Abstract
Zooplankton indicators based on rotifers (TSIROT) and an indicator based on Secchi disk visibility (TSISD) were used to assess trophic state changes in artificial, slow-flowing, and stagnant canal waters. The study was conducted in the summers of 2019, 2021, and 2022 in the Bydgoszcz Canal and the Noteć Canal (Poland). Water samples were taken from five sites once in the month of July and once in August. In terms of the species number and density, rotifers dominated among zooplankton (at almost all sites), which reflected the high trophic state. Our results showed statistically significant correlations between TSIROT and TSISD as well as between the individual indicators TSIROT1 (Rotifer numbers), TSIROT2 (Rotifer biomass), TSIROT3 (bacterivorous numbers of rotifers), TSIROT6 (rotifers indicating high trophic state), and TSISD. According to our study, the presented indices most likely determine the trends between TSIROT cumulative and TSISD in the studied canals. Similarly to previous studies performed on lakes and small reservoirs, zooplankton constituted a useful indicator of trophic state. It seems that, for stagnant and slowly flowing waters of canals such as the Bydgoszcz Canal and the Noteć Canal, the method of zooplankton trophic status indicators based on rotifers may be applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the end of the twentieth century and the beginning of the 21st, the eutrophication of stagnant or slow-flowing waters became a major anthropogenic threat not only in Polish surface waters but in most freshwater systems worldwide (Kundu et al., 2015; Bowes et al., 2016; Ochocka, 2021). Eutrophication is caused by intensive loading of nutrients, especially nitrogen (N) and phosphorous (P) (Dembowska et al., 2015).
An uncontrolled increase in N and P pollution leads to an increase in nutrient-rich wastewater discharge and more intense nutrient area runoff into aquatic ecosystems (Glibert et al., 2014; Paerl et al., 2016). Deteriorations in water quality can be especially dangerous for shallow, slow-flowing canals (Shields et al., 2010) because they are exposed to anthropogenic pollutants from huge river catchments (Biggs et al., 2016). Most studies have focused on water pollution problems in natural freshwaters, like rivers, streams, or lakes, with few data describing canals. A slight excess of nutrients (initial level) in fact stimulates primary production (macrophytes and algae growth) and positively affects the diversity of aquatic organisms. However, the overload of nutrients and resulting overproduction can lead to an excess of organic matter, which bacteria then break down, consuming oxygen in the process and thus leading to oxygen deficits. This can degrade environmental conditions. Excessive amounts of nutrients can cause the development of cyanobacteria, which can be toxic to aquatic organisms (Chislock et al., 2013; Paerl & Otten, 2013; Olsen et al., 2016).
The level of nutrients can be assessed by determining the trophic status of waters. Carlson (1977) introduced one of the first and most widely used classification schemes for lake trophic state assessment—the Trophic State Index (TSI). It is usually numerically assessed from data on Secchi disk visibility (SD), total phosphorus (TP), and the concentration of the phytoplankton pigment chlorophyll a (chl a). Carlson’s trophic state index is constructed on a scale of 0 to 100. A Trophic State Index increase of 10 units describes a halving of Secchi disk visibility and a doubling of phosphorus and chlorophyll concentrations. Equally, a trophic state can be indicated based on reaction of planktonic organisms. A change in water trophy may completely or partially change the zooplankton community by, for example, increasing biomass and abundance (Hanson & Peters, 1984), decreasing body size (Pace, 1986; Karpowicz et al., 2020), and reducing species diversity (Andronikova, 1996; Haberman & Haldna, 2014). According to several authors (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013; Ochocka & Pasztaleniec, 2016; Pociecha et al., 2018; Stamou et al., 2019; García-Chicote et al., 2019; Mashkova et al., 2020; Muñoz-Colmenares et al., 2021), zooplankton can be a useful indicator for monitoring and assessing trophic level in freshwater ecosystems. For example, rotifers seem to serve as good indicators of water quality (Wallace et al., 2015), e.g., short development time, high reproduction rate, and short lifespans (Ricci & Balsamo, 2000; Ejsmont-Karabin, 2019). Zooplankton organisms play an important role in the trophic web of aquatic habitats. Their position was introduced on the example of bottom-up control (shaped by algae population) and top-down control (the impact of planktivorous fish) (Jeppesen et al., 2011; Ochocka, 2021). The release of nutrients to the water column favors phytoplankton production (Persson, 1999; Kraska et al., 2013) and deeply impacts the composition of the zooplankton community (Xiao et al., 2020). Cyanobacteria and large inedible algae affect zooplankton by disturbing feeding, by being of poor nutritional quality as food, and by producing toxins (Vanni & Lampert, 1992; Ferrão-Filho & Kozlowsky-Suzuki, 2011).
Rotifers are among the groups of zooplankton most sensitive to trophic changes. Changes in rotifer abundance and species composition may be a good indicator of water quality associated with, e.g., total phosphorus and chlorophyll-a concentrations (May et al., 2014). Since the rotifers are cosmopolitan organisms suited to a range of habitats, they are ecologically resilient, short life cycles, and quick response to local environmental factors (Ejsmont-Karabin, 2019). Thus they may prove useful for biological monitoring (May & O’Hare, 2005; Lodi et al., 2011; Ejsmont-Karabin, 2012).
The European Water Framework Directive (WFD) (Directive 2000) presents requirements and assessments for controlling water quality and classifying waterbodies (“Ecological Status”). The fundamental goal of the WFD is to achieve good ecological status which is reflected by indicators that include biological quality elements (BQE) that are especially sensitive to nutrient loads (Lyche-Solheim et al., 2013; Dudley et al., 2013). Zooplankton is not listed among the BQEs, although it is sensitive to changes in nutrient concentrations and could be considered as a good indicator for assessing trophic status of aquatic ecosystems. Besides the WFD requirements and within new EU revisions, it is worth to strength the need of incorporating the zooplankton among biological components for monitoring of water quality and assessing trophic state in water bodies.
The aim of the study was to apply zooplankton indicators (TSIRot) to assess the trophic level in canals. We assumed that the rotifers index (TSIRot) would reflect trophic changes in artificial, slow-flowing, and stagnant canal waters, similarly as for lakes. We hypothesized that zooplankton is a useful indicator of trophic state in the stagnant and slow-flowing waters of the Bydgoszcz Canal and its tributary the Noteć Canal.
Materials and methods
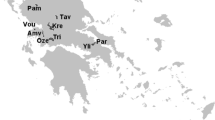
The research was conducted during the summers (July–August) of 2019, 2021, and 2022 in the Bydgoszcz Canal (part of which is located in the industrial area of Bydgoszcz city) and the Noteć Canal (located in the agricultural area near the town of Nakło). The study was performed in close proximity to locks—four in the Bydgoszcz Canal (Józefínki, Osowa Góra, Prądy, and Okole) and one in the Noteć Canal (Łochowo) (Fig. 1).
Map of investigated area. Bydgoszcz Canal: site 1—Józefinki; site 2—Osowa Góra; site 3—Prądy; site 4—Okole; and site 5—Noteć Canal, (Kolarova & Napiórkowski 2022—changed)
The Bydgoszcz Canal is a very important artificial waterway in north-west Poland. It is part of international waterway E70, which connects the two largest rivers in Poland—the Vistula and the Oder. The canal was built at the end of the eighteenth century. The total length of the Bydgoszcz Canal is 24.77 km, of which 15.7 km lies within the catchment of the Noteć River (a tributary of the Oder) and 9.0 km within the catchment of the Brda River (a tributary of the Vistula). The average depth of studied sites was 1.8 m depending on water level and the average width was 29 m (water flow was ~ 0.004 m/s).
The Noteć Canal comprises two sections (one simply referred to as the Noteć Canal and the other as the Upper Noteć Canal). The canal waterway covers the course of the Noteć river. The Upper Noteć Canal (where the samples were taken) is 25 km long and is strongly affected by anthropogenic contaminants due to human activities, including agriculture. At the study site, the canal was shallow, with an average depth of 1 m and an average width of about 15 m (water flow was ~ 0.14 m/s).
Water samples were collected at five sampling sites in two areas: Area 1: the Bydgoszcz Canal: (site 1) Józefinki upstream of the lock 53° 07′ 49.7′′ N 17° 38′ 23.9′′ E; (site 2) Osowa Góra upstream and downstream of the lock 53° 08′ 48.9′′ N 17° 52′ 49.2′′ E; (site 3) Prądy upstream and downstream of the lock 53° 08′ 38.6′′ N 17° 53′ 37.8′′ E; and (site 4) Okole downstream of the lock 53° 08′ 11.9′′ N 17° 58′ 06.1′′ E and Area 2: (site 5) the Noteć Canal—Łochowo upstream and downstream of the lock 53° 07′ 56.5′′ N 17° 51′ 18.1′′ E. Water samples were collected once a month in July and August of 2019, 2021, and 2022. Additional samples were taken from sites 2, 3, and 5 in 2022. A total of 36 samples were collected. Water samples were taken at different depths using a 1-L Patalas bucket. To obtain one qualitative and quantitative sample of zooplankton, 20 l of water was filtered through a plankton net, mesh size 25 μm. All samples were preserved with Lugol’s iodine solution (Wallace et al., 1993; Harris et al., 2000). The samples were prepared for counting under a microscope according to the methodology described previously by Wallace et al., (1993). Zooplankton density and biomass were calculated per 1 l of water.
The identification of zooplankton was performed using an Olympus BX 43 light microscope as well as an Olympus LC 30 soft imaging camera at ×10 magnification. The commonly available keys were used for taxonomical identification of zooplankton (Wallace et al., 1993; Radwan et al., 2004; Błędzki & Rybak, 2016). The rotifers biomass was calculated using the formula according to Radwan et al. (2004) and Ruttner-Kolisko (1977). At the same time as zooplankton sampling, the following environmental parameters of water were measured: water temperature (WT, °C), Secchi disk visibility (SD, m), conductivity (EC, µS/cm), oxygen concentration (DO, mg/l), chlorophyll (chl-a, µg/l), nitrates (NNO3−, mg/l), phosphates (PPO4 2−, mg/l), and pH (Table 1). Measurements were taken using Multimeter WTW Multi 3430SET F Xylem Analytics field probes (Weilheim, Germany). The surface water flow was measured during the sampling period using the electromagnetic hydrometric mill (Model 801).
Zooplankton seems to be a good indicator of trophic state in canals. This is because their slow water flow is similar to summer stagnant water period in lakes, for which zooplankton are proven to be an effective indicator. In the vicinity of the locks, the canals resembled small polymictic waterbodies. The season (July and August) was suggested by Ejsmont-Karabin (2012) as the best for comparative analysis of zooplankton because the stable summer communities are mainly influenced by trophic factors. A similar relationship was observed in previous studies conducted on the zooplankton of the Bydgoszcz Canal (Kolarova & Napiórkowski 2022). The water trophy in the canals was assessed based on the species composition, abundance, and biomass of rotifers.
In accordance with Ejsmont-Karabin (2012), the following indices were used to assess the Trophic State Index (TSIROT): (1) rotifer number; (2) total biomass of rotifer community; (3) percentage of bacterivores in total rotifer number; (4) ratio of biomass to number; (5) percentage of tecta form in the population of Keratella cochlearis (Gosse, 1851); and (6) contribution of species that indicate a high trophic state in the indicatory group’s number. TSIROT was obtained as the mean value of the mentioned indices. The small bacterivorous and detritophagous rotifers increase with trophic state (Obertegger & Wallace, 2023).
According to Carlson (1977), we used an index based on the Secchi disk visibility (SD) to determine the trophic state of the canals (TSISD). The TSISD was calculated using the formula: 60–14.41 ln(SD), where SD was measured in meters. According to some authors, TSISD is often used as an indicator for evaluating eutrophication in different types of water bodies (Jekatierynczuk-Rudczyk et al., 2012; Kordi et al., 2012; Haberman & Haldna, 2014; Heddam, 2016; Ochocka & Pasztaleniec, 2016).
Statistical analysis was performed using Statistica 14.0.0.15 software (TIBCO Software Inc., Palo Alto, CA, USA). Scatterplot with linear regression line was applied to analyze the relationship between the rotifer index TSIROT and the index based on the Secchi disk TSISD. Pearson simple correlation coefficient was calculated to compare the rotifers indices (TSIROT1-ROT6) and TSISD.
The level (P < 0.05) was indicated for statistically significant correlations. The normality of data distribution was tested by the Shapiro–Wilk W test (Shapiro & Wilk, 1965).
Results
Environmental characteristic
The water temperatures in the Bydgoszcz Canal and Noteć Canal were similar during the studies, averaging 21 °C. The average water transparency in the Bydgoszcz Canal was 1.5 m and increased from 0.9 m at Site 4 to 1.8 m at Site 2 and 3. Meanwhile, the average water transparency in the Noteć Canal came to 1.8 m and ranged from 1.3 to 2.0 m. The average water pH at all sites was slightly above 7, indicating alkaline conditions. The average value of dissolved oxygen concentration ranged from 6.5 mg/l in the Noteć Canal (site 5) up to 9.5 mg/l in the Bydgoszcz Canal (site 4). The conductivity was similar at all sites. The average chlorophyll concentration varied from 4.1 mg/l in the Noteć Canal up to 13.8 mg/l in the Bydgoszcz Canal at Site 4. There was no significant difference in the concentration of NNO3− and PPO42− between the Bydgoszcz Canal and the Noteć Canal (Table 1).
Rotifers characteristic
Samples analysis revealed the presence of 77 rotifer species, (i.e., 73% of all zooplankton species). In the Noteć Canal, 42 rotifer species were identified (i.e., 82% of all zooplankton species). The lowest number of rotifer species was recorded in the Bydgoszcz Canal at Site 1 (36 species). The highest number of rotifer species was recorded in the Bydgoszcz Canal at Site 4 (56 species). A list of all rotifers species at individual sites has been added as an attachment.
The highest average number of species in a single sample was observed at Site 4 (23 species) and the lowest at Site 1 (10 species). The highest and the lowest number of rotifer species were found at the same sites, like the average number of species, in one sample.
The average rotifer density was the highest in the Bydgoszcz Canal (148 ind/l) (ranging from 35 ind/l at Site 2 to 445 ind/l at Site 4) and lowest in the Noteć Canal—Site 5 (30 ind/l) (Table 2). Rotifers dominate in terms of the number of species and abundance in all studied sites. The dominant rotifer species were Anuraeopsis fissa (Gosse, 1851), Polyarthra remata Skorikov, 1896 and Brachionus calyciflorus Pallas, 1766.
The average rotifer biomass was five times higher in the Bydgoszcz Canal (0.049 mg/l) than in the Noteć Canal (0.009 mg/l). The rotifer biomass was lowest at site 5 in the Noteć Canal and highest at Site 4 in the Bydgoszcz Canal (0.150 mg/l) (Table 2).
During the study period, the α-diversity index (H′ = 2.49 ± 0.40) was highest at Site 2 in the Bydgoszcz Canal and lowest (H′ = 1.68 ± 0.41) at Site 1 in the Bydgoszcz Canal. The eveness index was highest (J′ = 0.72 ± 0.13) in the Bydgoszcz Canal, at Site 2 and lowest (J′ = 0.39 ± 0.20) at Site 4 in the Bydgoszcz Canal (Table 2).
Trophic State Index
Average TSISD value ranged from 51 at Site 2 to 62 at Site 4 in the Bydgoszcz Canal. According to the average value of TSISD, the trophic states of the sites in the Bydgoszcz Canal were classified from high mesotrophic (Site 2 and Site 3) to high eutrophic (Site 4) (Table 3). Thus, the average value of TSISD in the Bydgoszcz Canal was classified as eutrophic.
Average TSI value based on Chl-a varied from 32 in the Noteć Canal (Site 5) to 53 in the Bydgoszcz Canal (Site 3). The value of indices indicated from oligotrophic (Site 5) to meso-eutrophic state (Site 3). According to the trophic state value calculated based on Chl-a, the canals were classified from oligotrophic to high meso-eutrophic state (Table 3). During the study in 2019, 2021, and 2022, the average value of TSISD in the Bydgoszcz Canal increased from the rural agriculture area to the industrial area near Bydgoszcz city.
Average TSIROT (cumulative index) varied from 45 (Site 1, Site 2, Site 3) in the Bydgoszcz Canal) to 52 (Site 4—Bydgoszcz Canal). Most sites were classified from high mesotrophic to low meso-eutrophic state. Only Site 4 was classified as being in a high meso-eutrophic state. The average value of TSIROT in the Bydgoszcz Canal and in the Noteć Canal was 47 (Table 3). The highest values of rotifer indexes (TSIROT1-6) were at Site 4 (Bydgoszcz Canal). Only the value of TSIROT5 was higher at Site 1, Site 3 (Bydgoszcz Canal), and at Site 5 in the Noteć Canal than at Site 4 (Bydgoszcz Canal). The highest TSIROT3 (percentage of bacterivorous species; 59.3%) and TSIROT6 (species indicating high trophic state; 75.3%) in the total numbers of rotifers were found in the Bydgoszcz Canal at Site 4. The dominant species was A. fissa (56.2%).
Comparison of indices
The TSI was developed by Carlson based on water transparency (SD). Therefore, in order to verify the TSIROT in the analyzed canals, the authors first took into account TSISD. Our study showed that TSIROT correlates with the TSISD in slow-flowing and stagnant water of canals (r = 0.6037, P = 0.0001) (Fig. 2).
Relationship between rotifers index (TSIROT) and trophic state index calculated (acc. Carlson 1977) for Secchi disk visibility (TSISD), mean values at sites in the studied canals
During the analysis of individual indicators that make up the cumulative indicator TSIROT, it was noted that TSIROT1 based on the number of rotifers are statistically significant and correlate at the level of r = 0.6216 with trophic state index of Secchi disk visibility (TSISD) (P < 00001). TSIROT2 based on rotifer biomass depends on TSISD. Correlation coeficients are statistically significant at the level of r = 0.4849, P < 0.05. However, the relationship between the biomass and canal’s TSISD is weaker than the relationship between the numbers and TSISD. TSIROT3 (percentage of bacterivorous species—A. fissa, Filinia spp., Brachionus angularis Gosse, 1851, Keratella cochlearis (Gosse, 1851), Pompholyx sulcata Hudson, 1885 in the total number of rotifers) correlates with TSISD at the level of r = 0.6498, P < 0.0001. Rotifers index of individual indicator (TSIROT6) calculated on numbers of rotifer indicators of high trophic state in the indicative ecological group (IHT, %) is also well correlated with TSISD (r = 0.5656, P < 0.05) (Table 4).
Discussion
During the research, the trophic status of the canals was assessed on the basis of the species composition, abundance and biomass of zooplankton (rotifers), as well as on the basis of physical and chemical data.
The chlorophyll concentration decreased at most sites, which contributed to an increase in water transparency. The lowest Secchi disk visibility (0.9 m on average) was recorded at Site 4 and indicated high chlorophyll concentration (Table 1). Algal bloom coincided with high summer temperatures (21 °C on average). Many authors noted that the water transparency is a significant integrative indicator of phytoplankton biomass and primary production searched in the river basins (Kolarova & Napiórkowski, 2022; Shelyuk, 2019; Wilkerson et al., 2015). The concentration of oxygen was also the highest at Site 4. Algae are mainly responsible for the production of oxygen in water (Dembowska et al., 2012), which is confirmed by our research. Other physical and chemical parameters were at approximately uniform levels across all sites in the 3 years of the research (Table 1). During the study, rotifers dominated in species number (78%) and density (65%). Such a significant share of rotifers in zooplankton formation allows the use of this group as indicators (Ejsmont-Karabin, 2012). The highest number of rotifer species (23) and average density (445 ind/l) were found at Site 4, while the lowest number of rotifer species (10) at Site 1 and average density (30 ind/l) were found in the Noteć Canal (Table 2). Rotifer density increased from the sites outside of the city to the sites nearer to the city. Dembowska et al. (2015) suggest that rotifer density may be a more sensitive indicator of changes in trophic state than is species diversity. An increase in small-bodied rotifers and low individual biomasses indicate trophic conditions (Arndt, 1993; Radwan et al., 2004). For example, bacterioplankton-detritus-eating rotifers, which occur during the summer blooms, indicate high trophy (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Hillbricht-Ilkowska, 1994). A similar regularity was observed in the trophic gradient on the studied canals.
A previous study has noted that zooplankton density clearly correlates to Secchi disk visibility as a recommended indicator of trophic conditions (Ochocka, 2021). The results of our studies confirmed that rotifer density increased with TSISD. On the other hand, changes in TSISD seemed to have less influence on the number of rotifer species. At most sites, the rotifer index (TSIROT) increased along with rotifer density and number of species (Tables 2 and 3).
We found a positive correlation between TSIROT and TSISD in the studied canals (Fig. 2). It is clear that an increase in trophic pollution of waters causes an increase in TSISD and thus also an increase in TSIROT together with individual sub-indicators. Based on TSISD, most of the sites were classified as meso-eutrophic, whereas Site 4 was classified as high eutrophic. The sites with higher trophic status had lower transparency, which was the result of their exposure to higher-nutrient loads from anthropogenic sources. However, shallow water catchments are less resistant to eutrophication and pollution (Sługocki & Czerniawski, 2018). During the study, we found that the dominant species [K. cochlearis, Keratella tecta (Gosse, 1851), B. calyciflorus, and A. fissa] prefer high trophic states. The species found during our research were typical of eutrophic waters (Ejsmont-Karabin, 2012; Pociecha et al., 2018).
Based on TSIROT, the studied canals were characterized by low meso-eutrophy. TSIROT increased toward the city in the Bydgoszcz Canal. The part of the Bydgoszcz Canal catchment exposed to the city showed greater trophic pollution. At Site 4, the TSIROT value was highest (52—high meso-eutrophy). In this part of the canal, we observed the highest rotifer biomass and the highest chlorophyll-a concentration.
This study constitutes the first time that trophic state indices have ever been used in canals. Similar dependences have appeared only in research by Ejsmont-Kararbin (2012, 2013) but that was performed on lakes.
The results of our study showed that the rotifer indices are a functional and useful tool for assessing the trophic state of canals. We noticed that TSIROT correlates with the TSISD in slow-flowing and stagnant water of canals (Fig. 2, Table 4). Previous studies showed that rotifer indices are best for assessing the trophic state of ponds, dam reservoirs, coastal lagoons, or inland lakes (Ejsmont-Karabin, 2012; Gutkowska et al., 2013; Pociecha et al., 2018; Stamou et al., 2019; Kim et al., 2020; Kuczyńska-Kippen et al., 2021). Unfortunately, there is only a very small number of studies using rotifer indices to assess trophic conditions in slow-flowing waters (Sor et al., 2015; Liang et al., 2020). However, many authors suggest using zooplankton abundance, species composition, and biomass for assessing trophic conditions and controlling long-term changes in water quality (Montagud et al., 2019; Muñoz-Colmenares et al., 2021; Spoljar, 2013).
The European Water Framework Directive (WFD) requires the ecological quality of waters to be maintained based on the assessment of biological quality elements (BQE) and supported by a set of physical and chemical and hydro-morphological elements (Directive 2000). However, zooplankton have been omitted as a biological indicator from the water quality assessment. Nevertheless, zooplankton communities are an important component in the pelagic food web, as they respond quickly to environmental changes (Shurin et al., 2010). Thus, they may be an effective and useful indicator of water quality (Jeppesen et al., 2011), as our research shows.
Conclusion
Rotifers were the dominant group of zooplankton in the studied canals. Their taxonomic composition was typical of eutrophic and shallow waters.
Our research involved the first use of the rotifer index (TSIROT) and Secchi disk visibility index (TSISD) to assess trophic level in the slow-flowing and stagnant water of canals.
We found a positive correlation between TSIROT and TSISD. An increase in trophic pollution in waters causes an increase in TSISD and thus also increases in TSIROT and individual sub-indicators. We emphasized the importance of rotifers as indicators of trophic state in canals. Rotifers are functional groups of zooplankton species and could be included in the list of BQEs.
Data availability
Enquiries about data availability should be directed to the authors.
References
Andronikova, I., 1996. Zooplankton characteristics in monitoring of Lake Ladoga. Hydrobiologia 322: 173–179. https://doi.org/10.1007/BF00031824.
Arndt, H., 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)—a review. Hydrobiologia 255(256): 231–246. https://doi.org/10.1007/978-94-011-1606-0_31.
Biggs, J., S. Von Fumetti & M. Kelly-Quinn, 2016. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 793: 3–39. https://doi.org/10.1007/s10750-016-3007-0.
Błędzki, L. A. & J. I. Rybak, 2016. Freshwater Crustacean Zooplankton of Europe. Cladocera and Copepoda (Calanoida, Cyclopoida) Key to species identification, Springer, Cham: https://doi.org/10.1007/978-3-319-29871-9_7.
Bowes, M., P. Davison, M. Hutchins, S. McCall, C. Prudhomme, J. Sadowski, R. Soley, R. Wells & S. Willets, 2016. Climate change and eutrophication risk in English rivers. Technical Report No. SC140013/R, Environment Agency: Bristol, UK.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Chislock, M. F., E. Doster, R. A. Zitomer & A. E. Wilson, 2013. Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nature Education Knowledge 4(4): 10.
Dembowska, E., B. Głogowska & K. Dąbrowski, 2012. Dynamics of algae communities in an oxbow lake (Vistula River, Poland). Fisheries & Aquatic Life 20(1): 27–37. https://doi.org/10.2478/v10086-012-0004-4.
Dembowska, E. A., P. Napiórkowski, T. Mieszczankin & S. Józefowicz, 2015. Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecological Indicators 56: 15–22. https://doi.org/10.1016/j.ecolind.2015.03.019.
Dudley, B., M. Dunbar, E. Penning, A. Kolada, S. Hellsten, A. Oggioni, V. Bertrin, F. Ecke & M. Søndergaard, 2013. Measurements of uncertainty in macrophyte metrics used to assess European lake water quality. Hydrobiologia 704: 179–191. https://doi.org/10.1007/s10750-012-1338-z.
EC, 2000. European commission directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of European Communities Brussels L327: 1–72.
Ejsmont-Karabin, J., 2012. The usefulness of zooplankton as lake ecosystem indicators: Rotifer Trophic State Index. Polish Journal of Ecology 60(2): 339–350.
Ejsmont-Karabin, J., 2019. Does the world need faunists? Based on rotifer (Rotifera) occurrence reflections on the role of faunistic research in ecology. International Review of Hydrobiology 104(3–4): 49–56. https://doi.org/10.1002/iroh.201901991.
Ejsmont-Karabin, J. & A. Hillbricht-Ilkowska, 1994. Illustration of the eutrophication process: comparison of rotifers from Mikolajskie Lake in the years 1989–1990 and 1963–1964. Polskie Archiwum Hydrobiologii 41(4): 477–487.
Ejsmont-Karabin, J. & A. Karabin, 2013. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Polish Journal of Ecology 61(3): 561–573.
Ferrão-Filho, A. S. & B. Kozlowsky-Suzuki, 2011. Cyanotoxins: bioaccumulation and effects on aquatic animals. Marine Drugs. https://doi.org/10.3390/md9122729.
García-Chicote, J., X. Armengol & C. Rojo, 2019. Zooplankton species as indicators of trophic state in reservoirs from Mediterranean river basins. Inland Waters 9(1): 113–123. https://doi.org/10.1080/20442041.2018.1519352.
Glibert, P. M., D. C. Hinkle, B. Sturgis & R. V. Jesien, 2014. Eutrophication of a Maryland/Virginia coastal lagoon: a tipping point, ecosystem changes, and potential causes. Estuaries and Coasts 37(1): 128–146. https://doi.org/10.1007/s12237-013-9630-3.
Gutkowska, A., E. Paturej & E. Kowalska, 2013. Rotifer trophic state indices as ecosystem indicators in brackish coastal waters. Oceanologia 55(4): 887–899. https://doi.org/10.5697/oc.55-4.887.
Haberman, J. & M. Haldna, 2014. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Vőrtsjärv. Journal of Limnology. https://doi.org/10.4081/jlimnol.2014.828.
Hanson, J. M. & R. H. Peters, 1984. Empirical prediction of crustacean zooplankton biomass and profundal macrobenthos biomass in lakes. Canadian Journal of Fisheries and Aquatic Sciences 41: 439–445. https://doi.org/10.1139/f84-052.
Harris, R., P. Wiebe, J. Lenz, H. R. Skjoldal & M. Huntley, 2000. ICES zooplankton methodology manual, Academic Press, Cambridge:, 147–173. https://doi.org/10.1016/B978-0-12-327645-2.X5000-2.
Heddam, S., 2016. Secchi disk depth estimation from water quality parameters: artificial neural network versus multiple linear regression models? Environmental Processes 3(2): 525–536. https://doi.org/10.1007/s40710-016-0144-4.
Jekatierynczuk-Rudczyk, E., M. Grabowska, J. Ejsmont-Karabin & M. Karpowicz, 2012. Assessment of trophic state of four lakes in the Suwałki Landscape Park (NE Poland) based on the summer phyto–and zooplankton in comparison with some physicochemical parameters. Current advances in algal taxonomy and its applications. Institute of Botany Polish Academy of Sciences: 205–225.
Jeppesen, E., P. Noges, T. A. Davidson, J. Haberman, T. Noges, K. Blank, T. L. Lauridsen, M. Søndergaard, C. Sayer & R. Laugaste, 2011. Zooplankton as indicators in lakes: a scientific–based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 279–297. https://doi.org/10.1007/s10750-011-0831-0.
Karpowicz, M., Ł Sługocki, J. Kozłowska, A. Ochocka & C. Lopez, 2020. Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2020.106585.
Kim, S. K., J. C. Kim, G. J. Joo & J. Y. Choi, 2020. Response of the rotifer community to human–induced changes in the trophic state of a reservoir. Oceanological and Hydrobiological Studies 49(4): 329–344. https://doi.org/10.1515/ohs-2020-0029.
Kolarova, N. & P. Napiórkowski, 2022. How do specific environmental conditions in canals affect the structure and variability of the zooplankton community? Water 14(6): 979. https://doi.org/10.3390/w14060979.
Kordi, H., S. Hoseini, M. Sudagar & A. Alimohammadi, 2012. Correlation of chlorophyll–a with Secchi disk depth and water turbidity in aquaculture reservoirs a case study on Mohammadabad reservoirs, Gorgan, Iran. World Journal of Fish and Marine Sciences 4(4): 340–343. https://doi.org/10.5829/idosi.wjfms.2012.04.04.6345.
Kraska, M., P. Klimaszyk & R. Piotrowicz, 2013. Anthropogenic changes in properties of the water and spatial structure of the vegetation of the lobelia lake Lake Modre in the Bytów Lakeland. Oceanological and Hydrobiological Studies 42(3): 302–313. https://doi.org/10.2478/s13545-013-0087-6.
Kuczyńska-Kippen, N., M. Špoljar, M. Mleczek & C. Zhang, 2021. Elodeids, but not helophytes, increase community diversity and reduce trophic state: Case study with rotifer indices in field ponds. Ecological Indicators 128: 107829. https://doi.org/10.1016/j.ecolind.2021.107829.
Kundu, S., M. V. Coumar, S. Rajendiran, A. Rao & A. S. Rao, 2015. Phosphates from detergents and eutrophication of surface water ecosystem in India. Current Science 108: 1320–1325.
Liang, D., Q. Wang, N. Wei, C. Tang, X. Sun & Y. Yang, 2020. Biological indicators of ecological quality in typical urban river–lake ecosystems: the planktonic rotifer community and its response to environmental factors. Ecological Indicators 112: 106127. https://doi.org/10.1016/j.ecolind.2020.106127.
Lodi, S., L. C. G. Vieira, L. F. M. Velho, C. C. Bonecker, P. de Carvalho & L. M. Bini, 2011. Zooplankton community metrics as indicators of eutrophication in urban lakes. Natureza & Conservação 9(1): 87–92. https://doi.org/10.4322/natcon.2011.011.
Lyche-Solheim, A., C. K. Feld, S. Birk, G. Phillips, L. Carvalho, G. Morabito, U. Mischke, N. Willby, M. Søndergaard, S. Hellsten, A. Kolada, M. Mjelde, J. Böhmer, O. Miler, M. T. Pusch, C. Agrillier, E. Jeppesen, T. L. M. Lauridsen & S. Poikane, 2013. Ecological status assessment of European lakes: a comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 704(1): 57–74. https://doi.org/10.1007/s10750-012-1436-y.
Mashkova, I. V., A. Kostriykova, E. Shchelkanova, V. Trofimenko & A. Slavnaya, 2020. Study of the zooplankton community as an indicator of the trophic status of reservoirs. GEOMATE Journal 19(73): 57–63. https://doi.org/10.21660/2020.73.5337a.
May, L. & M. O’Hare, 2005. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. Hydrobiologia 546(1): 397–404. https://doi.org/10.1007/1-4020-4408-9_41.
May, L., B. M. Spears, B. J. Dudley & I. D. Gunn, 2014. The response of the rotifer community in Loch Leven, UK, to changes associated with a 60% reduction in phosphorus inputs from the catchment. International Review of Hydrobiology 99(1–2): 65–71. https://doi.org/10.1002/iroh.201301705.
Montagud, D., J. M. Soria, X. Soria-Perpiñà, T. Alfonso & E. Vicente, 2019. A comparative study of four indexes based on zooplankton as trophic state indicators in reservoirs. Limnetica 38(1): 291–302. https://doi.org/10.23818/limn.38.06.
Muñoz-Colmenares, M. E., J. M. Soria & E. Vicente, 2021. Can zooplankton species be used as indicators of trophic status and ecological potential of reservoirs? Aquatic Ecology 55(4): 1143–1156. https://doi.org/10.1007/s10452-021-09897-8.
Obertegger, U. & R. L. Wallace, 2023. Trait-based research on Rotifera: the Holy Grail or Just Messy? Water 15(8): 1459. https://doi.org/10.3390/w15081459.
Ochocka, A., 2021. ZIPLAS: Zooplankton Index for Polish Lakes’ Assessment: a new method to assess the ecological status of stratified lakes. Environmental Monitoring and Assessment 193(10): 1–15. https://doi.org/10.1007/s10661-021-09390-7.
Ochocka, A. & A. Pasztaleniec, 2016. Sensitivity of plankton indices to lake trophic conditions. Environmental Monitoring and Assessment 188(11): 1–16. https://doi.org/10.1007/s10661-016-5634-3.
Olsen, B. K., M. F. Chislock & A. E. Wilson, 2016. Eutrophication mediates a common off–flavor compound, 2–methylisoborneol, in a drinking water reservoir. Water Research 92: 228–234. https://doi.org/10.1016/j.watres.2016.01.058.
Pace, M. L., 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnology and Oceanography 31: 45–55. https://doi.org/10.4319/lo.1986.31.1.0045.
Paerl, H. W. & T. G. Otten, 2013. Harmful cyanobacterial blooms: causes, consequences, and controls. Microbial Ecology 65(4): 995–1010. https://doi.org/10.1007/s00248-012-0159-y.
Paerl, H. W., J. T. Scott, M. J. McCarthy, S. E. Newell, W. S. Gardner, K. E. Havens, D. K. Hoffman, S. W. Wilhelm & W. A. Wurtsbaugh, 2016. It takes two to tango: when and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environmental Science & Technology 50: 0805–10813. https://doi.org/10.1021/acs.est.6b02575.
Persson, L., 1999. Trophic cascades: abiding heterogeneity and the trophic level concept at the end of the road. Oikos. https://doi.org/10.2307/3546688.
Pociecha, A., I. Bielańska-Grajner, H. Kuciel & A. Z. Wojtal, 2018. Is zooplankton an indicator of the water trophic level in dam reservoirs? Oceanological and Hydrobiological Studies 47(3): 288–295. https://doi.org/10.1515/ohs-2018-0027.
Radwan, S., I. Bielańska-Grajner & J. Ejsmont-Karabin, 2004. Rotifers (Rotifera, Monogononta), Freshwater Fauna of Poland University of Lodz Press, Łódź, Poland:, 579.
Ricci, C. & M. Balsamo, 2000. The biology and ecology of lotic rotifers and gastrotrichs. Freshwater Biology 44(1): 15–28.
Ruttner-Kolisko, A., 1977. Suggestions for biomass calculations of planktonic rotifers. Archiv fur Hydrobiologie Beihefte 21: 71-76.
Shapiro, S. S. & M. B. Wilk, 1965. An analysis of variance test for normality (complete samples). Biometrika 52(3/4): 591–611.
Shelyuk, Y. S., 2019. Regularities of primary production formation in river ecosystems (the basins of the Pripyat’and Teterev Rivers, Ukraine). Hydrobiological Journal. https://doi.org/10.1615/HydrobJ.v55.i4.40.
Shields, F. D., Jr., R. E. Lizotte Jr., S. S. Knight, C. M. Cooper & D. Wilcox, 2010. The stream channel incision syndrome and water quality. Ecological Engineering 36(1): 78–90. https://doi.org/10.1016/j.ecoleng.2009.09.014.
Shurin, J. B., M. Winder, R. Adrian, W. Keller, B. Matthews, A. M. Paterson, B. Pinel-Alloul, A. Rusak & N. D. Yan, 2010. Environmental stability and lake zooplankton diversity–contrasting effects of chemical and thermal variability. Ecology Letters 13(4): 453–463. https://doi.org/10.1111/j.1461-0248.2009.01438.x.
Sługocki, Ł & R. Czerniawski, 2018. Trophic state (TSISD) and mixing type significantly influence pelagic zooplankton biodiversity in temperate lakes (NW Poland). PeerJ 6: e5731. https://doi.org/10.7717/peerj.5731/sup-1.
Sor, R., H. Segers & S. Meas, 2015. Rotifers as bio–indicators of freshwater quality: a case study from the upper Cambodian. Cambodian Journal of Natural History 2015: 148.
Spoljar, M., 2013. Microaquatic communities as indicators of environmental changes in lake ecosystems. Journal of Engineering Research 1(1): 29–42.
Stamou, G., M. Katsiapi, M. Moustaka-Gouni & E. Michaloudi, 2019. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 844(1): 83–103. https://doi.org/10.1007/s10750-018-3880-9.
Vanni, M. J. & W. Lampert, 1992. Food quality effects on life history traits and fitness in the generalist herbivore Daphnia. Oecologia 92(1): 48–57. https://doi.org/10.1007/BF00317261.
Wallace R.L., T.W. Snell, C. Ricci & T. Nogrady, 1993. Rotifera [Ed. Thomas Nogrady] Volume 1: Biology, Ecology and Systematics. In: Guides to the Identification of the Microinvertebrates of the Continental Waters of the World") [Ed. Henri J. Dumont], SPB Academic Publishing bv, The Hague.
Wallace, R. L., T. W. Snell & H. A. Smith, 2015. Phylum Rotifera, Thorp and Covich’s freshwater invertebrates Academic Press, Cambridge: 225–271.
Wilkerson, F. P., R. C. Dugdale, A. E. Parker, S. B. Blaser & A. Pimenta, 2015. Nutrient uptake and primary productivity in an urban estuary: using rate measurements to evaluate phytoplankton response to different hydrological and nutrient conditions. Aquatic Ecology 49(2): 211–233. https://doi.org/10.1007/s10452-015-9516-5.
Xiao, R., Q. Wang, M. Zhang, W. Pan & J. J. Wang, 2020. Plankton distribution patterns and the relationship with environmental gradients and hydrological connectivity of wetlands in the Yellow River Delta. Ecohydrology & Hydrobiology 20(4): 584–596. https://doi.org/10.1016/j.ecohyd.2020.01.002.
Funding
This study was funded by the Kazimierz Wielki University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska- Kippen / Diverse Rotifers in Diverse Ecosystems
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kolarova, N., Napiórkowski, P. Are rotifer indices suitable for assessing the trophic status in slow-flowing waters of canals?. Hydrobiologia 851, 3013–3023 (2024). https://doi.org/10.1007/s10750-023-05275-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05275-7