Abstract
Zooplankton may represent a considerable part of plankton in large rivers, but little is known about the factors that control it. We hypothesized that (1) significant longitudinal increase of zooplankton abundance, biomass, taxonomic richness, and diversity will occur along a free-flowing river section; (2) the residence time of water is more important for zooplankton population growth than environmental variables such as water temperature, oxygen saturation, and food concentration; and (3) the influence of tributaries on the longitudinal dynamics of zooplankton is insignificant or only has a local effect. A Lagrangian survey was applied in the free-flowing section of the River Elbe (Germany) in spring 2022. The abundances and biomass of the dominant rotifers as well as of cladocerans and copepods increased significantly downstream due to the population growth of zooplankton. The water residence time was the most important factor for zooplankton increment. One of the tributaries increased zooplankton abundance and biomass in the River Elbe, while other tributaries did not but the introduction of new species increased taxonomic richness and decreased the evenness of zooplankton in the main river so that diversity remained nearly constant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The River Continuum Concept predicts the development of zooplankton in the very downstream part of large rivers (Vannote et al., 1980). In the upper reaches of most rivers, downstream advection is so rapid that planktonic populations cannot develop (Hynes, 1970). In the lower reaches, a positive population increase is possible due to a longer residence time in the water. Residence time increases with the length of the river, which often leads to an increase in the abundance of plankton along the stream (Saunders & Lewis 1989; De Ruyter van Steveninck et al., 1992; Basu & Pick, 1996; Viroux, 1999; Zimmermann-Timm et al., 2007, etc.). Organisms with high reproduction rates have an advantage in flowing waters. It is assumed that ciliates and rotifers are typically most abundant in rivers mainly for this reason; they can increase their numbers faster than planktonic crustaceans (van Dijk & van Zanten, 1995; Lair, 2006). However, other zooplankton studies from rivers show different patterns of longitudinal dynamics and no consistent longitudinal trends in zooplankton development (Vadadi-Fülöp et al., 2009; Scherwass et al., 2010; Le Coz et al., 2017; Souley Adamou et al., 2022).
In addition to residence time, there are other factors which potentially regulate the spatial distribution of zooplankton in rivers. Thorp and Mantovani (2005) identified five abiotic environmental factors that should be particularly important for lotic zooplankton: turbidity (especially from suspended sediment), water turbulence, hydrological retention (which is influenced by stream discharge and access to sheltered, low-velocity sites, i.e., slackwaters), thermal conditions, and ultraviolet radiation. The importance of these and other abiotic variables (e.g., transparency, dissolved oxygen, reactive phosphorous, total dissolved solids, and organic carbon) has also been emphasized in studies on riverine zooplankton (Holland et al., 1983; Shiel, 1985; Pace et al., 1992; Thorp et al., 1994; Czerniawski & Pilecka-Rapacz, 2011; Bowszys et al., 2020, etc.). Biotic factors also contribute to the development of zooplankton in the river. A high nutrient load promotes the development of phytoplankton, which is the main food sources for zooplankton. The filtration activity of benthic filter feeders (Dreissena, Corbicula, Unionida) may affect the zooplankton negatively, both through direct predation and due to competition for food resources (e.g., algae, bacteria, detritus) (Basu & Pick, 1997; Ietswaart et al., 1999; Hardenbicker et al., 2016; Silaeva et al., 2016). Predation by fish (Jack & Thorp, 2002; Deosti et al., 2021) and interspecific competition in the zooplankton community (Brandl, 2005) influence the abundance, biomass, size structure, and diversity of zooplankton.
A third factor potentially affecting zooplankton in rivers is the input by tributaries. According to the Network Dynamics Hypothesis (Benda et al., 2004), tributary junctions represent locations in a network in which channel and valley morphology can change and local heterogeneity can be enhanced relative to the central tendency expected under the River Continuum Concept. Heterogeneity in resources and habitat may contribute to increased local species richness; therefore, tributary junctions may represent biological hotspots within a river network. However, the likelihood of morphologically significant perturbations to main stem channels increases with the ratio of tributary to main stem size (Benda et al., 2004). Usually one would expect a minor effect on zooplankton communities from tributaries which have a significantly lower discharge than that of the main stem. Furthermore, they are usually shorter, meaning that zooplankton have less time to multiply compared to the situation in the main river. However, many rivers are interrupted by weirs, dams, or other types of channel transformation (Shiel & Walker, 1984; Akopian et al., 2002; Havel et al., 2009; Napiórkowski & Napiórkowska, 2013). These impoundments strongly increase the residence time of water and may enable the growth of zooplankton upstream of weirs and in reservoirs. As a consequence, increased biomasses of zooplankton may be exported to downstream parts of the tributary and subsequently to the main river.
To disentangle the roles of residence time, other environmental factors, and of tributaries, we tested these hypotheses: (1) significant longitudinal increase of zooplankton abundance, biomass, taxonomic richness, and diversity will occur along a free-flowing river section; (2) the residence time of water is more important for zooplankton population growth than environmental variables, such as water temperature, oxygen saturation, and food concentration; and (3) the influence of tributaries on the longitudinal dynamics of zooplankton in the main river is insignificant or only has a local effect due to their relatively low water discharges and short lengths. For these tests, we investigated zooplankton within the almost 600 km long, free-flowing section of the River Elbe and its various tributaries (in Germany) and applied a Lagrangian survey according to water residence time in spring 2022.
Material and methods
Study area and sampling
Investigations were performed in the River Elbe in Central Europe, which is 1094 km long and drains a catchment area of 148,268 km2. Large parts of the River Elbe basin have the characteristics of a lowland river with a wide alluvial valley downstream of Dresden. The river has a rain‐snow‐type runoff regime, which usually shows high water levels in winter and spring, and low water levels in summer and autumn. The basin area has about 25 million inhabitants. Large cities and industrialized regions are usually accompanied by intensive point‐borne pollution in the river network. This pollution often stems from water treatment plants and industrial sites. More diffuse pollution is linked to agricultural areas, which comprise 42.8% of the area according to records of 2006. Nutrient pollution is an important problem in the Elbe basin, potentially affecting zooplankton development through food chains. The natural flow regime of the River Elbe and its tributaries is influenced by several anthropogenic measures, such as the creation of reservoirs, regulation of rivers, drainage of wetlands, and brown coal mining. Nevertheless, many sections of the main River Elbe in Germany are still free flowing and are not influenced by barrages. The originally broad floodplain areas around the middle and lower courses of the River Elbe are reduced and influenced by flood protection measures, leading to the disruption of the exchange of plankton biofunds in the river-floodplain system (Klöcking & Haberlandt, 2002; Hesse, 2019).
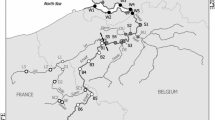
We sampled the free-flowing middle part of the River Elbe between Schmilka near the Czech-German border (km 4 according to German river kilometrage) and the weir Geesthacht near Hamburg just upriver of the tidal zone (km 586) (Fig. 1a, Table S1). We applied a Lagrangian approach using the research vessel Albis, i.e., sampling nearly the same water body as the vessel floated downstream. We adhered to the overall travel time of 8.3 days along the sampling stretch, one day of travel time representing 70 km of river. Sampling was conducted between April 25 and May 03, 2022 at a discharge of 428.0 m3 s−1 at Magdeburg. This level of water discharge was close to the maximum summer value (Fig. 1b). The flow velocity in this section of the River Elbe at average discharge is about 1 m s−1, decreasing in the lower reaches to about 0.9 m s−1 (Pusch & Fischer, 2006: p. 17). A detailed description of the hydrological conditions and habitat structure in the River Elbe is given by Pusch & Fischer (2006). Along that river stretch, four major tributaries flow into the Elbe: Schwarze Elster, Mulde, Saale, and Havel. The main characteristics of the tributaries, including the types of anthropogenic load, are presented in Table 1. Water discharge in the tributaries during the sampling period was 7.4 m3 s−1 in the Schwarze Elster, 43.8 m3 s−1 in the Mulde, 58.3 m3 s−1 in the Saale, and 43.5 m3 s−1 in the Havel. In the River Elbe, samples were taken from a water depth of 30 cm using a horizontal sampler. At each site, the water was sampled from the middle of the river as well as from the left and right parts of the channel. We also took one sample at the mouth of each tributary.
Sampling sites in the River Elbe (blue dots) and tributaries (red dots); the arrow marks the transition from river without groins to river with groins (a). Water discharge dynamics of River Elbe at Magdeburg during 2022 (MQ: mean discharge, MNQ: mean low discharge). The arrow marks the longitudinal sampling (b)
Environmental variables
Water temperature, oxygen saturation, pH, turbidity, and conductivity were measured using an YSI multiparameter probe (Exo2). Standard methods were applied for chemical sample preparations and analysis as described in Kamjunke et al. (2013), including all instructions of German standards. All filtrations were conducted immediately after sampling on board the vessel using quartz fiber filters (MN QR10, Macherey-Nagel) or glass fiber filters (GF/F, Whatman). After sampling, the filters were frozen and stored at 4 °C until analyses were conducted. Nitrate (NO3) and silicon (Si) were photometrically determined using the segmented flow technique. Soluble reactive phosphorus (SRP) was measured using the ammonium molybdate spectrometric method. Organic carbon (OC) concentration in the filtered and unfiltered original water samples was analyzed based on high-temperature oxidation using nondispersive infrared sensor detection (DIMATOC 2000, Dimatec Analysentechnik GmbH, Essen, Germany). For chlorophyll a analysis, samples were filtered onto glass fiber filters (GF-F, Whatman, Buckinghamshire, UK) immediately after sampling and the filters were frozen. Chlorophyll a was then determined using high performance liquid chromatography after ethanolic extraction (Kamjunke et al., 2021).
Zooplankton sample processing and analysis
Sixty-one zooplankton samples were collected during eight days of the Lagrangian survey. Eight liters of water were concentrated through a plankton net with a mesh size of 35 µm. Samples were fixed with Bouin’s solution (20% final concentration). Before processing the samples, the volume of each sample was brought to 100 ml in a graduated cylinder for the convenience of recalculating specimens. Samples were mixed before processing. Organisms were counted sequentially in 1 ml of the sample and then in 10 ml (excluding numerous species that were counted in 1 ml). The sediment of the remaining volume was examined for the presence and number of rare species. The calculation was done in a Bogorov counting chamber using a binocular microscope LEICA S6E (magnification up to × 80). Species identification was carried out using a Zeiss Axioscope (× 400 magnification) and the key literature (Kutikova, 1970; Monchenko, 1974; Borutsky et al., 1991; Smirnov, 1992, 1996; Nogrady et al., 1995; De Smet, 1996; Einsle, 1996; De Smet & Pourriot, 1997; Nogrady & Segers, 2002; Mirabdullayev & Defaye, 2004; Benzie, 2005).
Biomass (w) was calculated using the formula for the dependence of mass on body length:
where w—body weight; l—body length; and q—proportionality coefficient. Coefficients q for species were taken from literature (Balushkina & Vinberg, 1979, Gorbunov, 1983) or calculated using nomograms for determining the mass of aquatic organisms by body size and shape (Chislenko, 1968).
Most zooplankton taxa (77%) were identified to the species rank, except nauplinal and copepodid stages of copepods, harpacticoids, bdelloid rotifers, and some taxa of monogononta rotifers. Therefore, the term “lowest identified taxon” (LIT) was applied to describe taxonomic composition. Dominant taxa were defined as those taxa that together comprised ≥ 50% of the abundance or biomass.
The diversity of zooplankton communities was expressed using the Shannon–Wiener Index (H′), which was determined by abundance and biomass (Shannon, 1948):
where \({p}_{i}=\frac{{n}_{i}}{N}\), ni is the abundance/biomass of species i at a given station and N is the total abundance/biomass at that station.
The evenness (E) is calculated as follows:
where S is the number of taxa at the station.
The rate of change in zooplankton abundance was determined along a free-flowing river section without tributary influence. This net increase in abundance between the two sampling sites included losses to advection and predation. To calculate the net rate of increase along the river (r, d−1) and the doubling time (d, days), the following equations were used (Hardenbicker et al., 2016):
where N2—abundance (ind/m3) at sampling site 2; N1—abundance (ind/m3) at sampling site 1; and t—flow time between sampling sites 1 and 2 (days).
Statistical analyses
For all multivariate analyses, we calculated Bray–Curtis similarities based on 4th-root-transformed zooplankton abundances. We first tested if the position within the river, i.e., whether samples were taken from the left or right shore or from the middle of the river-affected zooplankton communities. A 2-way permutational analysis of similarities (PERMANOVA) showed that communities did not significantly differ with position (Pseudo F2,36 = 1.13, P = 0.293) but differed significantly with river km (Pseudo F18,36 = 5.19, P = 0.001). Hence, we averaged communities by river km for subsequent analysis. We conducted a cluster analysis to test if there are groups of sampling sites along the river that differed significantly in their community composition. We chose group average as the clustering mode and followed the cluster analysis with a SIMPROF analysis testing for a significant multivariate structure for each node of the dendrogram (Clarke et al. 2014). We conducted an indicator species analysis to test which species contributed significantly to cluster identity using the “indicspecies” package (v. 1.7.12, De Cáceres & Legendre, 2009) in R (R Core Team 2022). Relationships between zooplankton community composition and environmental factors were analyzed with a distance-based redundancy analysis (dbRDA). The analysis was conducted on a Bray–Curtis similarity matrix generated from 4th-root-transformed zooplankton abundance data and normalized environmental factors. Important environmental factors were selected with a stepwise selection based on the Akaike’s information criterion, modified for small numbers of samples relative to the number of predictors (Burnham & Anderson 2002). We excluded samples taken from tributaries as we were interested in longitudinal patterns within the River Elbe. All multivariate analyses were conducted in PRIMER (v. 7), and the PERMANOVA + add on (PRIMER-E Ltd, Devon, United Kingdom).
Results
Composition and taxonomic richness of zooplankton
Seventy-six zooplankton taxa (LIT) were found in the River Elbe, including 53 rotifers, 7 cladocerans, and 16 copepods. The number of zooplankton taxa was significantly lower at the mouths of the tributaries: 30 in Havel, 24 in Mulde, and 13 each in Schwarze Elster and Saale. Rotifers accounted for 63.3–96.0% of the species richness of the River Elbe, 70% in Havel, 85% in Schwarze Elster and Saale, and 96% in Mulde. The most abundant species of zooplankton in the River Elbe included the rotifers Keratella cochlearis (Gosse), Brachionus urceolaris Müll., Polyarthra dolichoptera Idels., Notholca squamula (Müll.), Synchaeta oblonga Ehrb., and Keratella quadrata (Müll.). In the mouth sections of the Schwarze Elster and Saale tributaries, Proales theodora (Gosse) was the most numerous zooplankton species. In the Mulde, S. oblonga and P. dolichoptera dominated, and in the Havel, P. dolichoptera, Nauplii Copepoda, and S. oblonga were dominant. In terms of biomass, the species which were most often dominant in the River Elbe were Brachionus calyciflorus Pall., B. urceolaris, P. dolichoptera, and K. quadrata. The following species dominated in biomass less frequently: N. squamula, S. oblonga, Synchaeta pectinata Ehrb., Asplanchna priodonta Gosse, and Daphnia galeata (Sars). In the mouth section of the Schwarze Elster, Nauplii Copepoda played the main role in the community biomass; in Mulde, S. pectinata and B. calyciflorus; in Saale, P. theodora; and in Havel, Nauplii Copepoda, P. dolichoptera, Chydorus sphaericus (O.F. Müll.), and K. quadrata.
The number of rotifer taxa in different parts of the river varied from 20 (155 km) to 30 (586 km). Copepods, which in the upper reaches consisted of 1–3 LIT and were represented mainly by specimens of copepod juvenile stages, reached 8 LIT in the lower reaches. C. sphaericus was the only cladoceran species in the upper reaches; the number of cladoceran species increased to 2–3 in the area from km 287 to 422 and 4–5 in the area of km 455–586. Thus, the taxonomic richness of all taxonomic groups of zooplankton in the River Elbe increased downstream. The number of zooplankton taxa in the tributaries was significantly lower than at the nearest Elbe sites upstream and downstream of their confluence. At the sites of the River Elbe below the confluence of the Schwarze Elster, Mulde, and Havel tributaries, the number of zooplankton species increased compared to the sites upstream of the confluence of the tributaries. It decreased below the confluence of the Saale (Fig. 2a).
Distribution of zooplankton abundance and biomass, ratio of taxonomic groups
The average values of zooplankton abundance in the sections of the river varied from 76 ± 13 ind. l−1 (km 155) to 849 ± 115 ind. l−1 (km 586); biomass varied from 0.04 ± 0.01 to 1.01 ± 0.11 mg l−1 in the same sections. The abundance and biomass of zooplankton in the mouth areas of the Schwarze Elster and Saale tributaries were significantly lower than in the nearby stations of the River Elbe, whereas the values in Mulde significantly exceeded those in the nearest stations of the River Elbe. The abundance of zooplankton in the Havel tributary was slightly lower than in neighboring stations of the River Elbe. However, the biomass was higher than in the Elbe due to the dominance of copepod nauplii in the tributary (Fig. 2b, c).
The predominance of rotifers characterized the taxonomic structure of zooplankton. In the River Elbe, rotifers accounted for 94.8–99.9% of the total abundance, the proportion of cladocerans did not exceed 0.6% and that of copepods 5.0%. The ratio of rotifera:cladocera:copepoda in terms of zooplankton abundance in the mouth area of Schwarze Elster was 62.0:1.0:37.0, in Mulde 99.5:0:0.5, in Saale 99.6:0.2:0.2, and in Havel 77.5:1.6:20.8. A similar ratio of taxonomic groups of zooplankton was found regarding biomass in the River Elbe and rotifers accounted for 73.3–99.7% of the total biomass. Cladocerans and copepods did not exceed 13.0% and 17.9%, respectively. In the mouth areas of the Mulde and Saale tributaries, rotifers also played the main role in the zooplankton biomass, with 99.0% and 92.0%, respectively. In the Schwarze Elster mouth section, the share of copepods reached 70.2%, a fact which was associated with developing individuals of the naupliar stages of copepods. In the mouth section of the Havel tributary, the proportions of rotifers and copepods were close to 44.1% and 42.1%, respectively.
The dynamics of the most numerous taxa (which accounted for more than 93% of the average abundance of the community) is shown in Fig. 3a. The lowest abundance values were on days 1.5–3.7, with a minimum on day 2.2 for most taxa. A significant increase in abundance could only be observed on day 4.1, probably downstream of the Mulde, since the number of rotifers at the next site downstream of the inflow of this tributary almost doubled (Fig. 3b). Planktonic crustaceans showed similar dynamics (Fig. 3c, d). Cladocerans were encountered only starting on day 4.5, after which they increased in numbers up to the lower reach (day 8.3). For copepods, the minimum values of abundance were observed on day 0.8. Then, there was a gradual increase in abundance, and starting on day 6.5 (downstream of the confluence of the Havel tributary), the abundance increased by more than five times compared to the previous site. Based on the obtained longitudinal dynamics, the increase in the zooplankton abundance in the River Elbe occurred mainly in the section of the middle and lower reaches, with varying intensities (Figs. 2, 3). Therefore, to calculate the doubling time, two free-flowing sections without the confluence of tributaries were chosen: km 318–422 and 455–586 (or days 4.5–6.0 and 6.5–8.3, respectively). Most species of rotifers had more intensive growth in the lower section of the river. Only P. dolichoptera showed more intensive growth in the area at km 318–422. The net growth rate of rotifers increased from 0.32 d−1 (doubling time of 2.1 days) to 0.47 d−1 (doubling time of 1.5 days) (Table 2). Among crustaceans, only Bosmina longirostris (O.F. Müll.) showed a more or less consistent longitudinal increase in abundance, which was greatest at the lowest site. The longitudinal dynamics of the abundance of other crustacean taxa was not consistent, although their total abundance increased in the lower stretch.
Abundance of the most numerous species of zooplankton (a), sum of rotifers (b), cladocerans (c), and copepods (d) in the River Elbe as a function of residence time during the Lagrangian survey in the period 25.04.22–03.05.22 (error bars are SD, exponential regressions were calculated using the River Elbe values only)
Similarity and structure of the communities
Cluster analysis showed a statistically significant division of sites into seven groups (colored differently in Fig. 4). The Schwarze Elster and Saale tributaries formed a cluster group, while the Mulde and Havel tributaries remained isolated. The sites within the River Elbe were divided into four groups at sections 4–258, 287–388, 422–455, and 475–586 km. The indicator species analysis conducted on the significant cluster groups showed that the cluster “river km 422–455” was characterized by taxa, such as Calanoida juv., Ilyocryptus agilis Kurz, Cephalodella forficula (Ehrb.), and F. passa (Müll.). Instead, the significant cluster “river km 475–586” was characterized by Notholca labis Gosse, D. galeata, C. sphaericus, Rhinoglena frontalis Ehrb., Alona guttata Sars, Eurytemora juv., B. longirostris, Cyclopoida juv., B. calyciflorus, Brachionus leydigii Cohn, Brachionus angularis Gosse, S. oblonga, N. squamula, and P. dolichoptera.
The similarity of groupings within the River Elbe was high (about 70%) (Fig. 4). The groupings were also similar in terms of Shannon diversity and composition of dominant complexes; changes were observed in the ranks of the dominants (Table 3). Thus, we can consider the zooplankton community in the free-flowing stretch of the River Elbe to be one single community with four modifications along the river stretch. In the River Elbe, taxonomic richness, abundance, and biomass of zooplankton were higher in each successive grouping downstream (Table 3). The groupings on the Elbe had a polydominant distribution structure, which became less pronounced toward the lower section due to an increase in the role of B. calyciflorus and B. urceolaris in the community biomass (Fig. S1). An increase in the role of dominating species in a community as well as the addition of rare species by tributaries reduces its evenness as observed in the River Elbe for both abundance and biomass-based data (Fig. 5). However, this did not lead to a decrease in Shannon diversity downstream, as it was offset by an increase in the number of zooplankton taxa (Fig. 5).
The zooplankton community from the Schwarze Elster and Saale rivers had a relatively low taxonomic richness, Shannon diversity, abundance and biomass, and had a monodominant distribution structure, i.e., the dominance curve had a steep slope and one taxon dominated. In the Mulde and Havel rivers, separate zooplankton communities were formed; these were quantitatively richer and had a greater Shannon diversity. The most pronounced polydominant community structure was in the River Havel (Table 3, Fig. S1).
Environmental variables and relationship with zooplankton
Water temperature increased along the river stretch, showing some daily fluctuations in the downstream part. These fluctuations were due to samplings in the morning and afternoon (Fig. 6a). The concentration of planktonic chlorophyll a started with relatively high values and increased only by a factor of two (Fig. 6b). In contrast, the concentrations in the tributaries were much lower in all cases. Simultaneously, oxygen saturation and pH increased due to algal photosynthesis; oxygen showed diurnal variability (Fig. 6c, d). As another consequence of phytoplankton growth, the concentrations of dissolved nutrients decreased to below the detection limit: phosphate from km 530 and silicate from km 390 (Fig. 6e, f). In contrast, these concentrations were mostly higher in the tributaries. The concentration of nitrate–N decreased longitudinally from 2.35 to 1.56 mg l−1 but was not consumed completely (Fig. S2). Conductivity increased after the inflow of the salty River Saale, turbidity, and POC concentration increased along the river stretch, whereas DOC concentration remained constant (Fig. S2).
The dbRDA analysis showed that communities were arranged along axis 1 explaining 59% of total community variation whereas axis 2 explained only 5% (Fig. 7). Hence, the dbRDA analysis selected residence time (Pseudo F = 21.95, P = 0.001) and pH (Pseudo F = 3.32, P = 0.001) as the two most important environmental variables structuring communities along the ordination axes.
Discussion
We investigated zooplankton along a free-flowing part of large River Elbe to disentangle the role of water residence time, other environmental factors, and tributaries. Hypotheses 1 and 3 were partially confirmed, and hypothesis 2 was fully confirmed. The abundances and biomass of the dominant rotifers but also of cladocerans and copepods increased significantly downstream due to the population growth of zooplankton in the river. The longitudinal increase in the taxonomic richness of zooplankton occurred simultaneously with a decrease in the evenness of communities in abundance and biomass, so that Shannon diversity remained nearly constant along the river (1). The water residence time was the most important factor for zooplankton increment (2). One of the tributaries increased zooplankton abundance and biomass in the River Elbe, while other tributaries did not (3).
Longitudinal zooplankton dynamics in the River Elbe (hypothesis 1)
Longitudinal increase of zooplankton abundance has been shown for several large rivers such as the Seine, the Marne (Akopian et al., 2002), the Rhine (De Ruyter van Steveninck et al., 1992), the Meuse (Viroux, 1999), the Hudson (Pace et. al., 1992), the Thames (May & Bass, 1998), and the Nakdong (Kim & Joo, 2000). An increase in the zooplankton abundance in the lower reaches of rivers is usually explained by an increase in the residence time (the retention time), i.e., time available for population growth (Hynes, 1970; Basu & Pick, 1996; Reckendorfer et al., 1999, etc.). The large lowland River Elbe, which has a fairly long stretch of free, unimpeded flow, and a water residence time of more than 10 days during the vegetation period, provides potentially favorable conditions for in-stream zooplankton population growth. In addition, the development of zooplankton in the river is favored by the availability of food and the low density of benthic filter feeders (Pusch & Fischer, 2006). This gave reason to hypothesize a significant longitudinal increase in zooplankton abundance along a free-flowing river section. The hypothesis (1) regarding the increase in zooplankton abundance downstream was confirmed (Figs. 2, 3). The increase in residence time in the lower section of the river, where the most intensive growth of plankton populations was observed (Fig. 3, Table 2), is also associated with a slight lessening of water flow velocity before the weir at Geesthacht. Similar trends in the dynamics of zooplankton abundance in the River Elbe were also noted in other seasons (Holst et al., 2001; Zimmermann-Timm et al., 2007; Hardenbicker et. al., 2016).
Assuming that zooplankton can reach high abundances as a result of population growth in the River Elbe, high flow velocity potentially limits the development of zooplankton in the channel. Flow velocities > 0.25 m s−1 are critical for limnic rotifer-crustacean zooplankton (Dubovskaya, 2009), which is the main reason for the decrease in the zooplankton abundance in river sections downstream of lakes and reservoirs. Small rotifers develop at flow rates of 0.5–0.8 m s−1 (Greze, 1957) but rotifers do not produce eggs at flow velocities > 1.5 m s−1 (Saunders & Lewis 1989). An increase in zooplankton abundance was detected at an average flow velocity of 0.6 m s−1 in the River Po, but not at 0.9 m s−1 (Bertani et al., 2016). In River Elbe, the distinct longitudinal population growth of zooplankton suggests that flow velocities of about 0.9–1.0 m s−1 are still sufficient for the zooplankton population growth of at least the dominant rotifer species. However, flow velocity is not evenly distributed with low values near shores, where zooplankton often develops in large numbers. The groin fields are the most common shore formations in the Middle Elbe. They are sites of low current and can provide plankton with additional residence time, contributing to population growth. Since groin fields are located in the section downstream from 120 km, this may be the reason for the delayed start of the plankton population growth in the channel. As our research has shown, an increase in abundance began only below 155 km or after day 2.2. A similar delay in the growth of plankton in the upper section of the Elbe was observed for low summer water of other years (Meister, 1994; Kamjunke et al., 2022, etc.).
The relationship between the retention time of a water body in running water and the reproduction rate of planktonic organisms is an important variable not only for the dynamics of large river communities but also for their structure (Viroux, 1997). Rotifers have an advantage over crustaceans in flowing waters due to their higher reproduction rates: it was usually the same species which multiplied, regardless of the geographical location of the river (Kim & Joo, 2000). These are mainly representatives of the genera Keratella, Brachionus, Polyarthra, and Synchaeta (Ferrari et. al., 1989; Saunders & Lewis, 1989; Thorp et al., 1994; Van Dijk & van Zanten, 1995; Lair & Reyes-Marchant, 1997; Speas, 2000, etc.). In the River Elbe, representatives of more or less the same genera predominated, which mainly ensured an increase in the abundance of zooplankton during downstream drift (Fig. 3). Overall, rotifers made up 94.8–99.9% of the total plankton population. The doubling time of rotifers in spring 2022 (Table 2) was in the range of values obtained for the summer of 1999 (39.4 h or 1.6 days), despite the fact that the dominant species were different at that time (Holst et al., 2002). Similar values were obtained for the River Rhine in May 1990, when rotifer densities almost doubled each day (De Ruyter van Steveninck et al., 1992). The predominance of rotifers over crustaceans in flowing waters can be explained not only by different growth rates, but also be the result of the different sensitivity of these two groups to turbulence and turbidity. An experiment carried out in August 2000 at Havelberg (the River Elbe, 423 km) demonstrated that rotifers benefit from turbulence whereas crustaceans are hindered (Pusch & Fischer, 2006). The proportion of plankton crustaceans increased in the lower reaches of the River Elbe; however, this did not significantly affect the community structure (Figs. 3, 5, S1). The most numerous crustaceans in the current were bosminids and the naupliar stages of copepods; these findings are typical for rivers (Saunders & Lewis, 1989; Pace et al., 1992; Thorp et al., 1994; Frutos et al., 2006; Gruberts et al., 2012, etc.). Zooplankton biomass increased along the river in accordance with the increase in the abundance of populations growing in the flow, which also confirms our hypothesis (1). The increase in biomass was also facilitated by a longitudinal restructuring of the zooplankton community toward the dominance of rotifer species with higher individual weight, as well as an increase in the abundance of crustaceans (Table 3, Fig. S1).
In addition to the autochthonous plankton developing in the flow, significant sources of river plankton are floodplain water bodies (lakes, arms, bays, etc.), including shore habitats with slow-flowing or stagnant water which represent “storage zones” or a refugium for plankton (Lancaster & Hildrew, 1993; Casper & Thorp, 2007, etc.). This source of zooplankton is especially important for rivers with high flow velocities and the associated high turbidity which limit the development of plankton in the channel, as has been shown for, e.g., the Australian Danube (Reckendorfer et al., 1999) or the River Desna (Sereda & Gromova, 2022). In such rivers where the main source of plankton is floodplain water bodies, the abundance and species richness of plankton in the riverbed increases during spring floods when there are better conditions for washing plankton out from the floodplain. In contrast, the abundance of plankton in the riverbed of the River Elbe is higher during the summer low-water period (Holst et al., 2001) when the riverbed is not connected to floodplain water bodies and zooplankton populations are growing in the main stem. The maximum rotifer abundance detected in rivers was found at high water temperature and intermediate discharge in River Elbe (Holst et al. 2001). In the present study, water temperature was still low and zooplankton abundance not maximal but the taxonomic richness was almost twice the values reported for the summer season (Meister, 1994). In addition, in the spring there was an increase in the taxonomic richness of zooplankton along the river (Figs. 2, 5), which also corresponds to our hypothesis (1). Tributaries (with the exception of the River Saale) contributed to the increase in the taxonomic richness of zooplankton in the River Elbe. The increase in zooplankton species richness downstream was accompanied by a decrease in community evenness, resulting in diversity that was fairly similar along the river (Fig. 5). Thus, the supposed longitudinal increase in zooplankton diversity was not confirmed (1).
Influence of environmental variables on the zooplankton composition in the River Elbe (hypothesis 2)
Residence time was the most important environmental variable structuring zooplankton community composition (Fig. 7). After passing the impoundments of the Elbe in the Czech Republic, zooplankton populations may grow for 8–9 days in the free-flowing part of the German freshwater Elbe, reaching its highest biomass at the most downstream sampling site. The role of pH as the second most important variable is less clear, and the relationship between zooplankton communities and pH is not direct. Zooplankton and phytoplankton biomass increase simultaneously along the river stretch, and pH increases due to the uptake of CO2 by algae during photosynthetic growth. Furthermore, high abundances of edible phytoplankton cells might promote zooplankton growth via a bottom-up control, i.e., high food concentration stimulates high grazer biomass. The increase in water turbidity in the river downstream (from 6.63 to 14.81 NTU) did not limit the development of zooplankton. Cladocerans, which are sensitive to turbidity, were more developed in the lower section of the river, although their abundance was not high. Thus, hypothesis (2) was confirmed: water residence time is more important for zooplankton growth than environmental variables, such as water temperature, oxygen saturation, and food concentration. Previously, such a hypothesis was confirmed for the semi-lentic conditions of a riverine lake (Burdis & Hirsch, 2017).
To estimate the potential role of the observed zooplankton community in the River Elbe, we calculated a possible grazing impact of zooplankton on phytoplankton. We considered their biomass changes during the last part of the river between km 570 and 585. Zooplankton biomass increased by 0.35 mg l−1 (from 0.66 to 1.01 mg l−1; Fig. 3c). Assuming a growth efficiency of 25% for rotifers, cladocerans, and copepods (Straile, 1997), this would result in a food demand of 1.4 mg l−1 d−1. On the other hand, POC concentration as a measure of phytoplankton decreased by 1.8 mg C l−1 (from 8.2 to 6.4 mg C l−1; Fig. S2) over the same stretch. Using previous data from the River Elbe, we established a high correlation between POC and phytoplankton biomass (POC = 0.53 × phytoplankton + 0.76, r2 = 0.93; Kamjunke et al. 2022), and the POC decrease was equivalent to a phytoplankton decrease of 3.4 mg l−1 (from 14.0 to 10.6 mg l−1). Consequently, zooplankton grazing (1.4 mg l−1) might explain about 40% of phytoplankton loss, whereas 60% might be attributed to sedimentation due to decreasing flow velocity. This calculation (conducted for the most downstream river section) represents a maximum estimation of the effect of zooplankton on phytoplankton in the River Elbe.
Influence of tributaries on zooplankton dynamics in the River Elbe (hypothesis 3)
The zooplankton communities of the mouths of the tributaries differed qualitatively and quantitatively from each other and from the Elbe community and were well separated in the cluster analysis (Fig. 4). Comparing the four tributaries, the highest abundance of rotifers was detected in the River Mulde. Regarding crustaceans, low abundances were found in the two tributaries with the highest toxicity for crustaceans (River Saale and River Mulde) and the highest abundance in a tributary with low toxicity (River Havel) (Table 1). Compared to the Elbe, the abundance of zooplankton in the tributaries was either 1) significantly lower (the Schwarze Elster, the Saale), 2) in the concentration range of the main river (the Havel) or 3) exceeded the abundance of the main river (the Mulde). The zooplankton communities most different from that of the River Elbe were those of the Schwarze Elster and the Saale tributaries, which were characterized by low taxonomic richness, diversity, and zooplankton abundance. At the same time, the Schwarze Elster did not have a noticeable influence on the zooplankton of the River Elbe, probably due to the low water discharge in the tributary. The Saale had a weak diluting effect in a limited section downriver of the tributary (km 318). The low abundance of zooplankton in the Saale is probably due to the high mineralization. Some studies even reported the absence of plankton in that tributary (Klapper, 1961). The number of rotifers of the Mulde tributary was more than two and a half times that of the Elbe upstream of its inflow, increasing the abundance of Elbe rotifer plankton downstream by a factor of almost two. In addition, the influence of the Mulde tributary was also reflected in the composition of the rotifers of the main river. In particular, the dominant S. pectinata in the Mulde was found in the River Elbe downstream of the tributary’s confluence. In contrast, planktonic crustaceans were more developed in the Havel tributary (63 ind. l−1) than in the River Elbe (0.25–13 ind. l−1). The greater abundance of crustaceans in the River Havel is apparently due to a high number of dammed river reaches with low flow velocities (Zimmermann-Timm et al., 2007). As a result, the abundance and biomass of crustaceans (copepods) in the River Elbe increased by a factor of five downstream of this tributary. The taxonomic richness of crustaceans also increased downstream of the River Havel. Despite this, crustaceans did not cause significant changes in the total abundance and structure of zooplankton in the River Elbe due to low abundance of crustaceans. According to previous studies, the River Havel as a whole had the greatest impact on middle Elbe zooplankton, increasing crustacean species richness and quantitative development (Klapper, 1961; Meister, 1994; Holst et al., 2001; 2002; Zimmermann-Timm et al., 2007; Hardenbicker et al., 2016). According to our research, the River Mulde with its abundant rotifers in the spring has a comparatively larger influence on the abundance of zooplankton in the River Elbe, despite the low water discharge and short length of this tributary. Thus, our hypothesis regarding the insignificant influence of tributaries on the longitudinal dynamics of zooplankton of the free-flowing section of the River Elbe (3) was partially confirmed: among the four tributaries, the River Mulde contributed noticeably to the abundance and biomass of plankton of the main river, while the influence of other tributaries was negligible.
Conclusion
The zooplankton of the River Elbe in the free-flowing stretch within Germany from Schmilka (river km 4 from the Czech-German border) to Geesthacht (river km 586) during the spring flood recession in 2022 was a continuum. As a result of the downstream drift of the rotifer-dominated zooplankton community (94.8–99.9% of the total population), its taxonomic richness, abundance, and biomass increased. Such longitudinal dynamics of zooplankton abundance increase in rivers are usually associated with the longer residence time available for population growth. The residence time along the free-flowing stretch of the River Elbe was more important for zooplankton community composition than environmental variables, such as water temperature, oxygen saturation, and food concentration. At the same time, no noticeable longitudinal increase of plankton abundance was observed in the upper section of the river (river km 4–258) or over the 3.7 days, which may be a consequence of both a shorter residence time and the absence of groin fields (shore structures with slow water exchange) in the upper reaches, which could probably contribute to the development of plankton. The increase in abundance and biomass of zooplankton in the River Elbe was also facilitated by tributaries that contributed rotifers (River Mulde) and, in smaller quantities, crustaceans (River Havel).
Considering the revealed features of the longitudinal dynamics of zooplankton and the huge role of shore habitats in the retention and development of zooplankton in rivers, in our opinion further studies of the longitudinal dynamics of zooplankton in the River Elbe should take shore biotopes, primarily groin fields, into account. Such studies could also assess the growth potential of zooplankton at sufficiently high flow rates in the river. Furthermore, the grazing pressure of zooplankton on phytoplankton is expected to be higher in summer at high temperatures and might be measured directly in feeding experiments to obtain a more precise estimation.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akopian, M., J. Garnier & R. Pourriot, 2002. Cinétique du zooplancton dans un continuum aquatique: de la Marne et son réservoir à l’estuaire de la Seine. Comptes Rendus. Biologies 325: 807–818. https://doi.org/10.1016/S1631-0691(02)01483-X.
Balushkina, E. V. & G. G. Vinberg, 1979. Relationship between body mass and length in planktonic animals. General foundations for the study of aquatic ecosystems. L.: Science. pp. 169–172 (in Russian).
Basu, B. K. & F. R. Pick, 1996. Factors regulating phytoplankton and zooplankton biomass in temperate rivers. Limnolodgy and Oceanography 41: 1572–1577. https://doi.org/10.4319/lo.1996.41.7.1572.
Basu, B. K. & F. R. Pick, 1997. Phytoplankton and zooplankton development in a lowland, temperate river. Journal of Plankton Research 19: 237–253. https://doi.org/10.1093/plankt/19.2.237.
Benda, L., L. R. Poff, D. Miller, T. Dunne, G. Reeves, G. Pess & M. Pollock, 2004. Network dynamics hypothesis: how channel networks structure riverine habitats. BioScience 54: 413–427. https://doi.org/10.1641/0006-3568(2004)054[0413:TNDHHC]2.0.CO;2.
Benzie, J. A. H., 2005. Cladocera: the genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). In Dumont, H. J. F. (ed), Guide to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 21. Backhuys Publishers, Leiden: 376.
Bertani, I., M. Del Longo, S. Pecora & G. Rossetti, 2016. Longitudinal variability in hydrochemistry and zooplankton community of a large river: a Lagrangian-based approach. River Research and Applications 32: 1740–1754. https://doi.org/10.1002/rra.3028.
Borutsky, E. V., L. A. Stepanova & M. S. Kos, 1991. Key to Calanoida fresh waters of the USSR, Nauka, St. Petersbur:, 504 (in Russian).
Bowszys, M., R. Tandyrak, I. Gołaś & E. Paturej, 2020. Zooplankton communities in a river downstream from a lake restored with hypolimnetic withdrawal. Knowledge & Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2020005.
Brandl, Z., 2005. Freshwater copepods and rotifers: predators and their prey. Hydrobiologia 546: 475–489. https://doi.org/10.1007/s10750-005-4290-3.
Burdis, R. M. & J. K. Hirsch, 2017. Crustacean zooplankton dynamics in a natural riverine lake, Upper Mississippi River. Journal of Freshwater Ecology 32: 247–265. https://doi.org/10.1080/02705060.2017.1279080.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multi-model inference: a practical information-theoretic approach, 2nd ed. Springer, New York: 488.
Casper, A. F. & J. Thorp, 2007. Diel and lateral patterns of zooplankton distribution in the St. Lawrence River. River Research and Applications 23: 73–85. https://doi.org/10.1002/rra.966.
Chislenko, L. L., 1968. Nomograms for determining the weight of aquatic organisms by body size and shape, Nauka, St. Petersbur: 108 (in Russian).
Clarke, K. R., R. N. Gorley, P. J. Somerfield & R. M. Warwick, 2014. Change in marine communities: an approach to statistical analysis and interpretation, 3rd ed. Plymouth, PRIMER-E:
Czerniawski, R. & M. Pilecka-Rapacz, 2011. Summer zooplankton in small rivers in relation to selected conditions. Central European Journal of Biology 6: 659–674. https://doi.org/10.2478/s11535-011-0024-x.
De Cáceres, M. & P. Legendre, 2009. Associations between species and groups of sites: indices and statistical inference. Ecology 90: 3566–3574. https://doi.org/10.1890/08-1823.1.
De Smet, W. H., 1996. The Proalidae (Monogononta), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 9. SPB Academic Publishing, Amsterdam: 102.
De Ruyter van Steveninck, E. D., W. Admiraal, L. Breebaart, G. M. J. Tubbing & B. van Zanten, 1992. Plankton in the River Rhine: structural and functional changes observed during downstream transport. Journal of Plankton Research 14: 1351–1368. https://doi.org/10.1093/plankt/14.10.1351.
De Smet, W. H. & R. Pourriot, 1997. Rotifera 5: The Dicranophoridae (Monogononta) and The Ituridae (Monogononta), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 12. SPB Academic Publishing, Amsterdam: 344.
Deosti, S., F. de Fátima Bomfim, F. M. Lansac-Tôha, B. A. Quirino, C. C. Bonecker & F. A. Lansac-Tôha, 2021. Zooplankton taxonomic and functional structure is determined by macrophytes and fish predation in a Neotropical river. Hydrobiologia 848: 1475–1490. https://doi.org/10.1007/s10750-021-04527-8.
Dubovskaya, O. P., 2009. Non-predator mortality of planktonic crustaceans, its possible causes (review). Journal of General Biology 70: 168–192 (in Russian).
Einsle, U., 1996. Genera Cyclops, Megacyclops, Acanthocyclops, Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 10. SPB Academic Publishing, Amsterdam: 82.
Ferrari, I., A. Farabegoli & R. Mazzoni, 1989. Abundance and diversity of planktonic rotifers in the Po River. Hydrobiologia 186: 201–208. https://doi.org/10.1007/BF00048913.
Frutos, S. M., A. S. G. Poi de Neiff & J. J. Neiff, 2006. Zooplankton of the Paraguay River: a comparison between sections and hydrological phases. Annales de Limnologie–International Journal of Limnology. 42: 277–288.
Gorbunov, A. K., 1983. On the method for determining the biomass of rotifers. Tez. II sympos. Trophic connections and their role in the productivity of natural water bodies. L., AS USSR: 122–124 (In Russian).
Greze, V. N., 1957. Food resources of fish of the Yenisei River and their use, Pishchepromizdat, Moscow:, 236 (in Russian).
Gruberts, D., J. Paidere, A. Škute & I. Druvietis, 2012. Lagrangian drift experiment on a large lowland river during a spring flood. Fundamental and Applied Limnology 179: 235–249. https://doi.org/10.1127/1863-9135/2012/0154.
Hardenbicker, P., M. Weitere, S. Ritz, F. Schöll & H. Fischer, 2016. Longitudinal plankton dynamics in the rivers Rhine and Elbe. River Research and Applications 32: 1264–1278. https://doi.org/10.1002/rra.2977.
Havel, J. E., K. A. Medley, K. D. Dickersonet, T. R. Angradi, D. W. Bolgrien, P. A. Bukaveckas & T. M. Jicha, 2009. Effect of main-stem dams on zooplankton communities of the Missouri River (USA). Hydrobiologia 628: 121–213. https://doi.org/10.1007/s10750-009-9750-8.
Hesse C., 2019. Integrated water quality modelling in meso‐ to large‐scale catchments of the Elbe River basin under climate and land use change. Kumulative Dissertation zur Erlangung des Akademischen Grades Dr. rer. nat. in der Wissenschaftsdisziplin “Geoökologie”, Universität Potsdam: 217. https://publishup.uni-potsdam.de/opus4-ubp/frontdoor/deliver/index/docId/42295/file/hesse_diss.pdf
Holland, L. E., C. F. Bryan & J. P. Newman, 1983. Water quality and the rotifer populations in the Atchafalaya River Basin, Louisiana. Hydrobiologia 98: 55–69. https://doi.org/10.1007/BF00019251.
Holst, H., H. Zimmermann, H. Kausch & W. Koste, 1998. Temporal and spatial dynamics of planktonic rotifers in the Elbe Estuary during spring. Estuarine, Coastal and Shelf Science 47: 261–273. https://doi.org/10.1006/ecss.1998.0364.
Holst, H., H. Zimmermann-Timm & H. Kausch, 2001. Zeitliche und räumliche Dynamik planktischer Rotatorien im Potamal der Elbe. Erweiterte Zusammenfassung der Deutschen Gesellschaft für Limnologie. Tagungsbericht 2000 (Magdeburg), Tutzing: 135–140.
Holst, H., H. Zimmermann-Timm & H. Kausch, 2002. Longitudinal and transverse distribution of plankton rotifers in the Potamal of the River Elbe (Germany) during late Summer. International Review of Hydrobiology 87: 267–280. https://doi.org/10.1002/1522-2632(200205)87.
Hynes H. B., 1970. The ecology of running waters. Liverpool: 555
Ietswaart, T., L. Breebaart, B. van Zanten & R. Bijkerk, 1999. Plankton dynamics in the River Rhine during downstream transport as influenced by biotic interactions and hydrological conditions. Hydrobiologia 410: 1–10. https://doi.org/10.1023/A:1003801110365.
Jack, J. D. & J. H. Thorp, 2002. Impacts of fish predation on an Ohio River zooplankton community. Journal of Plankton Research 24: 119–127. https://doi.org/10.1093/plankt/24.2.119.
Kamjunke, N., O. Büttner, C. G. Jäger, H. Marcus, W. von Tümpling, S. Halbedel, H. Norf, M. Brauns, M. Baborowski, R. Wild, D. Borchardt & M. Weitere, 2013. Biogeochemical patterns in a river network along a land use gradient. Environmental Monitoring and Assessment 185: 9221–9236. https://doi.org/10.1007/s10661-013-3247-7.
Kamjunke, N., M. Rode, M. Baborowski, J. V. Kunz, J. Zehner, D. Borchardt & M. Weitere, 2021. High irradiation and low discharge promote the dominant role of phytoplankton in riverine nutrient dynamics. Limnolodgy and Oceanography 66: 2648–2660. https://doi.org/10.1002/lno.11778.
Kamjunke, N., L.-M. Beckers, P. Herzsprung, W. von Tümpling, O. Lechtenfeld, J. Tittel, U. Risse-Buhl, M. Rode, A. Wachholz, R. Kallies, T. Schulze, M. Krauss, W. Brack, S. Comero, B. M. Gawlik, H. Skejo, S. Tavazzi, G. Mariani, D. Borchardt & M. Weitere, 2022. Lagrangian profiles of riverine autotrophy, organic matter transformation, and micropollutants at extreme drought. Science of the Total Environment 828: 154243. https://doi.org/10.1016/j.scitotenv.2022.154243.
Kim, H.-W. & G.-J. Joo, 2000. The longitudinal distribution and community dynamics of zooplankton in a regulated large river: a case study of the Nakdong River (Korea). Hydrobiologia 438: 171–184. https://doi.org/10.1023/A:1004185216043.
Klapper, H., 1961. Biologisches Gütebildder Elbe zwischen Schmilka und Boizenburg. Internationale Revue Der Gesamten Hydrobiologie 46: 51–64. https://doi.org/10.1002/iroh.19610460106.
Klöcking, B. & U. Haberlandt, 2002. Impact of land use changes on water dynamics—a case study in temperate meso and macroscale river basins. Physics and Chemistry of the Earth 27: 619–629. https://doi.org/10.1016/S1474-7065(02)00046-3.
Kutikova, L. A., 1970. Rotifers of the fauna of the USSR. L.: Nauka: 744 (in Russian).
Lair, N., 2006. A review of regulation mechanisms of metazoan planktonin riverine ecosystems: aquatic habitat versus biota. River Research and Applications 22: 567–593. https://doi.org/10.1002/rra.923.
Lair, N. & P. Reyes-Marchant, 1997. The potamoplankton of the Middle Loire and the role of the “moving littoral” in downstream transfer of algae and rotifers. Hydrobiologia 356: 33–52. https://doi.org/10.1023/A:1003127230386.
Lancaster, J. & A. G. Hildrew, 1993. Characterizing in-stream flow refugia. Canadian Journal of Fisheries and Aquatic Sciences 50: 1663–1675. https://doi.org/10.1139/f93-187.
Le Coz, M., S. Chambord, P. Meire, T. Maris, F. Azémar, J. Ovaert, E. Buffan-Dubau, J. C. Kromkamp, A. C. Sossou, J. Prygiel, G. Spronk, S. Lamothe, B. Ouddane, S. Rabodonirina, S. Net, D. Dumoulin, J. Peene, S. Souissi & M. Tackx, 2017. Test of some ecological concepts on the longitudinal distribution of zooplankton along a lowland water course. Hydrobiologia 802: 175–198. https://doi.org/10.1007/s10750-017-3256-6.
May, L. & J. A. B. Bass, 1998. A study of rotifers in the River Thames, England, April–October, 1996. Hydrobiologia 387: 251–257. https://doi.org/10.1023/A:1017073223382.
Meister, A., 1994. Untersuchung zum Plankton der Elbe und ihrer grosseren Nebenflusse. Limnologica 24: 153–171.
Mirabdullayev, I. M. & D. Defaye, 2004. On the taxonomy of the Acanthocyclops robustus species-complex (Copepoda, Cyclopidae): Acanthocyclops brevispinosus and A. einslei sp. n. Vestnik Zoologii 38: 27–37.
Monchenko, V. I., 1974. The cyclopidae (cyclopidae), Naukova dumka, Kyiv:, 452 (in Ukrainian).
Napiórkowski, P. & T. Napiórkowska, 2013. The diversity and longitudinal changes of zooplankton in the lower course of a large, regulated European river (the lower Vistula River, Poland). Biologia 68: 1163–1171. https://doi.org/10.2478/s11756-013-0263-6.
Nogrady, T. & H. Segers, 2002. Rotifera 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. In Priya, R. (ed), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 18. Backhuys Publishers, Leiden: 264.
Nogrady, T., R. Pourriot & H. Segers, 1995. Rotifera 3: The Notommatidae and The Scaridiidae. In Priya, R. (ed), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 8. SPB Academic Publishing, Amsterdam: 102.
Pace, M. L., S. E. G. Findlay & D. Lints, 1992. Zooplankton in advective environments: the Hudson River Community and a comparative analysis. Canadian Journal of Fisheries and Aquatic Sciences 49: 1060–1069. https://doi.org/10.1139/f92-117.
Pusch, M. & H. Fischer, 2006. Stoffdynamik und Habitatstruktur in der Elbe, Weißensee Verlag, Berlin:, 385.
R Core Team, 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Reckendorfer, W., H. Keckeis, G. Winkler & F. Schiemer, 1999. Zooplankton abundance in the River Danube, Austria: the significance of inshore retention. Freshwater Biology 41: 583–591. https://doi.org/10.1046/j.1365-2427.1999.00412.x.
Saunders, J. F. & W. M. Lewis, 1989. Zooplankton abundance in the lower Orinoco River, Venezuela. Limnology and Oceanography 34: 397–409. https://doi.org/10.4319/lo.1989.34.2.0397.
Scherwass, A., T. Bergfeld, A. Schöl, M. Weitere & H. Arndt, 2010. Changes in the plankton community along the length of the River Rhine: Lagrangian sampling during a spring situation. Journal of Plankton Research 32: 491–502. https://doi.org/10.1093/plankt/fbp149.
Sereda, T. M. & Yu. F. Gromova, 2022. Seasonal pattern of plankton drift in the estuarine section of the Desna River in the riverbed-floodplain system: mechanisms of biofund exchange. Hydrobiological Journal 58: 3–14. https://doi.org/10.1615/HydrobJ.v58.i1.10.
Shannon, C. E., 1948. A mathematical theory of communication. Bell System Technical Journal 27: 623–656.
Shiel, R. J., 1985. Zooplankton of the Darling River system, Australia. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 22: 2136–2140. https://doi.org/10.1080/03680770.1983.11897637.
Shiel, R. J. & K. F. Walker, 1984. Zooplankton of regulated and unregulated rivers: the Murray-Darling River system, Australia. In Lillehammer, A. & S. J. Saltveit (eds), Regulated rivers Universitets forlaget AS, Oslo: 263–270.
Silaeva, A. A., A. A. Protasov, T. N. Novoselova & Y. F. Gromova, 2016. Influence of filtration activity of Unionidae on the planktonic subsystem of a small river. Scientific Bulletin of Uzhhorod University. Series Biology 41: 44–47 (in Russian).
Smirnov, N. N., 1992. The Macrothricidae of the World, Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 1. SPB Academic Publishing, The Hague: 143.
Smirnov, N. N., 1996. The Chydoridae and Sayciinae (Chydoridae) of the World, Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. 11. SPB Academic Publishing, Amsterdam: 197.
Souley Adamou, H., B. Alhou, M. Tackx & F. Azémar, 2022. Zooplankton distribution and community structure as a function of environmental variables in the Niger River and its tributaries in Niger. African Journal of Aquatic Science. https://doi.org/10.2989/16085914.2022.2122391.
Speas, D. W., 2000. Zooplankton density and community composition following and experimental flood in the Colorado River, Grand Canyon, Arizona. Regulated Rivers Research & Management 16: 73–81. https://doi.org/10.1002/(SICI)1099-1646(200001/02)16:1%3C73::AID-RRR565%3E3.0.CO;2-%23.
Straile, D., 1997. Gross growth efficiencies of protozoan and metazoan zooplankton and their dependence on food concentration, predator-prey weight ratio, and taxonomic group. Limnology and Oceanography 42: 1375–1385.
Thorp, J. H. & S. Mantovani, 2005. Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshwater Biology 50: 1474–1491. https://doi.org/10.1111/j.1365-2427.2005.01422.x.
Thorp, J. H., A. R. Black, K. H. Haag & J. D. Wehr, 1994. Zooplankton assemblages in the Ohio River: seasonal, tributary, and navigation dam effects. Canadian Journal of Fisheries and Aquatic Sciences 51: 1634–1643. https://doi.org/10.1139/f94-164.
Vadadi-Fülöp, C., L. Hufnagel, G. Jablonszky & K. Zsuga, 2009. Crustacean plankton abundance in the Danube River and in its side arms in Hungary. Biologia 64: 1184–1195. https://doi.org/10.2478/s11756-009-0202-8.
Van Dijk, G. M. & B. van Zanten, 1995. Seasonal changes in zooplankton abundance in the lower Rhine during 1987–1991. Hydrobiologia 304: 29–38. https://doi.org/10.1007/BF02530701.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 370–377. https://doi.org/10.1139/f80-017.
Viroux, L., 1997. Zooplankton development in two large lowland rivers, the Moselle (France) and the Meuse (Belgium), in 1993. Journal of Plankton Research 19: 1743–1762. https://doi.org/10.1093/plankt/19.11.1743.
Viroux, L., 1999. Zooplankton distribution in flowing waters and its implications for sampling: case studies in the River Meuse (Belgium) and the River Moselle (France, Luxembourg). Journal of Plankton Research 21: 1231–1248. https://doi.org/10.1093/plankt/21.7.1231.
Zimmermann-Timm, H., H. Holst & H. Kausch, 2007. Spatial dynamics of rotifers in a large lowland river, the Elbe, Germany: how important are retentive shoreline habitats for the plankton community? Hydrobiologia 593: 49–58. https://doi.org/10.1007/s10750-007-9046-9.
Acknowledgements
We sincerely thank K. Rinke for his kind support. We thank the captain and crew of RV Albis, S. Bauth, H. Goreczka, and U. Link as well as H.-J. Dahlke, S. Willige, and F. Zander for their support in the field. A. Hoff, K. Lerche, I. Locker, I. Siebert, and M. Tibke contributed to the subsequent analyses in the laboratory, and Y. Rosenlöcher provided technical assistance during the processing of samples. We wish to show our appreciation W. von Tümpling for methodological help and Frederic Bartlett for correcting the English. Discharge data were kindly provided by the German Federal Waterways and Shipping Administration (WSV) and communicated by the German Federal Institute of Hydrology (BfG).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by funding from the Helmholtz Association in the framework of MOSES (Modular Observation Solutions for Earth Systems).
Author information
Authors and Affiliations
Contributions
Yuliia Hromova contributed to investigation, conceptualization, writing of the original draft, and design; Mario Brauns contributed to statistical analysis, design, writing, and editing of the manuscript; Norbert Kamjunke contributed to methodology, conceptualization, design, writing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Handling editor: María Florencia Gutierrez
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hromova, Y., Brauns, M. & Kamjunke, N. Lagrangian dynamics of the spring zooplankton community in a large river. Hydrobiologia 851, 3603–3621 (2024). https://doi.org/10.1007/s10750-024-05520-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05520-7