ATP and ROS, respectively) and impeding mitochondria-associated antiviral signaling [109]. Defective mitochondria are a potential source of ROS that can also lead to damage of healthy mitochondria. Therefore, disturbances of the rapid clearance of dysfunctional mitochondria create higher levels of ROS, promoting cell death [102, 104, 110, 111]. Afterwards, viruses (e.g., SARS-CoV-2) start to proliferate and propagate via changing potential targets, including NLRP3 inflammasome and autophagy [112].
In COVID-19-related sepsis, the SARS-CoV-2-host interaction releases the cytokine storm that ultimately leads to multiorgan failure [113]. The proinflammatory cytokine TNF-α increased mitochondrial ROS mediated by mitochondrial damage in human umbilical vein endothelial cells (HUVECs) [114]. Similarly, COVID-19 significantly upregulates TNF-α alongside other cytokines and chemokines (Figs. 1 and 3). Accordingly, SARS-CoV-2 presumably counteracts the antiviral response by upregulating TNF-α and causing mitochondrial ultrastructural abnormalities to produce higher amounts of ROS [115]. Viruses modulate mitochondria-mediated antiviral immune responses by altering autophagy, mitophagy, and cellular metabolism to facilitate their proliferation [112].
Autophagy is an essential target in SARS-COV-2-mediated COVID-19 [112]. The possible inhibition of autophagy by SARS-CoV might elaborate more the pathophysiological role of mitochondrial dysfunction during COVID-19. Cells adopt autophagy (i.e., a self-destruction mechanism) to remove dysfunctional and superfluous cellular components via the initiation and elongation of isolation membrane, autophagosomes formation, and fusion and degradation of autophagosome-lysosome [112]. Mitochondria regulate autophagy to remove harmful components by producing ROS, whereas autophagy controls mitochondrial homeostasis using mitophagy [116, 117]. The lack of normal autophagy due to viral infections leads to mitochondrial dysfunction and ROS generation (Fig. 2) [118]. Cardiovascular, neurodegenerative, chronic liver, and kidney diseases also confirmed the interaction between autophagy deterioration, mitochondrial dysfunction, and ROS generation [119,120,121,122]. These data support the fact that loss of normal autophagy might be one of the primary contributors to SARS-CoV-2 infection in disturbing the mitochondrial homeostasis. However, numerous studies reported that SARS-CoV, SARS-CoV-2, Middle East respiratory syndrome coronavirus (MERS-CoV), and mouse hepatitis virus (MHV) induce and inhibit autophagy. Further research on modulating autophagy (i.e., induction or inhibition of autophagy) would elaborate the consequences on SARS-CoV-2 treatment [123,124,125,126,127,128,129,130,131,132].
-
5.
Loss of Autophagy and ROS
Elderly COVID-19 patients exhibit a vulnerable antioxidant defence and an exaggerated oxidative damage. The onset of ARDS in COVID-19 patients requires the activation of the “ROS machinery” combined with innate immunity to facilitate NF-κB, exacerbating the proinflammatory host response (Fig. 2) [133]. The overproduction of ROS significantly disturbed the antioxidant system during the SARS-CoV pathogenesis, severity, and progression of the respiratory disease in vitro and in vivo [134, 135]. Humans share age-related loss of autophagy or shocking exposure to ROS. Autophagy may contribute to the ageing phenotype, denoting that ageing alters the adaptive immune response and the proinflammatory state of the host [136]. For example, older mice severely experienced SARS-CoV-induced lung lesions than younger mice [137]. Older macaques upregulate virus-host response with inflammation due to differential gene expression with NF-kB as a central player [137]. Elderly patients also had significantly higher incidence of multilobe lesions than young and middle-aged COVID-19 patients [138]. The concurrent decline in mitochondrial dysfunction due to the inhibition of autophagy and the predisposing comorbidities in elderly patients, might explain why old COVID-19 patients show severe clinical manifestations that eventually lead to multiorgan failure compared to younger patients (Fig. 2). The World Health Organization declared that currently approved medications (e.g., clozapine, glyburide, carbetapentane) could be used for the treatment of COVID-19, by targeting the NLRP3 inflammasome and autophagy to inhibit the propagation of SARS-CoV-2 [139,140,141,142,143].
-
6.
The Possible Crosstalk Between TLRs, NOX, and ROS
Evidence supports the association between NOX, ROS, inflammatory mediators, and SARS-CoV-2 pathogenesis as well as the relationship between ROS signalling with TLR4 activation during TLR4/NOX interaction (Fig. 2) [144, 145]. The administration of diphenyleneiodonium chloride (DPI) suppressed the upregulation of TLR2, 4, and 9 in alcohol-induced fatty liver injury [146]. Human cells highlighted the potential role of NOX2 inhibitors in viral infections. In respiratory syncytial virus, rhinovirus, and human immunodeficiency virus (HIV), TLR7 activates NOX2 to produce ROS and modifies the single cysteine residue of TLR7, inhibiting the key antiviral and humoral signalling [147]. The syncytial viral cytoplasmic components recognise TLR7 and other sensor molecules; the mitochondria produce large amounts of ·OH that oxidise mitochondrial DNA, driving the cascade from NLRP3 to the release of proinflammatory cytokines (Fig. 2) [72, 148].
Severe acute respiratory syndrome coronavirus 2 binds to TLRs to activate and regulate pro-IL-1, NLRP3, IL-1β, IL-6, IL-10, and TNF-α. Such cascade causes lung inflammation and fibrosis, suggesting that the TLR pathways are protective mechanisms in SARS-CoV infections [149,150,151]. Toll-like receptors (e.g., TLR3, 4, 7, 8, and 9) identify many viral conserved patterns where myeloid differentiation primary response 88 (MyD88)—an essential component of the TLR pathway—assembles NOX to generate ROS in neutrophils and macrophages (Fig. 2) [152, 153]. Myeloid differentiation primary response 88 activates the TIR-domain-containing adapter-inducing interferon (TRIF)‐dependent signalling to activate the IFN-1, NF‐kB, and mitogen-activated protein kinase (MAPK) pathway [154]. The activation of the TLR-MyD88 downstream signalling and NF-kB is a hallmark of SARS-CoV infections, where the inhibition of NF-kB significantly reduced respiratory coronavirus infection and increased survival in mice [151, 155].
Convalescent SARS-CoV-infected patients experienced mitochondrial- and ROS-responding gene upregulation [144]. For example, ROS/NF‐kB/TLR (mainly TL4) signalling pathways lead to ALI upon triggering by SARS-CoV. The TLR4-TRIF-TRAF6 pathogenic pathway mediates the severity of ALI. The loss of TLR4 or TRIF expression protected mice from H5N1-induced ALI, indicating that the severity of ALI depends on ROS and innate immunity.
A schematic representation of the interplay between mitochondria and inflammatory related factors with COVID-19 at different levels. ACE-2, Angiotensin-Converting Enzyme-2; ATII, Angiotensin II; IL, Interleukin; NF-κB, Nuclear factor kappa B; NOX, NADPH Oxidase; PARs, Protease-Activated Receptors; ROS, Reactive Oxygen Species; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; TF, Tissue Factor; TLR, Toll-Like Receptor; TNF-ɑ, Tissue Necrosis Factor-Alpha.
THE POTENTIAL ROLE OF THE NOX/ROS INTERPLAY IN MEDIATING HYPOXIA, ISCHAEMIC INJURY, THROMBOSIS, AND FIBROSIS IN COVID-19
Severe hypoxia occurring during the COVID-19 cytokine storm is the leading cause of myocardial and liver damage, toxic encephalopathy, extremity ischaemia, and abnormal coagulation [156,157,158,159]. Although the activation of NOX in pulmonary endothelium mediates an increase in ischaemia-mediated ROS, data remain scarce to support the role of the NOX family in hypoxia/ischaemia in COVID-19 patients [160, 161]. A murine model of coronary artery ligation showed that NOX2 led to adverse cardiac injury [162]. Rhinovirus, SARS-CoV, and the anoxia of human platelets generate NOX2-dependent ROS in vitro [163]. The genetic deletion of NOX2 quenched the cognitive deficits promoted by intermittent hypoxia and oxidative stress in mice [164, 165]. Mice transplanted with p47phox-deficient bone marrow had decreased levels of lung ischaemia and proinflammatory cytokines [166]. Apocynin—NOX2 inhibitor—reduced vascular permeability in sheep, and aborted ischaemic lung and hepatic injury, cell necrosis and tissue injury, cytokine release, and ROS production in different murine models [167,168,169,170,171,172,173]. These data highlight that the inhibition of NOX, ROS, or p47phox could hold promise for designing effective molecules to limit the ischaemic injury in COVID-19 patients [7, 103, 174].
-
1.
Brain Ischaemia
Patients with COVID-19 present with ischaemic strokes. Brain ischaemic stroke comprises more than 80% of all strokes and occurs due to an immediate halting of blood flow by middle cerebral artery blockade [175, 176]. The excessive production of ROS aggravates oxidative stress and contributes to brain damage during ischaemia, suggesting that decreasing ROS might be helpful in the management of cerebral stroke (Fig. 1) [177,178,179,180]. Studies demonstrated that NOX1, NOX2, NOX4, and NOX5 are associated with cerebral disorders and ROS release [181,182,183,184,185,186]. The genetic deletion of NOX2 had protective effects against cerebral stroke in middle cerebral artery occlusion (MCAO) model. Functional NOX2-deficient and NOX2 knockout (KO) mice had significant reduction of oedema, lesion volume, and blood–brain barrier (BBB) leakage, postischaemic inflammatory gene expression and oxidative stress markers, and better neurological function during cerebral ischaemia [187,188,189,190]. Mouse model of retinal ischaemia with NOX2-deficient hippocampal neurons experiences low ROS levels upon exposure to oxygen/glucose deprivation (OGD) with attenuated neuronal cell death [191]. Consequently, the treatment of stroke should adopt an effective NOX inhibitory strategy, especially NOX2. However, extensive research that simulates the human biological system is crucial to validate the data emerging from in vivo models given the small organs and the relatively large penumbra in the lesioned tissues.
-
2.
Thrombosis and Fibrosis
Microthrombosis, pulmonary embolism, endothelial failure, and disseminated intravascular coagulation (DIC) are reported in COVID-19 patients [7, 192,193,194,195,196]. Viruses activate the coagulation pathway to overproduce proinflammatory cytokines via proteinase-activated receptors (PAR1 and PAR2) mediated by mitochondrial ROS [196,197,198,199,200,201]. Both PAR1/PAR2—expressed on platelets, endothelial and epithelial cells, and vascular and nonvascular smooth muscles—are involved in inflammation [202,203,204,205]. The upregulation of the NOX subunit p22phox in endothelial cells generates ROS that promote PAR1- and PAR2-mediated tissue factor (TF) induction, causing acute and chronic inflammation (Fig. 2) [206,207,208,209]. During inflammation, iron (III) generate •OH that convert soluble plasma fibrinogen into abnormal fibrin clots in the form of dense matted deposits resistant to enzymatic degradation (i.e., blood coagulation) (Fig. 4) [210,211,212]. Tissue-plasminogen activator (tPA) downregulates both IL-1α and IL-1β in endothelial cells during inflammation [213, 214]. Three mechanically-ventilated COVID-19 patients demonstrated that tPA has a therapeutic role in ARDS, showing a transient improvement in the ratio of arterial oxygen partial pressure/fractional inspired oxygen [215]. However, this improvement is lost after the end of treatment due to the fact that NOX-dependent ROS inhibits tPA activity, leading to thrombosis [216, 217].
The biopsies of liver and lung injury in deceased COVID-19 patients showed severe inflammatory responses with higher levels of IL-2, IL-6, IL-8. IL-10, and IFN-γ [218]. Profibrotic responses are triggered upon the activation of PAR1 and PAR2, inducing the release of NF-kB and IL-6, IL-8, and MCP-1 that contribute to leucocyte recruitment during SARS-CoV-2 infection as well (Fig. 1) [219]. The direct upregulation of PAR (i.e., PAR2) in chronic liver disease and pulmonary fibrosis increases the production of ROS, enhancing fibrogenesis by inducing hepatocyte apoptosis, airway obstruction, and lung oedema [209, 220,221,222,223]. This is consistent with the fact that PAR-2-deficient mice showed reduced inflammation and improved survival [224, 225]. Therefore, it is expected that PAR-2-dependent ROS could contribute to lung and liver injuries in COVID-19 patients, especially with predisposing diseases such as liver disease, leading to immunosuppression and disease aggression [25, 226, 227].
COULD ROS SCAVENGERS BE EFFECTIVE AGAINST COVID-19?
Natural compounds such as lycopene, polyphenols, quercetin, phloretin, berberine, and sulforaphane show a preventive potential against SARS-CoV-2 infection [228,229,230,231]. The lecithinised superoxide dismutase (PC-SOD) enzyme possesses excellent bioavailability, safety (confirmed in phase I and II studies), and modulatory effect to reduce the harms of oxidative stress in COVID-19 [232,233,234,235,236]. It is a synthetic product with long-life and high bioavailability compared to non-lecithinsed forms of the enzyme [237, 238]. For example, the intravenous administration of PC-SOD was safe and suppressed pulmonary emphysema and fibrosis, lung inflammation or ARDS, and activation of proteases, and the expression in vitro and in animal models [234, 239,240,241,242,243]. The lecithinised superoxide dismutase reduced serum LDH and surfactant protein A in patients with stage III-IV idiopathic pulmonary fibrosis without significant side effects. It would exert a more pulmonary protective effect if administered earlier during the course of the disease [233].
CONCLUSIONS AND FUTURE DIRECTIONS
This review has shed light on the close relationship between mitochondrial dysfunction, NOX, ROS, NLRP3 inflammasome, TLRs, and NO as the “inflammatory circuit” of COVID-19. The lack of normal autophagy leads to central problems such as mitochondrial dysfunction and the production of ROS. Subsequently, there could be an interplay between autophagy and SARS-CoV-2, but the exact nature of such an interaction remains unclear.
The proposed crosstalk between ROS and NOX during SARS-CoV-2 infection unequivocally constitutes an emerging molecular analysis and drug design route for COVID-19. Other probable interfering signalling pathways (i.e., PAR, TLR-MyD88, ROS/NF‐kB/TLR, and TLR4/TRIF/TRAF6) take place during SARS-CoV-2 pathogenesis. The coronavirus proteases, especially 3C-like protease (Mpro or 3CLpro), are attractive antiviral drug targets because they are essential for coronaviral replication. Such antiviral drugs would inhibit viral replication and the dysregulation of signalling cascades in infected cells that may lead to the death of healthy cells [6].
Future investigations may unveil the mitochondrial innate antiviral signalling during COVID-19, SARS-CoV-2–host interactions, and how SARS-CoV-2 exploits alterations to the mitochondrial morphophysiology to its benefit [244, 245]. The inhibitors of NOS and ROX might be promising compounds to reduce the SARS-CoV-2-related hyperinflammatory states during the cytokine response, vascular hyperpermeability, microthrombosis, tissue injury/ischaemia and fibrosis, and multiorgan failure. Nevertheless, the essential functions of NOX and ROS in normal physiology should be considered. The use of antioxidants may face potential challenges, such as physiological interferences, biological functions of NOX/ROS, lack of target access, and the inability to attain adequate ROS concentrations.
DATA AVAILABILITY
Not applicable.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- ATII:
-
Angiotensin II
- BBB:
-
Blood-brain barrier
- CGD:
-
Chronic granulomatous disease
- COVID-19:
-
Coronavirus disease-19
- DIC:
-
Disseminated intravascular coagulation
- DPI:
-
Diphenyleneiodonium chloride
- HIV:
-
Human immunodeficiency virus
- HUVECs:
-
Human umbilical vein endothelial cells
- IFN-I:
-
Type I interferon
- IFN-α:
-
Interferon-alpha
- IFN-γ:
-
Interferon-gamma
- IL-6:
-
Interleukin-6
- KO:
-
Knockout
- LTB4:
-
Leukotriene B4
- MAPK:
-
Mitogen-activated protein kinase
- MAVS:
-
Mitochondrial antiviral signalling molecule
- MCAO:
-
Middle cerebral artery occlusion
- MI:
-
Myocardial infarction
- MyD88:
-
Myeloid differentiation primary response 88
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- NOX:
-
Nicotinamide adenine dinucleotide phosphate oxidases
- OGD:
-
Oxygen/glucose deprivation
- ORF-9b:
-
Open reading frame 9b
- PAI-1:
-
Plasminogen activator inhibitor-1
- PAR1:
-
Proteinase-activated receptor 1
- PAR2:
-
Proteinase-activated receptor 2
- PKC:
-
Protein kinase C
- ROS:
-
Reactive oxygen species
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TF:
-
Tissue factor
- TGF-β1:
-
Transforming growth factor-beta 1
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumour necrosis factor-alpha
- tPA:
-
Tissue-plasminogen activator
- TRIF:
-
TIR-Domain-containing adapter-inducing interferon
References
Rabi, F.A., M.S. Al Zoubi, A.D. Al-Nasser, G.A. Kasasbeh, and D.M. Salameh. 2020. Sars-cov-2 and coronavirus disease 2019: What we know so far. Pathogens 9 (3): 1–14.
Coronavirus disease (COVID-19). Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Singh, P.S., A. Bhatnagar, K.S. Singh, K.S. Patra, N. Kanwar, A. Kanwal, et al. 2022. SARS-CoV-2 Infections, Impaired Tissue, and Metabolic Health: Pathophysiology and Potential Therapeutics. Mini-Reviews in Medicinal Chemistry 22:1. Available from: http://www.eurekaselect.com/article/120610.
Khanmohammadi, S., and N. Rezaei. 2021. Role of Toll-like receptors in the pathogenesis of COVID-19. Journal of Medical Virology 93(5):2735–9. Available from: https://pubmed.ncbi.nlm.nih.gov/33506952.
Patra, R., N. Chandra Das, and S. Mukherjee. 2021. Targeting human TLRs to combat COVID-19: A solution? Journal of Medical Virology 93(2):615–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32749702.
Pratap, S.S. 2020. Clinical Application of the Main Viral Proteinase (Mpro or 3clpro) Inhibitors for Coronavirus Therapy. Biomedical Journal of Scientific and Technical Research 30 (3): 23352–23354.
Zhang, W., Y. Zhao, F. Zhang, Q. Wang, T. Li, Z. Liu, et al. 2020. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clinical Immunology 214:108393. Available from: https://pubmed.ncbi.nlm.nih.gov/32222466.
Campbell, A.M., M. Kashgarian, and M.J. Shlomchik. 2012. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Science Translational Medicine 4(157):157ra141–157ra141. Available from: https://pubmed.ncbi.nlm.nih.gov/23100627.
Kelkka, T., D. Kienhöfer, M. Hoffmann, M. Linja, K. Wing, O. Sareila, et al. 2014. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxidants and Redox Signaling 21(16):2231–45. Available from: https://pubmed.ncbi.nlm.nih.gov/24787605.
Bedard, K., and K.-H. Krause. 2007. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological Reviews 87(1):245–313. Available from: https://doi.org/10.1152/physrev.00044.2005.
Reshi, M.L.,Y.-C. Su, and J.-R. Hong. 2014. RNA Viruses: ROS-Mediated Cell Death. International Journal of Cell Biology 2014:467452. Available from: https://pubmed.ncbi.nlm.nih.gov/24899897.
Li, T. 2020. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0): Working Group of 2019 Novel Coronavirus, Peking Union Medical Colle. Emerging Microbes and Infections 9(1):582–5.
Chernyak, B.V., E.N. Popova, A.S. Prikhodko, O.A. Grebenchikov, L.A. Zinovkina, and R.A. Zinovkin. 2020. COVID-19 and Oxidative Stress. Biochemistry (Mosc). 85(12):1543–53. Available from: https://pubmed.ncbi.nlm.nih.gov/33705292.
Sedeek, M., R. Nasrallah, R.M. Touyz, and R.L. Hébert. 2013. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. Journal of the American Society of Nephrology 24(10):1512–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23970124.
Dariya, B., and G.P. Nagaraju. 2020. Understanding novel COVID-19: Its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine and Growth Factor Reviews 53:43–52. Available from: https://pubmed.ncbi.nlm.nih.gov/32409230.
Chen, G., D. Wu, W. Guo, Y. Cao, D. Huang, H. Wang, et al. 2020. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of the Clinical Investigation 130(5):2620–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32217835.
Chi, Y., Y. Ge, B. Wu, W. Zhang, T. Wu, T. Wen, et al. 2020. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. Journal of Infectious Diseases 222(5):746–54. Available from: https://pubmed.ncbi.nlm.nih.gov/32563194.
Han, Y., H. Zhang, S. Mu, W. Wei, C. Jin, C. Tong, et al. 2020. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY). 12(12):11245–58. Available from: https://pubmed.ncbi.nlm.nih.gov/32633729.
Wen, W., W. Su, H. Tang, W. Le, X. Zhang, Y. Zheng, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discovery 6:31. Available from: https://pubmed.ncbi.nlm.nih.gov/32377375.
Lucas, C., P. Wong, J. Klein, T.B.R. Castro, J. Silva, M. Sundaram, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584(7821):463–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32717743.
Huang, C., Y. Wang, X. Li, L. Ren, Zhao J, Hu Y, et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395(10223):497–506. Available from: https://pubmed.ncbi.nlm.nih.gov/31986264.
Ratajczak, M.Z., and M. Kucia. 2020. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 34(7):1726–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32483300.
Rodrigues, T.S., K.S.G. de Sá, A.Y. Ishimoto, A. Becerra, S. Oliveira, L. Almeida, et al. 2021. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 218(3):e20201707. Available from: https://pubmed.ncbi.nlm.nih.gov/33231615.
Damiano, S., C. Sozio, G. La Rosa, and M. Santillo. 2020. NOX-Dependent Signaling Dysregulation in Severe COVID-19: Clues to Effective Treatments. Frontiers in Cell Infectious Microbiology 10:608435. Available from: https://pubmed.ncbi.nlm.nih.gov/33384971.
Liu, B., M. Li, Z. Zhou, X. Guan, and Y. Xiang. 2020. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? Journal of Autoimmunity 111:102452. Available from: https://pubmed.ncbi.nlm.nih.gov/32291137.
Griendling, K.K., D. Sorescu, and M. Ushio-Fukai. NAD(P)H Oxidase. Circulation Research 86(5):494–501. Available from: https://doi.org/10.1161/01.RES.86.5.494.
Colling, M.E., and Y. Kanthi. COVID–19-associated coagulopathy: An exploration of mechanisms. Vascular Medical 25(5):471–8. Available from: https://doi.org/10.1177/1358863X20932640.
Cesari, M., M. Pahor, and R.A. Incalzi. 2010. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovascular Therapy 28(5):e72–91. Available from: https://pubmed.ncbi.nlm.nih.gov/20626406.
Qin, L., Y. Liu, X. Qian, J.-S. Hong, and M.L. Block. 2005. Microglial NADPH Oxidase Mediates Leucine Enkephalin Dopaminergic Neuroprotection. Annals of the New York of Academy Sciences 1053(1):107–20. Available from: https://doi.org/10.1111/j.1749-6632.2005.tb00016.x.
Ha, H., and H.I.B. Lee. 2005. Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology 10(s2):S7–10. Available from: https://doi.org/10.1111/j.1440-1797.2005.00448.x.
Salisbury, D., and U. Bronas. 2015. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nursing Research. 64(1). Available from: https://journals.lww.com/nursingresearchonline/Fulltext/2015/01000/Reactive_Oxygen_and_Nitrogen_Species__Impact_on.7.aspx.
Katsuyama, M., C. Fan, and C. Yabe-Nishimura. 2002. NADPH Oxidase Is Involved in Prostaglandin F2α-induced Hypertrophy of Vascular Smooth Muscle Cells: INDUCTION OF NOX1 BY PGF2α*. Journal of Biological Chemistry. 277(16):13438–42. Available from: https://www.sciencedirect.com/science/article/pii/S0021925819609312.
Chen, C., L. Li, H.J. Zhou, and W. Min. 2017. The Role of NOX4 and TRX2 in Angiogenesis and Their Potential Cross-Talk. Antioxidants (Basel, Switzerland). 6(2):42. Available from: https://pubmed.ncbi.nlm.nih.gov/28594389.
Seshiah, P.N., D.S. Weber, P. Rocic, L. Valppu, Y. Taniyama, K.K. Griendling. 2002. Angiotensin II Stimulation of NAD(P)H Oxidase Activity. Circulation Research. 91(5):406–13. Available from: https://doi.org/10.1161/01.RES.0000033523.08033.16.
Vlahos, R., and S. Selemidis. 2014. NADPH Oxidases as Novel Pharmacologic Targets against Influenza A Virus Infection. Molecular Pharmacology 86(6):747 LP – 759. Available from: http://molpharm.aspetjournals.org/content/86/6/747.abstract.
Rada, B.K., M. Geiszt, K. Káldi, C. Timár, and E. Ligeti. 2004. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 104(9):2947–53. Available from: https://www.sciencedirect.com/science/article/pii/S0006497120559715.
Frey, R.S., M. Ushio–Fukai, and A.B. Malik. 2008. NADPH Oxidase-Dependent Signaling in Endothelial Cells: Role in Physiology and Pathophysiology. Antioxidants and Redox Signaling 11(4):791–810. Available from: https://doi.org/10.1089/ars.2008.2220.
Zheng, J., and S. 2018. Perlman. Immune responses in influenza A virus and human coronavirus infections: an ongoing battle between the virus and host. Current Opinion Virology 28:43–52. Available from: https://www.sciencedirect.com/science/article/pii/S1879625717301190.
Vlahos, R., J. Stambas, and S. Selemidis. 2012. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends in Pharmacological Sciences 33(1):3–8. Available from: https://www.sciencedirect.com/science/article/pii/S0165614711001593.
Wu, F., S. Zhao, B. Yu, Y.-M. Chen, W. Wang, Z.-G. Song, et al. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579(7798):265–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32015508.
de Wit, E., N. van Doremalen, D. Falzarano, and V.J. Munster. 2016. SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Microbiology 14(8):523–34. Available from: https://pubmed.ncbi.nlm.nih.gov/27344959.
Haber, F., J. Weiss, and W.J. Pope. The catalytic decomposition of hydrogen peroxide by iron salts. Proceedings of the Royal Society of London Series A - Mathematical and Physical Sciences 147(861):332–51. Available from: https://doi.org/10.1098/rspa.1934.0221.
Verdecchia, P., C. Cavallini, A. Spanevello, and F. Angeli. 2020. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. European Journal Internal Medicine 76:14–20. Available from: https://pubmed.ncbi.nlm.nih.gov/32336612.
Turner, A.J., J.A. Hiscox, and N.M. Hooper. ACE2: from vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences 25(6):291–4. Available from: https://pubmed.ncbi.nlm.nih.gov/15165741.
Delgado-Roche, L., and F. Mesta. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Archives of Medical Research 51(5):384–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32402576.
Khomich, O.A., S.N. 2018. Kochetkov, B. Bartosch, and A.V. Ivanov. Redox Biology of Respiratory Viral Infections. Viruses 10(8):392. Available from: https://pubmed.ncbi.nlm.nih.gov/30049972.
Barciszewska, A.-M. 2021. Elucidating of oxidative distress in COVID-19 and methods of its prevention. Chemico-Biological Interaction 344:109501. Available from: https://pubmed.ncbi.nlm.nih.gov/33974898.
Camini, F.C., C.C. da Silva Caetano, L.T. Almeida, and C.L. de Brito Magalhães. 2017. Implications of oxidative stress on viral pathogenesis. Archives of Virology 162(4):907–17. Available from: https://doi.org/10.1007/s00705-016-3187-y.
Ivanov, A.V., V.T. Valuev-Elliston, O.N. Ivanova, S.N. Kochetkov, E.S. Starodubova, B. Bartosch, et al. 2016. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxidative Medical and Cellular Longevity 2016:8910396. Available from: https://pubmed.ncbi.nlm.nih.gov/27829986.
Mehta, P., D.F. McAuley, M. Brown, E. Sanchez, R.S. Tattersall, J.J. Manson, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 395(10229):1033–4. Available from: https://pubmed.ncbi.nlm.nih.gov/32192578.
Waris, G., and Ahsan, H. 2006. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of Carcinogenesis 5:14. Available from: https://pubmed.ncbi.nlm.nih.gov/16689993.
Sorokin, A.V., S.K. Karathanasis, Z.-H. Yang, L. Freeman, K. Kotani, A.T. Remaley. 2020. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB Journal 34(8):9843–53. Available from: https://pubmed.ncbi.nlm.nih.gov/32588493.
Valko, M., C.J. Rhodes, J. Moncol, M. Izakovic, and M. Mazur. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions 160(1):1–40. Available from: https://www.sciencedirect.com/science/article/pii/S0009279705004333.
Kell, D.B., and E. Pretorius. 2014. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 6(4):748–73. Available from: https://doi.org/10.1039/c3mt00347g.
Nakabeppu, Y. 2014. Cellular levels of 8-oxoguanine in either DNA or the nucleotide pool play pivotal roles in carcinogenesis and survival of cancer cells. International Journal of Molecular Sciences 15(7):12543–57. Available from: https://pubmed.ncbi.nlm.nih.gov/25029543.
Habtemariam, S. 2019. Modulation of Reactive Oxygen Species in Health and Disease. Antioxidants (Basel, Switzerland). 8(11):513. Available from: https://pubmed.ncbi.nlm.nih.gov/31717825.
Zorov, D.B., M. Juhaszova, and S.J. Sollott. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Review 94(3):909–50. Available from: https://pubmed.ncbi.nlm.nih.gov/24987008.
Petlicki, J., and T.G.M. van de Ven. 1998. The equilibrium between the oxidation of hydrogen peroxide by oxygen and the dismutation of peroxyl or superoxide radicals in aqueous solutions in contact with oxygen. Journal of Chemical Society, Faraday Transactions 94(18):2763–7. Available from: https://doi.org/10.1039/A804551H.
Bedwell, S., R.T. Dean, and W. Jessup. 1989. The action of defined oxygen-centred free radicals on human low-density lipoprotein. Biochemical Journal 262(3):707–12. Available from: https://pubmed.ncbi.nlm.nih.gov/2556107.
Chatgilialoglu, C., C. Ferreri, M.G. Krokidis, A. Masi, and M.A. Terzidis. 2021. On the relevance of hydroxyl radical to purine DNA damage. Free Radical Research 55(4):384–404. Available from: https://doi.org/10.1080/10715762.2021.1876855.
Montgomery, E.B. 1995. Heavy metals and the etiology of Parkinson’s disease and other movement disorders. Toxicology 97(1):3–9. Available from: https://www.sciencedirect.com/science/article/pii/0300483X9402962T.
Schipper, H.M., R. Vininsky, R. Brull, L. Small, and J.R. Brawer. 1998. Astrocyte Mitochondria: A Substrate for Iron Deposition in the Aging Rat Substantia Nigra. Experimental Neurology 152(2):188–96. Available from: https://www.sciencedirect.com/science/article/pii/S0014488698968546.
Schipper, H.M. 2004. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Research Reviews 3(3):265–301. Available from: https://www.sciencedirect.com/science/article/pii/S156816370400011X.
Acton, R.T., J.C. Barton, L.V. Passmore, P.C. Adams, G.D. McLaren, C. Leiendecker-Foster, et al. Accuracy of family history of hemochromatosis or iron overload: the hemochromatosis and iron overload screening study. Clinical Gastroenterology and Hepatology 6(8):934–8. Available from: https://pubmed.ncbi.nlm.nih.gov/18585964.
Buxton, G.V., C.L. Greenstock, W.P. Helman, and A.B. Ross. 1988. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. Journal of Physical and Chemical Reference Data 17(2):513–886. Available from: https://doi.org/10.1063/1.555805.
Wardman, P. 1989. Reduction Potentials of One Electron Couples Involving Free Radicals in Aqueous Solution. Journal Physical and Chemical Reference Data 18(4):1637–755. Available from: https://doi.org/10.1063/1.555843.
Ozaki, E., M. Campbell, and S.L. Doyle. 2015. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. Journal of Inflammation Research 8:15–27. Available from: https://pubmed.ncbi.nlm.nih.gov/25653548.
Swanson, K.V., M. Deng, and J.P.-Y. Ting. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews Immunology 19(8):477–89. Available from: https://pubmed.ncbi.nlm.nih.gov/31036962.
Mangan, M.S.J., E.J. Olhava, W.R. Roush, H.M. Seidel, G.D. Glick, and E. Latz. 2018. Targeting the NLRP3 inflammasome in inflammatory diseases. Nature Reviews Drug Discovery 17(8):588–606. Available from: https://doi.org/10.1038/nrd.2018.97.
Hauenstein, A.V., L. Zhang, and H. Wu. 2015. The hierarchical structural architecture of inflammasomes, supramolecular inflammatory machines. Current Opinion in Structural Biology 31:75–83. Available from: https://pubmed.ncbi.nlm.nih.gov/25881155.
Hosseinian, N., Y. Cho, R.F. Lockey, and N. Kolliputi. 2015. The role of the NLRP3 inflammasome in pulmonary diseases. Therapeutic Advances in Respiratory Disease 9(4):188–97. Available from: https://doi.org/10.1177/1753465815586335.
Hirano, S.-I., Y. Ichikawa, B. Sato, H. Yamamoto, Y. Takefuji, and F. Satoh. 2021. Potential Therapeutic Applications of Hydrogen in Chronic Inflammatory Diseases: Possible Inhibiting Role on Mitochondrial Stress. International Journal of Molecular Sciences 22(5):2549. Available from: https://pubmed.ncbi.nlm.nih.gov/33806292.
Tschopp, J. 2011. Mitochondria: Sovereign of inflammation? European Journal of Immunology 41(5):1196–202. Available from: https://doi.org/10.1002/eji.201141436.
Ren, J.-D., X.-B. Wu, R. Jiang, D.-P. Hao, and Y. Liu. 2016. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochimica et Biophysica Acta-Molecular Cell Research 1863(1):50–5. Available from: https://www.sciencedirect.com/science/article/pii/S0167488915003651.
Ohsawa, I., M. Ishikawa, K. Takahashi, M. Watanabe, K. Nishimaki, K. Yamagata, et al. 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine 13(6):688–94. Available from: https://doi.org/10.1038/nm1577.
Guan, W.-J., C.-H. Wei, A.-L. Chen, X.-C. Sun, G.-Y. Guo, X. Zou, et al. 2020. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. Journal of Thoracic Disease 12(6):3448–52. Available from: https://pubmed.ncbi.nlm.nih.gov/32642277.
Yang, L., Y. Guo, X. Fan, Y. Chen, B. Yang, K.-X. Liu, et al. 2020. Amelioration of Coagulation Disorders and Inflammation by Hydrogen-Rich Solution Reduces Intestinal Ischemia/Reperfusion Injury in Rats through NF-κB/NLRP3 Pathway. Mediators of Inflammation 2020:4359305. Available from: https://pubmed.ncbi.nlm.nih.gov/32587471.
Channappanavar, R., and S. Perlman. 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology 39(5):529–39. Available from: https://pubmed.ncbi.nlm.nih.gov/28466096.
Fraser, D.D., E.K. Patterson, M. Slessarev, S.E. Gill, C. Martin, M. Daley, et al. 2020. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Critical Care Explorations 2(9):e0194–e0194. Available from: https://pubmed.ncbi.nlm.nih.gov/32904031.
Becker, R.C. 2020. COVID-19 update: Covid-19-associated coagulopathy. Journal of Thrombosis Thrombolysis 50(1):54–67. Available from: https://pubmed.ncbi.nlm.nih.gov/32415579.
Ozdemir, B., and A. Yazici. 2020. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Medical Hypotheses 144:109970. Available from: https://pubmed.ncbi.nlm.nih.gov/32534341.
Amraei, R., and N. Rahimi. 2020. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 9(7):1652. Available from: https://pubmed.ncbi.nlm.nih.gov/32660065.
Fang, W., J. Jiang, L. Su, T. Shu, H. Liu, S. Lai, et al. 2021. The role of NO in COVID-19 and potential therapeutic strategies. Free Radical and Biology Medicine 163:153–62. Available from: https://pubmed.ncbi.nlm.nih.gov/33347987.
Bohlen, H.G. 2015. Nitric Oxide and the Cardiovascular System. Comprehensive Physiology 2015. p. 803–28. (Major Reference Works). Available from: https://doi.org/10.1002/cphy.c140052.
Teixeira, R., M. Santos, and V. Gil. 2020. COVID-19 and cardiovascular comorbidities: An update. Revista Portuguesa de Cardiologia 39(8):417–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32718858.
Banu, N., S.S. Panikar, L.R. Leal, and A.R. Leal. 2020. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sciences 256:117905. Available from: https://pubmed.ncbi.nlm.nih.gov/32504757.
Boscá, L., M. Zeini, P.G. Través, and S. Hortelano. 2005. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology 208(2):249–58. Available from: https://www.sciencedirect.com/science/article/pii/S0300483X04006237.
Li, H., Z. Liu, and J. Ge. 2020. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. Journal of Cellular Molecular Medicine 24(12):6558–70. Available from: https://pubmed.ncbi.nlm.nih.gov/32320516.
Varga, Z., A.J. Flammer, P. Steiger, M. Haberecker, R. Andermatt, A.S. Zinkernagel, et al. 2020. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–8. Available from: https://www.sciencedirect.com/science/article/pii/S0140673620309375.
Song, P., W. Li, J. Xie, Y. Hou, and C. You. 2020. Cytokine storm induced by SARS-CoV-2. Clinical Chimica Acta 509:280–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32531256.
Shenoy, S. 2020. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflammation Research 69(11):1077–85. Available from: https://pubmed.ncbi.nlm.nih.gov/32767095.
Urso, C., and G. Caimi. 2011. [Oxidative stress and endothelial dysfunction]. Minerva Medicolegale 102(1):59–77. Available from: http://europepmc.org/abstract/MED/21317849.
Akerström, S., M. Mousavi-Jazi, J. Klingström, M. Leijon, A. Lundkvist, and A. Mirazimi. 2005. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. Journal of Virology 79(3):1966–9. Available from: https://pubmed.ncbi.nlm.nih.gov/15650225.
Akerström, S., V. Gunalan, C.T. Keng, and Y.-T. Tan, A. 2009. Mirazimi. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology 395(1):1–9. Available from: https://pubmed.ncbi.nlm.nih.gov/19800091.
Báez-Santos, Y.M., S.E. St John, and A.D. Mesecar. 2015. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Research 115:21–38. Available from: https://pubmed.ncbi.nlm.nih.gov/25554382.
Snijder, E.J., E. Decroly, and J. Ziebuhr. 2016. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Advances in Virus Research 96:59–126. Available from: https://pubmed.ncbi.nlm.nih.gov/27712628.
Mannick. J.B. 1995. The antiviral role of nitric oxide. Research Immunology 146(9):693–7. Available from: https://www.sciencedirect.com/science/article/pii/0923249496849200.
Pambuccian, S.E. 2020. The COVID-19 pandemic: implications for the cytology laboratory. Journal of the American Society of Cytopathology 9(3):202–11. Available from: https://doi.org/10.1016/j.jasc.2020.03.001.
Lu, R., X. Zhao, J. Li, P. Niu, B. Yang, H. Wu, et al. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–74. Available from: https://www.sciencedirect.com/science/article/pii/S0140673620302518.
Stefano, G.B., T. Esch, and R.M. Kream. 2020. Potential Immunoregulatory and Antiviral/SARS-CoV-2 Activities of Nitric Oxide. Medical Science Monitor 26:e925679–e925679. Available from: https://pubmed.ncbi.nlm.nih.gov/32454510.
Letko, M., A. Marzi, and V. Munster. 2020. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiology 5(4):562–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32094589.
Harrois, A., O. Huet, and J. Duranteau. 2009. Alterations of mitochondrial function in sepsis and critical illness. Current Opinion in Anesthesiology 22(2). Available from: https://journals.lww.com/co-anesthesiology/Fulltext/2009/04000/Alterations_of_mitochondrial_function_in_sepsis.3.aspx.
Ma, J., P. Xia, Y. Zhou, Z. Liu, X. Zhou, J. Wang, et al. 2020. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clinical Immunology 214:108408. Available from: https://pubmed.ncbi.nlm.nih.gov/32247038.
Murphy, M.P. 2013. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metabolism 18(2):145–6. Available from: https://www.sciencedirect.com/science/article/pii/S1550413113002957.
McBride, H.M., M. Neuspiel, and S. Wasiak. 2006. Mitochondria: More Than Just a Powerhouse. Current Biology 16(14):R551–60. Available from: https://www.sciencedirect.com/science/article/pii/S0960982206017817.
Chan, D.C. 2006. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell 125(7):1241–52. Available from: https://www.sciencedirect.com/science/article/pii/S0092867406007689.
Kim, S.-J., M. Khan, J. Quan, A. Till, S. Subramani, and A. Siddiqui. 2013. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathogens 9(12):e1003722–e1003722. Available from: https://pubmed.ncbi.nlm.nih.gov/24339771.
Kim, S.-J., G.H. Syed, and A. Siddiqui. 2013. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. PLoS Pathogens 9(3):e1003285–e1003285. Available from: https://pubmed.ncbi.nlm.nih.gov/23555273.
Seth, R.B., L. Sun, C.-K. Ea, and Z.J. Chen. 2005. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein that Activates NF-κB and IRF3. Cell 122(5):669–82. Available from: https://www.sciencedirect.com/science/article/pii/S0092867405008160.
Frank, M., S. Duvezin-Caubet, S. Koob, A. Occhipinti, R. Jagasia, A. Petcherski, et al. 2012. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochimica et Biophysica Acta - Molecular Cell Research 1823(12):2297–310. Available from: https://www.sciencedirect.com/science/article/pii/S0167488912002315.
Sena, L.A., and N.S. Chandel. 2012. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell 48(2):158–67. Available from: https://pubmed.ncbi.nlm.nih.gov/23102266.
Singh, S.P., S. Amar, P. Gehlot, S.K. Patra, N. Kanwar, and A. Kanwal. 2021. Mitochondrial Modulations, Autophagy Pathways Shifts in Viral Infections: Consequences of COVID-19. International Journal of Molecular Sciences 22(15):8180. Available from: https://pubmed.ncbi.nlm.nih.gov/34360945.
Li, H., L. Liu, D. Zhang, J. Xu, H. Dai, N. Tang, et al. 2020. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet (London, England) 395(10235):1517–20. Available from: https://pubmed.ncbi.nlm.nih.gov/32311318.
Chen, X., B.T. Andresen1, M. Hill, J. Zhang, F. Booth, and C. Zhang. 2008. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Current Hypertension Reviews 4(4):245–55. Available from: https://pubmed.ncbi.nlm.nih.gov/20559453.
Li, J., X. Gong, Z. Wang, R. Chen, T. Li, D. Zeng, et al. 2020. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Research 286:198043. Available from: https://pubmed.ncbi.nlm.nih.gov/32502551.
Lee, J., S. Giordano, and J. Zhang. 2012. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochemical Journal 441(2):523–40. Available from: https://pubmed.ncbi.nlm.nih.gov/22187934.
Okamoto, K., and N. Kondo-Okamoto. 2012. Mitochondria and autophagy: Critical interplay between the two homeostats. Biochimica Biophysica Acta - General Subjects 1820(5):595–600. Available from: https://www.sciencedirect.com/science/article/pii/S030441651100184X.
Venco, P., M. Bonora, C. Giorgi, E. Papaleo, A. Iuso, H. Prokisch, et al. 2015. Mutations of C19orf12, coding for a transmembrane glycine zipper containing mitochondrial protein, cause mis-localization of the protein, inability to respond to oxidative stress and increased mitochondrial Ca2+. Frontiers in Genetics 6:185. Available from: https://www.frontiersin.org/article/10.3389/fgene.2015.00185.
Ueno, T., and M. Komatsu. 2017. Autophagy in the liver: functions in health and disease. Nature Reviews Gastroenterology & Hepatology 14(3):170–84. Available from: https://doi.org/10.1038/nrgastro.2016.185.
Menzies, F.M., A. Fleming, A. Caricasole, C.F. Bento, S.P. Andrews, A. Ashkenazi A, et al. 2017. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 93(5):1015–34. Available from: https://www.sciencedirect.com/science/article/pii/S0896627317300466.
Lin, T.-A., V.C.-C. Wu, and C.-Y. Wang. 2019. Autophagy in Chronic Kidney Diseases. Cells 8(1):61. Available from: https://pubmed.ncbi.nlm.nih.gov/30654583.
Bravo-San Pedro, J.M., G. Kroemer, and L. Galluzzi. 2017. Autophagy and Mitophagy in Cardiovascular Disease. Circulation Research 120(11):1812–24. Available from: https://doi.org/10.1161/CIRCRESAHA.117.311082.
Kindrachuk, J., B. Ork, B.J. Hart, S. Mazur, M.R. Holbrook, M.B. Frieman, et al. 2015. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents and Chemotheraphy 59(2):1088–99. Available from: https://pubmed.ncbi.nlm.nih.gov/25487801.
Reggiori, F., I. Monastyrska, M.H. Verheije, T. Calì, M. Ulasli, S. Bianchi, et al. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host and Microbe 7(6):500–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20542253.
Prentice, E., W.G. Jerome, T. Yoshimori, N. Mizushima, M.R. Denison. 2003. Coronavirus replication complex formation utilizes components of cellular autophagy. Journal of Biological Chemistry 279(11):10136–41. Available from: https://pubmed.ncbi.nlm.nih.gov/14699140.
Vincent, M.J., E. Bergeron, S. Benjannet, B.R. Erickson, P.E. Rollin, T.G. Ksiazek, et al. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal 2(1):69. Available from: https://doi.org/10.1186/1743-422X-2-69.
Gassen, N.C., Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. 2019. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nature Communications 10(1):5770. Available from: https://pubmed.ncbi.nlm.nih.gov/31852899.
Keyaerts, E., L. Vijgen, P. Maes, J. Neyts, and M. Van Ranst. 2004. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochemical and Biophysics Research Communications 323(1):264–8. Available from: https://pubmed.ncbi.nlm.nih.gov/15351731.
Zhu, J., W. Yu, B. Liu, Y. Wang, J. Shao, J. Wang, et al. 2017. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death and Disease 8(10):e3113–e3113. Available from: https://pubmed.ncbi.nlm.nih.gov/29022891.
Yao, X., F. Ye, M. Zhang, C. Cui, B. Huang, P. Niu, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical Infectious Disease 71(15):732–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32150618.
Wu, C.-Y., J.-T. Jan, S.-H. Ma, C.-J. Kuo, H.-F. Juan, Y-S.E. Cheng, et al. 2004. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proceedings of the National Academy of Sciences U S A 101(27):10012–7. Available from: https://pubmed.ncbi.nlm.nih.gov/15226499.
Klein, B., K. Wörndl, U. Lütz-Meindl, and H.H. Kerschbaum. 2011. Perturbation of intracellular K+ homeostasis with valinomycin promotes cell death by mitochondrial swelling and autophagic processes. Apoptosis 16(11):1101. Available from: https://doi.org/10.1007/s10495-011-0642-9.
Smith, J.T., N.J. Willey, and J.T. Hancock. 2012. Low dose ionizing radiation produces too few reactive oxygen species to directly affect antioxidant concentrations in cells. Biology Letters 8(4):594–7. Available from: https://pubmed.ncbi.nlm.nih.gov/22496076.
Lin. C.-W., K.-H. Lin, T.-H. Hsieh, S.-Y. Shiu, J.-Y. Li. 2006. Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis. FEMS Immunology and Medical Microbiology 46(3):375–80. Available from: https://pubmed.ncbi.nlm.nih.gov/16553810.
van den Brand, J.M.A., B.L. Haagmans, D. van Riel, A.D.M.E. Osterhaus, and T. Kuiken. 2014. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. Journal of Comparative Pathology 151(1):83–112. Available from: https://pubmed.ncbi.nlm.nih.gov/24581932.
Rubinsztein, D.C., G. Mariño, and G. Kroemer. 2011. Autophagy and Aging. Cell 146(5):682–95. Available from: https://www.sciencedirect.com/science/article/pii/S0092867411008282.
Smits, S.L., A. de Lang, J.M.A. van den Brand, L.M. Leijten, W.F. van IJcken, M.J.C. Eijkemans, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathogens 6(2):e1000756–e1000756. Available from: https://pubmed.ncbi.nlm.nih.gov/20140198.
Liu, K., Y. Chen, R. Lin, and K. Han. 2020. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. Journal of Infection 80(6):e14–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32171866.
Albayrak, Y., and K. Hashimoto. 2017. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders BT - Sigma Receptors: Their Role in Disease and as Therapeutic Targets. In: Smith SB, Su T-P, editors. Cham: Springer International Publishing; p. 153–61. Available from: https://doi.org/10.1007/978-3-319-50174-1_11.
Vucicevic, L., M. Misirkic-Marjanovic, L. Harhaji-Trajkovic, N. Maric, and V. Trajkovic. 2018. Mechanisms and therapeutic significance of autophagy modulation by antipsychotic drugs. Cell Stress 2(11):282–91. Available from: https://pubmed.ncbi.nlm.nih.gov/31225453.
Maurice, T. 2016. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behavior Brain Research 296:270–8. Available from: https://www.sciencedirect.com/science/article/pii/S0166432815301935.
Zahid, A., B. Li, A.J.K. Kombe, T. Jin, and T. Tao. 2019. Pharmacological Inhibitors of the NLRP3 Inflammasome. Frontiers in Immunology. Vol. 10 Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.02538.
Xu, S., X. Li, Y. Liu, Y. Xia, R. Chang, and C. Zhang. 2019. Inflammasome inhibitors: promising therapeutic approaches against cancer. Journal of Hematology and Oncology 12(1):64. Available from: https://pubmed.ncbi.nlm.nih.gov/31242947.
Shao, H., D. Lan, Z. Duan, Z. Liu, J. Min, L. Zhang, et al. 2006. Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients. Journal of Clinical Immunology 26(6):546–54. Available from: https://pubmed.ncbi.nlm.nih.gov/17024565.
Gill, R., A. Tsung, and T. Billiar. 2010. Linking oxidative stress to inflammation: Toll-like receptors. Free Radical Biology and Medicine 48(9):1121–32. Available from: https://pubmed.ncbi.nlm.nih.gov/20083193.
Gustot, T., A. Lemmers, C. Moreno, N. Nagy, E. Quertinmont, C. Nicaise, et al. 2006. Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43(5):989–1000. Available from: https://doi.org/10.1002/hep.21138.
To, E.E., R. Vlahos, R. Luong, M.L. Halls, P.C. Reading, P.T. King, et al. 2017. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nature Communications 8(1):69. Available from: https://doi.org/10.1038/s41467-017-00057-x.
Shimada, K., T.R. Crother, J. Karlin, J. Dagvadorj, N. Chiba, S. Chen, et al. 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36(3):401–14. Available from: https://pubmed.ncbi.nlm.nih.gov/22342844.
Lu, H. 2020. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Bioscience Trends 14 (1): 69–71.
Jin, Y.-H., L. Cai, Z.-S. Cheng, H. Cheng, T. Deng, Y.-P. Fan, et al. 2020. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res [Internet]. 2020 Feb 6;7(1):4. Available from: https://pubmed.ncbi.nlm.nih.gov/32029004.
Totura, A.L., A. Whitmore, S. Agnihothram, A. Schäfer, M.G. Katze, M.T. Heise, et al. 2015. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. MBio 6(3):e00638. Available from: https://pubmed.ncbi.nlm.nih.gov/26015500.
Wu, J., and Z.J. Chen. 2014. Innate Immune Sensing and Signaling of Cytosolic Nucleic Acids. Annual Review of Immunology 32(1):461–88. Available from: https://doi.org/10.1146/annurev-immunol-032713-120156.
Laroux, F.S., X. Romero, L. Wetzler, P. Engel, and C. Terhorst. 2005. Cutting Edge: MyD88 Controls Phagocyte NADPH Oxidase Function and Killing of Gram-Negative Bacteria. Journal of Immunology 175(9):5596 LP – 5600. Available from: http://www.jimmunol.org/content/175/9/5596.abstract.
DeDiego, M.L., J.L. Nieto-Torres, J.A. Regla-Nava, J.M. Jimenez-Guardeño, R. Fernandez-Delgado, C. Fett, et al. 2013. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. Journal of Virology 88(2):913–24. Available from: https://pubmed.ncbi.nlm.nih.gov/24198408.
McLetchie, S., B.D. Volpp, M.C. Dinauer, J.S. Blum. 2015. Hyper-responsive Toll-like receptor 7 and 9 activation in NADPH oxidase-deficient B lymphoblasts. Immunology 146(4):595–606. Available from: https://pubmed.ncbi.nlm.nih.gov/26340429.
Xiong, T.-Y. 2020. Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. European Heart Journal 41(19):1798–800. Available from: https://pubmed.ncbi.nlm.nih.gov/32186331.
Tan, W., and J. Aboulhosn. 2020. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. International Journal of Cardiology 309:70–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32248966.
Li, J., and J.-G. Fan. 2020. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. Journal of Clinical and Translational Hepatology 8(1):13–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32274341.
Zhang, Y., L. Zheng, L. Liu, M. Zhao, J. Xiao, and Q. Zhao. 2020. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver International 40(9):2095–103. Available from: https://doi.org/10.1111/liv.14455.
Al-Mehdi, A.B., G. Zhao, C. Dodia, K. Tozawa, K. Costa, and V. Muzykantov, et al. 1998. Endothelial NADPH Oxidase as the Source of Oxidants in Lungs Exposed to Ischemia or High K+. Circulation Research 83(7):730–7. Available from: https://doi.org/10.1161/01.RES.83.7.730.
Weissmann, N., A. Sydykov, H. Kalwa, U. Storch, B. Fuchs, M. Mederos y Schnitzler, et al. 2012. Activation of TRPC6 channels is essential for lung ischaemia–reperfusion induced oedema in mice. Nature Communications 3(1):649. Available from: https://doi.org/10.1038/ncomms1660.
Looi, Y.H., D.J. Grieve, A. Siva, S.J. Walker, N. Anilkumar, A.C. Cave, et al. 2008. Involvement of Nox2 NADPH Oxidase in Adverse Cardiac Remodeling After Myocardial Infarction. Hypertension 51(2):319–25. Available from: https://doi.org/10.1161/HYPERTENSIONAHA.107.101980.
Basili, S., P. Pignatelli, G. Tanzilli, E. Mangieri, R. Carnevale, C. Nocella, et al. 2011. Anoxia-Reoxygenation Enhances Platelet Thromboxane A2 Production via Reactive Oxygen Species–Generated NOX2. Arteriosclerosis, Thrombosis, and Vascular Biology 31(8):1766–71. Available from: https://doi.org/10.1161/ATVBAHA.111.227959.
Zhao, X., J.M. Nicholls, and Y.-G. Chen. 2008. Severe Acute Respiratory Syndrome-associated Coronavirus Nucleocapsid Protein Interacts with Smad3 and Modulates Transforming Growth Factor-β Signaling*. Journal of Biological Chemistry 283(6):3272–80. Available from: https://www.sciencedirect.com/science/article/pii/S0021925820697903.
Nair. D., E.A. Dayyat, S.X. Zhang, Y. Wang, and D. Gozal. 2011. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 6(5):e19847–e19847. Available from: https://pubmed.ncbi.nlm.nih.gov/21625437.
Yang, Z., A.K. Sharma, M. Marshall, I.L. Kron, and V.E. Laubach. 2009. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. American Journal of Respiratory Cell and Molecular Biology 40(3):375–81. Available from: https://pubmed.ncbi.nlm.nih.gov/18787174.
Dodd-O, J.M., and D.B. Pearse. 2000. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. American Journal of Physiology Circulatory Physiology 279(1):H303–12. Available from: https://doi.org/10.1152/ajpheart.2000.279.1.H303.
Pearse, D.B., and J.M. Dodd-o. 1999. Ischemia-Reperfusion Lung Injury Is Prevented by Apocynin, a Novel Inhibitor of Leukocyte NADPH Oxidase. Chest 116:55S-56S. Available from: https://www.sciencedirect.com/science/article/pii/S001236921530670X.
Liu, P.-G., S.-Q. He, Y.-H. Zhang, and J. Wu. 2008. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World Journal of Gastroenterology 14(18):2832–7. Available from: https://pubmed.ncbi.nlm.nih.gov/18473406.
Shiotani, S., M. Shimada, A. Taketomi, Y. Soejima, T. Yoshizumi, K. Hashimoto, et al. 2007. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/reperfusion-induced acute lethal liver injury: effect on hepatocellular NADPH oxidase system. Gene Therapy 14(19):1425–33. Available from: https://doi.org/10.1038/sj.gt.3303000.
Csányi, G., E. Cifuentes-Pagano, I. Al Ghouleh, D.J. Ranayhossaini, L. Egaña, L.R. Lopes, et al. 2011. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radical Biology and Medicine 51(6):1116–25. Available from: https://pubmed.ncbi.nlm.nih.gov/21586323.
Dorman, R.B., C. Wunder, H. Saba, J.L. Shoemaker, L.A. MacMillan-Crow, and R.W. Brock. 2006. NAD(P)H oxidase contributes to the progression of remote hepatic parenchymal injury and endothelial dysfunction, but not microvascular perfusion deficits. American Journal of Physiology Liver Physiology 290(5):G1025–32. Available from: https://doi.org/10.1152/ajpgi.00246.2005.
Paterniti, I., M. Galuppo, E. Mazzon, D. Impellizzeri, E. Esposito, P. Bramanti, et al. 2010. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. Journal of Leukocyte Biology 88(5):993–1003. Available from: https://doi.org/10.1189/jlb.0610322.
Loukogeorgakis, S.P., M.J. van den Berg, R. Sofat, D. Nitsch, M. Charakida, B. Haiyee, et al. 2010. Role of NADPH Oxidase in Endothelial Ischemia/Reperfusion Injury in Humans. Circulation 121(21):2310–6. Available from: https://doi.org/10.1161/CIRCULATIONAHA.108.814731.
Avula, A., K. Nalleballe, N. Narula, S. Sapozhnikov, V. Dandu, S. Toom, et al. 2020. COVID-19 presenting as stroke. Brain, Behavior and Immunity 87:115–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32360439.
Hacke, W., M. Kaste, E. Bluhmki, M. Brozman, A. Dávalos, D. Guidetti, et al. 2008. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. New England Journal of Medicine 359(13):1317–29. Available from: https://doi.org/10.1056/NEJMoa0804656.
Marnett, L.J., J.N. Riggins, and J.D. West. 2003. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. Journal of Clinical Investigation 111(5):583–93. Available from: https://pubmed.ncbi.nlm.nih.gov/12618510.
Brennan, A.M., S.W. Suh, S.J. Won, P. Narasimhan, T.M. Kauppinen, H. Lee, et al. 2009. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature Neuroscience 12(7):857–63. Available from: https://pubmed.ncbi.nlm.nih.gov/19503084.
Gürsoy-Özdemir, Y., A. Can, and T. Dalkara. 2004. Reperfusion-Induced Oxidative/Nitrative Injury to Neurovascular Unit After Focal Cerebral Ischemia. Stroke 35(6):1449–53. Available from: https://doi.org/10.1161/01.STR.0000126044.83777.f4.
Adibhatla, R.M., and J.F. Hatcher. 2009. Lipid Oxidation and Peroxidation in CNS Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants and Redox Signaling 12(1):125–69. Available from: https://doi.org/10.1089/ars.2009.2668.
Lambeth, J.D. 2007. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radical Biology and Medicine 43(3):332–47. Available from: https://pubmed.ncbi.nlm.nih.gov/17602948.
Sorce, S., and K.-H. Krause. 2009. NOX Enzymes in the Central Nervous System: From Signaling to Disease. Antioxidants and Redox Signaling 11(10):2481–504. Available from: https://doi.org/10.1089/ars.2009.2578.
Montezano, A.C., D. Burger, T.M. Paravicini, A.Z. Chignalia, H. Yusuf, M. Almasri, et al. 2010. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circulation Research 106(8):1363–73. Available from: https://pubmed.ncbi.nlm.nih.gov/20339118.
Chrissobolis, S., and F.M. Faraci. 2008. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends in Molecular Medicine 14(11):495–502. Available from: https://pubmed.ncbi.nlm.nih.gov/18929509.
Infanger, D.W., R.V. Sharma, and R.L. Davisson. NADPH Oxidases of the Brain: Distribution, Regulation, and Function. Antioxidants and Redox Signaling 8(9–10):1583–96. Available from: https://doi.org/10.1089/ars.2006.8.1583.
Jackman, K.A., A.A. Miller, G.R. Drummond, and C.G. Sobey. 2009. Importance of NOX1 for angiotensin II-induced cerebrovascular superoxide production and cortical infarct volume following ischemic stroke. Brain Research 1286:215–20. Available from: https://www.sciencedirect.com/science/article/pii/S0006899309012736.
Kahles, T., P. Luedike, M. Endres, H.-J. Galla, H. Steinmetz, R. Busse, et al. 2007. NADPH Oxidase Plays a Central Role in Blood-Brain Barrier Damage in Experimental Stroke. Stroke 38(11):3000–6. Available from: https://doi.org/10.1161/STROKEAHA.107.489765.
Chen, H., Y.S. Song, and P.H. Chan. 2009. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. Journal Cerebral Blood Flow and Metabolism 29(7):1262–72. Available from: https://pubmed.ncbi.nlm.nih.gov/19417757.
Chen, H., G.S. Kim, N. Okami, P. Narasimhan, and P.H. Chan. 2011. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiology of Disease 42(3):341–8. Available from: https://pubmed.ncbi.nlm.nih.gov/21303700.
Yoshioka, H., K. Niizuma, M. Katsu, N. Okami, H. Sakata, G.S. Kim, et al. 2010. Nadph Oxidase Mediates Striatal Neuronal Injury after Transient Global Cerebral Ischemia. Journal of Cerebral Blood Flow and Metabolism 31(3):868–80. Available from: https://doi.org/10.1038/jcbfm.2010.166.
Yokota, H., S.P. Narayanan, W. Zhang, H. Liu, M. Rojas, Z. Xu, et al. 2011. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Investigative Ophthalmology and Visual Science 52(11):8123–31. Available from: https://pubmed.ncbi.nlm.nih.gov/21917939.
Dhaunsi, G.S., M.K. Paintlia, J. Kaur, and R.B. Turner. 2004. NADPH Oxidase in Human Lung Fibroblasts. Journal of Biomedical Science 11(5):617–22. Available from: https://www.karger.com/DOI/10.1159/000079674.
Fox, S.E., A. Akmatbekov, J.L. Harbert, G. Li, J. Quincy Brown, and R.S. Vander Heide. 2020. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respiratory Medicine 8(7):681–6. Available from: https://pubmed.ncbi.nlm.nih.gov/32473124.
Barton, L.M., E.J. Duval, E. Stroberg, S. Ghosh, and S. Mukhopadhyay. 2020. COVID-19 Autopsies, Oklahoma, USA. American Journal of Clinical Pathology 153(6):725–33. Available from: https://pubmed.ncbi.nlm.nih.gov/32275742.
Zhou, F., T. Yu, R. Du, G. Fan, Y. Liu, Z. Liu, et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 395(10229):1054–62. Available from: https://pubmed.ncbi.nlm.nih.gov/32171076.
Tang, N., D. Li, X. Wang, and Z. Sun. 2020. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 18(4):844–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32073213.
Han, H., L. Yang, R. Liu, F. Liu, K. Wu, J. Li, et al. 2020. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine 58(7):1116–20. Available from: https://doi.org/10.1515/cclm-2020-0188.
Yang, X., Y. Yu, J. Xu, H. Shu, J. Xia, H. Liu, et al. 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Medicine 8(5):475–81. Available from: https://pubmed.ncbi.nlm.nih.gov/32105632.
Perrone, L.A., J.A. Belser, D.A. Wadford, J.M. Katz, and T.M. Tumpey. 2013. Inducible Nitric Oxide Contributes to Viral Pathogenesis Following Highly Pathogenic Influenza Virus Infection in Mice. Journal of Infectious Disease 207(10):1576–84. Available from: https://doi.org/10.1093/infdis/jit062.
Meduri, G.U., G. Kohler, S. Headley, E. Tolley, F. Stentz, and A. Postlethwaite. 1995. Inflammatory Cytokines in the BAL of Patients With ARDS: Persistent Elevation Over Time Predicts Poor Outcome. Chest 108(5):1303–14. Available from: https://www.sciencedirect.com/science/article/pii/S0012369216357087.
José, R.J., A.E. Williams, and R.C. Chambers. 2014. Proteinase-activated receptors in fibroproliferative lung disease. Thorax [Internet]. 2014 Feb 1;69(2):190 LP – 192. Available from: http://thorax.bmj.com/content/69/2/190.abstract.
R. D’Andrea, M., C.K. Derian, D. Leturcq, S.M. Baker SM, Brunmark A, Ling P, et al. 1998. Characterization of Protease-activated Receptor-2 Immunoreactivity in Normal Human Tissues. Journal of Histochemistry and Cytochemistry 46(2):157–64. Available from: https://doi.org/10.1177/002215549804600204.
Vu, T.-K.H., D.T. Hung, V.I. Wheaton, and S.R. Coughlin. 1991. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64(6):1057–68. Available from: https://www.sciencedirect.com/science/article/pii/009286749190261V.
Coughlin, S.R., and E. Camerer. 2003. PARticipation in inflammation. Journal of Clinical Investigations 111(1):25–7. Available from: https://pubmed.ncbi.nlm.nih.gov/12511583.
Yin, Y.-J., Z. Salah, S. Grisaru-Granovsky, I. Cohen, S.C. Even-Ram, M. Maoz, et al. 2003. Human Protease-Activated Receptor 1 Expression in Malignant Epithelia. Arteriosclerosis, Thrombosis and Vascular Biology 23(6):940–4. Available from: https://doi.org/10.1161/01.ATV.0000066878.27340.22.
Djordjevic, T., A. Pogrebniak, R.S. BelAiba, S. Bonello, C. Wotzlaw, H. Acker, et al. 2005. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radical Biology and Medicine 38(5):616–30. Available from: https://www.sciencedirect.com/science/article/pii/S0891584904008317.
Moons, A.H.M., M. Levi, and R.J.G. Peters. 2002. Tissue factor and coronary artery disease. Cardiovascular Research 53(2):313–25. Available from: https://doi.org/10.1016/S0008-6363(01)00452-7.
Ott, I. 2003. Tissue Factor in Acute Coronary Syndromes. Semin Vasc Med. 03 (02): 185–192.
Banfi, C., M. Brioschi, S.S. Barbieri, S. Eligini, S. Barcella, E. Tremoli, et al. 2009. Mitochondrial reactive oxygen species: a common pathway for PAR1- and PAR2-mediated tissue factor induction in human endothelial cells. Journal of Thrombosis and Haemostasis 7(1):206–16. Available from: https://doi.org/10.1111/j.1538-7836.2008.03204.x.
Pretorius, E., J. Bester, N. Vermeulen, and B. Lipinski. 2013. Oxidation inhibits iron-induced blood coagulation. Current Drug Targets 14(1):13–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23170793.
Schaer, D.J., P.W. Buehler, A.I. Alayash, J.D. Belcher, and G.M. Vercellotti. 2013 Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121(8):1276–84. Available from: https://pubmed.ncbi.nlm.nih.gov/23264591.
Jankun, J., P. Landeta, E. Pretorius, E. Skrzypczak-Jankun, and B. Lipinski. 2014. Unusual clotting dynamics of plasma supplemented with iron(III). International Journal of Molecular Medicine 33(2):367–72. Available from: https://doi.org/10.3892/ijmm.2013.1585.
Bevilacqua, M.P., R.R. Schleef, M.A. Gimbrone Jr, and D.J. Loskutoff. 1986. Regulation of the fibrinolytic system of cultured human vascular endothelium by interleukin 1. Journal of Clinical Investigations 78(2):587–91. Available from: https://pubmed.ncbi.nlm.nih.gov/3090105.
Nachman, R.L., K.A. Hajjar, R.L. Silverstein, and C.A. Dinarello. 1986. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. Journal of Experimental Medicine 163(6):1595–600. Available from: https://doi.org/10.1084/jem.163.6.1595.
Wang, J., N. Hajizadeh, E.E. Moore, R.C. McIntyre, P.K. Moore, L.A. Veress, et al. 2020. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. Journal of Thrombosis and Haemostasis 18(7):1752–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32267998.
Chow, C.-W., M.T. Herrera Abreu, T. Suzuki, and G.P. Downey. 2003. Oxidative Stress and Acute Lung Injury. American Journal of Respiratory Cell and Molecular Biology 29(4):427–31. Available from: https://doi.org/10.1165/rcmb.F278.
Van Guilder, G.P., G.L. Hoetzer, J.J. Greiner, B.L. Stauffer, and C.A. DeSouza. 2008. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. Journal of Physiology 586(14):3525–35. Available from: https://pubmed.ncbi.nlm.nih.gov/18499730.
Rabaan, A.A., S.H. Al-Ahmed, J. Muhammad, A. Khan, A.A. Sule, R. Tirupathi, et al. 2021. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 9(5):436. Available from: https://pubmed.ncbi.nlm.nih.gov/33946736.
Riewald, M., and W. Ruf. 2002. Orchestration of Coagulation Protease Signaling by Tissue Factor. Trends in Cardiovascular Medicine 12(4):149–54. Available from: https://www.sciencedirect.com/science/article/pii/S1050173802001536.
Borensztajn, K., J.H. von der Thüsen, M.P. Peppelenbosch, and C.A. Spek. 2010. The coagulation factor Xa/protease activated receptor-2 axis in the progression of liver fibrosis: a multifaceted paradigm. Journal of Cell and Molecular Medicine 14(1–2):143–53. Available from: https://pubmed.ncbi.nlm.nih.gov/19968736.
Grandaliano, G., P. Pontrelli, G. Cerullo, R. Monno, E. Ranieri, M. Ursi, et al. 2003. Protease-Activated Receptor-2 Expression in IgA Nephropathy: A Potential Role in the Pathogenesis of Interstitial Fibrosis. Journal of American Society of Nephrology 14(8):2072 LP – 2083. Available from: http://jasn.asnjournals.org/content/14/8/2072.abstract.
Cederqvist, K., C. Haglund, P. Heikkilä, M.D. Hollenberg, R. Karikoski, and S. Andersson. 2005. High Expression of Pulmonary Proteinase-activated Receptor 2 in Acute and Chronic Lung Injury in Preterm Infants. Pediatric Research 57(6):831–6. Available from: https://doi.org/10.1203/01.PDR.0000161416.63314.70.
Hofmann, C., M. Völkers, and H.A. Katus. 2019. Targeting coagulation in heart failure with preserved ejection fraction and cardiac fibrosis. European Heart Journal 40(40):3333–5. Available from: https://doi.org/10.1093/eurheartj/ehz450.
Blackhart, B.D., K. Emilsson, D. Nguyen, W. Teng, A.J. Martelli, S. Nystedt, et al. 1996. Ligand Cross-reactivity within the Protease-activated Receptor Family*. Journal of Biological Chemistry 271(28):16466–71. Available from: https://www.sciencedirect.com/science/article/pii/S0021925818318970.
Takizawa, T., M. Tamiya, T. Hara, J. Matsumoto, N. Saito, T. Kanke, et al. 2005. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. Journal of Pharmacological Sciences 98(1):99–102. Available from: http://europepmc.org/abstract/MED/15879675.
Xu, Z., L. Shi, Y. Wang, J. Zhang, L. Huang, C. Zhang, et al. 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Medicine 8(4):420–2. Available from: https://pubmed.ncbi.nlm.nih.gov/32085846.
Miossec, P., and J.K. Kolls. 2012. Targeting IL-17 and TH17 cells in chronic inflammation. Nature Reviews Drug Discovery 11(10):763–76. Available from: https://doi.org/10.1038/nrd3794.
Cavezzi, A., E. Troiani, and S. Corrao. 2020. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clinical Practice 10(2):1271. Available from: https://pubmed.ncbi.nlm.nih.gov/32509258.
Xian, Y., J. Zhang, Z. Bian, H. Zhou, Z. Zhang, Z. Lin, et al. 2020. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharmaceutica Sinica B 10(7):1163–74. Available from: https://pubmed.ncbi.nlm.nih.gov/32834947.
Pruimboom, L. 2020. Methylation Pathways and SARS-CoV-2 Lung Infiltration and Cell Membrane-Virus Fusion Are Both Subject to Epigenetics. Frontiers in Cell and Infection Microbiology 10:290. Available from: https://pubmed.ncbi.nlm.nih.gov/32574283.
Grabowska, M., D. Wawrzyniak, K. Rolle, P. Chomczyński, S. Oziewicz, S. Jurga, et al. 2019. Let food be your medicine: nutraceutical properties of lycopene. Food Functions 10(6):3090–102. Available from: http://dx.doi.org/10.1039/C9FO00580C.
Farella, I., R. Panza, M. Capozza, and N. Laforgia. 2021. Lecithinized superoxide dismutase in the past and in the present: Any role in the actual pandemia of COVID-19? Biomedical Pharmacotherapy 141:111922. Available from: https://pubmed.ncbi.nlm.nih.gov/34323703.
Kamio, K., A. Azuma, K. Ohta, Y. Sugiyama, T. Nukiwa, S. Kudoh, et al. 2014. Double-blind controlled trial of lecithinized superoxide dismutase in patients with idiopathic interstitial pneumonia - short term evaluation of safety and tolerability. BMC Pulmonary Medicine 14:86. Available from: https://pubmed.ncbi.nlm.nih.gov/24886036.
Broeyer, F.J.F., B.E. van Aken, J. Suzuki, M.J.B. Kemme, H.C. Schoemaker, A.F. Cohen, et al. 2008. The pharmacokinetics and effects of a long-acting preparation of superoxide dismutase (PC-SOD) in man. Brirtish Journal of Clinical Pharmacology 65(1):22–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17610527.
Suzuki, J., F. Broeyer, A. Cohen, M. Takebe, J. Burggraaf, and Y. Mizushima. 2008. Pharmacokinetics of PC-SOD, a Lecithinized Recombinant Superoxide Dismutase, After Single- and Multiple-Dose Administration to Healthy Japanese and Caucasian Volunteers. Journal of Clinical Pharmacology 48(2):184–92. Available from: https://doi.org/10.1177/0091270007309705.
Suzuki, Y., T. Matsumoto, S. Okamoto, and T. Hibi. 2008. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Color Disease 10(9):931–4. Available from: https://doi.org/10.1111/j.1463-1318.2008.01487.x.
Hangaishi, M., H. Nakajima, J. Taguchi, R. Igarashi, J. Hoshino, K. Kurokawa, et al. 2001. Lecithinized Cu, Zn-Superoxide Dismutase Limits the Infarct Size Following Ischemia-Reperfusion Injury in Rat Hearts in Vivo. Biochemical and Biophysical Research Communications 285(5):1220–5. Available from: https://www.sciencedirect.com/science/article/pii/S0006291X01953197.
Koo, D.D.H., K.I. Welsh, N.E.J. West, K.M. Channon, A.J. Penington, J.A. Roake, et al. 2001. Endothelial cell protection against ischemia/reperfusion injury by lecithinized superoxide dismutase. Kidney International 60(2):786–96. Available from: https://www.sciencedirect.com/science/article/pii/S0085253815479256.
Tanaka, K.-I., Y. Tanaka, Y. Miyazaki, T. Namba, K. Sato, K. Aoshiba, et al. 2011. Therapeutic Effect of Lecithinized Superoxide Dismutase on Pulmonary Emphysema. Journal of Pharmacological and Experimental Therapeutics 338(3):810 LP – 818. Available from: http://jpet.aspetjournals.org/content/338/3/810.abstract.
Tanaka, K.-I., T. Ishihara, A. Azuma, S. Kudoh, M. Ebina, T. Nukiwa, et al. 2009. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. American Journal of Physiology Cellular and Molecular Physiology 298(3):L348–60. Available from: https://doi.org/10.1152/ajplung.00289.2009.
Tanaka, K.-I., K. Sato, K. Aoshiba, A. Azuma, and T. Mizushima. 2012. Superiority of PC-SOD to other anti-COPD drugs for elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice. American Journal of Physiology Cellular and Molecular Physiology 302(12):L1250–61. Available from: https://doi.org/10.1152/ajplung.00019.2012.
Tanaka, K., F. Tamura, T. Sugizaki, M. Kawahara, K. Kuba, Y. Imai, et al. 2016. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. American Journal of Respiratory Cellular and Molecular Biology 56(2):179–90. Available from: https://doi.org/10.1165/rcmb.2016-0158OC.
Tanaka, K.-I., A. Azuma, Y. Miyazaki, K. Sato, and T. Mizushima. 2012. Effects of Lecithinized Superoxide Dismutase and/or Pirfenidone Against Bleomycin-Induced Pulmonary Fibrosis. Chest 142(4):1011–9. Available from: https://www.sciencedirect.com/science/article/pii/S0012369212605728.
Vasquez-Bonilla, W.O., R. Orozco, V. Argueta, M. Sierra, L.I. Zambrano, F. Muñoz-Lara, D.S. López-Molina, K. Arteaga-Livias, Z. Grimes, C. Bryce, A. Paniz-Mondolfi, and A.J. Rodríguez-Morales. 2020. A review of the main histopathological findings in coronavirus disease 2019. Human Pathology 105:74–83. https://doi.org/10.1016/j.humpath.2020.07.023. Epub 2020 Aug 2. PMID: 32750378; PMCID: PMC7395947.
Iqbal Yatoo, M., Z. Hamid, O.R. Parray, A.H. Wani, A. Ul Haq, A. Saxena, S.K. Patel, M. Pathak, R. Tiwari, Y.S. Malik, R. Sah, A.A. Rabaan, A.J. Rodriguez Morales, and K. Dhama. 2020. COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Human Vaccines and Immunotherapeutics 16(12):2891–2904. https://doi.org/10.1080/21645515.2020.1788310. Epub 2020 Jul 23. PMID: 32703064; PMCID: PMC8641591.
ACKNOWLEDGEMENTS
None
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MMA conceived the idea of the manuscript and developed the first draft. RS and AJRM contributed with development of figures. All authors contributed with editing and revision of subsequent versions of the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwar, M.M., Sah, R., Shrestha, S. et al. Disengaging the COVID-19 Clutch as a Discerning Eye Over the Inflammatory Circuit During SARS-CoV-2 Infection. Inflammation 45, 1875–1894 (2022). https://doi.org/10.1007/s10753-022-01674-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01674-5