Abstract
Detailed knowledge on habitat use by marine mammals is critical to understand their role in the ecosystem. The stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) have been widely used to study the trophic ecology of marine mammals, but the stable isotope ratios of other elements such as sulfur (δ34S) and oxygen (δ18O) can better inform about habitat use in areas with strong salinity and redox gradients. The Río de la Plata estuary represents the largest freshwater runoff in the south-western Atlantic Ocean and supports a rich community of marine mammals. Here, we analyzed δ34S values in bone from seven marine mammal species inhabiting the estuary and the adjacent Atlantic Ocean, in order to complement previous isotopic data (δ13C, δ15N and δ18O) and compare their resolution as habitat tracers. As expected, δ34S and δ18O offered relevant insights into the characterization of the habitat used by marine mammals and allowed a better delineation of habitat partitioning between them. Bottlenose dolphins, South American sea lions and South American fur seals seem to be frequent users of the less saline areas of the estuary, whereas Burmeister´s porpoises, franciscana dolphins and false killer whales seemed to prefer the saltier marine waters close to the bottom. Fraser´s dolphins were the only inhabitants of true offshore waters. Our findings demonstrate how the integration of different stable isotope ratios can help disentangle fine habitat partitioning between marine mammals living in a complex ecosystem such as Río de la Plata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge on the habitat use of the different marine mammal species is key to understand their ecological function in an ecosystem, to evaluate direct and indirect interactions with anthropogenic activities, and to ensure a proper management of the marine biodiversity (Barlow 2018; Roman and Ester 2018). Río de la Plata is one of the largest estuaries in South America. It not only has a relevant role in the regional circulation pattern, but also supports a rich community of marine mammals of disparate origins (Guerrero et al. 1997; Miloslavich et al. 2011). The franciscana dolphin (Pontoporia blainvillei) is an endemic species to Río de la Plata and adjoining regions in the southwestern Atlantic Ocean, whereas the South American sea lion (Otaria flavescens), the South American fur seal (Arctocephalus australis), and the Burmeister’s porpoise (Phocoena spinipinnis) are widespread in the temperate and cold regions of South America, from southern Brazil to Peru. On the other hand, the Fraser’s dolphin (Lagenodelphis hosei), the bottlenose dolphin (Tursiops truncatus), and the false killer whale (Pseudorca crassidens) have circumtropical distributions, although the latter two also occur in temperate regions, and the false killer whale may even reach the sub-Antarctic tip of South America (Reeves et al. 2002).
Species such as bottlenose dolphins, South American sea lions, and fur seals have been intensively studied, and there is no doubt that they make an extended use of the Río de la Plata estuary, although sea lions and fur seals may also forage in adjoining coastal waters (Wells and Scott 2018; Cárdenas-Alayza 2018a; Drago et al. 2021). On the contrary, the Fraser’s dolphin is a poorly studied species, although the scarce information available suggests an oceanic niche (Dolar 2018; Drago et al. 2021). Moreover, the habitat preferences of false killer whales, franciscana dolphins and Burmeister’s porpoises in the region, are not completely understood, because contradictory information has been reported in the literature about their distribution in the estuary, and no good model of habitat partitioning exists to date in the regions where they coexist (Baird 2018; Cárdenas-Alayza 2018b; Crespo 2018; Reyes 2018; Drago et al. 2020, 2021).
Detailed information on the habitat use of marine mammals is difficult to collect, because direct observations are often challenging, and satellite tracking is of limited utility for the study of small cetaceans (Balmer et al. 2014). This is why the stable isotopes of several chemical elements have been used increasingly since the 1970s as intrinsic markers to study the trophic ecology and habitat use patterns of marine mammals (Newsome et al. 2010; Ramos and González-Solís 2012).
The stable isotopes of carbon (C) and nitrogen (N) are the most widely used elements in studies related to the trophic ecology of marine mammals, but the interest on the stable isotopes of sulfur (S) and oxygen (O) has increased recently (Rubenstein and Hobson 2004; Newsome et al. 2010; Ramos and González-Solís 2012; Drago et al. 2020; Borrell et al. 2021). The C stable isotope ratio (13C/12C; δ13C) is informative about the primary source of carbon in a specific food web, mostly due to a different discrimination against the heavier 13C isotope during photosynthesis between primary producers (Peterson and Howarth 1987; Michener and Lajtha 2007). In general, the highest δ13C values are observed in species with inshore benthic habits, and the lowest in offshore epipelagic consumers (Rubenstein and Hobson 2004; Newsome et al. 2010). On the other hand, the N stable isotope ratio (15N/14N; δ15N) increases consistently along the food web due to the trophic enrichment caused by the preferential use and excretion of the light 14N isotope, providing a convenient and simple method to assess the trophic position of the species (Post 2002).
Although popular as proxies for diet reconstruction, the use of δ13C and δ15N as habitat tracers can be hindered by geographic shifts in diet and the isotopic baseline (Rubenstein and Hobson 2004; Michener and Lajtha 2007; Newsome et al. 2010). For this reason, the use of the S stable isotope ratio (34S/32S; δ34S) as a habitat tracer in marine mammals has increased in the last decade, often in combination with δ13C and δ15N (Croisetière et al. 2009; Pinzone et al. 2019; Borrell et al. 2021). Differences in δ34S values are caused by the variability in sources of inorganic sulfur available to primary producers, with little to no trophic discrimination (Peterson et al. 1985). In estuarine food webs, both terrestrial plants and aquatic primary producers using anoxic sediments with intense sulfate reduction (i.e., seagrasses and marsh plants) have lower δ34S values than marine phytoplankton and benthic macroalgae using 34S-enriched sulfates from the water-column (Peterson et al. 1985; Peterson 1999; Croisetière et al. 2009). As fractionation during uptake and assimilation is minimal (Peterson et al. 1985), the δ34S values in animal tissues reflect their food sources and allow to position them along the redox gradient existing from reduced, anoxic sediments found often inshore and in benthic regions, to the more oxidizing conditions found within the water column in offshore and pelagic regions (Peterson et al. 1985; Rubenstein and Hobson 2004; Ramos and González-Solís 2012).
On the contrary, the O stable isotope ratio (18O/16O; δ18O) has been rarely used in ecological studies on marine mammals, despite being relatively common in other research areas such as paleontology (Seyboth et al. 2018; Newsome et al. 2010). The δ18O values in the marine environment are positively and linearly correlated with the salinity of the water (Gat 1996; Conroy et al. 2014), and therefore, it can be a useful habitat tracer in areas where an abundant freshwater input creates marked horizontal and vertical salinity gradients (Guerrero et al. 1997; Conroy et al. 2014; Belem et al. 2019). The δ18O values in animal tissues often reflect with great precision those of the body of water where they feed (Yoshida and Miyazaki 1991; Ben-David and Flaherty 2012), allowing to discriminate between species with estuarine, coastal, and marine habits (Clementz and Koch 2001; Rubenstein and Hobson 2004; Newsome et al. 2010; Matthews et al. 2016; Drago et al. 2020).
The present study combines previously published values of δ13C, δ15N (Drago et al. 2021), and δ18O (Drago et al. 2020), with unpublished δ34S values from the bone tissue of seven marine mammal species, in order to provide a fine resolution model of habitat partition between marine mammals in estuarine habitats. In addition, it was aimed to assess the usefulness of the less common δ34S and δ18O values to obtain reliable information of habitat use in these ecosystems.
Methods
Study Area
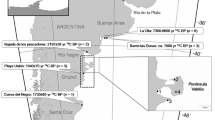
The Río de la Plata estuary is located at approximately 35° S and 55° W, in the south-western Atlantic Ocean (Fig. 1). It is 320 km long (Framiñan and Brown 1996) and has an average discharge of 2.0–2.5 × 104 m3 s−1, representing the biggest fresh-water inflow and terrestrial run-off to the region (Guerrero et al. 1997; Miloslavich et al. 2011). In addition, the confluence of two major currents off the estuary–the cold, nutrient-rich Falkland/Malvinas current and the warm, nutrient-poor Brazil current–creates a series of oceanographic structures (i.e., eddies, marine fronts) that increase the biological production in the area (Miloslavich et al. 2011).
Study area and sampling locations. The black dashed lines show the sampling area along the Uruguayan coast for the skulls of the seven marine mammal species included in this study; the gray dashed lines delineate the boundaries between the zones with different salinities: (I) the inner estuarine zone, defined by the bottom salinity front; (II) the estuary/mixohaline zone, defined between the surface and bottom salinity fronts; and (III) the marine zone. Sea surface salinity and bottom salinity values (Guerrero et al. 1997; Moreira and Simionato 2019) are reported in practical salinity units (psu). The red line represents the modal position of the maximum turbidity zone for the estuary (Acha et al. 2008)
Primary production is particularly high at the estuary, considered one of the most productive environments in the world, and it plays a major role in supporting the rich biodiversity found along the Uruguayan coast (Acha et al. 2004; Guerrero et al. 1997; Miloslavich et al. 2011; Ortega and Martínez 2007). This includes several marine mammal species that feed and breed along its large basin (Bastida et al. 2007; Miloslavich et al. 2011).
The bathymetry of the relatively shallow waters of the Río de la Plata estuary creates a unique hydrographic system. Since the freshwater input exceeds local evaporation, this area can be defined as a “positive estuary,” with a strong horizontal gradient as salinity increases steadily towards oceanic waters (Pritchard 1952; Guerrero et al. 1997). In addition, the estuary is characterized by an almost constant vertical stratification, with the saltier marine waters moving upstream along the bottom of the estuary, and the freshwater layer originating from the river discharge remaining atop the water column, thus creating a salinity wedge in between the two water masses (Guerrero et al. 1997; Acha et al. 2008). A bottom salinity front is defined at the upstream reach of the salt wedge by the topography, and a surface salinity front results from the convergence of estuarine and marine waters combined with the direction and velocity of the winds, among other factors (Fig. 1; Framiñan and Brown 1996; Guerrero et al. 1997; Acha et al. 2008). Typically, salinity increases with the distance from the inner estuary, varying from 0 psu at the river mouth to 33 psu at the marine zone, and with depth, more evident towards the estuarine zone (Fig. 1; Framiñan and Brown 1996; Guerrero et al. 1997; Acha et al. 2008; Moreira and Simionato 2019).
A well-developed turbidity front, also known as the maximum turbidity zone, is formed by the flocculation of suspended matter at the tip of the salinity wedge, and the resuspension of sediment due to the tidal stirring over the Barra del Indio shoal, between Punta Piedras and Montevideo (Fig. 1). Furthermore, the food webs within the estuary seem to be supported by at least three sources of organic matter: terrestrial detritus, marsh plant detritus, and phytoplankton (Framiñan and Brown 1996; Acha et al. 2008; Botto et al. 2011), although their distribution is not equal. At the innermost part of the estuary, around the maximum turbidity zone, there is a high influence of terrestrial and freshwater marsh plant detritus due to light limitations. Immediately offshore these turbidity front and within the estuary/mixohaline zone, the influence of the salt marsh detritus coming from Samborombón Bay (Fig. 1) increases, as well as the phytoplankton concentration as the light becomes more available. Finally, the marine zone is dominated mostly by phytoplankton (Carreto et al. 2003, 2008; Acha et al. 2008; Botto et al. 2011). This distribution of resources will determine the distinct isotopic values found along the Río de la Plata estuary (Botto et al. 2011).
Sampling
A total of 133 bone samples of seven marine mammal species were analyzed, including two otariids, the South American sea lion Otaria flavescens (n = 11 ♀, 10 ♂), and the South American fur seal Arctocephalus australis (n = 14 ♀, 13 ♂); and five odontocetes, the pontoporiid franciscana dolphin Pontoporia blainvillei (n = 11 ♀, 11 ♂), the phocoenid Burmeister’s porpoise Phocoena spinipinnis (n = 1 ♀, 4 ♂, 5 unknown), and the delphinids Fraser’s dolphin Lagenodelphis hosei (n = 3 ♀, 2 ♂, 5 unknown), false killer whale Pseudorca crassidens (n = 3 ♀, 3 ♂, 4 unknown), and bottlenose dolphin Tursiops truncatus (n = 5 ♂, 5 unknown). Bone tissue has a relatively slow turnover rate and hence, the values reported here integrate information on the habitat use of each individual over several years (Hobson et al. 2010; Schoeninger 2010; Fahy et al. 2017).
Every specimen included in this study was found dead stranded along the Uruguayan coastline or was incidentally caught by Uruguayan fishermen between 1958 and 2016. All bone samples were collected from skulls in the scientific collection of the Museo Nacional de Historia Natural (MNHN) and the Facultad de Ciencias of the Universidad de la República (UdelaR) at Montevideo (Uruguay), and consisted of a small fragment of crushed bone from the nasal cavity (turbinate bone) for the otariids, and the maxilla for the odontocetes.
It should be noted that, based on their skull characteristics, all bottlenose dolphins included in this study belong to specimens of the subspecies T. t. gephyreus (Lahille’s bottlenose dolphin), the coastal ecotype (Costa et al. 2016; Wickert et al. 2016). All the skulls from South American sea lions, South American fur seals, and franciscana dolphins were considered to belong to adult or physically mature specimens (see Drago et al. 2017, 2018 for details on age determination). The age and standard length of the individuals of the remaining species were unknown, but the condylobasal length of each skull was measured to ensure that only specimens of similar body size were included and thus avoid any age-related bias (Drago et al. 2018, 2020). The condylobasal length ranged 55–59 cm for bottlenose dolphins, 27–29 cm for Burmeister’s porpoises, 39–44 cm for Fraser’s dolphins, and 61–65 cm for false killer whales (Drago et al. 2020).
Stable Isotope Analysis
Bone samples were cleaned with distilled water, oven dried at 60 °C for 36 h, and ground into a fine powder using a mortar and pestle. For the δ34S, no pre-treatment was applied to the bone prior to the analysis in order to avoid eliminating amino acids that contain this element. Approximately 10 mg of each bone sample was weighed into a tin capsule, and vanadium pentoxide (V2O5) was added as catalyst to accelerate the combustion and reduce variability (Nehlich and Richards 2009). Samples were loaded and combusted at 1035 °C and analyzed with an elemental analyzer (Carlo Erba 1108) coupled to a Delta Plus XP mass spectrometer through a ConFlow III interface (both from Thermofisher) at Centres Cientifics i Tecnologics (CCiT-UB) of the University of Barcelona, Spain. International isotope secondary standards distributed by the International Atomic Energy Agency (IAEA) of known 34S/32S ratios, in relation to the Vienna- Canyon Diablo Troilite (VCDT) were used. These consisted in barium sulfate (NBS-127: δ34S = +21.2‰, IAEA SO-5: δ34S = +0.5‰ and IAEA SO-6: δ34S = −34.1‰) and YCEM (δ34S = +12.8), and they were employed once every 12 samples. Analytical precision for repeat measurements of the reference material, run in parallel with the bone samples, was 0.1‰.
The δ18O, δ13C, and δ15N values from the same samples were compiled from Drago et al. (2020, 2021).
The Suess effect (Keeling 1979) correction was applied to all the original values of δ13C to compensate for the increment of atmospheric CO2 over time and to allow for comparison of δ13C values of specimens from different periods (see Drago et al. 2021 for details on Suess effect correction factor determination). Furthermore, because δ18O values in animal studies are more commonly presented relative to the Vienna Standard Mean Oceanic Water (V-SMOW) index, δ18O values were converted from PDB to SMOW according to the following equation (Koch et al. 1997):
Stable isotope abundances are expressed in delta (δ) notation, with the relative variations of stable isotope ratios expressed in per mil (‰) deviations from predefined international standards, and they were calculated as follows:
where jX is the heavier isotope (13C, 15N, 18O, or 34S), and iX is the lighter isotope (12C, 14N, 16O or 32S) in the analytical sample and international measurement standard (Bond and Hobson 2012).
Data Analysis
Normality was tested by means of the Lilliefors test, and homoscedasticity by means of the Levene test, with only δ34S and δ15N values showing homogeneity of variances. Differences between the stable isotope ratios (δ18O, δ34S, δ13C, and δ15N) of males and females of the three species with the largest sample size (franciscana dolphins, South American sea lions, and South American fur seals) were assessed through a Permutational Multivariate Analysis of Variance (PERMANOVA; Anderson 2001, 2014), using the vegan package version 2.5–7 with 999 permutations (Oksanen et al. 2020). Since differences were found between sexes for the two otariids (see Results for details), nine groups were considered for further analysis: the five odontocetes species plus females and males of the two otariid species. Boxplots of the original values for each stable isotope ratio were built to compare the average values and ranges of each species using one-way ANOVA followed by a Scheffe post-hoc test.
In order to identify differences in the isotopic niches of the considered marine mammal groups, the δ18O, δ34S, δ13C, and δ15N values were combined using a PERMANOVA test followed by a pairwise multilevel comparison with the Bonferroni correction for multiple comparisons (Chen et al. 2017), using the package pairwiseAdonis with 999 permutations (Martinez Arbizu 2020). However, since the pairwise comparison seemed to account more for the similarities than the differences between species (i.e., if two species had similar values in at least two stable isotope ratios, they were sometimes considered “similar”), two-dimensional plots were built using the package “SIBER” (Stable Isotope Bayesian Ellipses; Jackson et al. 2011) to estimate the isotopic niche width and overlaps between the marine mammal groups for the different pairs of stable isotope ratios. This was also used to recognize the dimensions in which those “similar” species differed, and to estimate the best isotopic niche discriminator among the analyzed stable isotope ratios. Two complementary approaches were used to estimate the isotopic niche width (Jackson et al. 2011): the standard ellipse areas corrected for small sample size (SEAc) were used to plot the isotopic niche of each species within the isotopic space (isospace) and to calculate the overlap among species, and the Bayesian standard ellipse areas (SEAb) were used to obtain an unbiased estimate of the isotopic niche width with 95% credibility intervals.
All statistical analyses and plots were carried out using R Statistical Software v 4.1.2 (R Core Team 2021). Statistical results are reported according to Smith (2020).
Results
Given the small difference between the average values of δ34S, δ18O, δ15N, and δ13C of female and male franciscana dolphins (0.07‰, 0.09‰, 0.24‰, and 0.23‰, respectively; Table 1), both sexes were pooled in one group for later analysis. On the contrary, the average δ34S and δ15N values of South American sea lions were higher in males than in females (1.68‰ and 1.10‰, respectively) and hence, considered biologically different (Table 1). Therefore, both sexes were considered independently in further analysis. Similarly, the average values of δ34S, δ18O, and δ15N of male South American fur seals were higher than those of females (1.15‰, 0.34‰, and 0.87‰, respectively; Table 1), and both sexes were also treated as separate groups in further analysis.
The mean values of the nine groups considered here (five odontocetes species plus females and males of the two otariid species) were scattered along a gradient for the four stable isotope ratios analyzed (Fig. 2). In each case, either bottlenose dolphins or sea lions were at one extreme and Fraser’s dolphins at the other (Fig. 2), with differences between the average values at both extremes of 3.78‰ for δ34S, 2.32‰ for δ18O, 2.71‰ for δ13C, and 6.06‰ for δ15N. According to the δ18O values, South American sea lions (both females and males), bottlenose dolphins, and female South American fur seals presented the lowest mean δ18O values; male South American fur seals, franciscana dolphins, and false killer whales had intermediate values, whereas Burmeister’s porpoises and Fraser´s dolphins showed the highest δ18O (Fig. 2). Similar placements between species were found for the δ13C values, although in this case, female South American fur seals presented intermediate values (Fig. 2). Moreover, bottlenose dolphins, Burmeister´s porpoises, female sea lions, and Franciscans dolphins had similarly low mean δ34S, whereas the Fraser’s dolphins and male fur seals presented the highest δ34S (Fig. 2). In addition, the largest differences found between females and males for both otariids were in the δ34S values, with males having considerably higher mean values than the respective females (Fig. 2). Lastly, the Fraser’s dolphins had the lowest δ15N values and appear to be different from the remaining species, whereas Burmeister’s porpoises, franciscana dolphins, and South American sea lions (females and males) showed the highest δ15N values (Fig. 2).
Boxplots of the isotopic values (δ18O, δ34S, δ13C, and δ15N) of the considered marine mammal species from Río de la Plata estuary and adjacent areas, organized by the mean values indicated in each case by a red “x”. Groups with different superscript (lower case letters) are statistically different in their mean values, according to the Scheffe post-hoc test following nested ANOVA. Boxes represent the first and third quartile, lines the median, and whiskers 95% confidence interval. A general description of the habitat and trophic habits suggested by both, the values for each stable isotope ratio and the origin of resources described by Botto et al. (2011) and Peterson and Howarth (1987) is showed underneath the boxes, with POM, particulate organic matter; and T.P, trophic position. Sample sizes and species/groups: female South American sea lions (Of ♀, n = 11), male South American sea lions (Of ♂, n = 10), female South American fur seals (Aa ♀, n = 14), male South American fur seals (Aa ♂, n = 13), bottlenose dolphins (Tt, n = 10), franciscana dolphins (Pb, n = 22), false killer whales (Pc, n = 10), Burmeister’s porpoises (Ps, n = 10), and Fraser’s dolphins (Lh, n = 10). δ13C values are corrected for Suess effect
The PERMANOVA and pairwise multilevel comparison comprising the four stable isotope ratios and the seven marine mammal species together, indicated differences between most of the considered groups (Table S1). On one side, female and male sea lions shared similar isotopic niches (R2 = 0.227, adjusted p value = 0.216; n = 11 and n = 10, respectively), but only males showed similarities with those of false killer whales (R2 = 0.357, adjusted p value = 0.072; n = 10 and n = 10, respectively) and Burmeister’s porpoises (R2 = 0.337, adjusted p value = 0.072). Likewise, female and male fur seals also showed similar isotopic niches (R2 = 0.203, adjusted p value = 0.072; n = 14 and n = 13, respectively), but only that of females was similar to that of Burmeister’s porpoises (R2 = 0.234, adjusted p value = 0.072; n = 14 and n = 10, respectively). Other groups with similar isotopic niches, according to this analysis, were female and male fur seals and false killer whales (R2 = 0.086, adjusted p value = 1.000; and R2 = 0.128, adjusted p value = 1.000, respectively) and Burmeister’s porpoises with bottlenose dolphins (R2 = 0.263, adjusted p value = 0.072; n = 10 and n = 10, respectively), franciscana dolphins (R2 = 0.095, adjusted p value = 1.000; n = 10 and n = 22, respectively), and false killer whales (R2 = 0.220, adjusted p value = 0.072; n = 10 and n = 10, respectively). However, when comparing these results with the differences found initially with the ANOVA and Scheffe test (Fig. 2), some groups considered here as “similar” had different mean values for at least one stable isotope ratio. One clear example was between Burmeister’s porpoises and bottlenose dolphins, considered similar by the pairwise comparison (Table S1) despite the differences in δ18O and δ13C (Fig. 2) that already showed biologically relevant differences in their isotopic niches.
A better view of these differences between isotopic niches was obtained through the estimation of the area of the standard ellipse of each marine mammal group (Fig. 3) and the overlapped area between each pair for the different isotopic dimensions (Table S2). Most groups did not overlap in at least one pair of stable isotope ratios and hence were considered to exploit different isotopic niches. However, the following seven pairs showed constant overlap of isotopic niche areas in all the considered dimensions: males and females for both otariids; franciscana dolphins and Burmeister´s porpoises; and false killer whales with: Fraser’s dolphins, fur seals (females and males), and franciscana dolphins (Fig. 3; Table S2).
Standard ellipses of the marine mammal groups from Río de la Plata estuary, corrected for small sample size (SEAc), in the isospace defined by δ18O, δ34S, δ13C, and δ15N. The colored arrows indicate the general interpretation of the isotopic values for each element as written, where blue corresponds to carbon (δ13C), purple to oxygen (δ18O), orange to nitrogen (δ15N), and green to sulfur (δ34S) stable isotope ratios. Sample sizes and species/groups: female South American sea lions (Of ♀, n = 11), male South American sea lions (Of ♂, n = 10), female South American fur seals (Aa ♀, n = 14), male South American fur seals (Aa ♂, n = 13), bottlenose dolphins (Tt, n = 10), franciscana dolphins (Pb, n = 22), false killer whales (Pc, n = 10), Burmeister’s porpoises (Ps, n = 10), and Fraser’s dolphins (Lh, n = 10). δ.13C values are corrected for Suess effect. The percentage of overlapped area between each pair of species/groups is available in Table S2
Discussion
The results reported here revealed fine niche partitioning between the seven species of marine mammals studied, and demonstrated the utility of combining the stable isotope ratios of four elements to characterize the isotopic niche of consumers inhabiting estuaries with strong gradients of salinity and redox potential.
Assessing the Method
The addition of δ34S and δ18O to the initial δ13C–δ15N isospace described by Drago et al. (2021) provided with unique and useful information about the habitat use of the seven species of marine mammals considered here, allowing a better characterization of their individual isotopic niches (Franco-Trecu et al. 2017).
Each stable isotope ratio (δ18O, δ34S, δ13C, and δ15N) characterized a different dimension of the isotopic niche of each group and provided complementary information on their habitat preferences and trophic position. The isotopic niches revealed by combining four stable isotope ratios allowed not only to confirm some of the information reported in the literature, but also to gain new knowledge on the habitat use of some species, as well as to infer differences between apparently similar groups. It is worth noting that species with similar isotopic niches do not necessarily have the same distribution, as isotopic similarity only indicates similar preferences for certain environmental conditions, which could be found in different areas along the estuary (Acha et al. 2008). Furthermore, bone tissue acts as a long-term integrator of the stable isotope ratios due to its relatively slow turnover rate, which makes it useful to obtain information over extended periods of time (Hobson et al. 2010; Schoeninger 2010; Fahy et al. 2017; Skedros et al. 2013; Matsubayashi et al. 2017; Tomaszewicz et al. 2018; Matsubayashi and Tayasu 2019). Accordingly, the values reported here for each individual are averages integrating habitat use over a period of several years, but lack resolution on shorter time scales (weeks to months).
The pairwise multilevel comparison employed after the PERMANOVA has been used in different studies to compare three or more stable isotope ratios between different species of marine mammals (Borrell et al. 2021). It uses random permutations in order to analyze the relationship between the stable isotope ratios and the species, and hence, slightly different results can be expected every time the test is run (Anderson 2001, 2014). Moreover, based on the results obtained here, this statistical analysis seems to account more for the similarities between groups and not as much for the differences, which was the main interest of the present study. Therefore, to define the isotopic niche of different species in an ecosystem, the Stable Isotope Bayesian Ellipses (Jackson et al. 2011) were considered more appropriate.
Origin of Resources According to δ34S, δ18O, δ13C, and δ15N
The variation in stable isotope ratios within an ecosystem depends mostly on the physical and ecological processes that determine the origin of each chemical element (Peterson and Howarth 1987; Rubenstein and Hobson 2004). In the Río de la Plata estuary, these processes are mainly driven by the constant inflow of a fresh-water surface layer with terrestrial particulate organic matter (POM) over the salty shelf waters that, combined with wind stress and topography, create a series of well-characterized inshore and marine fronts and a strong stratification with marked vertical and horizontal salinity gradients (Fig. 4; Guerrero et al. 1997; Acha et al. 2004; Botto et al. 2011). Here, the main source of δ18O variation between the marine mammal groups is the salinity of the water where they feed, as demonstrated by the strong positive correlation between these two factors (Conroy et al. 2014; Belem et al. 2019). Indeed, although data for the Río de la Plata estuary are limited, a general increase in δ18O values is observed from inshore (δ18O = ~−1‰ PDB; ~29.8‰ SMOW) to offshore (δ18O = ~+2‰ PDB; ~32.9‰ SMOW) marine environments in the nearby Atlantic Ocean (LeGrande and Schmidt 2006; McMahon et al. 2013). Additionally, the almost permanent vertical salinity stratification in the mixohaline zone leads to the formation of a salt wedge and the occurrence of salty marine waters with higher δ18O values just above the bed of the estuary (Fig. 4; Guerrero et al. 1997).
Graphical representation of the estimated habitat preferences of the considered marine mammal groups from the Río de la Plata estuary, based on the combination of δ18O, δ34S, δ13C, and δ.15N values reported here, the physical and ecological processes of the estuary explained by Acha et al. (2008), and the origin of resources affecting the different zones of the estuary and their respective isotopic values reported by Botto et al. (2011) and Peterson and Howarth (1987). The short arrows show the general tendency of the respective stable isotope ratio in a given zone, where upward arrows indicate high values (enriched) and downward arrows indicate low values (depleted); POM, particulate organic matter. Species/groups: bottlenose dolphins (Tt), female South American sea lions (Of ♀), male South American sea lions (Of ♂), female South American fur seals (Aa ♀), male South American fur seals (Aa ♂), false killer whales (Pc), Burmeister’s porpoises (Ps), franciscana dolphins (Pb), and Fraser’s dolphins (Lh)
On the other hand, δ13C and δ34S values are directly affected by the source of the primary producers and plant detritus reaching the estuary (Peterson and Howarth 1987; Connolly et al. 2004). In the Río de la Plata estuary, there are three known sources of organic C: (1) the 13C-depleted terrestrial and freshwater marsh plants with a C3 metabolism, brought into the estuary by the river; (2) the 13C-enriched salt marsh plants with a C4 metabolism, found mainly at the southern shore of the estuary along Samborombón Bay (Fig. 1), mostly Spartina spp.; and (3) the marine phytoplankton, also 13C-depleted (Peterson and Howarth 1987; Botto et al. 2011; Bergamino et al. 2017). The first two sources reach the estuary mostly as detritus and the presence of phytoplankton is light-dependent; as a result, the relevance of each source is spatially variable (Botto et al. 2011).
At the innermost part of the estuary, the maximum turbidity zone (Figs. 1 and 4) is characterized by a high concentration of suspended particles originated from terrestrial plants and freshwater marsh plants, since water turbidity does not allow phytoplankton growth (Framiñan and Brown 1996; Acha et al. 2003; Botto et al. 2011). Here, the base of the food web seems to be supported by this allochthonous detritus with low δ13C values (Fig. 4; Peterson 1999; Botto et al. 2011). Once in the estuary/mixohaline zone, most of the suspended POM seems to originate from the salt marshes with high δ13C values, representing the only known source of 13C-enriched particles for the consumers in the area (Fig. 4; Botto et al. 2011). Nonetheless, a considerable increase in phytoplankton concentration has been found inside the estuary following the maximum turbidity zone (Carreto et al. 2008), so it is also possible to find consumers with low δ13C values associated to this zone. In the marine waters, the food web is supported mostly by phytoplankton and hence consumers are characterized by low δ13C values (Fig. 4; Peterson 1999; Botto et al. 2011). In contrast, the variation in δ34S values depends on the source of inorganic S (Peterson et al. 1985). Terrestrial and marsh plants use 34S-depleted sulfides originated, respectively, from precipitation and anoxic sediments with high sulfate reduction (Peterson et al. 1985; Peterson 1999). As a result, the consumers associated to the maximum turbidity zone are expected to have low δ34S values, as well as those from the estuary/mixohaline zone (Fig. 4). On the other hand, planktonic organisms use the 34S-enriched seawater sulfates product of the sulfide oxidation within the water-column, and are responsible for the high δ34S typical of offshore/pelagic species (Fig. 4; Peterson and Howarth 1987; Peterson 1999).
Habitat Use According to δ34S, δ18O, δ13C, and δ15N
From the section above, results that the isotopic niche of each marine mammal species inhabiting the Río de la Plata estuary and adjoining waters can be used to characterize their habitat use pattern (Fig. 4).
The differences between females and males for both otariid species (South American sea lions and fur seals) were consistent with their sexual dimorphism and distinct behavior (Cárdenas-Alayza 2018a, b). In both cases, females usually stay closer to the coast and near to their breeding grounds (Rodríguez et al. 2013; González Carman et al. 2016), whereas males often perform long-distance foraging trips further from their rookeries and reach more marine waters with less terrestrial influence (Giardino et al. 2016; de Lima et al. 2022), as evidenced here by the higher δ34S values found in males (Connolly et al. 2004). Nonetheless, the low δ18O values (Fig. 2) reported for these four groups indicated a preference for low salinity foraging grounds, suggesting that even though males use more marine habits than females, the estuarine zone above the salt wedge is still an important feeding ground for both sexes in both species. Moreover, the higher δ13C values of sea lions of both sexes compared to fur seals indicated a higher reliance of the former on the food web supported by salt marsh detritus, while the combination of low δ13C and high δ34S values typical of fur seals, particularly males, better fits with a phytoplankton-based food web (Fig. 4; Saporiti et al. 2016; Drago et al. 2017). This is consistent with the prevalence of pelagic and demersal-pelagic fishes and squids in the diet of fur seals (Naya et al. 2002; Franco-Trecu et al. 2014; de Lima et al. 2022), compared to the prevalence of benthic prey in the diet of sea lions throughout the year, although they increase the consumption of more pelagic prey during the pre-breeding season (Franco-Trecu et al. 2014; Drago et al. 2015). The different trophic positions of the two otariid species is further supported by the higher δ15N values of sea lions (Drago et al. 2021).
Bottlenose dolphins shared similarities with female sea lions on three out of the four stable isotope ratios analyzed (Fig. 2), suggesting similar preferences of habitat but differences in their trophic positions. The low δ18O and δ34S values indicated the use of low salinity areas with high terrestrial influence, and the high δ13C confirmed an affinity for the mixohaline zone in areas with high influence of salt marsh detritus (Fig. 4; Connolly et al. 2004; Botto et al. 2011). These results are consistent with the demerso-pelagic diet described for bottlenose dolphins in the Río de la Plata estuary (Botta et al. 2012; Wells and Scott 2018), although some individuals might be using the marine waters at the bottom of the estuary as they presented high δ18O values, similar to those found in marine species (Fig. 2; Acha et al. 2008; Secchi et al. 2017).
False killer whales had a broad isotopic niche, overlapping with marine species such as the Fraser´s dolphins, as well as with species associated with the estuary/mixohaline zone such as fur seals and franciscana dolphins (Fig. 3, Table S2). This was likely due to the wide range of values presented by the species for most stable isotope ratios, especially δ18O, δ13C, and δ15N. In Southern Brazil, Botta et al. (2012) also reported intraspecific variation for the false killer whales and divided them into two groups, one similar to the local inshore predators (bottlenose dolphins and killer whales) and another one with lower δ13C and δ15N values that suggested offshore/oceanic habits. Likewise, based on the different δ18O values found for Río de la Plata, there might be at least two groups of false killer whales with different feeding habits, one using the upper layers of the estuary/mixohaline zone above the salinity wedge, and another group using the waters below the salinity wedge, although a larger sample size is needed to test this hypothesis (Fig. 4). It is worth noting that bottlenose dolphins and false killer whales have similar isotopic niches within the δ13C–δ15N isospace (Bisi et al. 2013; Drago et al. 2021) but differed largely when δ34S and δ18O are considered (Figs. 3 and 4).
On the other hand, the high δ18O values reported for the Burmeister’s porpoise combined with relatively low δ13C values, previously led to report offshore habits for this species in Río de la Plata (Drago et al. 2020, 2021). However, the Burmeister’s porpoise is generally described as a coastal species based on sightings and incidental catches (Reyes 2018), and diet often includes inshore prey, either demersal or pelagic (Reyes and Van Waerebeek 1995; Molina-Schiller et al. 2005; Riccialdelli et al. 2010; Reyes 2018). Considering the low mean δ34S values reported here (Fig. 2), the combination of high δ18O and low δ13C values can be explained by the use of benthic areas close to the bottom salinity front and below the salt wedge in the mixohaline zone (Fig. 4). Here, there is a high influence of 18O- and 34S-enriched marine waters entering the estuary from the bottom, the formation of the maximum turbidity zone with high influence of 13C- and 34S-depleted terrestrial POM, and the presence of 13C-enriched detritus from salt marshes associated with the estuary/mixohaline zone (Peterson and Howarth 1987; Guerrero et al. 1997; Acha et al. 2008; Botto et al. 2011). Although the preference for areas with high salinity is clear, the merging of all these different isotopic sources in a relatively small area is likely the reason for the wide intraspecific variation of δ13C and δ34S found for the Burmeister’s porpoise.
In the case of the franciscana dolphin, both females and males seem to have similar foraging habits (Crespo 2018), consistent with the similarities found here between their isotopic values. Moreover, the high δ18O combined with relatively high δ13C and low δ34S indicated the use of the mixohaline zone below the salinity wedge, likely with the influence of salt marsh detritus as C and S sources, although some individuals might be using areas with higher influence of terrestrial POM (Fig. 4; Peterson and Howarth 1987; Acha et al. 2008; Botto et al. 2011). These descriptions of habitat use also appear to be consistent with the demersal and benthopelagic diet described for the southern populations of this species through stomach content analysis (Franco-Trecu et al. 2017; Tellechea et al. 2017; Botta et al. 2022).
Opposite to the other groups, the isotopic niche of the Fraser’s dolphin is consistent with an offshore, pelagic feeding, most likely supported by a food chain relying on phytoplankton using water-column sulfates (Fig. 4; Drago et al. 2021). This species is often found in deep waters, from 250 to up to 3500 m deep, probably following the distribution of their preys (Dolar 2018), and stomach content analysis of stranded and incidentally caught individuals has confirmed a preference for mesopelagic and deep-water fishes and squids (Dolar et al. 2003; Wang et al. 2011; Dolar 2018). Previous studies have also reported constantly low δ13C values for Fraser’s dolphins in different regions (Bisi et al. 2013; Botta et al. 2012; Costa et al. 2020), and their offshore/marine habits are further confirmed here with the high δ34S and δ18O values (Peterson and Howarth 1987; Carreto et al. 2008; Belem et al. 2019).
Finally, δ15N values are used as a proxy of the trophic position through the estimation of a trophic discrimination factor, which vary between 1.57 and 2.7‰ for different species of marine mammals (Borrell et al. 2012; Aurioles-Gamboa et al. 2013; Beltran et al. 2016; Giménez et al. 2016). If so, the range reported here, from 12.62‰ in one of Fraser’s dolphins to 22.73‰ in one of franciscana dolphins, would suggest that the assemblage is foraging along more than three trophic levels. This is highly unlikely and certainly an artifact caused by the high input of sewage reaching Río de la Plata estuary from Montevideo and Buenos Aires, and the resulting shift in the δ15N baseline between estuarine and oceanic waters (McClelland and Valiela 1998; Botto et al. 2011; McMahon et al. 2013; Troina et al. 2020, 2021).
Conclusions
Our isotopic data suggest the extended use of the estuarine/mixohaline zone as a foraging ground by several marine mammal species inhabiting the Río de la Plata estuary and adjacent south-western Atlantic waters. On one side, bottlenose dolphins, South American sea lions, and fur seals showed a higher affinity to the low salinity layers above the salt wedge, characterized also by distinct C and S sources: POM from salt marshes and phytoplankton (Fig. 4). A second group of species, including the Burmeister´s porpoises, franciscana dolphins, and false killer whales, seemed to prefer the saltier marine waters close to the bottom of the estuary. However, the porpoises showed affinity for the maximum turbidity zone with high terrestrial influence, whereas the other two species showed a higher influence from salt marsh detritus (Fig. 4). Finally, the Fraser’s dolphin was the only species that showed an affinity for the marine zone, an area with high salinity and phytoplankton and water-column sulfates as C and S sources, respectively (Fig. 4). Considering the disparate distribution ranges of the seven species studied here (Reeves et al. 2002), the pattern of habitat partitioning reported is unlikely to have resulted from coevolution, but emerges probably because of differences in their fundamental niches and the possible role of competition. The actual relevance of competitive exclusion has yet to be assessed, but the restriction of bottlenose dolphins to the less saline areas of the estuary is striking, as the species inhabits mostly coastal waters elsewhere in the south-western Atlantic Ocean (Lodi et al. 2016).
In conclusion, the use of δ18O and δ34S provided additional and important insights into the habitat use of the considered marine mammal species in an estuarine setting, allowing to differentiate between species using different salinity ranges and food webs with different contributions of terrestrial detritus. Both stable isotope ratios worked as complementary habitat tracers, without discarding the useful information on trophic relationships provided by δ13C and δ15N. Furthermore, the combination of at least three habitat tracers allowed a better visualization of the different dimensions that make up the whole ecosystem, thus improving the understanding of habitat partitioning between marine mammal species.
Data Availability
Data available from the University of Barcelona Digital Repository https://doi.org/10.34810/data675.
References
Acha, E.M., H.W. Mianzan, O. Iribarne, D.A. Gagliardini, C. Lasta, and P. Daleo. 2003. The role of the Río de la Plata bottom salinity front in accumulating debris. Marine Pollution Bulletin 46 (2): 197–202. https://doi.org/10.1016/s0025-326x(02)00356-9.
Acha, E.M., H.W. Mianzan, R.A. Guerrero, M. Favero, and J. Bava. 2004. Marine fronts at the continental shelves of austral South America: physical and ecological processes. Journal of Marine Systems 44 (1–2): 83–105.
Acha, E.M., H. Mianzan, R. Guerrero, J. Carreto, D. Giberto, N. Montoya, and M. Carignan. 2008. An overview of physical and ecological processes in the Rio de la Plata Estuary. Continental Shelf Research 28 (13): 1579–1588. https://doi.org/10.1016/j.csr.2007.01.031.
Anderson, M.J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Anderson, M.J. 2014. Permutational multivariate analysis of variance (PERMANOVA). In Wiley statsref: statistics reference online, 1–15. John Wiley & Sons, Inc. https://doi.org/10.1002/9781118445112.stat07841.
Aurioles-Gamboa, D., M.Y. Rodríguez-Pérez, L. Sánchez-Velasco, and M.F. Lavín. 2013. Habitat, trophic level, and residence of marine mammals in the Gulf of California assessed by stable isotope analysis. Marine Ecology Progress Series 488: 275–290. https://doi.org/10.3354/meps10369.
Baird, R.W. 2018. False killer whale. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 347–349. San Diego: Academic Press.
Balmer, B.C., R.S. Wells, L.E. Howle, A.A. Barleycorn, W.A. McLellan, D. Ann Pabst, T.K. Rowles, L.H. Schwacke, F.I. Townsend, A.J. Westgate, and E.S. Zolman. 2014. Advances in cetacean telemetry: a review of single-pin transmitter attachment techniques on small cetaceans and development of a new satellite-linked transmitter design. Marine Mammal Science 30 (2): 656–673.
Barlow, J. 2018. Management. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 555–558. San Diego: Academic Press.
Bastida, R., D. Rodríguez, E.R. Secchi, and V. da Silva. 2007. Mamíferos acuáticos de Sudamérica y Antártida. Buenos Aires: Vazquez Mazzini Editores.
Belem, A.L., C. Caricchio, A.L.S. Albuquerque, I.M. Venancio, M. Zucchi, T.H.R. Santos, and Y.G. Alvarez. 2019. Salinity and stable oxygen isotope relationship in the Southwestern Atlantic: constraints to paleoclimate reconstructions. Anais da Academia Brasileira de Ciências 91: e20180226.
Beltran, R.S., S.H. Peterson, E.A. McHuron, C. Reichmuth, L.A. Hückstädt, and D.P. Costa. 2016. Seals and sea lions are what they eat, plus what? Determination of trophic discrimination factors for seven pinniped species. Rapid Communications in Mass Spectrometry 30 (9): 1115–1122. https://doi.org/10.1002/rcm.7539.
Ben-David, M., and E.A. Flaherty. 2012. Stable isotopes in mammalian research: a beginner´s guide. Journal of Mammalogy 93 (2): 312–328. https://doi.org/10.1644/11-MAMM-S-166.1.
Bergamino, L., M. Schuerch, A. Tudurí, S. Carretero, and F. García-Rodríguez. 2017. Linking patterns of freshwater discharge and sources of organic matter within the Río de la Plata estuary and adjacent marshes. Marine and Freshwater Research 68 (9): 1704–1715.
Bisi, T.L., P.R. Dorneles, J. Lailson-Brito, G. Lepoint, A.D.F. Azevedo, L. Flach, O. Malm, and K. Das. 2013. Trophic relationships and habitat preferences of delphinids from the southeastern Brazilian coast determined by carbon and nitrogen stable isotope composition. PLoS ONE 8 (12): e82205.
Bond, A.L., and K.A. Hobson. 2012. Reporting stable-isotope ratios in ecology: recommended terminology, guidelines and best practices. Waterbirds 35 (2): 324–331.
Borrell, A., N. Abad-Oliva, E. Gómez-Campos, J. Giménez, and A. Aguilar. 2012. Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Communications in Mass Spectrometry 26 (14): 1596–1602. https://doi.org/10.1002/rcm.6267.
Borrell, A., M. Gazo, A. Aguilar, J.A. Raga, E. Degollada, P. Gozalbes, and R. García-Vernet. 2021. Niche partitioning amongst northwestern Mediterranean cetaceans using stable isotopes. Progress in Oceanography 193: 102559.
Botta, S., A.A. Hohn, S.A. Macko, and E.R. Secchi. 2012. Isotopic variation in delphinids from the subtropical western South Atlantic. Journal of the Marine Biological Association of the United Kingdom 92 (8): 1689–1698.
Botta, S., M. Bassoi, and G.C. Troina. 2022. Chapter 2 - Overview of franciscana diet. In The franciscana dolphin: on the edge of survival, ed. P.C. Simões-Lopes and M.J. Cremer, 15–48. Academic Press. https://doi.org/10.1016/B978-0-323-90974-7.00003-3. ISBN 9780323909747.
Botto, F., E. Gaitán, H. Mianzan, M. Acha, D. Giberto, A. Schiariti, and O. Iribarne. 2011. Origin of resources and trophic pathways in a large SW Atlantic estuary: an evaluation using stable isotopes. Estuarine, Coastal and Shelf Science 92 (1): 70–77. https://doi.org/10.1016/j.ecss.2010.12.014.
Cárdenas-Alayza, S. 2018a. South American Sea Lion (Otaria byronia). In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 907–910. San Diego: Academic Press.
Cárdenas-Alayza, S. 2018b. South American Fur Seal (Arctocephalus australis). In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 905–907. San Diego: Academic Press.
Carreto, J.I., N.G. Montoya, H.R. Benavides, R.A. Guerrero, and M.O. Carignan. 2003. Characterization of spring phytoplankton communities in the Río de la Plata maritime front using pigment signatures and cell microscopy. Marine Biology 143 (5): 1013–1027.
Carreto, J.I., N. Montoya, R. Akselman, M.O. Carignan, R.I. Silva, and D.A. Cucchi Colleoni. 2008. Algal pigment patterns and phytoplankton assemblages in different water masses of the Río de la Plata maritime front. Continental Shelf Research 28 (13): 1589–1606. https://doi.org/10.1016/j.csr.2007.02.012.
Chen, S.Y., Z. Feng, and X. Yi. 2017. A general introduction to adjustment for multiple comparisons. Journal of Thoracic Disease 9 (6): 1725–1729. https://doi.org/10.21037/jtd.2017.05.34.
Clementz, M.T., and P.L. Koch. 2001. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 129 (3): 461–472.
Connolly, R.M., M.A. Guest, A.J. Melville, and J.M. Oakes. 2004. Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia, 138(2), 161–167. https://doi.org/10.1007/s00442-003-1415-0.
Conroy, J.L., K.M. Cobb, J. Lynch-Stieglitz, and P.J. Polissar. 2014. Constraints on the salinity–oxygen isotope relationship in the central tropical Pacific Ocean. Marine Chemistry 161: 26–33.
Costa, A.P.B., P.E. Rosel, F.G. Daura-Jorge, and P.C. Simoes-Lopes. 2016. Offshore and coastal common bottlenose dolphins of the western South Atlantic face-to-face: what the skull and the spine can tell us. Marine Mammal Science 32 (4): 1433–1457.
Costa, A.F., S. Botta, S. Siciliano, and T. Giarrizzo. 2020. Resource partitioning among stranded aquatic mammals from Amazon and Northeastern coast of Brazil revealed through Carbon and Nitrogen Stable Isotopes. Scientific Reports 10 (1): 1–13.
Crespo, E.A. 2018. Franciscana dolphin Pontoporia blainvillei. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 388–392. San Diego: Academic Press.
Croisetière, L., L. Hare, A. Tessier, and G. Cabana. 2009. Sulphur stable isotopes can distinguish trophic dependence on sediments and plankton in boreal lakes. Freshwater Biology 54 (5): 1006–1015.
de Lima, R.C., V. Franco-Trecu, T.S. Carrasco, P. Inchausti, E.R. Secchi, and S. Botta. 2022. Segregation of diets by sex and individual in South American fur seals. Aquatic Ecology 56 (1): 251–267.
Dolar, M.L. 2018. Fraser´s dolphin Lagenodelphis hosei. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 392–395. San Diego: Academic Press.
Dolar, M.L.L., W.A. Walker, G.L. Kooyman, and W.F. Perrin. 2003. Comparative feeding ecology of spinner dolphins (Stenella longirostris) and Fraser’s dolphins (Lagenodelphis hosei) in the Sulu Sea. Marine Mammal Science 19 (1): 1–19.
Drago, M., V. Franco-Trecu, L. Zenteno, D. Szteren, E.A. Crespo, F.R. Sapriza, L. de Oliveira, R. Machado, P. Inchausti, and L. Cardona. 2015. Sexual foraging segregation in South American sea lions increases during the pre-breeding period in the Río de la Plata plume. Marine Ecology Progress Series 525: 261–272.
Drago, M., L. Cardona, V. Franco-Trecu, E.A. Crespo, D.G. Vales, F. Borella, L. Zenteno, E.M. Gonzáles, and P. Inchausti. 2017. Isotopic niche partitioning between two apex predators over time. Journal of Animal Ecology 86 (4): 766–780. https://doi.org/10.1111/1365-2656.12666.
Drago, M., V. Franco-Trecu, A.M. Segura, M. Valdivia, E.M. González, A. Aguilar, and L. Cardona. 2018. Mouth gape determines the response of marine top predators to long-term fishery-induced changes in food web structure. Scientific Report. 8 (1): 1–12.
Drago, M., M. Valdivia, D. Bragg, E.M. González, A. Aguilar, and L. Cardona. 2020. Stable oxygen isotopes reveal habitat use by marine mammals in the Río de la Plata estuary and adjoining Atlantic Ocean. Estuarine, Coastal and Shelf Science 238: 106708.
Drago, M., M. Signaroli, M. Valdivia, E.M. González, A. Borrell, A. Aguilar, and L. Cardona. 2021. The isotopic niche of Atlantic, biting marine mammals and its relationship to skull morphology and body size. Scientific Reports 11 (1): 1–14.
Fahy, G.E., C. Deter, R. Pitfield, J.J. Miszkiewicz, and P. Mahoney. 2017. Bone deep: variation in stable isotope ratios and histomorphometric measurements of bone remodelling within adult humans. Journal of Archaeological Science 87: 10–16.
Framiñan, M.B., and O.B. Brown. 1996. Study of the Río de la Plata turbidity front, Part 1: spatial and temporal distribution. Continental Shelf Research 16 (10): 1259–1282.
Franco-Trecu, V., D. Aurioles-Gamboa, and P. Inchausti. 2014. Individual trophic specialization and niche segregation explain the contrasting population trends of two sympatric otariids. Marine Biology 161 (3): 609–618.
Franco-Trecu, V., M. Drago, P. Costa, C. Dimitriadis, and C. Passadore. 2017. Trophic relationships in apex predators in an estuary system: a multiple-method approximation. Journal of Experimental Marine Biology and Ecology 486: 230–236.
Gat, J.R. 1996. Oxygen and hydrogen isotopes in the hydrologic cycle. Annual Review of Earth and Planetary Sciences 24 (1): 225–262.
Giardino, G.V., M.A. Mandiola, J. Bastida, P.E. Denuncio, R.O. Bastida, and D.H. Rodríguez. 2016. Travel for sex: long-range breeding dispersal and winter haulout fidelity in southern sea lion males. Mammalian Biology 81 (1): 89–95.
Giménez, J., F. Ramírez, J. Almunia, M.G. Forero, and R. de Stephanis. 2016. From the pool to the sea: applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). Journal of Experimental Marine Biology and Ecology 475: 54–61. https://doi.org/10.1016/j.jembe.2015.11.001.
González Carman, V., A. Mandiola, D. Alemany, M. Dassis, J.P. Seco Pon, L. Prosdocimi, A. Ponce de León, H. Mianzan, E.M. Acha, D. Rodríguez, and M. Favero. 2016. Distribution of megafaunal species in the Southwestern Atlantic: key ecological areas and opportunities for marine conservation. ICES Journal of Marine Science 73 (6): 1579–1588.
Guerrero, R.A., E.M. Acha, M.B. Framin, and C.A. Lasta. 1997. Physical oceanography of the Río de la Plata Estuary. Argentina. Continental Shelf Research 17 (7): 727–742.
Hobson, K.A., R. Barnett-Johnson, and T. Cerling. 2010. Chapter 13: using isoscapes to track animal migration. In Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping, ed. J.B. West, G.J. Bowen, T.E. Dawson, and K.P. Tu, 273–298. Dordrecht: Springer Science & Business Media.
Jackson, A.L., R. Inger, A.C. Parnell, and S. Bearhop. 2011. Comparing isotopic niche widths among and within communities: SIBER Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80 (3): 595–602.
Keeling, C.D. 1979. The Suess effect: 13Carbon-14Carbon interactions. Environment International 2 (4–6): 229–300.
Koch, P.L., N. Tuross, and M.L. Fogel. 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxyapatite. Journal of Archaeological Science 24 (5): 417–429.
LeGrande, A.N., and G.A. Schmidt. 2006. Global gridded data set of the oxygen isotopic composition in seawater. Geophysical Research Letters 33 (12): L12604.
Lodi, L., C. Domit, P. Laporta, J.C. Di Tullio, C.C.A. Martins, and E. Vermeulen. 2016. Report of the working group on the distribution of Tursiops truncatus in the Southwest Atlantic Ocean. Latin American Journal of Aquatic Mammals 11 (1–2): 29–46.
Martinez Arbizu, P. 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4. https://github.com/pmartinezarbizu/pairwiseAdonis. Accessed 13 June 2022.
Matsubayashi, J., and I. Tayasu. 2019. Collagen turnover and isotopic records in cortical bone. Journal of Archaeological Science 106: 37–44.
Matsubayashi, J., Y. Saitoh, Y. Osada, Y. Uehara, J. Habu, T. Sasaki, and I. Tayasu. 2017. Incremental analysis of vertebral centra can reconstruct the stable isotope chronology of teleost fishes. Methods in Ecology and Evolution 8 (12): 1755–1763.
Matthews, C.J., F.J. Longstaffe, and S.H. Ferguson. 2016. Dentine oxygen isotopes (δ18O) as a proxy for odontocete distributions and movements. Ecology and Evolution 6 (14): 4643–4653.
McClelland, J.W., and I. Valiela. 1998. Changes in food web structure under the influence of increased anthropogenic nitrogen inputs to estuaries. Marine Ecology Progress Series 168: 259–271.
McMahon, K.W., L.L. Hamady, and S.R. Thorrold. 2013. A review of ecogeochemistry approaches to estimating movements of marine animals. Limnology and Oceanography 58 (2): 697–714.
Michener, R.H., and K. Lajtha. 2007. Stable isotopes in ecology and environmental science, 2nd ed. Malden: Blackwell publishing.
Miloslavich, P., E. Klein, J.M. Díaz, C.E. Hernández, G. Bigatti, L. Campos, F. Artigas, J. Castillo, P.E. Penchaszadeh, P.E. Neill, A. Carranza, M.V. Retana, J.M. Díaz de Astarloa, M. Lewis, Y. Yorio, M.L. Piriz, D. Rodríguez, Y. Yoneshigue-Valentin, L. Gamboa, and A. Martín. 2011. Marine biodiversity in the Atlantic and Pacific coasts of South America: knowledge and gaps. PLoS ONE 6 (1): e14631.
Molina-Schiller, D., S.A. Rosales, and T.R.O. De Freitas. 2005. Oceanographic conditions off Coastal South America in relation to the distribution of Burmeister´s porpoise, Phocoena spinipinnis. Latin American Journal of Aquatic Mammals 4 (2): 141–156.
Moreira, D., and C.G. Simionato. 2019. Hidrología y circulación del estuario del Río de la plata. Meteorologica 44 (1): 1–30.
Naya, D.E., M. Arim, and R. Vargas. 2002. Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos. Uruguay. Marine Mammal Science 18 (3): 734–745.
Nehlich, O., and M.P. Richards. 2009. Establishing collagen quality criteria for sulphur isotope analysis of archaeological bone collagen. Archaeological and Anthropological Sciences 1 (1): 59–75.
Newsome, S.D., M.T. Clementz, and P.L. Koch. 2010. Using stable isotope biogeochemistry to study marine mammal ecology. Marine Mammal Science 26 (3): 509–572.
Oksanen, J., G.F. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, M.H.H. Stevens, E. Szoecs, and H. Wagner. 2020. Package ‘vegan’, version 2.5–7. Community Ecology Package. http://CRAN.Rproject.org/package=vegan. Accessed 13 June 2022.
Ortega, L., and A. Martínez. 2007. Multiannual and seasonal variability of water masses and fronts over the Uruguayan shelf. Journal of Coastal Research 23 (3): 618–629.
Peterson, B.J. 1999. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecologica 20 (4): 479–487.
Peterson, B.J., and R.W. Howarth. 1987. Sulfur, carbon, and nitrogen isotopes used to trace organic matter flow in the salt-marsh estuaries of Sapelo Island, Georgia 1. Limnology and Oceanography 32 (6): 1195–1213.
Peterson, B.J., R.W. Howarth, and R.H. Garritt. 1985. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 227 (4692): 1361–1363. https://doi.org/10.1126/science.227.4692.1361.
Pinzone, M., F. Damseaux, L.N. Michel, and K. Das. 2019. Stable isotope ratios of carbon, nitrogen and sulphur and mercury concentrations as descriptors of trophic ecology and contamination sources of Mediterranean whales. Chemosphere 237: 124448.
Post, D.M. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83 (3): 703–718.
Pritchard, D.W. 1952. Estuarine hydrography. In Advances in geophysics, vol. 1, 243–280. Elsevier.
R Core Team. 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org. Accessed 13 June 2022.
Ramos, R., and J. González-Solís. 2012. Trace me if you can: the use of intrinsic biogeochemical markers in marine top predators. Frontiers in Ecology and the Environment 10 (5): 258–266.
Reeves, R.R., B.S. Stewart, P.J. Clapham, and J.A. Powell. 2002. Guide to marine mammals of the world, 528. NY: Knopf.
Reyes, J.C. 2018. Burmeister’s porpoise Phocoena spinipinnis Burmeister, 1865. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 146–148. San Diego: Academic Press.
Reyes, J.C., and K. Van Waerebeek. 1995. Aspects of the biology of Burmeister’s porpoise from Peru. Rep. Int. Whal. Commn (special Issue) 16: 349–364.
Riccialdelli, L., S.D. Newsome, M.L. Fogel, and R.N.P. Goodall. 2010. Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Marine Ecology Progress Series 418: 235–248.
Rodríguez, D.H., M. Dassis, A.P. de León, C. Barreiro, M. Farenga, R.O. Bastida, and R.W. Davis. 2013. Foraging strategies of southern sea lion females in the La Plata river estuary (Argentina-Uruguay). Deep Sea Research Part II: Topical Studies in Oceanography 88: 120–130.
Roman, J., and J.A. Ester. 2018. Ecology. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 299–303. San Diego: Academic Press.
Rubenstein, D.R., and K.A. Hobson. 2004. From birds to butterflies: animal movement patterns and stable isotopes. Trends in Ecology and Evolution 19 (5): 256–263. https://doi.org/10.1016/j.tree.2004.03.017.
Saporiti, F., S. Bearhop, D.G. Vales, L. Silva, L. Zenteno, M. Tavares, E.A. Crespo, and L. Cardona. 2016. Resource partitioning among air-breathing marine predators: are body size and mouth diameter the major determinants? Marine Ecology 37 (5): 957–969. https://doi.org/10.1111/maec.12304.
Schoeninger, M.J. 2010. Chapter 15: Toward a δ13C isoscape for primates. In Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping, ed. J.B. West, G.J. Bowen, T.E. Dawson, and K.P. Tu, 319–333. Dordrecht: Springer Science & Business Media. https://doi.org/10.1007/978-90-481-3354-3_15.
Secchi, E.R., S. Botta, M.M. Wiegand, L.A. Lopez, P.F. Fruet, R.C. Genoves, and J.C. Di Tullio. 2017. Long-term and gender-related variation in the feeding ecology of common bottlenose dolphins inhabiting a subtropical estuary and the adjacent marine coast in the western South Atlantic. Marine Biology Research 13 (1): 121–134.
Seyboth, E., S. Botta, and E. Secchi. 2018. Using chemical elements to the study of trophic and spatial ecology in marine mammals of the Southwestern Atlantic Ocean. In Advances in marine vertebrate research in Latin America: technological innovation and conservation, 221–248. Cham: Springer.
Skedros, J.G., A.N. Knight, G.C. Clark, C.M. Crowder, V.M. Dominguez, S. Qiu, D.M. Mulhern, S.W. Donahue, B. Busse, B.I. Hulsey, and M. Zedda. 2013. Scaling of Haversian canal surface area to secondary osteon bone volume in ribs and limb bones. American Journal of Physical Anthropology 151 (2): 230–244.
Smith, E.P. 2020. Ending reliance on statistical significance will improve environmental inference and communication. Estuaries and Coasts 43: 1–6.
Tellechea, J.S., W. Perez, D. Olsson, M. Lima, and W. Norbis. 2017. Feeding habits of franciscana dolphins (Pontoporia blainvillei): Echolocation or passive listening? Aquatic Mammals 43 (4): 430–438. https://doi.org/10.1578/AM.43.4.2017.430.
Tomaszewicz, C.N.T., J.A. Seminoff, L. Avens, L.R. Goshe, J.M. Rguez-Baron, S.H. Peckham, and C.M. Kurle. 2018. Expanding the coastal forager paradigm: long-term pelagic habitat use by green turtles Chelonia mydas in the eastern Pacific Ocean. Marine Ecology Progress Series 587: 217–234.
Troina, G.C., F. Dehairs, S. Botta, J.C. Di Tullio, M. Elskens, and E.R. Secchi. 2020. Zooplankton-based δ13C and δ15N isoscapes from the outer continental shelf and slope in the subtropical western South Atlantic. Deep Sea Research Part I: Oceanographic Research Papers 159: 103235. https://doi.org/10.1016/j.dsr.2020.103235.
Troina, G.C., P. Riekenberg, M.T. van der Meer, S. Botta, F. Dehairs, and E.R. Secchi. 2021. Combining isotopic analysis of bulk-skin and individual amino acids to investigate the trophic position and foraging areas of multiple cetacean species in the western South Atlantic. Environmental Research 201: 111610. https://doi.org/10.1016/j.envres.2021.111610.
Wang, M.C., K.T. Shao, S.L. Huang, and L.S. Chou. 2011. Food partitioning among three sympatric odontocetes (Grampus griseus, Lagenodelphis hosei, and Stenella attenuata). Marine Mammal Science 28 (2): E143–E157.
Wells, R.S., and M.D. Scott. 2018. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Encyclopedia of marine mammals, 3rd ed., ed. B. Würsig, J.G.M. Thewissen, and K.M. Kovacs, 118–125. San Diego: Academic Press.
Wickert, J.C., S.M. von Eye, L.R. Oliveira, and I.B. Moreno. 2016. Revalidation of Tursiops gephyreus Lahille, 1908 (Cetartiodactyla: Delphinidae) from the southwestern Atlantic Ocean. Journal of Mammalogy 97 (6): 1728–1737.
Yoshida, N., and N. Miyazaki. 1991. Oxygen isotope correlation of cetacean bone phosphate with environmental water. Journal of Geophysical Research: Oceans 96 (C1): 815–820.
Acknowledgements
We thank the Museo Nacional de Historia Natural (MNHN, Uruguay) and the Facultad de Ciencias of the Universidad de la República (UdelaR, Uruguay) for allowing us access to their scientific collections.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The study was funded by the Fundació Barcelona Zoo (Spain) through the Research and Conservation Programme–PRIC (309998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Steven Litvin
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cani, A., Cardona, L., Valdivia, M. et al. Niche Partitioning Among Marine Mammals Inhabiting a Large Estuary as Revealed by Stable Isotopes of C, N, S, and O. Estuaries and Coasts 46, 1083–1097 (2023). https://doi.org/10.1007/s12237-023-01193-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01193-y