Abstract
The mixing regime, the spatial distribution of nutrients and light determine the distribution of phytoplankton in lakes to a large extent. Linear stratification is a unique phenomenon among the various forms the lakes can stratify, representing a continuous and gradual water temperature decrease with depth. Here, we aimed to understand how mixing, nutrient and light affect the vertical distribution of phytoplankton in the case of linear water column stratification using the taxonomic and functional group approaches. We sampled phytoplankton and physical and chemical variables in the Malom-Tisza oxbow lake (Hungary) monthly from May to September between 2007 and 2009. Our results revealed that multiple biomass peaks of taxa belonging to distinct phytoplankton functional groups could develop in response to the strong linear stratification of the water column. Although several different species represented the functional groups, only one or two species developed the peaks. Light irradiance did not influence the vertical distribution of biomass and taxonomic richness of phytoplankton, but the depth of the euphotic zone determined the number of distinct biomass peaks. We found that diversity indices could not reflect the phytoplankton compositional differences well in the case of linear stratification, but similarity indices calculated among water column layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of the solar radiation penetrating the water rapidly attenuates in a thin layer, resulting in the absorption of heat in the uppermost part of the lakes’ water column (Wetzel, 2001). This phenomenon and the temperature dependence of water density can result in the thermal stratification of standing waters (Dodds & Whiles, 2010). Differences in the individual characteristics of lakes, like heating and mixing by diverse climatic variations, the volume of inflow and outflow in relation to the volume of the basin, surface area and fetch length, or differences in the wind speed, can result in distinct stratification patterns both at temporal and spatial scales (Wetzel, 2001; Kalff, 2002).
Although phytoplankton biomass concentrates usually in the uppermost, well-illuminated and well-mixed epilimnion, depending on the light availability, phytoplankton occasionally develops persistent assemblages in the metalimnion and even in the hypolimnion of stratified oligotrophic lakes (Wetzel, 2001; O’Sullivan & Reynolds, 2008). The strong water density gradient in the metalimnion can result in large physical stability. Accordingly, disruption of the metalimnion requires a substantial amount of energy and it functions like other strong barriers including for example the lake bottom in shallow, fertile lakes (Gliwicz, 1979). This relative strong stability enables the development of other clinal patterns beyond the thermocline, such as chemocline or oxycline. In the metalimnion (or hypolimnion) of oligotrophic stratified lakes, a so-called deep chlorophyll maximum (DCM) can develop if the euphotic depth exceeds the depth of the epilimnion and reaches the metalimnion or the upper layer of hypolimnion (Camacho, 2006). The DCMs are mainly formed by species able to actively regulate their position in the water column by flagella (e.g. cryptophytes, dinoflagellates) or by other buoyancy regulating mechanisms like creation of gas vesicles (e.g. Cyanobacteria). Interestingly, besides Cyanobacteria (Pannard et al., 2015; Selmeczy et al., 2016) and cryptophytes (Camacho et al., 2001), non-motile species, e.g. diatoms (Coon et al., 1987; Barbiero & Tuchman, 2001) or chlorococcaleans (Larson et al., 1987; Krienitz & Scheffler, 1994) can also develop DCMs. According to Camacho (2006), different mechanisms can underlie DCM formation. The main driver is water column stability, which determines the thermal, density and chemical gradients in a lake (Kalff, 2002), as well as the persistent stratification with opposing vertical gradients of light and nutrients (Klausmeier & Litchman, 2001; Pannard et al., 2015). Further possible mechanisms are grazing (Pannard et al., 2015), the passive sinking of epilimnetic species (Camacho, 2006), in situ growth of phototrophs (Camacho, 2006) and the symbiotic association of algae with protozoa (Queimaliños et al., 1999).
In dimictic lakes, the metalimnion is usually a relatively thin layer, but this is not necessarily the case in all lakes. Small lakes with relatively short fetch, large depth, high seasonal temperature variation and with the lack of diurnal temperature fluctuation, a so-called linear stratification pattern may develop (Wetzel, 2001). It is, however, a rarely observed phenomenon in nature. In the case of linear stratification, the well-mixed epilimnion is missing and water temperature gradually decreases with depth. Accordingly, the entire water column belongs to the metalimnion (Wetzel, 2001) with stable spatial segregation in environmental variables (Fig. 1; Kufel & Kalinowska, 1997). For photosynthetic organisms, linear layering practically means distinct niche spaces, in which various phytoplankton species may find their optima for growth. It may coincide with species replacements and abrupt phytoplankton compositional changes in the composition of the adjacent vertical layers (Borics et al., 2011).
While most dimictic lakes have a classical three-layer stratification pattern in Hungary, linear stratification can also develop in some cases (Borics et al., 2015). One excellent example for the linear stratification pattern is the summer water temperature profile of the hypertrophic Malom-Tisza oxbow (max. depth: ~ 10 m; Krasznai et al., 2010) with diverse phytoplankton assemblages (Görgényi et al., 2019). Temperature profiles of the Malom-Tisza oxbow imply a near-linear stratification of the water column in summer (Borics et al., 2015). The thickness of the surface mixed layer is negligible and almost the whole water column may belong to the metalimnion. Water column stability measured by the calculation of the Lake Number—‘LN’ (Read et al., 2011), which is calculated based on the vertical temperature profile, indicated an extremely stable summer stratification for the Malom-Tisza oxbow (LN ~ 120).
Former studies revealed a very diverse microflora for Malom-Tisza oxbow (Krasznai et al., 2010; Görgényi et al., 2019), which refers to large habitat diversity within the lake. Patchy distribution of some taxa was shown previously (Borics et al., 2011) but the rate of species or functional group turnover between the adjacent layers, and the temporal pattern of the dominating groups in the various layers have not been analysed numerically.
In this study, we aimed to highlight a strong relationship between the linear stratification pattern and the compositional differences of phytoplankton among layers. Since linear stratification may enable the development of persistent vertical environmental gradients, we hypothesised that
-
(i)
the physically stable metalimnion enables the spatial segregation of coexisting assemblages;
-
(ii)
nutrients and irradiance strongly influence biomass and species richness of phytoplankton in the various layers;
-
(iii)
there is a relationship between the diversity and the rate of community turnover (hereafter community change rate) of the phytoplankton in the vertical layers; and finally,
-
(iv)
the thickness of the photic zone determines the number of biomass peaks created by characteristic and spatially distinct assemblages.
Material and methods
Study site
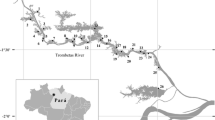
Malom-Tisza oxbow is a 4.6-km-long, hypertrophic and wind-sheltered water body with an average depth of 3 m. It is the middle section of the Tiszadob oxbow system (11-km-long) and located in the middle part of the Tisza valley (Carpathian Basin, Hungary; Fig. 2).
Although at the eastern part floating islands developed (Calamagrostido-Salicetum cinereae, Borhidi et al., 2014), extended open water area characterises the most part of the Malom-Tisza oxbow. Strong linear stratification characterised the oxbow during the samplings in each year (Borics et al., 2015).
Sampling
A single water column was sampled for physical, chemical and phytoplankton analyses monthly from May to August in 2008 and from May to September in 2007 and 2009 at the deepest point of the oxbow (N 48° 01′ 14.35″; E 21° 11′ 26.50″) (7 m). Vertical samples were collected by a Ruttner sampler (HYDRO-BIOS) at each 1-m interval. We used the thermometer of the sampler to measure water temperature (T–°C). The conductivity (COND–µS cm−1), pH and dissolved oxygen concentration (DO–mg l−1) were measured in the field by a portable-multiparameter digital meter (HQ30d, Germany) in each sampled layer. For further chemical analyses, water samples were kept at 4 °C in a cooler bag during transportation to the laboratory.
The following chemical parameters were measured in the laboratory, according to international and Hungarian guidelines: ammonium-nitrogen (NH4+-N in mg l−1; MSZ ISO 7150–1:1992), dissolved inorganic nitrogen (DIN in mg l−1; MSZ 1484–13:2009), phosphate (PO43−-P in µg l−1; MSZ EN ISO 6878:2004) and chlorophyll a (Chl-a in µg l−1, MSZ ISO 10260:1993).
We measured the light penetration in the water column with a Secchi disc. The euphotic layer of pelagial zone was calculated from the Secchi depth (2.5 × depth of Secchi disc, Poikane, 2009). The Secchi depth (SD) was used to calculate light extinction coefficient (k) applying the Pool and Atkins (1929) formula: k = 1.7/SD. Irradiance at a given depth was calculated using the following equation:
where Id is irradiance at d depth, I0 is midday irradiance at the surface (2000 µE/m2/sec) (Falkowski & Raven, 1997), d is depth, k is light extinction coefficient, e is Euler number (2.718).
Both quantitative and qualitative phytoplankton samples were collected. Phytoplankton samples were preserved with Lugol’s solution following sampling. Quantitative phytoplankton samples were collected by a Ruttner sampler (HYDRO-BIOS, Altenholz, Germany) at one-meter intervals of the whole water column (7 m) during every sampling occasion. In May and June 2007, because of technical limitations, phytoplankton samples were taken only from the upper 4 m. To achieve accurate species identification, using a tube sampler we also took qualitative phytoplankton samples from the upper 2 ms of the water column at each sampling occasion, and filtered these samples through a 10-µm mesh size plankton net. Phytoplankton species of the net samples were studied with upright microscope (Leica DMRB) at 100–1000 × magnification.
Study of the phytoplankton
We studied the phytoplankton samples at 100 × and 630 × magnification using a LEICA DMIL inverted microscope. Species were documented by Canon EOS digital camera. At least 400 units (cells, colonies and filaments) were counted in each sample. For the estimation of the biomass, we measured the linear dimensions of 20 specimens of each taxon. Phytoplankton biovolume and surface area were calculated using realistic 3D algal models following Borics et al. (2021). Accepted names of phytoplankton species were based on the AlgaeBase (Guiry & Guiry, 2023).
Functional classification of phytoplankton
Since we supposed that the different vertical layers provided distinct habitats for planktic organisms, we assigned phytoplankton taxa into Reynolds’ functional groups (a.k.a. codon, coda in plural Reynolds et al., 2002; Padisák et al., 2009). Functional groups sensu Reynolds contain species sharing similar responses to the environment, and this represents functional group-specific habitat templates. Accordingly, Reynolds’ functional groups can also be considered functional response groups (Violle et al., 2007; Abonyi et al., 2018), and therefore, they provide an appropriate approach to test our hypotheses.
Statistical analyses
To analyse the vertical pattern of phytoplankton diversity, we calculated Shannon diversity for each sampled layer. To assess the relationship between phytoplankton biomass, irradiance and diversity measures, we calculated Spearman rank correlation (Zar, 2009). We also used the taxonomic richness within each sampled layer as a measure of diversity.
To evaluate compositional differences among water layers at a given time, we calculated Bray–Curtis similarity (Bray & Curtis, 1957). Low similarity values refer to large community change rate. The sum of the absolute values of differences in Bray–Curtis similarities between the layers at a given time was considered as water column level community change rate. Thus, small values indicate low similarities, i.e. high level of vertical niche segregation, while large numbers refer to small differences in niche characteristics in the water column.
We considered “biomass peaks” here as the maxima of the biomass of a given taxa or FG in the water column, if their relative biomass > 20% of total biomass. Peaks with lower relative biomass were not considered, because the low numbers of observed individuals in samples can also incur higher counting error.
Results
Environmental variables
Physical and chemical parameters showed distinct vertical changes along the depth of the water column (Table 1). Clinal and abrupt changes could also be observed. Below 3 m, the dissolved oxygen markedly decreased (from 5–10.2 to under 1.9 mg l−1), while the pH continuously decreased from the surface (mean: 8.44) to the bottom (mean: 7.37). The conductivity decreased in the upper 2 m (from 364 to 348 µS cm−1) then increased (from 351 to 430). The concentration of nutrients (P and N) increased with depth (DIN from 0.31 to 2.36 mg l−1; PO43−-P from 12 to 97.5 µg l−1) and were above the concentrations considered limiting for phytoplankton (Reynolds, 2006). The actual chlorophyll-a concentrations (including both the bacterio- and phytoplankton chlorophyll) increased with depth at each sample occasion (mean values of the layers ranged from 13.4 to 36.3 µg l−1, see in Table 1).
Phytoplankton composition
The qualitative analysis of phytoplankton revealed a species rich microflora for the oxbow. We identified 332 taxa altogether (9 anoxygenic autotrophic bacteria, 47 oxygenic photoautotrophic Cyanobacteria and 270 eukaryotic algae). Eukaryotic algae were characterised by a high number of Chlorophyta (113 species), followed by Bacillariophyta (44 species), Ochrophyta (40 species), Euglenozoa (25 species) and Charophyta (21 species). The most frequently occurring taxa were Aphanocapsa delicatissima West & G. S. West, Planktolyngbya limnetica (Lemmermann) Komárková–Legnerová & Cronberg (Cyanobacteria), Monoraphidium tortile (West & G. S. West) Komárková–Legnerová, Monoraphidium contortum (Thuret) Komárková–Legnerová, Tetraëdron minimum (A. Braun) Hansgirg (Chlorophyta) and Peridinium gatunense Nygaard (Miozoa), which were present in more than 80% of the samples. The detailed species list with average biovolume data and species classifications can be found in a Supplementary Material (Supplementary Table 1).
Vertical distribution of phytoplankton and spatial segregation of coexisting species
The spatio-temporal biomass profile of phytoplankton (including Cyanobacteria and eukaryotic algae) and bacterioplankton showed considerable vertical differences (Fig. 3). High phytoplankton biomass peaks were observed in July below the euphotic zone every year. Before and after July, these peaks appeared above the euphotic zone. Photosynthetic sulphur bacteria (Thiopedia rosea Winogradsky and cf. Leptothrix pseudovacuolata Skuja) were characteristic for the most part of the aphotic layer (< 4 m) throughout the study period.
Cyanobacterial blooms developed in the water column every mid-summer (in July) throughout the study period (Fig. 4). In contrast, in the spring and autumn periods several other planktic groups characterised the euphotic zone (see in Supplementary Figs. 1–4), while the aphotic zone (below 3–4 m) was dominated by photosynthetic sulphur bacteria (V codon).
Vertical profiles of dominant functional groups in Malom-Tisza oxbow in the three consecutive years of the study, 2007, 2008 and 2009. Dominant coda are shown in Table 2
Altogether 27 coda sensu Reynolds were found in the samples. Although their distribution in the water column was not homogeneous, because of the possible counting error, only coda with relative biomass > 20% are detailed in Fig. 4.
In 2007, only 1 or 2 coda (Lo, J, S1, T) dominated the euphotic and aphotic zone in each month, then in 2008, distinct peaks were observed (D, Lo, S1, Q, T) (Supplementary Figs. 1, 2). In 2008, the depth of the euphotic zone was more stable than in the previous year. Different coda were responsible for the peaks both in the euphotic (S1, Q) and aphotic zones (T, V) in July. In contrast to the previous years, different assemblages peaked in 2009. The number of coda that attained the 20% relative biomass was much higher (9) than in the previous years (~ 4–5) (Supplementary Figs. 3, 4). For example, in August, taxa belonging to H1, T, K, Y, X2 and D coda created peaks below each other. Interestingly, in September, a non-photosynthetic iron bacterium cf. Ochrobium sp. appeared in the euphotic zone. Although many species occurred in some coda, only 1 or 2 species were systematically responsible for the biomass peaks (detailed in Supplementary Figs. 1–4). The habitat templates of taxa providing the dominant coda based on biomass peaks are summarised in Table 2.
Effect of irradiance on the vertical distribution of phytoplankton
Several biomass peaks produced by taxa belonging to diverse coda appeared below the compensation depth (CD) calculated by the CD = 2.5 × Secchi transparency formula. Therefore, we calculated the level of irradiance in the studied layers (Cal. Id). The calculated irradiance at a given depth (Supplementary Table 2) systematically exceeded the critical irradiance value (Ic) of species found in the literature. The Ic is the minimum irradiance required for a population to survive. However, several species such as Kephyrion sp., Merismopedia glauca (Ehrenberg) Kützing, Mougeotia sp., Pediastrum duplex Meyen, Pseudopediastrum boryanum (Turpin) Hegewald formed peaks in the deeper layers (< 4 m depth), where their Ic values exceeded the level of measured irradiance. Because of its very low Ic value, Thiopedia rosea had multiple peaks between 4 and 7 m, where the calculated irradiance indicated limiting light conditions for the growth of the majority of phytoplankton species.
Diversity and community change rate
The vertical profile of taxonomic richness (S) and Shannon diversity (H) of Malom-Tisza oxbow showed a notable decreasing trend with depth. However, the values did not change gradually. Median values of species richness showed a peak at 4-m depth (Fig. 5) and decreased further with depth. The vertical profile for Shannon diversity was similar, but H values increased only slightly between the surface and the peak at 3–4 m depth.
The Spearman rank-order correlation revealed a significantly negative correlation between total phytoplankton biomass and both diversity measures (S, H) in the upper 4 m of the water column (Table 3). We did not find any significant relationship among these parameters in the deeper layer. There was no significant relationship between the calculated irradiance values and diversity, neither in the upper (0–4 m), nor in the lower (5–7) water column.
We experienced large fluctuations in the Bray–Curtis similarity values between water column layers for both in terms of taxonomic richness and number of functional groups. Values of the two metrics showed simultaneous changes (Fig. 6), i.e. the rate and direction of changes were mostly identical.
Low (< 0.5) similarity values were observed in each sample occasion, which indicates high compositional differences, i.e. large differences in the identity of dominant taxa or FGs between the underlying layers. However, we found no significant relationship between Bray–Curtis similarity values and diversity, neither for taxa, nor for functional groups (Fig. 7, P > 0.05 in all cases).
Impact of the photic zone depth on the number of spatially distinct assemblages
A positive (but marginally significant, P = 0.01) relationship occurred between the euphotic depth and the number of biomass peaks in the water column (Fig. 8). When the euphotic zone was < 3 m, 1 or 2 biomass peaks developed. The number of biomass peaks increased significantly if the euphotic depth exceeded 3 m, and the number of biomass peaks ranged between 1 and 4.
Discussion
Vertical distribution of phytoplankton and spatial segregation of coexisting species
Spatial arrangement of phytoplankton in water is neither vertically nor horizontally homogenous (Reynolds, 2006). The mixing regimes, spatial differences in growth-limiting nutrients (mainly P) and light jointly determine the distribution of phytoplankton in lakes (Longhi & Beisner, 2009). Thus, we hypothesised that a physically stable metalimnion enables the spatial segregation of coexisting phytoplankton assemblages.
There were altogether 13 coda (with relative biomass > 20%), in which taxa produced distinct peaks in the linearly stratified water column of Malom-Tisza oxbow during the sampling period. It occasionally occurred that almost in each of the sampled layers of the water column had a biomass peak make up by taxa belonging to different coda, demonstrating that the stable metalimnion enables the spatial segregation of coexisting species. Interestingly, taxa that produced biomass dominance within coda have wide range of habitat preferences, i.e. they have different habitat templates. Based on the literature, some of them occurred in shallow, enriched lakes ponds and lakes (D, J, Y) (Reynolds et al., 2002; Padisák et al., 2009). Some of the peak-created coda are sensitive to mixing (H1, X2, K), while some of them tolerated the low light (C, T, Y, V). While taxa of one codon, which dominated the deeper part of the water column, tolerate mixing (X2), other taxa are sensitive to it (codon C). One codon is typical in summer epilimnia in mesotrophic lakes (LO), while other in small, oligotrophic lakes (E). The V codon is characteristic in the metalimnia of eutrophic stratified lakes, which explains well its distribution in Malom-Tisza oxbow. The above findings support our assumption that stable spatial segregation of environmental variables in the water column provide different habitats for phytoplankton in each layer.
It has long been recognised that the mixing depth-to-photic depth ratio is influential in the development of phytoplankton under well-mixed conditions (Sverdrup, 1953; Huisman et al., 1999). In the Malom-Tisza oxbow, water column stratification remained stable through the whole study period. Thus, species characterised with different critical light intensity might coexist if they change or keep their position in the most suitable layer of the water column. Majority of species that displayed biomass peaks in some of the vertical layers of the water column in Malom-Tisza oxbow have the capability of buoyancy regulation (e.g. Aphanizomenon sp.) or active swimming by flagella (e.g. Peridinium gatunense, Cryptomonas sp.). Representatives of this latter group can successfully compete for light and nutrients and besides cyanobacteria appear as subdominants in eutrophic waters (De Melo & Huszar, 2000). We note here that metalimnetic dominance of Aphanizomenon flos-aquae has also been formerly described for phytoplankton in the Lake Stechlin (Selmeczy et al., 2016).
Since our samplings give a snapshot of a dynamic pattern, it is reasonable to suppose that the flagellates do not have a constant position in the water column and can migrate during the day. Diel vertical migration of Peridinium gatunense (Usvyatsov & Zohary, 2006) in the Lake Kinneret or Cryptomonas spp. in a stratified mesotrophic reservoir (Kansas, USA) have also been reported (deNoyelles et al., 2016). There are several other flagellates like Gonyostomum semen (Raphidophyceae) (Salonen & Rosenberg, 2000) and Dinobryon spp. (Chrysophyceae) (Heinze et al., 2013) that have characteristic circadian migration rhythms. Vertical differences in the availability of light and phosphorus governed the circadian vertical migration of Ceratium hirundinella (O. F. Müller) Dujardin moving > 4 m in the water (James et al., 1992). These taxa were also present in the Malom-Tisza oxbow, but their abundance was low, therefore their vertical differences in the water column could not be demonstrated. Vertical migration of most phytoplankton taxa shows the same pattern: daytime ascent and night-time descent. Migration enables species to exploit the nutrient sources of the aphotic deep layers at night and enjoy the well-illuminated regions in the daytime, where they reduce the respiratory losses and avoid zooplankton grazers. Besides the direct stimuli such as light, chemical gradients or temperature (Clegg et al., 2007), importance of endogenous circadian rhythms in the vertical migration of phytoplankton has also been suggested by several authors (Sournia, 1974; Frempong, 1983; Prézelin, 1992; Gervais, 1997a, b). The flagellates that produced peaks in the different layers of Malom-Tisza oxbow might have been influenced by the actual strength of the above factors and by their specific sensitivity to them.
Interestingly, occasionally species with no capability for buoyancy regulation or active swimming created biomass peaks in the deeper layers. These peaks must have been resulted by the sinking of the species that produced peaks in the upper layers, but they were not able to maintain their position in the water column (Camacho, 2006). In Malom-Tisza oxbow, the non-motile taxa, Fragilaria spp., Pediastrum spp., Romeria sp., Mougeotia sp. showed a deep layer biomass peak in the water column.
Effects of nutrients and irradiance on the vertical distribution of phytoplankton
While in a well-mixed water column where nutrients are homogenously distributed the light availability can be responsible for the inhomogeneity in phytoplankton biomass (Mellard et al., 2011), in a poorly mixed water column, like in the Malom-Tisza oxbow, both light and nutrients are important drivers of the phytoplankton community composition (Klausmeier & Litchman, 2001). Here, we revealed that vertical differences in light and nutrients are linked to an almost complete linear summer stratification of a deep oxbow lake.
Concerning the vertical changes of nutrients, we found that direction of changes in the concentration of the dissolved oxygen and NH4+ ion, pH or conductivity values of Malom-Tisza oxbow corresponded well with the clinal changes described for stratified lakes (Wetzel, 2001). The higher soluble reactive phosphorus concentration in the deep layer must have been produced by the internal load, i.e. liberation of phosphorus from redox sensitive iron compounds in low redox environment (Sondergaard et al., 2001). Although these changes resulted in considerable differences in the concentrations of available nutrients in Malom-Tisza oxbow, the concentration ranges where the inorganic nitrogen and phosphorus vary were much higher than considered as limiting (IP: 3 µg l−1; DIN: 20 µg l−1) for phytoplankton (Sas, 1990; Wetzel, 2001; Reynolds, 2006). Therefore, contrary to our second hypothesis, nutrient limitation was not likely to occur in the studied oxbow, and thus it plays a negligible role in shaping the observed vertical changes in phytoplankton composition.
Since field studies and laboratory experiments proved that light was a crucial variable for vertical migration of phytoflagellates evoking a positive phototaxis (Sommer, 1982), we hypothesised that the irradiance strongly influenced biomass and species richness of phytoplankton assemblages in the water column. However, this idea was not supported by our results because none of the two variables showed significant relationship with irradiance. One of the explanation is that the irradiance entering the upper water layer is strong enough to cause photoinhibition for phytoplankton (Hsu et al., 2013). Majority of phytoplankters, therefore, avoid excessive radiation by moving towards deeper layers (Goss et al., 1999). In the Malom-Tisza oxbow phytoplankton never peaked in the uppermost well-illuminated layer. Several phytoplankton groups contain accessory pigments that enable the absorption of wide range of light spectrum and the different light intensities, which allow them to optimise their vertical position in the water column (Falkowski & Raven, 1997). Despite the poor light conditions below the euphotic zone at about 3–4 m depth, the calculated irradiance values indicated that there was still plenty of light for many species to grow at this depth in Malom-Tisza oxbow. Even the sinking populations of Fragilaria sp., Pediastrum duplex, Mougeotia sp. at 3 m and Romeria sp., Pseudopediastrum boryanum at 4 m depths had enough light to grow and to create biomass peaks. Falkowski & Raven (1997) found that the capability of buoyancy regulation or active swimming and the differences in the light absorption abilities allowed the coexistence of a number of species, which phenomenon was also supported by our results.
One additional explanation for the lack of relationship was that we found a large biomass sulphur bacteria population in the deepest water layer. Field studies (Caldwell & Tiedje, 1975; Steenbergen et al., 1989; Banens, 1990) demonstrate that dense populations of phototrophic bacterial communities can develop in the anoxic meta- and hypolimnion of stratified lakes. The characteristic member of this bacterial community is the bloom-forming, sulphur bacteria Thiopedia rosea, which is sensitive to moderate light intensities, since its optimum light intensity is 100 µE m−2 s−1 and its compensation point is between 0.5 and 2 µE m−2 s−1 (Eichler & Pfennig, 1991). This is in line with the distribution of T. rosea that we encountered in Malom-Tisza oxbow. The anoxic part of the linearly stratified water column and the appropriate light level provided good conditions for this species to proliferate and flourish.
In addition to this species, the other characteristic member of the bacterial community in an anoxic metalimnion can be the iron bacteria, Ochrobium sp. This species with its gas vacuoles is able to move up in the water column (Jones, 1981), but its capsule encrusted by ferric ions, which gives an excess density to the cell enabling its downward movement towards the bottom (Robbins & Iberall, 1991). At the start of mixing, this species peaked at 2–3 m in September 2009, in a well-oxygenated part of the water column of Malom-Tisza oxbow. Similar phenomenon has also been reported from a Karelian stratified lake (Dubinina & Kuznetsov, 1976).
Diversity and community change rate
The strong thermal stratification provides wide range of vertical microhabitats for phytoplankton and the spatial niche segregation contributes to the maintenance of a high diversity (Longhi & Beisner, 2010). Investigating the microscale differences in a shallow lake metalimnion, Gasol et al. (1991) found drastic changes in oxygen and hydrogen sulphide concentrations within a < 0.5 m part of the water column. Since these differences have a significant impact on the composition of microalgal assemblages, we expected that the higher the community change rate, the higher the diversity. The results, however, did not support our expectations. Our results proved that despite finding low Bray–Curtis similarity values between the underlying neighbouring layers of the water column (i.e. high community change rate), these changes showed no relationship with the diversity of phytoplankton. The explanation of this strange phenomenon is that differences in the community change rates have been attributed to changes in the identity of the dominant species, and not by changes in the species pool. This latter can be accounted for by the continuous sedimentation of species that eliminates the otherwise considerable differences among the underlying layers. These results imply that similarity or dissimilarity metrics calculated between the adjacent vertical layers are better descriptors of compositional differences than diversity metrics, because diversity metrics are sensitive to the uneven distribution of species, but do not for the identity of species creating the uneven distribution. Thus, the different layers might have similar diversity values besides completely different species pools, or besides identical species but different dominance relations.
Impact of the photic zone depth on number of characteristic spatially segregated assemblages
In accordance with the above findings, we found that there was no correlation between diversity and irradiance in the upper water column. In the studied lake, parallel to the increase in depth and the reduction of light, a slight increase in the median values of taxonomic richness could be observed. This result did not support the idea that the irradiance strongly influences community species richness in a linearly stratified water column. However, our results demonstrated that the depth of the photic zone had a significant effect on the number of peaks in the water column, i.e. with increasing photic zone depth, the number of peaks also increased. When the photic layer was < 3 m, the number of peaks was significantly smaller than in cases when it exceeded 3 m. In lakes with a three-layer stratification, no more than two biomass peaks may develop: one in the epilimnetic surface layer, called surface chlorophyll maximum (SCM) (Simmonds et al., 2015), and the other the deep chlorophyll maximum (DCM) in the meta- or hypolimnion (Camacho, 2006). In the hypertrophic Malom-Tisza oxbow, however, under appropriate light conditions, occasionally multiple biomass peaks developed. These peaks were produced by functionally distinct taxa: flagellated mixotrophic species, strictly autotrophic species with or without buoyancy regulation. They all require different habitat templates and are characteristic for a given lake type or for a given successional period (Reynolds et al., 2002). The fact that species from different FGs can produce distinct vertical peaks simultaneously support our hypothesis that the linearly stratified water column enables a spatial niche segregation, and coincides to the large functional and species diversity of the oxbow.
Conclusions
Understanding the proximate reasons of why and how a given species creates a peak in a certain layer of the water column would require the implementation of high frequency close-interval sampling and measurement of various factors. Therefore, in the present study, we did not aim to give an exhaustive explanation for the development of phytoplankton biomass peaks vertically. However, we revealed the importance of light gradient as a potential driver of the vertical distribution of phytoplankton, and described how a linear stratification could contribute to the diversity and compositional differences of the phytoplankton in a linearly stratifying oxbow.
Here, we demonstrated that in the various layers of the linearly stratified water column, taxa belonging to several distinct functional groups could prevail and create multiple biomass peaks. We demonstrated that the number of these peaks depended on the euphotic depth. We found no evidence for associations between diversity and the strength of irradiance or community change rate. Our results imply that the vertical distribution of phytoplankton manifests in the number of functionally distinct species (or FGs) that can dominate the community.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abonyi, A., Z. Horváth & R. Ptacnik, 2018. Functional richness outperforms taxonomic richness in predicting ecosystem functioning in natural phytoplankton communities. Freshwater Biology 63(2): 178–186. https://doi.org/10.1111/fwb.13051.
Banens, R. J., 1990. Occurrence of hypolimnetic blooms of the purple sulfur bacterium, Thiopedia rosea, and the green sulfur bacterium, Chlorobium limicola, in an australian reservoir. Marine and Freshwater Research 41(2): 223–235. https://doi.org/10.1071/MF9900223.
Barbiero, R. P. & M. L. Tuchman, 2001. Results from the US EPA’s biological open water surveillance program of the Laurentian Great Lakes: II—Deep chlorophyll maxima. Journal of Great Lakes Research 27(2): 155–166. https://doi.org/10.1016/S0380-1330(01)70628-4.
Borhidi, A., B. Kevey & G. Lendvai, 2014. Plant communities of Hungary, Akadémiai Kiadó, Budapest.
Borics, G., A. Abonyi, E. Krasznai, G. Várbíró, I. Grigorszky, S. Szabó, Cs. Deák & B. Tóthmérész, 2011. Small-scale patchiness of the phytoplankton in a lentic oxbow. Journal of Plankton Research 33(6): 973–981. https://doi.org/10.1093/plankt/fbq166.
Borics, G., A. Abonyi, G. Várbíró, J. Padisák & E. T-Krasznai, 2015. Lake stratification in the Carpathian basin and its interesting biological consequences. Inland Waters 5(2): 173–186. https://doi.org/10.5268/IW-5.2.702
Borics, G., V. Lerf, E. T-Krasznai, I. Stanković, L. Pickó, V. Béres & G. Várbíró, 2021. Biovolume and surface area calculations for microalgae, using realistic 3D models. Science of the Total Environment 773: 145538. https://doi.org/10.1016/j.scitotenv.2021.145538.
Bray, J. R. & J. T. Curtis, 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27(4): 326–349.
Caldwell, D. E. & J. M. Tiedje, 1975. The structure of anaerobic bacterial communities in the hypolimnia of several Michigan lakes. Canadian Journal of Microbiology 21(3): 377–385. https://doi.org/10.1139/m75-052.
Camacho, A., E. Vicente & M. R. Miracle, 2001. Ecology of Cryptomonas at the chemocline of a karstic sulfate-rich lake. Marine and Freshwater Research 52(5): 805–815. https://doi.org/10.1071/MF00097.
Camacho González, A., 2006. On the occurrence and ecological features of deep chlorophyll maxima (DCM) in Spanish stratified lakes. Limnetica 25(1–2): 453–478. https://doi.org/10.23818/limn.25.32
Clegg, M. R., S. C. Maberly & R. I. Jones, 2007. Behavioral response as a predictor of seasonal depth distribution and vertical niche separation in freshwater phytoplanktonic flagellates. Limnology and Oceanography 52(1): 441–455. https://doi.org/10.4319/lo.2007.52.1.0441.
Coon, T. G., M. M. Lopez, P. J. Richerson, T. M. Powell & C. R. Goldman, 1987. Summer dynamics of the deep chlorophyll maximum in Lake Tahoe. Journal of Plankton Research 9(2): 327–344. https://doi.org/10.1093/plankt/9.2.327.
De Melo, S. & V. L. M. Huszar, 2000. Phytoplankton in an Amazonian flood-plain lake (Lago Batata, Brasil): diel variation and species strategies. Journal of Plankton Research 22(1): 63–76. https://doi.org/10.1093/plankt/22.1.63.
deNoyelles Jr., F., V. H. Smith, J. H. Kastens, L. Bennett, J. M. Lomas, C. W. Knapp, S. P. Bergin, S. L. Dewey, B. R. K. Chapin & D. W. Graham, 2016. A 21-year record of vertically migrating subepilimnetic populations of Cryptomonas spp. Inland Waters 6(2): 173–184. https://doi.org/10.5268/IW-6.2.930.
Dodds, W. K. & M. R. Whiles, 2010. Freshwater ecology: Concepts and environmental applications of limnology, Elsevier Academic Press.
Dubinina, G. A. & S. I. Kuznetsov, 1976. The ecological and morphological characteristics of microorganisms in Lesnaya Lamba (Karelia). Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 61(1): 1–19. https://doi.org/10.1002/iroh.19760610102.
Eichler, B. & N. Pfennig, 1991. Isolation and characteristics of Thiopedia rosea (neotype). Archives of Microbiology 155(3): 210–216. https://doi.org/10.1007/BF00252202.
Falkowski, P. G. & J. A. Raven, 1997. Aquatic photosynthesis, Blackwell, Oxford.
Frempong, E., 1983. A laboratory simulation of diel changes affecting cellular composition of the dinoflagellate Ceratium hirundinella. Freshwater Biology 13: 129–138. https://doi.org/10.1111/j.1365-2427.1983.tb00665.x.
Gasol, J. M., J. García-Cantizano, R. Massana, F. Peters, R. Guerrero & C. Pedrós-Alió, 1991. Diel changes in the microstratification of the metalimnetic community in Lake Cisó. Hydrobiologia 211(3): 227–240. https://doi.org/10.1007/BF00008536.
Gervais, F., 1997a. Light-dependent growth, dark survival, and glucose uptake by cryptophytes isolated from a freshwater chemocline. Journal of Phycology 33(1): 18–25. https://doi.org/10.1111/j.0022-3646.1997.00018.x.
Gervais, F., 1997b. Diel vertical migration of Cryptomonas and Chromatium in the deep chlorophyll maximum of a eutrophic lake. Journal of Plankton Research 19(5): 533–550. https://doi.org/10.1093/plankt/19.5.533.
Gliwicz, Z.M. 1979. Metalimnetic gradients and trophic state of lake epilimnia. Memorie dell’Istituto Italiano di Idrobiologia. Dr. Marco de Marchi. Verbania Pallanza (Italy).
Görgényi, J., B. Tóthmérész, G. Várbíró, A. Abonyi, E. T-Krasznai, V. B-Béres & G. Borics, 2019. Contribution of phytoplankton functional groups to the diversity of a eutrophic oxbow lake. Hydrobiologia 830(1): 287–301. https://doi.org/10.1007/s10750-018-3878-3.
Goss, R., H. Mewes & C. Wilhelm, 1999. Stimulation of the diadinoxanthin cycle by UV-B radiation in the diatom Phaeodactylum tricornutum. Photosynthesis Research 59: 73–80. https://doi.org/10.1023/A:1006169901482.
Guiry, M.D. & G.M. Guiry. 2023. AlgaeBase: World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 23 January 2023.
Heinze, A. W., C. L. Truesdale, S. B. DeVaul, J. Swinden & R. W. Sanders, 2013. Role of temperature in growth, feeding, and vertical distribution of the mixotrophic chrysophyte Dinobryon. Aquatic Microbial Ecology 71(2): 155–163. https://doi.org/10.3354/ame01673.
Hsu, S. B., C. J. Lin, C. H. Hsieh & K. Yoshiyama, 2013. Dynamics of phytoplankton communities under photoinhibition. Bulletin of Mathematical Biology 75: 1207–1232. https://doi.org/10.1007/s11538-013-9852-3.
Huisman, J., P. van Oostveen & F. J. Weissing, 1999. Species dynamics in phytoplankton blooms: incomplete mixing and competition for light. The American Naturalist 154(1): 46–68. https://doi.org/10.1086/303220.
James, W. F., W. D. Taylor & J. W. Barko, 1992. Production and vertical migration of Ceratium hirundinella in relation to phosphorus availability in Eau Galle Reservoir, Wisconsin. Canadian Journal of Fisheries and Aquatic Sciences 49(4): 694–700. https://doi.org/10.1139/f92-078.
Jones, J. G., 1981. The population ecology of iron bacteria (genus Ochrobium) in a stratified eutrophic lake. Microbiology 125(1): 85–93. https://doi.org/10.1099/00221287-125-1-85.
Kalff, J., 2002. Limnology: Inland water ecosystems, Prentice-Hall, Upper Saddle River.
Klausmeier, C. A. & E. Litchman, 2001. Algal games: The vertical distribution of phytoplankton in poorly mixed water columns. Limnology and Oceanography 46(8): 1998–2007. https://doi.org/10.4319/lo.2001.46.8.1998.
Krasznai, E., G. Borics, G. Várbíró, A. Abonyi, P. Padisák, C. Deák & B. Tóthmérész, 2010. Characteristics of the pelagic phytoplankton in shallow oxbows. Hydrobiologia 639(1): 173–184. https://doi.org/10.1007/s10750-009-0027-z.
Krienitz, L. & W. Scheffler, 1994. The Selenastraceae of the oligotrophic Lake Stechlin. Biologia Bratislava 49(4): 463–471.
Kufel, L. & K. Kalinowska, 1997. Metalimnetic gradients and the vertical distribution of phosphorus in a eutrophic lake. Archiv Für Hydrobiologie 140(3): 309–320.
Larson, D. W., C. N. Dahm & N. S. Geiger, 1987. Vertical partitioning of the phytoplankton assemblage in ultraoligotrophic Crater Lake, Oregon, USA. Freshwater Biology 18(3): 429–442. https://doi.org/10.1111/j.1365-2427.1987.tb01328.x.
Longhi, M. L. & B. E. Beisner, 2009. Environmental factors controlling the vertical distribution of phytoplankton in lakes. Journal of Plankton Research 31(10): 1195–1207. https://doi.org/10.1093/plankt/fbp065.
Longhi, M. L. & B. E. Beisner, 2010. Patterns in taxonomic and functional diversity of lake phytoplankton. Freshwater Biology 55(6): 1349–1366. https://doi.org/10.1111/j.1365-2427.2009.02359.x.
Mellard, J. P., K. Yoshiyama, E. Litchman & C. A. Klausmeier, 2011. The vertical distribution of phytoplankton in stratified water columns. Journal of Theoretical Biology 269(1): 16–30. https://doi.org/10.1016/j.jtbi.2010.09.041.
MSZ 1484–13:2009. 2006. Water quality: Part 12—Determination of nitrate and nitrite by spectrophotometric method. In Hungarian
MSZ EN ISO 6878:2004. 2004. Water quality: Determination of phosphorus—Ammonium molybdate spectrometric method (ISO 6878:2004).
MSZ ISO 10260:1993. 1993. Water quality: Measurement of biochemical parameters—Spectrometric determination of the chlorophyll–a concentration. In Hungarian.
MSZ ISO 7150–1:1992. 1992: Water quality: Determination of ammonium—Part 1: Manual spectrometric method. In Hungarian
O’Sullivan, P. & C. S. Reynolds (eds), 2008. The lakes handbook, volume 1: limnology and limnetic ecology. Vol. 1. John Wiley & Sons.
Padisák, J., L. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19. https://doi.org/10.1007/s10750-008-9645-0.
Pannard, A., D. Planas & B. E. Beisner, 2015. Macrozooplankton and the persistence of the deep chlorophyll maximum in a stratified lake. Freshwater Biology 60(8): 1717–1733. https://doi.org/10.1111/fwb.12604.
Poikane, S., 2009. Water framework directive intercalibration technical report: Part 2—Lakes. EUR 28838EN/2, Office for Official Publications of the European Communities, Luxembourg.
Poole, H. H. & W. R. G. Atkins, 1929. Photo-electric measurements of submarine illumination throughout the year. Journal of the Marine Biological Association of the United Kingdom 16(1): 297–324.
Prézelin, B. B., 1992. Diel periodicity in phytoplankton productivity. Hydrobiologia 238: 1–35. https://doi.org/10.1007/978-94-011-2805-6_1.
Queimaliños, C. P., B. E. Modenutti & E. G. Balseiro, 1999. Symbiotic association of the ciliate Ophrydium naumanni with Chlorella causing a deep chlorophyll a maximun in an oligotrophic South Andes lake. Journal of Plankton Research 21: 167–178. https://doi.org/10.1093/plankt/21.1.167.
Read, J. S., D. P. Hamilton, I. D. Jones, K. Muraoka, L. A. Winslow, R. Kroiss, C. H. Wu & E. Gaiser, 2011. Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environmental Modelling & Software 26(11): 1325–1336. https://doi.org/10.1016/j.envsoft.2011.05.006.
Reynolds, C. S., 2006. The ecology of phytoplankton, Cambridge University Press.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428. https://doi.org/10.1093/plankt/24.5.417.
Robbins, E. I. & A. S. Iberall, 1991. Mineral remains of early life on Earth? On Mars? Geomicrobiology Journal 9(1): 51–66. https://doi.org/10.1080/01490459109385985
Salonen, K. & M. Rosenberg, 2000. Advantages from diel vertical migration can explain the dominance of Gonyostomum semen (Raphidophyceae) in a small, steeply-stratified humic lake. Journal of Plankton Research 22(10): 1841–1853. https://doi.org/10.1093/plankt/22.10.1841.
Sas, H., 1990. Lake restoration by reduction of nutrient loading: expectations, experiences, extrapolations. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 24(1): 247–251. https://doi.org/10.1080/03680770.1989.11898731.
Selmeczy, G. B., K. Tapolczai, P. Casper, L. Krienitz & J. Padisák, 2016. Spatial-and niche segregation of DCM-forming cyanobacteria in Lake Stechlin (Germany). Hydrobiologia 764(1): 229–240. https://doi.org/10.1007/s10750-015-2282-5.
Simmonds, B., S. A. Wood, D. Özkundakci & D. P. Hamilton, 2015. Phytoplankton succession and the formation of a deep chlorophyll maximum in a hypertrophic volcanic lake. Hydrobiologia 745: 297–312. https://doi.org/10.1007/s10750-014-2114-z.
Sommer, U., 1982. Vertical niche separation between two closely related planktonic flagellate species (Rhodomonas lens and Rhodomonas minuta v. nannoplanctica). Journal of Plankton Research 4: 137–142. https://doi.org/10.1093/plankt/4.1.137.
Sondergaard, M., P. J. Jensen & E. Jeppesen, 2001. Retention and internal loading of phosphorus in shallow, eutrophic lakes. The Scientific World Journal 1: 427–442. https://doi.org/10.1100/tsw.2001.72.
Sournia, A., 1974. Circadian periodicities in natural populations of marine phytoplankton. Advances in Marine Biology 12: 325–389. https://doi.org/10.1016/S0065-2881(08)60460-5.
Steenbergen, C. L. M., H. J. Korthals, A. L. Baker & C. J. Watras, 1989. Microscale vertical distribution of algal and bacterial plankton in Lake Vechten (The Netherlands). FEMS Microbiology Ecology 5(4): 209–219. https://doi.org/10.1111/j.1574-6968.1989.tb03695.x.
Sverdrup, H. U., 1953. On conditions for the vernal blooming of phytoplankton. Journal De Conseil 18(3): 287–295.
Usvyatsov, S. & T. Zohary, 2006. Lake Kinneret continuous time-depth chlorophyll record highlights major phytoplankton events. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 29(3): 1131–1134.
Violle, C., M. L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional! Oikos 116(5): 882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x.
Wetzel, R. G., 2001. Limnology: Lake and river ecosystems, Academic Press, NY.
Zar, J. H., 2009. Biostatistical analysis, 5th ed. Prentice Hall, New Jersey.
Acknowledgements
We are grateful to Prof. Frantisek Hindák (†) for his advice with identifying the algae, and to Tibor Kisantal for his graphical assistance.
Funding
Open access funding provided by ELKH Centre for Ecological Research. This work was supported by Hungarian Scientific Research Fund (NKFIH OTKA) project no.: K-132150. AA was supported by FK 142485 and by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, while PT by KKP 144068 (National Research, Development and Innovation Office, Hungary) during the manuscript preparation.
Author information
Authors and Affiliations
Contributions
ET-K, AA, PT, GV and GB collected the samples. ET-K and VB-B identified and counted the phytoplankton. GB and ET-K developed the structure and wrote the manuscript. VL, PT and GV carried out the statistical analyses. All authors contributed to revisions substantially and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No human participants and animals were involved in the research.
Additional information
Handling editor: Sidinei M. Thomaz
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait-Based Approaches in Micro-Algal Ecology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2023_5327_MOESM1_ESM.eps
Supplementary file1 (EPS 1263 KB) Supplementary Figure 1. Vertical profiles of functional groups which relative biomass frequency exceeded the 20% and the species that occurred therein of Malom-Tisza oxbow in 2007.
10750_2023_5327_MOESM2_ESM.eps
Supplementary file2 (EPS 843 KB) Supplementary Figure 2. Vertical profiles of functional groups which relative biomass frequency exceeded the 20% and the species that occurred therein of Malom-Tisza oxbow in 2008.
10750_2023_5327_MOESM3_ESM.eps
Supplementary file3 (EPS 782 KB) Supplementary Figure 3. Vertical profiles of functional groups which relative biomass frequency exceeded the 20% and the species that occurred therein of Malom-Tisza oxbow in 2009 from May to July.
10750_2023_5327_MOESM4_ESM.eps
Supplementary file4 (EPS 693 KB) Supplementary Figure 4. Vertical profiles of functional groups which relative biomass frequency exceeded the 20% and the species that occurred therein of Malom-Tisza oxbow in 2009 from August to September.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
T-Krasznai, E., B-Béres, V., Lerf, V. et al. Linear water column stratification and euphotic depth determine the number of phytoplankton taxa that create biomass peaks in a hypertrophic oxbow lake. Hydrobiologia 851, 767–783 (2024). https://doi.org/10.1007/s10750-023-05327-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05327-y