Abstract
The common bottlenose dolphin (Tursiops truncatus) is a top marine predator widely dispersed in coastal and pelagic habitats and with a generalist feeding behavior. Yet, information on the trophic ecology of animals inhabiting pelagic environments is still scarce. Using carbon (δ13C: 13C/12C) and nitrogen (δ15N: 15N/14N) stable isotope ratios, we identified and quantified the main groups of prey assimilated by bottlenose dolphins inhabiting an oceanic habitat (Madeira Island, East Atlantic). Bottlenose dolphins assimilated pelagic, schooling fish (such as blue jack mackerel, Trachurus picturatus) and mesopelagic and demersal squids, which reinforces the pelagic dietary composition of insular/oceanic dolphins. Also, intra-seasonal differences were found in their stable isotope ratios, which suggest intraspecific variability in the feeding behavior among individuals living in the same area. Sex was not the main factor contributing to these differences, suggesting the lack of trophic niche segregation between adult males and females in this offshore environment. Nonetheless, further studies including different life stages and information on the ecophysiological requirements are necessary to disclose the factors responsible for the observed variability. This study showed that insular dolphins fed primarily on economically important pelagic prey, highlighting the need of developing management strategies that integrate conservation in fisheries plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet information on marine mammals is necessary to describe their role in marine ecosystems (Bowen, 1997; Gulka et al., 2017). Given their generally large sizes and ecological position (as high trophic level predators), they can have regulative effects on species at lower levels in marine food webs. Moreover, given their high degree of exposure to human-induced threats (Maxwell et al., 2013), this information is of paramount importance to design effective management and conservation strategies (Prato et al., 2013).
Characterizing variability of the feeding habits of marine mammals can increase the knowledge on how they explore their habitat, both temporally and spatially (horizontally and vertically in the water column). Such variability can be caused by seasonal fluctuations in the availability of food resources (e.g., O'Toole et al., 2015; Guerra et al., 2020), by interspecific competition for resources (e.g., Aurioles-Gamboa et al., 2013; Young et al., 2017), or by intraspecific characteristics like sex or age class (e.g., Bolnick et al., 2011; Fernández et al., 2011; Quérouil et al., 2013). However, investigating the trophic interactions and diet of marine mammals, such as cetaceans, remains challenging given that some species are endangered or inhabit difficult-to-access environments (e.g., Hays et al., 2016).
Stable carbon and nitrogen isotope analysis has increasingly been used to investigate the trophic interactions within marine predator communities (including marine mammals), as well as spatial, ontogenetic, and sex variations of the diet of predators across a diversity of marine ecosystems (e.g., Hobson et al., 1996; Walker et al., 1999; Kiszka et al., 2014). Stable isotopes ratios provide a time-integrated signal of the food sources in the ecosystem that were incorporated into the consumers' structural components and energy reserves (Peterson & Fry, 1987). Moreover, stable isotope ratios in the consumers' tissues predictably reflect those of their prey, demonstrating an average trophic fractionation (i.e., the difference between the consumer and its diet) of approximately 0.4‰ δ13C and 3.4‰ δ15N per trophic level (Vander Zanden & Rasmussen, 2001; Caut et al., 2009). A major limitation of stable isotope analysis is that it does not provide a detailed description of the diet of consumers (i.e., taxonomy, size), which is typically obtained through the analysis of stomach (Spitz et al., 2011; Jansen et al., 2013) and fecal contents (Ford et al., 2016). However, mass-balanced isotopic models partially address this problem by estimating the dietary composition of a consumer based on the isotopic composition of candidate prey (Moore & Semmens, 2008; Parnell et al., 2013; Stock et al., 2018).
The common bottlenose dolphin Tursiops truncatus (Montagu, 1821) (hereafter, bottlenose dolphin) is undoubtedly among the most studied odontocete species because it is generally abundant in temperate and tropical marine waters around the world (reviewed in Wells & Scott, 2018). Its trophic ecology has been widely studied through several methods (e.g., stomach contents, fatty acids, stable isotopes; Barros & Wells, 1998; Samuel & Worthy, 2004; Mèndez-Fernandez et al., 2012; Kiszka et al., 2014; Bode et al., 2022), yet such studies are unbalanced in favor of coastal habitats where prey type and abundance can differ from those available in pelagic habitats.
Madeira is among the most isolated archipelagos in the North Atlantic, offering a privileged location to study the ecology of pelagic predators in insular habitats. The island lies in a warm temperate latitude, is part of the Macaronesia biogeographical region (Spalding et al., 2007), and is characterized by a narrow continental shelf, steep submarine canyons, and deep waters (Geldmacher et al., 2000). This oceanic island benefits from complex geophysical wake flow such as fronts and eddies that can enhance primary productivity (known as island mass effect phenomena; Caldeira et al., 2002; Couvelard et al., 2012) attracting prey and consumers of all trophic levels (Kaufmann et al., 2015; Friedlander et al., 2017; Alves et al., 2018). Although surrounded by oligotrophic waters, the archipelago hosts rich marine biodiversity comprising several coastal and epipelagic fish species of economic and ecological interest (e.g., mackerel and tuna species; Wirtz et al., 2008; Hermida & Delgado, 2016; Tejerina et al., 2019), as well as a high number of species of megafauna (Ramos et al., 2016; Alves et al., 2018; Freitas et al., 2018; McIvor et al., 2022). The bottlenose dolphin is among Madeira's most frequently encountered cetacean species (Dinis et al., 2016a, b; Alves et al., 2018), where island-associated and transient animals are known to co-occur year-round (Dinis et al., 2016a, 2018; Fernandez et al., 2021). No genetic differentiation was found between animals sampled in Madeira and Azores, suggesting that bottlenose dolphins in Macaronesia belong to a large oceanic population (Quérouil et al., 2007). This is supported by a photographic-identification study showing individual movements between Madeira and the neighboring archipelagos of the Azores and the Canaries (Dinis et al., 2021).
Recently, a study based on nucleic acid-derived indices suggested that bottlenose dolphins occurring in Madeira waters are in good nutritional condition (Alves et al., 2020). Yet, that study provided only a general insight into feeding ecology without any inferences regarding diet. Further, there is no available information on stomach contents from stranded animals in Madeira and only one animal was analyzed in the neighboring Canary Islands (Fernández et al., 2009). Thus, the present study aimed to identify the main groups of prey assimilated by insular bottlenose dolphins from Madeira and investigate potential seasonal differences in their diet. For that, carbon and nitrogen stable isotope ratios were combined with Bayesian mixing models to identify and quantify the groups of prey assimilated by bottlenose dolphins between 2017 and 2018. This will contribute to enlightening the trophic ecology of a worldwide distributed top predator in a poorly studied habitat.
Materials and methods
Data collection
Samples were collected off the south coast of Madeira Island, Portugal (Fig. 1). To investigate seasonal variability in the feeding habits of bottlenose dolphins, tissue samples of wild animals were obtained during autumn (November) 2017 and spring (March and April) 2018 using a biopsy darting system (150-lb crossbow, with arrows and darts specially designed for small cetaceans by Finn Larsen, Ceta-Dart; Mathews et al., 1988). Biopsies were collected by experienced researchers carrying legal permits (see 'Ethics Approval') and targeted the flanks of large and robust animals with no signs of emaciation or carrying calves. Biopsy samples were stored in a liquid nitrogen container on board and kept at – 80 °C before being processed.
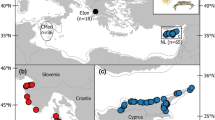
Location of Madeira Archipelago (with the contour of its EEZ—Economic Exclusive Zone in the inset picture) and of biopsied common bottlenose dolphins (Tursiops truncatus) during autumn 2017 and spring 2018 (basemap credits: Esri, Garmin, GEBCO, NOOA NGDC, and other contributors). Small pelagic fishes and squids used in this study were sampled in the same general area where biopsies were taken (i.e., between the coastline and 20 km off the south coast of Madeira Island), whereas tuna were sampled across the Madeira EEZ. Species illustration by E. Berninsone
Bottlenose dolphins are generalist predators feeding on a large variety of pelagic fish and pelagic and demersal squid species (e.g., González et al., 1994; Barros et al., 2000). Therefore, several species (Table S1) of the most abundant demersal and pelagic squids and small schooling fish (Clarke & Lu, 1995; Clarke, 2006; Hermida & Delgado, 2016; Tejerina et al., 2019) were also sampled, because previous studies suggested they will likely prey on the locally available species (Barros & Odell, 1990; Hernandez-Milian et al., 2015). Additionally, two abundant tuna species (Hermida & Delgado, 2016, Table S1) were also considered as potential prey based on personal communication by local tuna fishers, which reported seeing dolphins preying on tunas in this area. Prey specimens were collected in the same general area as dolphins' biopsies (i.e., between the coastline and 20 km off the South coast of Madeira Island), except for tuna that were collected across the Madeira Exclusive Economic Zone (Fig. 1). Fish were collected on board fishing boats between the summer of 2017 and spring of 2018, and squids were bought from fishermen or captured directly from the coastline during 2018 and 2019 (Table S1). All prey samples were kept at – 20 °C before being processed.
Laboratory analyses
For stable isotope analyses (SIA) skin samples from bottlenose dolphins, and muscle (fish) and mantle (squids) samples of their potential prey, were dried in an oven at 60 °C and ground to a fine powder with a mortar and pestle. Stable isotope ratios were measured using a Flash EA 1112 Series elemental analyser coupled online via Finnigan ConFlo III interface to a Thermo delta V S mass spectrometer (Marefoz, University of Coimbra, Portugal). Stable isotope ratios are reported in δ notation, δX = (Rsample/Rstandard − 1) × 103, where X is the C (carbon) or N (nitrogen) stable isotope, and R is the ratio of heavy: light stable isotopes. The δ13C and δ15N are expressed in units per mill (‰) relative to Vienna Pee Dee Belemnite and air, respectively. The analytical precision was better than 0.1‰ for δ13C and 0.3‰ for δ15N. To control for sample processing quality, samples with an SD between replicates (i.e., two sub-samples of the same sample) 0.2‰ δ13C or δ15N were not included in subsequent data analyses.
For sex determination, genomic DNA from bottlenose dolphins was extracted from the skin samples using a standard high-salt protocol as outlined in Sambrook et al. (1989). Single PCR reactions with only one set of primers (thus two PCR reactions per sample) were carried out to amplify both ZFX and SRY gene fragments (Bérube & Palsbøll, 1996) using Phusion Flash High-Fidelity PCR Master Mix (Thermo Scientific™) in 20 μl reactions. The amplification conditions used in this study are detailed in Alves et al. (2020). Several electrophoresis bands from different samples were sequenced to confirm whether the desired genes were amplified. The PCR products were cut from the gel, purified with the NZYGelpure (NZYTech), and sent to direct sequencing (Sanger sequencing) using the light run sequencing service of GATC Biotech. The DNA sequences were analyzed using the BioEdit Sequence Alignment Editor version 7.0.4.1 (Hall, 1999) and aligned against reference sequences from GenBank.
Data analysis
The relative contribution of the most likely prey to the diet of bottlenose dolphins was quantified using the Bayesian stable isotope mixing model MixSIAR v3.1.12 (Stock & Semmens, 2016). The δ13C and δ15N values were adjusted for one trophic level using the trophic fractionation estimates (Δδ13C = 1.01 ± 0.37‰, Δδ15N = 1.57 ± 0.52‰) derived from the most extended feeding experiment available on bottlenose dolphins (350 days; following Giménez et al., 2016).
An exploratory analysis revealed that sex alone did not explain the observed intra-seasonal variability in bottlenose dolphins' stable isotope ratios because models including sex as a fixed factor showed multiplicative errors much greater than one (ε > 3). This suggests that sources could be missing or that important consumer population structure was absent from the model (Stock et al., 2018). Thus, mixing polygon simulations were constructed to check the adequacy of the food sources and trophic fractionation values used for each season (Smith et al., 2013). If consumers fall within the 95% mixing region, that indicates that a mathematical solution can be found that satisfies the geometry of mixing models (Smith et al., 2013). Because no additional information was available on the consumers other than sex (e.g., age or size; although all the sampled dolphins were adults), we performed a hierarchical cluster analysis (linkage—Ward), which is a method of pattern mining (Han et al., 2011), to identify potential grouping in the bottlenose dolphins' stable isotopes by season. For that, the function hclust available in the package stats was used. The best number of clusters was determined using the function NbClust available in the package NbClust (Charrad et al., 2014).
Four models were fitted to the bottlenose dolphins' data: null (i.e., consider that all individuals in the population share the same diet), sex, group, and sex and group. The relative support for each model was evaluated with leave-one-out cross-validation (the best model should have dLOOic = 0), and the corresponding Akaike weights were inspected (Vehtari et al., 2017). To run the models, the stable isotope ratios of bottlenose dolphins and their most likely prey were input as raw data, using non-informative priors. Model convergence was assessed via Gelman–Rubin and Geweke diagnostics (Stock & Semmens, 2016). Posterior distributions obtained from the MixSIAR analyses are expressed as median and 95% credibility intervals.
When dealing with generalist predators that feed on multiple species, a reduced set of prey species or consolidating prey species is necessary due to overlapping δ13C and δ15N values (Jansen et al., 2013). In this case, prey were grouped according to their taxonomic group (fish or squids) and habitat. Thus, the Atlantic chub mackerel (Scomber colias), European pilchard (Sardina pilchardus), sardines (Sardinella spp.), bogue (Boops boops), and slender snipefish (Macroramphosus gracilis) were grouped as 'small pelagic fish A'. The blue jack mackerel (Trachurus picturatus) formed the group 'small pelagic fish B'. This species is commonly observed in Madeira close to the surface (Tejerina et al., 2019), but ranges in depth to at least 370 m (Vasconcelos et al., 2006). Their 13C- and 15N-enriched composition, when compared to those from 'small pelagic fish A', suggest they may obtain their prey from deeper or coastal habitats, or that they feed on prey from higher trophic levels than those individuals comprising the 'small pelagic fish A' group. The skipjack tuna (Katsuwonus pelamis) and juvenile bigeye tuna (Thunnus obesus) were grouped as 'large pelagic fish'. The European squid (Loligo vulgaris) formed the group 'demersal squids', the webbed flying squid (Ommastrephes caroli) formed the group 'pelagic squids A', and the orangeback squid (Sthenoteuthis pteropus) formed the group 'pelagic squids B'. Although the last two squid species occupy similar habitats (Table S1), the difference in their mean δ15N and δ13C values of ca. 3‰ and 0.6‰, respectively, suggests that the orangeback squid occupies a higher trophic position than the webbed flying squid (e.g., Vander Zanden & Rasmussen, 2001). For that reason, they were separated into different prey groups.
Due to a correlation between ‘small pelagic fish A', 'demersal squids', and 'pelagic squids A' stable isotope values, the model could not fully discriminate between those groups. Therefore, we excluded the group 'small pelagic fish A' from the mixing models because previous studies based on stomach content analyses estimated a small contribution of epipelagic fish to the diet of bottlenose dolphins from coastal (Santos et al., 2001, 2007; Hernandez-Milian et al., 2015; Giménez et al., 2017; Milani et al., 2018) and offshore areas (Mead & Potter, 1995).
The most likely prey assimilated by bottlenose dolphins in each season were identified using δ13C and δ15N bi-plots, where bottlenose dolphins' δ13C and δ15N values (after adjusting for trophic fractionation) were compared to prey δ13C and δ15N values. Prey specimens captured in summer and autumn were included in the diet analysis for the 'summer/autumn' season, while those caught in winter and spring were included in the 'winter/spring' season. This approach was used because cetacean skin has a half-life of 30–40 days (Giménez et al., 2016).
All the δ13C values were corrected for lipid content because lipids are depleted in 13C compared to protein and carbohydrates, which usually causes an inverse relationship between the C:N and δ13C values in the muscle tissues of aquatic animals (DeNiro & Epstein, 1977). A correction factor of 1.5‰ was applied to the bottlenose dolphins' δ13C values, following Wilson et al. (2014), because the mean (± SD) C:N was lower than 4.5 (4.2 ± 0.2). Prey tissue values were also corrected for lipid content following the mass balance correction for fish muscle tissue proposed by Hoffman and Sutton (2010, Eq. 6), which uses estimates of C:Nprotein and Δδ13Clipid similar to those from the muscle tissue found in other fish (e.g., Sweeting et al., 2006) and taxonomic groups (e.g., shrimp and zooplankton; Fry & Allen, 2003; Smyntek et al., 2007).
Standard deviation (± SD) was used as a measure of data dispersion when reporting mean values. All the analyses were performed using the open-source statistical language R software (R Core Team 2020).
Results
Thirty-four samples of bottlenose dolphins were analyzed in this study. It included 14 samples (six males and eight females) obtained during seven encounters (one to four samples per encounter) in autumn 2017 and 20 samples (11 males and nine females) during 12 encounters (one to five samples per encounter) in spring 2018 (Table 1).
Hierarchical cluster analysis of bottlenose dolphins' data identified three distinct clusters in the season 'summer/autumn' (Fig. 2). The first branch separates 15N-enriched individuals (group A) from 15N-depleted (groups B and C) individuals: 10.3 ± 0.3‰ (group A), 9.5 ± 0.2‰ (group B), and δ15N: 9.4 ± 0.2‰ (group C). The mean δ13C values of the individuals from groups A, B, and C were − 16.9 ± 0.3‰, − 17.2 ± 0.2‰, and − 16.6 ± 0.3‰, respectively.
Dendrogram showing Ward's hierarchical clustering by season (autumn 2017 and spring 2018) for δ13C and δ15N values of bottlenose dolphins. Each leaf corresponds to the sex of each individual: M—male and F—female. Bottlenose dolphins’ groups obtained with the cluster analysis are represented by capital letters A to E
In 'winter/spring', two clusters were identified, of which group D comprised six individuals and group E, 14 individuals (Fig. 2). The first branch separates 13C- and 15N- enriched individuals (group D; δ15N: 11.0 ± 0.3‰, δ13C: − 16.8 ± 0.5‰) from 13C- and 15N- depleted individuals (group E; δ15N: 9.9 ± 0.4‰, δ13C: − 17.3 ± 0.3‰).
Overall, the δ13C and δ15N values of bottlenose dolphins analyzed for the seasons 'summer/autumn' and 'winter/spring'—after adjusting for trophic fractionation—were intermediate between several potential prey groups, indicating reliance on multiple sources (Fig. 3). The low δ15N values of individuals analyzed for 'summer/autumn' suggest the assimilation of 15N- depleted prey such as demersal squids, pelagic fish, and pelagic squids. The range of δ13C values of bottlenose dolphins also suggests the assimilation of pelagic fish belonging to the group 'small pelagic fish B' (Fig. 3). On the other hand, the individuals analyzed for the season 'winter/spring' showed high variability in their δ15N values, suggesting the assimilation of 15N- enriched prey such as 'large pelagic fish' (Fig. 3).
Mean (± SD) δ13C and δ15N values (‰) of each group of bottlenose dolphins formed by the hierarchical clustering (following Fig. 2), adjusted for one trophic level fractionation (δ13C = 1.01 ± 0.37‰, δ15N = 1.57 ± 0.52‰, Giménez et al. 2016), and their potential prey in 'summer/autumn' (top) and 'winter/spring' (bottom). Bottlenose dolphins' groups (following Fig. 2) in 'summer/autumn' include A (open squares), B (open triangles), and C (open circles), and in 'winter/spring' include D (closed inverted triangles—females, open inverted triangles—males) and E (closed diamonds—females, open diamonds—males). The 'small pelagic fish A' comprises the Atlantic chub mackerel, European pilchard, sardines, bogue, and slender snipefish, whereas the 'small pelagic fish B' is formed by the blue jack mackerel. The 'large pelagic fish' comprises the skipjack and bigeye tuna. The 'demersal squids' is formed by the European squid, the 'pelagic squids A' by the webbed flying squid, and the 'pelagic squids B' by the orangeback squid (see ‘Materials and Methods’ for details)
After correcting for trophic fractionation, bottlenose dolphins' δ13C and δ15N values fell within the simulated mixing polygons calculated with the selected most likely prey groups (Fig. S2). Overall, models predicted that the blue jack mackerel (i.e., 'small pelagic fish B') and squids were the main prey assimilated by the various groups of bottlenose dolphins, although with some differences between seasons (Figs. 4 and 5). No sex-specific differences in the prey contributions were apparent in 'summer/autumn'. The model showing the highest probability of making the best predictions on new data (55%), conditional on the set of models considered, was the model where only group was considered as a factor, followed by the model where group and sex were included as factors (45%). The 'pelagic fish B' was the main prey assimilated by individuals from groups A and C, while 'pelagic squids A' was the most relevant prey for individuals from group B (Fig. 4). On the other hand, for the individuals analyzed for 'winter/spring', the model showing the highest probability of making the best predictions on new data was the model where group and sex were included as factors (85%), followed by the model with group only (15%). Nonetheless, the main differences in the type of prey assimilated were found between groups, whereas males and females differed in the relative contribution of each prey group. Individuals from group D assimilated mostly fish, whereas, for individuals from group E, squids were the most relevant prey (Fig. 5).
Relative contribution of each type of prey to bottlenose dolphins’ groups (A, B, and C, following Fig. 2) during 'summer/autumn' based on the stable isotope mixing models. The types of prey included in the model were ‘demersal squids’ (DSquids), ‘small pelagic fish B’ (PFish B), and ‘pelagic squids A’ (PSquids A). Closed circles indicate the median value and lines indicate the 95% Bayesian credibility intervals
Relative contribution of each type of prey to bottlenose dolphins’ groups (D and E, following Fig. 2) during 'winter/spring' based on the stable isotope mixing models. The types of prey included in the model were ‘demersal squids’ (DSquids), ‘large pelagic fish’ (LpFish), ‘small pelagic fish B’ (PFish B), and ‘pelagic squids A’ (PSquids A). Closed circles indicate the median value and lines indicate the 95% Bayesian credibility intervals
Discussion
Bottlenose dolphins feed on a broad spectrum of fish and squid species due to their generalist feeding behavior, individual specialization, or habitat use (Santos et al., 2001, 2007; Wells & Scott, 2018). Although the diet of offshore populations has been previously analyzed (Mead & Potter, 1990; Walker et al., 1999; Barros et al., 2000), most studies covered animals inhabiting the outer region of the continental shelf (i.e., up to ~ 200 m depth, neritic habitat), which contrast with the present study that sampled dolphins in a truly pelagic environment (i.e., between 500 and 2500 m isobaths, Dinis et al., 2016b; Fernandez et al., 2021). As found in neritic habitats, bottlenose dolphins sampled off the coast of Madeira Island exhibited a generalist feeding behavior preying on pelagic school fish (such as blue jack mackerel) and mesopelagic and demersal squids. This finding reinforces the pelagic dietary composition of insular/oceanic bottlenose dolphins.
This study showed intra-seasonal variability in the stable isotope ratios of bottlenose dolphins, suggesting differences in the relative contribution of assimilated prey. Based on the selected potential prey, it was found that while some groups assimilated mainly squids, others assimilated mostly small, schooling fish. Conspecifics may vary in their diet and space use patterns, leading to niche partitioning within populations (Bolnick et al., 2003; Nicholson et al., 2021). In social species, such as bottlenose dolphins (e.g., Connor et al., 2001; Lusseau & Newman, 2004; Möller et al., 2006), resource partitioning may correspond to population social structure as highly associated individuals occupy the same habitat and encounter the same resources (Semmens et al., 2009; Nicholson et al., 2021). The present study and others (e.g., Barros & Odell, 1990) suggest that sex does not fully explain intrapopulation differences in diet, while others identified differences between the diet of males and females. For instance, on the Israeli coast, it was found that males ate a more diverse diet than females (Sheinin et al., 2014), whereas, in the western Mediterranean, males ate larger but fewer fish than females (Blanco et al., 2001). Off the west coast of Florida (USA), females showed broader foraging habits than males due to habitat specialization (Rossman et al., 2015). Moreover, compared to inshore ecosystems, pelagic environments such as the waters surrounding Madeira lack multi-habitat specificities, and thus other factors are likely contributing to the isotopic differences observed between individuals.
Recently, it was found that individual foraging variation can drive social organization in bottlenose dolphins (Methion & Díaz López, 2019). Based on social network analysis, it was concluded that bottlenose dolphins prefer to affiliate with individuals displaying similar foraging strategies, which likely promotes segregation of the population into behaviourally distinct groups (Methion & Díaz López, 2019). For instance, individuals facing a patchy and irregular prey distribution may benefit from increased cooperation and reduced intragroup competition leading to strong intragroup associations (Methion & Díaz López, 2019). Our sample strategy consisted of sampling only large and robust animals with no signs of emaciation nor carrying calves. However, we lacked associated information about the sampled individuals (e.g., age, residency patterns, feeding tactics) which impairs further conclusions about the role of behavior, or other factors such as metabolic and physiological requirements or habitat use, on their feeding ecology. Notwithstanding it, differences were found between the diet of bottlenose dolphins living in coastal and in offshore/oceanic habitats, with the latter ecotype consuming higher relative proportions of pelagic squids (Mead & Potter, 1995; Hernandez-Milian et al., 2015). Thus, given the observed intra-seasonal variability in the stable isotope values, it is possible that individuals of different residency patterns were analyzed in this study (Dinis et al., 2016a, 2018). Yet, the lack of photographic-identification of the biopsied animals does not allow discussing it, nor the possibility of duplicates influencing the results. To test this hypothesis, further studies should complement photographic-identification with the stable isotope values of the baselines in both coastal and offshore habitats to investigate the origin of the prey assimilated by the bottlenose individuals in Madeira. These findings highlight the need to investigate this topic further, given that the data analysis was consistent with distinct feeding groups within the assemblage sampled and that bottlenose dolphins' feeding strategies may expose them differently to local human threats (e.g., fisheries, pollution).
The main seasonal difference between the resources assimilated by bottlenose dolphins was the relative contribution of pelagic fish, which was higher during 'winter/spring' than during 'summer/autumn'. This increase was driven by the apparent contribution of small tunas to their diet during 'winter/spring', which coincides with the seasonal increase in their availability in Madeira, especially bigeye tuna (Gouveia et al., 2017). To the best of our knowledge, no study reported the consumption of tuna by bottlenose dolphins, suggesting caution when interpreting this result. However, previous studies have identified similar-sized fishes from the family Scombridae (e.g., bonito Sarda spp., little tunny Euthynnus spp., king mackerel Scomberomorus cavalla) in their diet in other regions of the Atlantic (reviewed in Mead & Potter, 1990). This possibility was also considered based on in situ observations made by local fishermen, which reported dolphins preying on skipjack tuna and juveniles from bigeye tuna (fish of 4–5 kg). There is also the possibility that access to this prey may result not from direct attacks but from opportunistic associations with other species that feed on tuna. Nonetheless, we cannot exclude the possibility that individuals sampled during spring could have recently migrated from coastal or other geographic areas to the area where sampling took place or that other 15N- enriched prey are missing in our analyses.
One critical assumption of investigating the trophic ecology of bottlenose dolphins in Madeira was that the seasonal variability in their stable isotope ratios resulted from seasonal differences in the prey assimilated. A previous study conducted with captive bottlenose dolphins estimated half-life turnover rates in skin tissue of 24.16 ± 8.19 days for δ13C and 47.63 ± 19 days for δ15N (Giménez et al., 2016). Such rates enabled us to properly investigate seasonal variations in the dietary habits of this consumer, which has been rarely approached in the trophic ecology of cetaceans (but see Guerra et al., 2020). Also, for modeling purposes, the stable isotope ratios from bogue, European squid, and webbed flying squid collected during 'summer/autumn' were used in the analysis conducted for 'winter/spring'. While inter-seasonal variability is likely to occur in the stable isotopes of these prey, it was expected to be low and similar to that observed in other prey species collected during this study. For example, the mean (± SD) δ13C and δ15N values of the small pelagic European pilchard, which is zooplanktivore (e.g., Costalago & Palomera, 2014; Hure & Mustac, 2020), were similar throughout the studied period (Table S1). The mean (± SD) δ13C and δ15N values of the individuals collected between summer and autumn were − 19.3 ± 0.3‰ and 7.6 ± 0.4‰, respectively, while the mean (± SD) δ13C and δ15N values of those collected between winter and spring were − 18.9 ± 0.4‰ and 7.6 ± 0.4‰, respectively. These prey species are expected to reflect changes occurring at the base of the food web as a result of their feeding behavior. Thus, the minor differences observed in their δ13C and δ15N values suggest that there were no major isotopic changes at the base of the pelagic food web throughout the studied period or that they were not prolonged enough in time to translate into major changes in the stable isotope ratios of the European pilchard. Small changes in the mean (± SD) δ13C and δ15N values between seasons were also observed for epipelagic (i.e., blue jack mackerel, and Atlantic chub mackerel) and other pelagic species (i.e., Sardinella spp.) (Table S1), thus suggesting small temporal changes in the isotopic composition at the base of the food webs they rely upon.
As an overview, this study was the first attempt to analyze bottlenose dolphins' trophic ecology in a truly pelagic environment. We suggest that bottlenose dolphins living off the coast of Madeira assimilated prey of intermediate (i.e., mackerels) and high (i.e., tunas) trophic levels and that their relative contribution varied between seasons. Also, intra-seasonal differences were found in their stable isotope ratios, which suggest intraspecific variability in the feeding behavior among individuals living in the same area, although the origin for that variability remains unknown. Future studies should include coastal and other demersal prey species and additional information on individuals (e.g., age, habitat use, physiological condition) and groups of individuals (social organization) to identify the main factors responsible for the diet's intrapopulation variability observed in Madeira. Moreover, DNA analysis on stomach contents from stranded bottlenose dolphins or biologgers equipped with cameras to observe their feeding behavior in situ, would allow obtaining detailed information about their prey, especially to disclose if they prey on tunas. Finally, this study suggests that bottlenose dolphins assimilated small pelagic fish that are among the most commercially important captured species in Madeira (Hermida & Delgado, 2016; Tejerina et al., 2019). This outcome highlights the need for developing monitoring programs to evaluate their interaction with fisheries and the potential to be caught due to their feeding activities.
Data availability
Available as Electronic Supplementary Material S3.
References
Alves, F., R. Ferreira, M. Fernandes, Z. Halicka, L. Dias & A. Dinis, 2018. Analysis of occurrence patterns and biological factors of cetaceans based on long-term and fine-scale data from platforms of opportunity: Madeira Island as a case study. Marine Ecology 39: e12499. https://doi.org/10.1111/maec.12499.
Alves, F., M. Dromby, V. Baptista, R. Ferreira, A. M. Correia, M. Weyn, R. Valente, E. Froufe, M. Rosso, I. Sousa-Pinto, A. Dinis, E. Dias & M. A. Teodósio, 2020. Ecophysiological traits of highly mobile large marine predators inferred from nucleic acid derived indices. Scientific Reports 10: 4752. https://doi.org/10.1038/s41598-020-61769-7.
Aurioles-Gamboa, D., M. Y. Rodríguez-Pérez, L. Sánchez-Velasco & M. F. Lavín, 2013. Habitat, trophic level, and residence of marine mammals in the Gulf of California assessed by stable isotope analysis. Marine Ecology Progress Series 488: 275–290. https://doi.org/10.3354/meps10369.
Barros, N. B. & D. K. Odell, 1990. Food habits of bottlenose dolphins in the Southeastern United States. In Leatherwood, S. & R. R. Reeves (eds), The Bottlenose Dolphin Academic Press, San Diego: 309–328.
Barros, N. B. & R. S. Wells, 1998. Prey and feeding patterns of resident bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Journal of Mammalogy 79: 1045–1059. https://doi.org/10.2307/1383114.
Barros, N. B., E. C. M. Parsons & T. A. Jefferson, 2000. Prey of offshore bottlenose dolphins from the South China Sea. Aquatic Mammal 26: 2–6.
Bérube, M. & P. Palsbøll, 1996. Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Molecular Ecology 5: 283–287. https://doi.org/10.1111/j.1365-294x.1996.tb00315.x.
Blanco, C., O. Salomón & J. A. Raga, 2001. Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 81: 1053–1058. https://doi.org/10.1017/S0025315401005057.
Bode, A., C. Saavedra, M. Álvarez-González, M. Arregui, M. Arbelo, A. Fernández, L. Freitas, M. A. Silva, R. Prieto, J. M. N. Azevedo, J. Giménez, G. J. Pierce & M. B. Santos, 2022. Trophic position of dolphins tracks recent changes in the pelagic ecosystem of the Macaronesian region (NE Atlantic). Marine Ecology Progress Series 699: 167–180. https://doi.org/10.3354/meps14176.
Bolnick, D. I., R. Svanbäck, J. A. Fordyce, L. H. Yang, J. M. Davis, C. D. Hulsey & M. L. Forister, 2003. The ecology of individuals: incidence and implications of individual specialization. American Naturalist 161: 1–28. https://doi.org/10.1086/343878.
Bolnick, D. I., P. Amarasekare, M. Araújo, R. Bürger, J. Levine, M. Novak, V. Rudolf, S. Schreiber, M. Urban & D. Vasseur, 2011. Why intraspecific trait variation matters in community ecology. Trends in Ecology & Evolution 26: 183–192. https://doi.org/10.1016/j.tree.2011.01.009.
Bowen, W. D., 1997. Role of marine mammals in aquatic ecosystems. Marine Ecology Progress Series 158: 267–274. https://doi.org/10.3354/meps158267.
Caldeira, R. M. A., S. Groom, P. Miller, D. Pilgrim & N. Nezlin, 2002. Sea-surface signatures of the island mass effect phenomena around Madeira Island, Northeast Atlantic. Remote Sensing of Environment 80: 336–360. https://doi.org/10.1016/S0034-4257(01)00316-9.
Caut, S., E. Angulo & F. Courchamp, 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453. https://doi.org/10.1111/j.1365-2664.2009.01620.x.
Charrad, M., N. Ghazzali, V. Boiteau & A. Niknafs, 2014. NbClust: an R package for determining the relevant number of clusters in a data set. Journal of Statistical Software 61: 1–36.
Clarke, M. R., 2006. Oceanic cephalopod distribution and species diversity in the eastern north Atlantic. Arquipélago, Life and Marine Sciences 23A: 27–46.
Clarke, M. R. & C. C. Lu, 1995. Cephalopoda of Madeiran waters. Boletim Do Museu Municipal Do Funchal 4: 181–200.
Connor, R. C., M. R. Heithaus & L. M. Barre, 2001. Complex social structure, alliance stability and mating access in a bottlenose dolphin “Super-Alliance.” Proceedings: Biological Sciences 268: 263–267.
Costalago, D. & I. Palomera, 2014. Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: from late larvae to adults. Sci Mar 78: 41–54. https://doi.org/10.3989/scimar.03898.06D.
Couvelard, X., R. M. A. Caldeira, I. B. Araújo & R. Tomé, 2012. Wind mediated corticity-generation and eddy-confinement, leeward of the Madeira Island: 2008 numerical case study. Dynamics of Atmospheres and Oceans 58: 128–149. https://doi.org/10.1016/j.dynatmoce.2012.09.005.
DeNiro, M. J. & S. Epstein, 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197: 261–263. https://doi.org/10.1126/science.327543.
Dinis, A., F. Alves, C. Nicolau, C. Ribeiro, M. Kaufmann, A. Cañadas & L. Freitas, 2016a. Bottlenose dolphin Tursiops truncatus group dynamics, site fidelity, residency and movement patterns in the Madeira Archipelago (North-East Atlantic). African Journal of Marine Science 38: 151–160. https://doi.org/10.2989/1814232X.2016.1167780.
Dinis, A., A. Carvalho, F. Alves, C. Nicolau, C. Ribeiro, M. Kaufmann, A. Cañadas & L. Freitas, 2016b. Spatial and temporal distribution of bottlenose dolphins, Tursiops truncatus, in the Madeira archipelago, NE Atlantic. Arquipelago, Life and Marine Sciences 33: 45–54.
Dinis, A., F. Alves, C. Nicolau, C. Ribeiro, M. Kaufmann, A. Cañadas & L. Freitas, 2018. Social structure of a population of bottlenose dolphins (Tursiops truncatus) in the oceanic archipelago of Madeira, Portugal. Journal of the Marine Biological Association of the United Kingdom 98: 1141–1149. https://doi.org/10.1017/S0025315417000650.
Dinis, A., C. Molina, M. Tobeña, A. Sambolino, K. Hartman, M. Fernandez, S. Magalhães, R. P. dos Santos, F. Ritter, V. Martín, N. Aguilar de Soto & F. Alves, 2021. Large-scale movements of common bottlenose dolphins in the Atlantic: dolphins with an international courtyard. PeerJ 9: e11069. https://doi.org/10.7717/peerj.11069.
Fernández, R., M. B. Santos, M. Carrillo, M. Tejedor & G. J. Pierce, 2009. Stomach contents of cetaceans stranded in the Canary Islands 1996–2006. Journal of the Marine Biological Association of the United Kingdom 89: 873–883. https://doi.org/10.1017/S0025315409000290.
Fernández, R., S. García-Tiscar, M. B. Santos, A. López, J. A. Martínez-Cedeira, J. Newton & G. J. Pierce, 2011. Stable isotope analysis in two sympatric populations of bottlenose dolphins Tursiops truncatus: evidence of resource partitioning? Marine Biology 158: 1043–1055. https://doi.org/10.1007/s00227-011-1629-3.
Fernandez, M., F. Alves, R. Ferreira, J.-C. Fischer, P. Thake, N. Nunes, R. Caldeira & A. Dinis, 2021. Modeling fine-scale cetaceans’ distributions in oceanic islands: Madeira Archipelago as a case study. Frontiers in Marine Science 8: 688248. https://doi.org/10.3389/fmars.2021.688248.
Fernández-Álvarez, F. Á., H. E. Braid, C. M. Nigmatullin, K. S. R. Bolstad, M. Haimovici, P. Sánchez, K. K. Sajikumar, N. Ragesh & R. Villanueva, 2020. Global biodiversity of the genus Ommastrephes (Ommastrephidae: Cephalopoda): an allopatric cryptic species complex. Zoological Journal of the Linnean Society 190: 460–482. https://doi.org/10.1093/zoolinnean/zlaa014.
Ford, M. J., J. Hempelmann, M. B. Hanson, K. L. Ayres, R. W. Baird, C. K. Emmons, J. I. Lundin, G. S. Schorr, S. K. Wasser & L. K. Park, 2016. Estimation of a Killer Whale (Orcinus orca) population’s diet using sequencing analysis of DNA from Feces. PLoS ONE 11: e0144956. https://doi.org/10.1371/journal.pone.0144956.
Freitas, C., R. Caldeira, J. Reis & T. Dellinger, 2018. Foraging behaviour of juvenile loggerhead sea turtles in the open ocean: from Lévy exploration to area-restricted search. Marine Ecology Progress Series 595: 203–215.
Friedlander, A. M., E. Ballesteros, S. Clemente, E. J. Gonçalves, A. Estep, P. Rose & E. Sala, 2017. Contrasts in the marine ecosystem of two Macaronesian islands: A comparison between the remote Selvagens Reserve and Madeira Island. PLoS ONE 12: e0187935. https://doi.org/10.1371/journal.pone.0187935.
Fry, B. & Y. C. Allen, 2003. Stable isotopes in zebra mussels as bioindicators of river–watershed linkages. River Research and Applications 19: 683–696. https://doi.org/10.1002/rra.715.
Geldmacher, J., P. Van Den Bogaard, K. Hoernle & H. U. Schmincke, 2000. The 40Ar/39Ar age dating of the Madeira Archipelago and hotspot track (eastern North Atlantic). Geochem Geophy Geosy 1: 1999GC000018. https://doi.org/10.1029/1999GC000018.
Giménez, J., F. Ramirez, J. Almunia, M. G. Forero & R. Stephanis, 2016. From the pool to the sea: applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). Journal of Experimental Marine Biology and Ecology 475: 54–61. https://doi.org/10.1016/j.jembe.2015.11.001.
Giménez, J., A. Marçalo, F. Ramírez, P. Verborgh, P. Gauffier, R. Esteban, L. Nicolau, E. González-Ortegón, F. Baldó, C. Vilas, J. Vingada, M. G. Forero & R. de Stephanis, 2017. Diet of bottlenose dolphins (Tursiops truncatus) from the Gulf of Cadiz: Insights from stomach content and stable isotope analyses. PLoS ONE 12: e0184673. https://doi.org/10.1371/journal.pone.0184673.
González, A. F., A. López, A. Guerra & A. Barreiro, 1994. Diets of marine mammals stranded on the northwestern Spanish Atlantic coast with special reference to Cephalopoda. Fisheries Research 21: 179–191. https://doi.org/10.1016/0165-7836(94)90103-1.
Gouveia, L., A. Amorim, A. Alves & M. Hermida, 2017. Updated fishery statistics for bigeye, skipjack and albacore tunas from Madeira Archipelago. Collective Volume of Scientific Papers 73: 1547–1560.
Guerra, M., L. Wing, S. Dawson & W. Rayment, 2020. Stable isotope analyses reveal seasonal and inter-individual variation in the foraging ecology of sperm whales. Marine Ecology Progress Series 638: 207–219. https://doi.org/10.3354/meps13255.
Gulka, J., P. C. Carvalho, E. Jenkins, K. Johnson, L. Maynard & G. K. Davoren, 2017. Dietary niche shifts of multiple marine predators under varying prey availability on the Northeast Newfoundland coast. Frontiers in Marine Science 4: 324. https://doi.org/10.3389/fmars.2017.00324.
Hall, T. A., 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT. Nucleic Acids Symposium Series 41: 9598.
Han, J., M. Kamber & J. Pei, 2011. Data Mining: Concepts and Techniques, Elsevier, New York:
Hays, G. C., L. C. Ferreira, A. M. M. Sequeira, et al., 2016. Key questions in marine megafauna movement ecology. Trends in Ecology & Evolution 31: 463–475. https://doi.org/10.1016/j.tree.2016.02.015.
Hermida, M. & J. Delgado, 2016. High trophic level and low diversity: would Madeira benefit from fishing down? Marine Policy 73: 130–137. https://doi.org/10.1016/j.marpol.2016.07.013.
Hernandez-Milian, G., S. Berrow, M. B. Santos, D. Reid & E. Rogan, 2015. Insights into the trophic ecology of bottlenose dolphins (Tursiops truncatus) in Irish Waters. Aquatic Mammal 41: 226–239. https://doi.org/10.1578/AM.41.2.2015.226.
Hobson, K. A., D. M. Schell, D. Renouf & E. Noseworthy, 1996. Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Canadian Journal of Fisheries and Aquatic Sciences 53: 528–533. https://doi.org/10.1139/f95-209.
Hoffman, J. C. & T. Sutton, 2010. Lipid correction for carbon stable isotope analysis of deep-sea fishes. Deep Sea Research I 57: 956–964. https://doi.org/10.1016/j.dsr.2010.05.003.
Hure, M. & B. Mustac, 2020. Feeding ecology of Sardina pilchardus considering co-occurring small pelagic fish in the eastern Adriatic Sea. Marine Biodiversity 50: 40. https://doi.org/10.1007/s12526-020-01067-7.
Jansen, O. E., L. Michel, G. Lepoint, K. Das, A. S. Couperus & P. J. Reijnders, 2013. Diet of harbor porpoises along the Dutch coast: a combined stable isotope and stomach contents approach. Marine Mammal Science 29: E295–E311. https://doi.org/10.1111/j.1748-7692.2012.00621.x.
Kaufmann, M. J., F. Santos & M. Maranhão, 2015. Checklist of nanno- and microphytoplankton off Madeira Island (Northeast Atlantic) with some historical notes. Nova Hedwigia 101: 205–232. https://doi.org/10.1127/nova_hedwigia/2015/0265.
Kiszka, J. J., P. Méndez-Fernandez, M. R. Heithaus & V. Ridoux, 2014. The foraging ecology of coastal bottlenose dolphins based on stable isotope mixing models and behavioural sampling. Marine Biology 161: 953–961. https://doi.org/10.1007/s00227-014-2395-9.
Lusseau, D. & M. E. J. Newman, 2004. Identifying the role that animals play in their social networks. Proceedings of the Royal Society of London. Series b: Biological Sciences 271: S477–S481. https://doi.org/10.1098/rsbl.2004.0225.
Mathews, E. A., S. Keller & D. B. Weiner, 1988. A method to collect and process skin biopsies for cell culture from the free-ranging gray whales (Eschrichtius robustus). Marine Mammal Science 4: 1–12. https://doi.org/10.1111/j.1748-7692.1988.tb00178.x.
Maxwell, S., E. Hazen, S. Bograd, et al., 2013. Cumulative human impacts on marine predators. Nature Communications 4: 2688. https://doi.org/10.1038/ncomms3688.
McIvor, A. J., C. T. Williams, F. Alves, A. Dinis, M. P. Pais & J. Canning-Clode, 2022. The status of marine megafauna research in Macaronesia: a systematic review. Frontiers in Marine Science 9: 819581. https://doi.org/10.3389/fmars.2022.819581.
Mead, J. G. & C. W. Potter, 1990. Natural history of bottlenose dolphins along the central Atlantic coast of the United States. In Leatherwood, S. & R. Reeves (eds), The Bottlenose Dolphin Academic Press, San Diego: 129–139.
Mead, J. G. & C. W. Potter, 1995. Recognizing two populations of the bottlenose dolphin (Tursiops truncatus) off the Atlantic coast of North America: Morphologic and ecologic considerations. IBI Reports (internship Marine Biology Research Institute, Kamogawa, Japan) 5: 31–44.
Mèndez-Fernandez, P., P. Bustamante, A. Bode, T. Chouvelon, M. Ferreira, A. López, G. J. Pierce, M. B. Santos, J. Spitz, J. V. Vingada & F. Caurant, 2012. Foraging ecology of five toothed whale species in the Northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. Journal of Experimental Marine Biology and Ecology 413: 150–158. https://doi.org/10.1016/j.jembe.2011.12.007.
Methion, S. & B. Díaz López, 2019. Natural and anthropogenic drivers of foraging behaviour in bottlenose dolphins: influence of shellfish aquaculture. Aquatic Conservation 29: 927–937. https://doi.org/10.1002/aqc.3116.
Milani, C. B., A. Vella, P. Vidoris, A. Christidis, E. Koutrakis, A. Frantzis, A. Miliou & A. Kallianiotis, 2018. Cetacean stranding and diet analyses in the North Aegean Sea (Greece). Journal of the Marine Biological Association of the United Kingdom 98: 1011–1028. https://doi.org/10.1017/S0025315417000339.
Möller, L. M., L. B. Beheregaray, S. J. Allen & R. G. Harcourt, 2006. Association patterns and kinship in female Indo-Pacific bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Behavioral Ecology and Sociobiology 61: 109–117. https://doi.org/10.1007/s00265-006-0241-x.
Moore, J. W. & B. X. Semmens, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11: 470–480. https://doi.org/10.1111/j.1461-0248.2008.01163.x.
Nicholson, K., L. Bejder & N. Loneragan, 2021. Niche partitioning among social clusters of a resident estuarine apex predator. Behavioral Ecology and Sociobiology 75: 160. https://doi.org/10.1007/s00265-021-03091-4.
O’Toole, M. D., M. A. Lea, C. Guinet, R. Schick & M. A. Hindell, 2015. Foraging strategy switch of a top marine predator according to seasonal resource differences. Frontiers in Marine Science 2: 21. https://doi.org/10.3389/fmars.2015.00021.
Parnell, A. C., D. L. Phillips, S. Bearhop, B. X. Semmens, E. J. Ward, J. W. Moore, et al., 2013. Bayesian stable isotope mixing models. Environmetrics 14: 387–399. https://doi.org/10.1002/env.2221.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology, Evolution, and Systematics 18: 293–320.
Prato, G., P. Guidetti, F. Bartolini, L. Mangialajo & P. Francour, 2013. The importance of high-level predators in marine protected area management: consequences of their decline and their potential recovery in the Mediterranean context. Advances in Oceanography and Limnology 4: 176–193. https://doi.org/10.1080/19475721.2013.841754.
Quérouil, S., M. A. Silva, L. Freitas, R. Prieto, S. Magalhães, A. Dinis, F. Alves, J. A. Matos, D. Mendonça, P. Hammond & R. S. Santos, 2007. High gene flow in oceanic bottlenose dolphins (Tursiops truncatus) of the North Atlantic. Conservation Genetics 8: 1405–1419. https://doi.org/10.1007/s10592-007-9291-5.
Quérouil, S., J. Kiszka, A. R. Cordeiro, I. Cascão, L. Freitas, A. Dinis, F. Alves, R. S. Santos & N. M. Bandarra, 2013. Investigating stock structure and trophic relationships among island-associated dolphins in the oceanic waters of the North Atlantic using fatty acid and stable isotope analyses. Marine Biology 160: 1325–1337. https://doi.org/10.1007/s00227-013-2184-x.
R Development Core Team, 2020. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Ramos, R., I. Ramírez, V. H. Paiva, T. Militão, M. Biscoito, D. Menezes, R. A. Phillips, F. Zino & J. González-Solís, 2016. Global spatial ecology of three closely-related gadfly petrels. Scientific Reports 6: 23447. https://doi.org/10.1038/srep23447.
Rossman, S., E. Berens McCabe, N. B. Barros, H. Gandhi, P. H. Ostrom, C. A. Stricker & R. S. Wells, 2015. Foraging habits in a generalist predator: sex and age influence habitat selection and resource use among bottlenose dolphins (Tursiops truncatus). Marine Mammal Science 31: 155–168. https://doi.org/10.1111/mms.12143.
Sambrook, J., E. F. Fritsch & T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual, Cold Harbor Spring Press, New York:
Samuel, A. M. & G. A. J. Worthy, 2004. Variability in fatty acid composition of bottlenose dolphin (Tursiops truncatus) blubber as a function of body site, season, and reproductive state. Canadian Journal of Zoology 82: 1933–1942. https://doi.org/10.1139/z05-001.
Santos, M. B., G. J. Pierce, R. J. Reid, L. A. P. Patterson, H. M. Ross & E. Mente, 2001. Stomach contents of bottlenose dolphins (Tursiops truncatus) in Scottish waters. Journal of the Marine Biological Association of the United Kingdom 81: 873–878. https://doi.org/10.1017/S0025315401004714.
Santos, M. B., R. Fernandez, A. López, J. A. Martínez & G. J. Pierce, 2007. Variability in the diet of bottlenose dolphin, Tursiops truncatus, in Galician waters, north-western Spain, 1990–2005. Journal of the Marine Biological Association of the United Kingdom 87(2): 31–241. https://doi.org/10.1017/S0025315407055233.
Semmens, B. X., E. J. Ward, J. W. Moore & C. T. Darimont, 2009. Quantifying inter-and intra-population niche variability using hierarchical Bayesian stable isotope mixing models. PLoS ONE 4: e6187. https://doi.org/10.1371/journal.pone.0006187.
Sheinin, A. P., D. Kerem, S. Lojen, J. Liberzon & E. Spanier, 2014. Resource partitioning between common bottlenose dolphin (Tursiops truncatus) and the Israeli bottom trawl fishery? Assessment by stomach contents and tissue stable isotopes analysis. Journal of the Marine Biological Association of the United Kingdom 94: 1203–1220. https://doi.org/10.1017/S0025315414001015.
Smith, J. A., D. Mazumder, I. M. Suthers & M. D. Taylor, 2013. To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods in Ecology and Evolution 4: 612–618. https://doi.org/10.1111/2041-210X.12048.
Smyntek, P. M., M. A. Teece, K. L. Schulz & S. J. Thackeray, 2007. A standard protocol for stable isotope analysis of zooplankton in aquatic food web research using mass balance correction models. Limnology and Oceanography 52: 2135–2146. https://doi.org/10.4319/lo.2007.52.5.2135.
Spalding, M. D., H. E. Fox, G. R. Allen & N. C. Davidson, 2007. Marine ecoregions of the world: a bioregionalisation of coastal and shelf areas. BioScience 57: 573–583. https://doi.org/10.1641/B570707.
Spitz, J., Y. Cherel, S. Bertin, J. Kiszka, A. Dewez & V. Ridoux, 2011. Prey preferences among the community of deep-diving odontocetes from the Bay of Biscay, Northeast Atlantic. Deep Sea Research 58: 273–282. https://doi.org/10.1016/j.dsr.2010.12.009.
Stock, B. C. & B. X. Semmens, 2016. Unifying error structures in commonly used biotracer mixing models. Ecology 97: 2562–2569. https://doi.org/10.1002/ecy.1517.
Stock, B. C., A. L. Jackson, E. J. Ward, A. C. Parnell, D. L. Phillips & B. X. Semmens, 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6: e5096. https://doi.org/10.7717/peerj.5096.
Sweeting, C. J., N. V. C. Polunin & S. Jennings, 2006. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Communications in Mass Spectrometry 20: 595–601. https://doi.org/10.1002/rcm.2347.
Tejerina, R., M. Hermida, G. Faria & J. Delgado, 2019. The purse-seine fishery for small pelagic fishes off the Madeira Archipelago. African Journal of Marine Science 41: 373–383. https://doi.org/10.2989/1814232X.2019.1678520.
Vander Zanden, M. J. & J. B. Rasmussen, 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46: 2061–2066. https://doi.org/10.4319/lo.2001.46.8.2061.
Vasconcelos, J., A. Alves, E. Gouveia & G. Faria, 2006. Age and growth of the blue jack mackerel, Trachurus picturatus Bowdich, 1825 (Pisces: Teleostei) off the Madeira archipelago. Arquipélago-Life and Marine Sciences 23A: 47–57.
Vehtari, A., A. Gelman & J. Gabry, 2017. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing 27: 1413–1432. https://doi.org/10.1007/s11222-016-9696-4.
Walker, J. L., C. W. Potter & S. A. Macko, 1999. The diet of modern and historic bottlenose dolphin populations reflected through stable isotopes. Marine Mammal Science 15: 335–350. https://doi.org/10.1111/j.1748-7692.1999.tb00805.x.
Wells, R. S. & M. D. Scott, 2018. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Würsig, B., J. G. M. Thewissen & K. M. Kovacs (eds), Encyclopedia of Marine Mammals 3rd ed. Academic Press, New York: 118–125.
Wilson, R. M., J. P. Chanton, B. C. Balmer & D. P. Nowacek, 2014. An evaluation of lipid extraction techniques for interpretation of carbon and nitrogen isotope values in bottlenose dolphin (Tursiops truncatus) skin tissue. Marine Mammal Science 30: 85–103. https://doi.org/10.1111/mms.12018.
Wirtz, P., R. Fricke & M. J. Biscoito, 2008. The coastal fishes of Madeira Island new records and an annotated check-list. Zootaxa 1715: 1–26. https://doi.org/10.11646/zootaxa.1715.1.1.
Young, H., K. Nigro, D. J. McCauley, L. T. Balance, E. M. Oleson & S. Baumann-Pickering, 2017. Limited trophic partitioning among sympatric delphinids off a tropical oceanic atoll. PLoS ONE 12: e0181526. https://doi.org/10.1371/journal.pone.0181526.
Acknowledgements
To Mafalda Correia (CIIMAR), Maria Paola Tomasino (CIIMAR), Annalisa Sambolino (MARE), and Roi Martínez-Escauriaza (OOM) for helping with data collection, to Margarida Hermida for helping with data collection and revision of the manuscript, to Marisa Fernandes (University of Madeira), Nereida Cordeiro (University of Madeira) and Graça Faria (Direção Regional de Pescas da Madeira) for laboratory facilities, to Thomas Dellinger (University of Madeira) for logistic support, to Raúl Valente (CIIMAR), Elsa Froufe (CIIMAR) and Manuela Gouveia (University of Madeira) for helping with genetic analysis, to Gustavo Silva (OOM) for help with the creation of the map, and to Alejandro Escánez Pérez (MARE) for helping with squid species identification.
Funding
Open access funding provided by FCT|FCCN (b-on). This study is a result of the project MARCET (MAC/1.1b/149) supported by MAC 2014–2020 program under the Interreg fund and of the project MarInfo (NORTE-01-0145-FEDER-000031) supported by NORTE 2020 under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund (ERDF). This study had the support of the Oceanic Observatory of Madeira throughout the project M1420-01-0145-FEDER-000001-OOM, and of the Portuguese Foundation for Science and Technology (FCT) throughout the strategic projects UIDB/04292/2020 granted to MARE, LA/P/0069/2020 granted to the Associate Laboratory ARNET, UID/Multi/04326/2020 granted to CCMAR, and UIDB/04423/2020 and UIDP/04423/2020 granted to CIIMAR. R.F. and A.G. were partially supported by the FCT grants SFRH/BD/147225/2019 and PD/BD/150603/2020, respectively. A.D. was funded by ARDITI throughout the project M1420-09-5369-FSE-000002.
Author information
Authors and Affiliations
Contributions
FA, AD, MAT, and ED conceived the study, FA, AD, MAT, and IS-P obtained funding for data collection and laboratory analyses, MD, RF, ÁG, RT, MR, AD, and FA collected the data, MD, JCH, and ED analyzed the data, LFCC performed genetic sexing, MD, ED, and FA drafted the manuscript, and all authors critically reviewed the manuscript and approved the version for publication.
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Ethical approval
Biopsies were obtained following the relevant guidelines and regulations imposed by Instituto de Florestas e Conservação da Natureza IP-RAM under sampling permits 308 1.856/2017, 508/2018, and 10661/2018.
Additional information
Handling editor: M. Grazia Pennino
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dias, E., Dromby, M., Ferreira, R. et al. Trophic ecology of common bottlenose dolphins in a pelagic insular environment inferred by stable isotopes. Hydrobiologia 850, 4227–4241 (2023). https://doi.org/10.1007/s10750-023-05294-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05294-4