Abstract
Urbanization is one of the most serious threats to stream ecosystems worldwide. It is crucial to understand its effects on stream organisms as a prerequisite for the mitigation of urban degradation. Our aim was to investigate the general effects of urbanization in a moderately urbanized landscape and to assess the relationship between local environmental variables and biotic attributes of macroinvertebrate assemblages. Multiple sites at low-order streams flowing from natural forested areas to moderately urbanized landscapes were surveyed. We found that local habitat properties presented degraded conditions at urban sites. Urbanization had a negative effect on the richness and Shannon diversity of macroinvertebrate assemblages, and altered assemblage composition. Biotic assemblage parameters showed negative, neutral or positive relationships with local physical parameters. Concrete cover was one of the most important variables, which explained a decreasing richness and diversity of macroinvertebrates. In natural conditions, microhabitat-level environmental variables significantly impacted community variation, while in degraded conditions microhabitat and site-level environmental variables had a substantial impact together. Individual streams showed considerable variability under natural conditions, as well as in their response to urban effects. Subsequently, the mitigation of the effects of urbanization might also need considerable variability in the type of actions required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human population is growing annually with the total number of people living on Earth reaching eight billion (UN DESA, 2022), which draws greater attention to the effects of urbanization on the natural world. Urbanization is the movement of people from rural to urban areas and the conversion of rural environment to urban leading to increased population size in cities, the expansion of urban land area, and growing number of settlements (UN DESA, 2019). In 1950 only 30% of the world’s population lived in cities while today it is 55% and expected to increase 68% by 2050 (UN DESA, 2019). Locally, urbanization is a complex environmental process with changes causing detrimental effects including alteration of the local climate, pollution, removal of vegetation, drainage and extraction of freshwater, habitat loss and fragmentation which result in damaged and degraded ecosystems (Grimm et al., 2008). Urbanization is considered as a major driving force of biodiversity and species loss (Savard et al., 2000; McKinney, 2002; Pauchard et al., 2006; Simkin et al., 2022), and urban development causes some of the largest local extinctions among native species (Kowarik, 1995; McKinney, 2002).
Urbanization has become a major interest of stream ecology because many of the rivers and streams flowing through populated areas experience increased pressures from anthropogenic sources. The ‘urban stream syndrome’ (Meyer et al., 2005; Walsh et al., 2005) presents a complex biological, physical and chemical impact system on streams affected by urbanization. The symptoms include changes in flow velocity, more frequent flash floods, elevated concentrations of nutrients and contaminants, altered channel morphology and stability. These changes have a strong impact on stream-living animals leading to reduction in biotic richness and increased dominance of tolerant and often non-native taxa. One of the most affected groups are aquatic macroinvertebrates, which play an important role in stream ecosystems by feeding on algae, coarse detritus, fine particulate organic matter and other aquatic organisms as predators (Cummins, 1974), by engineering (Mermillod-Blondin, 2011; Statzner, 2012) and by providing food for animals at higher trophic levels, such as amphibians (Nery & Schmera, 2016) or fish (Covich et al., 1999). The syndrome causes alterations in species composition, resulting in sensitive groups (e.g. Ephemeroptera, Plecoptera, Trichoptera) becoming less abundant or even absent in urban stream sections, while tolerant taxa, for example Oligochaeta and Diptera (mainly Chironomidae), become dominant (Walsh et al., 2005). Urbanization has an overall negative effect on the species diversity of freshwater macroinvertebrates (Gál et al., 2019).
There has been a general need to understand that what level of urban degradation causes detectable alterations in stream assemblages. For instance, fish communities showed fast degradation as impervious surface cover of the catchment exceeded 10–15% (Klein, 1979; Wang et al., 2000, 2001). However, Utz et al. (2010) suggested that even the lowest levels of urban degradation may cause fish biodiversity loss and the sensitivity to urbanization can vary depending on the species and also on the biogeographic region. For macroinvertebrates, Walsh et al. (2001) suggested significantly lower values in taxon richness once connected catchment imperviousness was above 10%. Later, Schueler et al. (2009) and Fitzgerald et al. (2012) suggested that this limit should be as low as 5%, while Cuffney et al. (2010) indicated that any level of impervious surface cover can cause considerable degradation and limits of 10% or 5% do not provide a significant safeguard for protection. On the other hand, local-scale characteristics have proven to be the most important determinants for macroinvertebrate assemblages over different spatial scales (Luo et al., 2018), while Gál et al. (2020) demonstrated that local human-made structures, such as road crossings or culverts, can cause a rapid degradation of native macroinvertebrate communities. Relatively short generation times and presence in a wide range of trophic positions mean that stream macroinvertebrates can react rapidly to environmental alterations and even small changes in local habitat properties can affect their presence or abundance (Collier & Clements, 2011).
Urbanization, therefore, involves not only land-use alterations, but also changes in local physical parameters. These changes include microhabitat-level (i.e. substrate-related) changes, as well as site-level changes in hydromorphology and in riparian vegetation. Microhabitat complexity is essential in the structuring and functioning of freshwater assemblages (Taniguchi & Tokeshi, 2004; Willis et al., 2005). According to the ‘habitat heterogeneity hypotheses’, high variety of habitats leads to high species diversity (MacArthur & MacArthur, 1961). Individual species of benthic macroinvertebrates are often associated with particular microhabitat types (Giller & Malmqvist, 1998; Leitner et al., 2015; Vilenica et al., 2018), and their selection is determined by the characteristics of the streambed substrate (Rempel et al., 2000; Vilenica et al., 2018; Doretto et al., 2022). The quality and heterogeneity of mineral and organic substrate define the number of microhabitats; therefore, substrate is the primary physical variable affecting macroinvertebrate richness and abundance (Erman & Erman, 1984; Duan et al., 2008). In urban areas, a sudden degradation of the streambed often occurs, resulting in poor quality substrates consequently microhabitat and heterogeneity loss.
In terms of site-level changes, urbanization is often associated with concrete channelization, including straightening and streambank fixation, which increases surface imperviousness (Paul & Meyer, 2001; Walsh et al., 2005). These channels suffer from extreme high-flow events with fast periodic ascending and descending phases (Walsh et al., 2005) with increasing the chance of macroinvertebrates drifting downstream or causing direct mortality (Konrad & Booth, 2005). A concrete stream bottom prevents the connection of surface water to groundwater, hence causing a general degradation of the hyporheic zone. The interstitial spaces of the hyporheic zone are important for their unique fauna (Williams & Hynes, 1974) as well as for other macroinvertebrates in early development stages (Williams, 1984; Stubbington, 2012). Interstitial spaces are also crucial during high storm events or desiccation by providing refuge for individuals and by aiding the recolonization process (Stubbington, 2012; Lewandowski et al., 2019). The hyporheic zone plays an important role in nutrient cycling of streams and also in mitigating contamination (Hancock, 2002; Lewandowski et al., 2019). Another important change for macroinvertebrates is the complete removal or thinning of the riparian vegetation along urban stream sections (Paul & Meyer, 2001). Riparian vegetation forms an interface between stream and terrestrial ecosystems and has an essential role by providing food and refuge for stream macro-fauna and by buffering temperature, solar radiation and flow regimes (Pusey & Arthington, 2003; Sargac et al., 2021). Under natural conditions, riparian vegetation has an important effect on microhabitat heterogeneity and hydromorphology. It decreases riparian erosion, prevents the accumulation of fine sediment in the streambed, determines the proportion of different grain sizes and regulates channel width and water depth. Conversely, it allows the accumulation of leaf litter and woody debris, thereby increasing microhabitat complexity and regulating current velocity.
The Pannon Biogeographic Region, Central Europe, has a dense network of flowing surface waters with a diverse stream invertebrate fauna including endemic species, while it has been experiencing rapid urban sprawl (Kovács et al., 2019; Lennert et al., 2020). Recent studies (Bozóki et al., 2018; Szita et al., 2019; Tóth et al., 2019; Czeglédi et al., 2020; Erős et al., 2020; Gál et al., 2020) found ambiguous results, and thus, further studies were recommended to better understand the effects of urban development and degradation on stream ecology in this region. The Balaton Uplands (Hungary, Central Europe), which is located in the region, is part of a National Park with a wide range of valuable aquatic ecosystems. It is characterized by small settlements and an associated low level of urban development and impervious surfaces (e.g. roads). Most of the settlements in the area are concentrated along the shore of Lake Balaton, one of the main destinations for tourism in Hungary. This indicates periodically increasing urban stress during the tourist season on influent streams. Furthermore, according to projections, Balaton Uplands is one of the few areas in the country where population growth (due to population reallocation) is expected in the near future (Lennert, 2019). Hence, it is crucial to understand the effects of urbanization in this area to estimate the changes on biodiversity due to increasing urban stress and to recommend actions to mitigate the impacts on stream ecosystems.
The objective of this study was to examine the effect of urbanization on low-order streams in the Pannon Biogeographic Region. To achieve this, we compared the abiotic and biotic parameters of streams flowing from natural areas through a moderately urbanized landscape and examined how local environmental variables explained biotic changes. Particularly, we were interested in the following questions: (1) Which environmental variables are altered in urban streams when comparing with natural ones in the Pannon Ecoregion? (2) Does urbanization affect macroinvertebrate community attributes such as taxa richness and Shannon diversity, as well as abundance and assemblage composition? (3) Which environmental variables explain changes in community attributes? (4) Which group of environmental variables explain the highest amount of community variation?
Materials and methods
Study area and sites
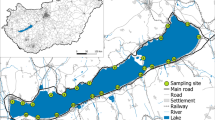
The study area was located in the Balaton Uplands, Hungary (Suppl. Table 1). The climate of this area is continental with a slight Mediterranean aspect. The annual mean temperature is around 10 °C, and the typical annual precipitation is between 600 and 700 mm. Balaton Uplands area is covered mostly by natural deciduous forests, vineyards and agricultural areas. There are small (wetted width < 5 m) low-order streams flowing through small towns and villages towards Lake Balaton, which is the largest lake in Central Europe and plays a significant role in providing ecosystem services such as recreation (tourism, fishing, bathing, sailing), food and water supply, and regulation of climate. These streams are mostly short in length and characterized by a small catchment area and low level of catchment urbanization (urban land cover is under 10%). Five streams (Aszófői, Burnót, Cserkúti, Koloska, Örvényesi) were selected based on the criteria of having both natural and urban sections along their course (Fig. 1). Along streams, six sites were selected in natural sections and six sites in urban sections, respectively. The length of sampled sites varied between 20 and 50 m (usually shorter in urban areas), and the distance between sites was at least 20 m. The average spatial distance between urban and natural sampling sites within streams was 1.50 km (min. in Örvényesi: 0.57 km, max. in Koloska: 3.59 km). Hence, based on this sampling design we used 5 [streams] × 2 [land use type: natural and urban] × 6 [sites] = 60 samples.
a Hungary in Central Europe with the position of Lake Balaton, b Lake Balaton and the studied streams (1: Burnót, 2: Cserkúti, 3: Örvényesi, 4: Aszófői, 5: Koloska), c Schematic representation of the study design showing Aszófői stream as an example. Land cover types based on the Ecosystem Base Map of Hungary (Ministry of Agriculture, 2019). Urban: any type of buildings, roads, railways, including green areas within human settlements; Rural: any tape of agricultural area, Grassland: all grassland, Forest: all forest type. Map was made using QGIS software with version 3.8.3—Zanzibar (QGIS Development Team, 2019)
Environmental variables
The Stream Visual Habitat Assessment (SVHA) protocol was used for evaluating abiotic parameters at each site. Wetted width at three cross-sections was recorded. Physical habitat parameters at three randomly selected points were measured [water depth and water velocity (using Valeport Model 801 (Flat) EM Flow Meter)] and the streambed material [bedrock, boulder (> 25.6 cm), cobble (6.4–25.6 cm), pebble (1.6–6.4 cm), gravel (0.2–1.6 cm), sand (0.0125–0.2 cm), silt (< 0.0125 cm) and concrete] and organic [algae, particulate organic matter (POM), wood, macrophytes, terrestrial plants] substrate composition was assessed as percentage. To assess the different components of riparian vegetation, the presence of trees, shrubs and grasses at both sides of the stream was recorded. Then, we summed the presence of the six riparian vegetation components into a riparian condition index (RCI, ranging between 0 and 6). Canopy coverage of the sites was assessed at the peak of the growing season in mid-summer, estimated as percentage of covering. To consider the human impact of the sites, we assessed the presence of stagnation, bank fixation (left, right, bed), litter, sedimentation, pollution, straightening and transverse concrete structures at each site. In order to quantify the human impact, we summed the nine human impact components into a human impact index (HII, ranging between 0 and 9). Environmental variables were divided into two groups according to microhabitat level (mineral and organic substrate: proportion of bedrock, boulder, cobble, pebble, gravel, sand, silt, concrete, algae, POM, wood, macrophytes, terrestrial plants) and site level including variables of hydromorphology (wetted width, water depth, current velocity), riparian vegetation (canopy cover and RCI), and human impact index (HII).
Sampling and identification of macroinvertebrates
Aquatic macroinvertebrates were collected in April 2018. A multihabitat sampling method and “kick and sweep” technique with a 500-μm mesh size hand-net was used (AQEM Consortium, 2002). At each site, ten subsamples were taken, by disturbing an area of 0.25 × 0.25 m. Subsamples were pooled in the field and taken into consideration as a composite sample. Collected animals were preserved in alcohol (70% ethanol and Patosolv©) and transported to the laboratory for identification. Macroinvertebrates were identified under stereomicroscope to the lowest possible taxonomic level (usually species) by experts using the EUTAXA software (Lechthaler & Stockinger, 2005; Lechthaler, 2009; Bauernfeind & Lechthaler, 2014) and relevant identification keys (Hynes, 1977; Savage, 1989; Bass, 1998; Disney, 1999; Csabai, 2000; Bauernfeind & Humpesch, 2001; Csabai et al., 2002; Askew, 2004; Waringer & Graf, 2011; Ambrus et al., 2018). Protected taxa are listed in the valid 100/2012. (IX.28.) decree of Ministry of Rural Development, while species classified as non-native based on DAISIE—Delivering Alien Invasive Species Inventories for Europe (DAISIE, 2009).
Statistical analyses
Spearman’s rank correlation tests were used to analyse the relationships between environmental variables. Standardized principal component analysis [PCA; (Podani, 2000)] and permutational multivariate analysis of variance using distance matrices (PERMANOVA or ADONIS in R, hereafter ADONIS) (Anderson, 2001) with Euclidean distance were used to assess differences in environmental variables between urban and natural sites.
Linear mixed-effects models (LME models) were used to test the effect of urbanization on the number of taxa (taxa richness), Shannon diversity and the number of individuals (abundance, log-transformed) for the whole macroinvertebrate and EPT (Ephemeroptera, Plecoptera and Trichoptera) communities. Taxa richness, Shannon diversity and macroinvertebrate abundance were used as response variables, stream section (2 levels: natural, urban; and 30 replicates per level) was a fixed factor, and stream identity (5 levels: Aszófői, Burnót, Cserkúti, Koloska, Örvényesi; and 12 replicates per level) was a random factor, while the number of samples was 60 (N = 60). EPT abundances were dominated by a single taxon; thus, the analysis of abundance of EPT was conducted without Baetis rhodani (Pictet, 1843). Therefore, the abundance of EPT was marked as Abu-EPT-Br (-Br subindex refers to assemblage without B. rhodani). The percentage of EPT species from all macroinvertebrate species (relative richness, EPT%) was determined for each site, and an LME model was used to test how urbanization affected the relative richness of EPT species. LME models were used to test the effect of urbanization on each dominant taxon. Abundances of B. rhodani, Electrogena ujhelyii (Sowa, 1981) and Chironomidae were log(x + 1)-transformed, while abundances of Gammarus roeselii Gervais, 1835 and G. fossarum Koch, 1836 were square-root-transformed to better meet the assumptions of normality. Similar to previous analyses, abundance was used as a response variable, while stream section (natural, urban) was used as a fixed factor. We included stream identity (Aszófői, Burnót, Cserkúti, Koloska, Örvényesi) in the models as a random factor.
Non-metric multidimensional scaling (NMDS, Kruskal, 1964) and ADONIS (Anderson, 2001) with Bray–Curtis distance was used to test the community-based separation of natural (N = 30) and urban (N = 30) sections. We used all macroinvertebrate taxa, as well as only EPT taxa in a separate analysis as dependent variables, while stream section and identity were independent variables. Spearman’s rank correlation tests were used to evaluate relationships between environmental variables and macroinvertebrate taxa richness, abundance and Shannon diversity for the entire macroinvertebrate and separately for EPT assemblages. The response of the most abundant taxa was also analysed.
Variance partitioning (VP; Borcard et al., 1992) with redundancy analysis (RDA) was used to examine how microhabitat-level as well as site-level parameters explained assemblage variation of the entire macroinvertebrate assemblage, and EPT separately. We examined natural and urban sections separately. Negative variances were considered as zero variances (Legendre, 2008). The following variables were excluded from variance partitioning analyses due to their zero variance: the proportion of terrestrial plants and concrete in natural sections, and the proportion of bedrock, boulder and wood in urban sections. Stepwise (both forward and backward) model selection methods were used to derive the best fit model for both microhabitat level and site level (Blanchet et al., 2008). An analysis of variance (ANOVA)-like permutation test for redundancy analysis was used to test the significance of the different partitions such as the RDA models of microhabitat-level and site-level parameters. Statistical analyses were performed in R (R Development Core Team, 2020) using packages ‘vegan’ (Oksanen et al., 2019), ‘lmerTest’ (Kuznetsova et al., 2020) and ‘PerformanceAnalytics’ (Peterson et al., 2020).
Results
Habitat properties
Spearman correlations showed that the proportion of algae was highly (ρ > 0.75) correlated with the proportion of concrete, current velocity and the HII index, while the proportion of concrete and the HII index were highly correlated. The HII index showed highly negative correlations with the proportion of sand and canopy cover (Suppl. Table 2). The PCA showed a marked separation of urban and natural sites (Fig. 2). Natural sites are positioned at the positive values of the first PCA axis, while urban sites at the negative values of the first PCA axis. Urban sites were characterized by a high proportion of concrete, algae, plants and higher current velocity. Natural sites showed considerable variability. Some sites showed a high proportion of particulate organic matter (POM) and wood (negative values of the second PCA axis), while other natural sites were wider with cobble and boulder substrates (positive values of the second PCA axis). A higher proportion of canopy cover and better condition of riparian vegetation also characterized natural sites. Individual streams offered unique habitats for macroinvertebrates. ADONIS showed a significant difference between natural and urban sites (df = 1, SS = 202,389, R2 = 0.297, F = 24.533, P < 0.001) and between streams (df = 4, SS = 195,821, R2 = 0.288, F = 5.551, P < 0.001). Section and stream identity explained very similar amounts of variation of environmental variables.
General effect of urbanization
Altogether 108 taxa were identified from the collected 29,691 individuals (Suppl. Table 3). The most abundant (> 1,000 individuals) taxa were Gammarus roeselii (11,267 individuals), Baetis rhodani (6,067 individuals), Gammarus fossarum (4,408 individuals), Chironomidae (2,032 individuals) and Electrogena ujhelyii (1,136 individuals). We identified 3 protected [Astacus astacus (Linnaeus, 1758), Calopteryx virgo Linnaeus, 1758, Orthetrum brunneum Fonscolombe, 1837] and one non-native, invasive [Potamopyrgus antipodarum (Gray, 1843)] taxa. The invasive snail was present only in one stream with low abundance (40 individuals). Trichoptera was represented by the highest number of taxa (33), while there was only one species of Decapoda [Astacus astacus (Linnaeus, 1758)] and Megaloptera (Sialis fuliginosa Pictet, 1836).
Taxa richness of macroinvertebrate community ranged between 6 and 28 taxa per site. Taxa richness was higher for natural sites (natural: mean = 16.400, sd. = 4.731, N = 30; urban: mean = 12.733, sd. = 4.394, N = 30). The LME model for taxa richness showed that urbanization had a negative effect on the number of taxa (Welch–Satterthwaite t-test: urban section estimate = − 3.667, s.e. = 1.039, df = 54, t = − 3.529, P < 0.001) and stream identity as a random factor explained 26% of the total variance. Streams showed considerable variability around the effect of urbanization (Fig. 3). Shannon diversity indices ranged between 0.666 and 2.289 (natural: mean = 1.499, sd. = 0.350, N = 30; urban: mean = 1.297, sd. = 0.247, N = 30). The LME model showed that local urbanization had a negative effect on Shannon diversity (Welch–Satterthwaite t-test: urban section estimate = − 0.203, s.e. = 0.062, df = 54, t = − 3.246, P = 0.002). Stream identity as a random factor explained 41% of the total variance. Shannon diversity also showed substantial heterogeneity in different streams (Fig. 4). The number of individuals ranged between 78 and 1,818 per site and was higher in urban sites (natural: mean = 391.733, sd. = 178.093, N = 30; urban: mean = 597.967, sd. = 425.908, N = 30). The statistical model for abundance data showed that local urbanization had no effect on the number of individuals (Welch–Satterthwaite t-test: urban section estimate = 0.266, s.e. = 0.157, df = 54, t = 1.690, P = 0.097). Stream identity as a random factor explained 25% of the total variance. Abundances varied in different streams (Fig. 5).
Ephemeroptera, Plecoptera and Trichoptera (EPT) were present with 44 taxa and 9,562 individuals. EPT richness ranged between 1 and 13 species per site (natural: mean = 7.300, sd. = 2.706, N = 30; urban: mean = 5.100, sd. = 2.496, N = 30). The LME models showed that urbanization had a negative effect on the richness (Rich-EPT), as well as on the number of individuals (Abu-EPT-Br) and the Shannon diversity of EPT (Sha-EPT), while the relative richness of EPT (EPT%) showed no relationship with urbanization (Table 1). Among the dominant species, urbanization showed negative (Electrogena ujhelyii), neutral (Gammarus fossarum, Chironomidae) and positive (Baetis rhodani, Gammarus roeselii) effects on the number of collected individuals (Table 1).
NMDS (Fig. 6) showed a clear separation of the samples among the macroinvertebrate assemblages collected at natural and urban sites. Natural sites were mainly associated with negative values of the second NMDS axis, while urban sites were associated with positive values of the second NMDS axis. ADONIS showed that stream identity had a greater ability to partition sites than stream section (Table 2). It appeared that streams possessed unique community members, which were present only in a given stream, and however, ordination hulls showed that sites were also separated according to section within streams. According to the NMDS plot, the assemblages of certain streams were more similar; for example, Cserkúti and Örvényesi or Koloska and Aszófői stream, while Burnót stream was distinctly separated from the other streams. The urban sites were more clustered within and more separated between streams, while natural sites were more scattered and less separated. The NMDS performed exclusively on EPT assemblages showed a somewhat different result (Suppl. Fig. 1, Suppl. Table 4). Natural and urban sites were separated mostly along the first NMDS axis. Contrary to the results of the entire macroinvertebrate assemblages, streams were not well separated.
Relationship between local environmental variables and biotic attributes of macroinvertebrate assemblages
Taxa richness of the entire macroinvertebrate assemblage (Rich-Ent) was negatively correlated with the proportion of concrete and algae (Table 3) at microhabitat level, while it was positively correlated with wetted width at site level. The abundance of the entire macroinvertebrate assemblage (Abu-Ent) did not correlate strongly with the any of environmental variables (Table 3). Shannon diversity (Sha-Ent) showed a strong positive correlation with wetted width (Table 3).
Considering only EPT, taxa richness (Rich-EPT) showed a positive correlation with POM, wood and canopy cover, and negative correlation with the proportion of concrete, algae and current velocity (Table 3). The abundance of EPT without Baetis rhodani (Abu-EPT-Br) was positively correlated with the proportion of gravel, wetted width and water depth, while negatively correlated with the proportion of concrete. Shannon diversity (Sha-EPT) showed a strong positive correlation with the proportion of cobble, gravel, sand, POM, wood, wetted width and canopy cover. Sha-EPT was negatively correlated with the proportion of concrete, the proportion of algae, current velocity and the HII index. The proportion of EPT within the entire macroinvertebrate assemblage (EPT%) did not show strong correlations (Table 3).
The abundance of Baetis rhodani was strongly negatively correlated with the proportion of gravel, sand and wetted width, and strongly positively correlated with the proportion of concrete. It was positively correlated with the HII index. The abundance of Electrogena ujhelyii was strongly positively correlated with the proportion of boulder, cobble, pebble, gravel, sand and the proportion of POM and canopy cover. It was strongly negatively correlated with the proportion of concrete, the proportion of algae, current velocity and the HII index. E. ujhelyii abundance was positively correlated with the proportion of wood and wetted width. The abundance of Gammarus roeselii was strongly negatively correlated with the RCI index and negatively correlated with the proportion of pebble and silt. It was positively correlated with the proportion of macrophytes and wood, and water depth. The abundance of G. fossarum showed a similar pattern but in the opposite direction: positively correlated with the proportion of pebble, while negatively correlated with the proportion of wood and water depth. The abundance of Chironomidae was strongly negatively correlated with wetted width and water depth and showed a positive correlation with the proportion of silt and the RCI index (Table 3).
Community variation
Regarding the entire macroinvertebrate assemblage of natural sections, variance partitioning showed that microhabitat level explained the highest pure variation (21%), while site-level parameters had limited pure explanation power (1%, Table 4). The shared effect of microhabitat- and site-level parameters explained 17% of total variance. The total explained variance was 39%. When the assemblages of urban sections were analysed, variance partitioning showed that both microhabitat and site-level parameters explained purely a large variation (35% and 26%, respectively), followed by a shared effect (9%, Table 4). The total explained variance was 70%, resulting in remarkably less residual variance than in natural section. All RDA models were significant.
Microhabitat-level variables explained the highest variation (27%) within the EPT assemblages of natural sections, followed by a shared effect of microhabitat- and site-level parameters (3%) and a pure effect of site-level parameters (2%, Table 4). The total explained variance was 32%. When the assemblages of urban sections were analysed, site-level parameters explained the largest amount of community variation (25%). The shared effect of microhabitat- and site-level variables explained 36%, while microhabitat-level variables had a limited explanatory power (4%, Table 4). The total explained variance was 65%. All RDA models were significant.
Discussion
The ecological consequences of urban development are not fully understood. Scientific evidence suggests that even a moderate degree of urbanization might have a negative effect on the diversity and composition of stream organisms (Cuffney et al., 2010; Collier & Clements, 2011; Richardson, 2019). It is not clear, however, whether and how the urbanization-related alterations caused by the local physical habitat degradation drive these changes. We found that low-order streams suffer from considerable local degradation while flowing from natural forested areas to moderately urbanized landscapes. The habitat of natural and urban stream sections differed regarding microhabitat- and site-level parameters. These differences in habitat parameters contributed to a reduced richness and an altered composition of macroinvertebrates. Our analyses revealed that stream identity explained a remarkable amount of community variation. This finding suggests that more attention needs to be paid to the individual response of streams to urbanization.
In our study, urban and natural sections were different in terms of local environmental variables. Substrate heterogeneity displayed a shift in response to urbanization. Urban sites were characterized by a straight stream channel, less variable mineral substrate and by the dominance of concrete surface. Furthermore, streams were narrower, shallower and flowing faster in these sites, which is common in urban streams because of the channelization and concrete lining of the streambed (Paul & Meyer, 2001; Walsh et al., 2005). Riparian vegetation was generally reduced along the urban sites, which allowed the growth of herbaceous plants and algae due to the lack of light limitation by the canopy (Taylor et al., 2004; Sargac et al., 2021). This growth might also indicate nutrient-limited conditions in natural sites, while the urban sites receive a higher nutrient load (Taylor et al., 2004; Walsh et al., 2005). Leaf litter and other allochthonous organic matter input were limited in urban sites similar to the findings of Sargac et al. (2021). In our case, urbanization influenced each local stream environment similarly, implying a direct link with habitat degradation. This contradicts with results by Czeglédi et al. (2020) who did not find any relationship between urbanization-related variables and local stream environmental variables in the same ecoregion.
The macroinvertebrate community differed significantly between the urban and natural sites, but each stream had unique communities even in the urban sections. It seems that the effect of local (individual stream) species pools cannot be overridden by the effect of urbanization. This was previously shown on fish assemblages (Stoll et al., 2014; Czeglédi et al., 2020) and in tropical macroinvertebrate communities (Al-Shami et al., 2014). The well-documented negative effects of urbanization, such as biotic homogenization, were less apparent in our study. Similarly, Utz et al. (2010) showed substantial differences regarding the effects of urbanization depending on the geographic region, regional species pool, and moreover, the local species composition. We postulate that the lack of a uniform response by local species pools could be due to the relatively low level of urbanization in our study area. Presumably, when urban degradation is more intense (i.e. the amount of artificial surface cover is higher, pollution is greater and hydromorphology is even more altered), the effect of urbanization may be greater than the effect of stream identity. Communities under such conditions may be less resilient, and the original species pool would be less determining. Szita et al. (2019), however, did not find significant separation of assemblage structure between urban and reference sites in the same region, though their study area included a larger city. One of the reasons could be that their studied communities were already more degraded than those in this study. Alternatively, it could be because they compared sites upstream and downstream of the city, which implies that the community might regenerated after urban effect. For instance, the study of Gál et al. (2020) indicated that macroinvertebrates can recover relatively quickly after alteration. On the other hand, we found weaker responses of EPT assemblages to urbanization compared with the entire macroinvertebrate assemblage. Moreover, EPT compositions were comparatively similar in different streams. Different taxonomic groups of EPT respond differently to human alterations and even responses on a species level can affect the results greatly. The level of urban degradation in our sites may favour tolerant taxa, but it might not have reached the threshold to filter the more sensitive taxa.
Taxa richness and Shannon diversity were significantly lower in urban sites than in natural sites. This finding is similar to other studies reporting a negative relationship of richness and diversity with urbanization (Walsh et al., 2005; Stepenuck et al., 2008; Cuffney et al., 2010; Wang et al., 2012; Gál et al., 2019; Gao et al., 2022), though another study in the Pannon Biogeographic Region found a positive correlation between Shannon diversity and urbanization level at the landscape scale (Bozóki et al., 2018). Similar to the results of Gál et al. (2019), we observed that stream identity explained a large amount of variance, indicating high heterogeneity in the effect of urbanization on macroinvertebrate diversity between streams.
While richness and diversity showed a decrease due to urbanization, we did not find a significant difference between the abundance of macroinvertebrates in natural and urban sites. Other studies showed that macroinvertebrate abundance can significantly increase downstream along the urban gradient as pollution increases (Álvarez-Cabria et al., 2011), especially in tropical areas where carbon sources are mostly autochthonous (Lau et al., 2009; Wiederkehr et al., 2020; Gao et al., 2022), whereas at temperate climates abundance decreases downstream (Paul & Meyer, 2001). At higher levels of urbanization, increased abundance can be caused by high numbers of Chironomidae and Oligochaeta (Paul & Meyer, 2001; Walsh et al., 2005). However, in our study Chironomidae abundance showed no significant difference between natural and urban sites, whereas the abundance of Oligochaeta was negligible at all sites. The high number of individuals in urban sites was explained by Gammarus roeselii and Baetis rhodani. Although Gammarus species tend to be generalists in European streams and have a wide dietary breadth while Baetis rhodani is one of the most widespread mayfly species, they are far less tolerant than Oligochaeta or Chironomidae (Gabriels et al., 2010).
Though tolerant macroinvertebrate taxa tend to increase in number due to urbanization, EPT taxa usually appear to be more sensitive. Because of this, they are often used in water quality assessments as indicators of pollution-free, well-oxygenated waters (Lenat, 1988; Hamid & Rawi, 2017). Hence, our finding of significantly lower species richness, Shannon diversity and abundance in the urbanized sites is in accord with previous studies (Walsh et al., 2007; Cuffney et al., 2010; Álvarez-Cabria et al., 2011; Collier & Clements, 2011; Wang et al., 2012; Luo et al., 2018). However, the relative richness of EPT did not change between urban and natural sites, remaining around 40% in both types of sections. This could be due to some of the more tolerant EPT taxa remaining in the community, while other, non-EPT taxa decreased in richness at the urban sites.
The analysis on individual taxa indicated differences in their abundances between natural and urban sites. An example for this could be the mayflies Baetis rhodani and Electrogena ujhelyii. Both species belong to the functional feeding group of scrapers (Cummins & Klug, 1979) grazing on periphytic algae as their main food source (Schmedtje & Colling, 1996; Tachet et al., 2000). As Baetis rhodani showed a higher abundance in urban sections, Electrogena ujhelyii was more abundant in natural sections, which might indicate the replacement of one species by the other depending on the level of urbanization. For instance, Baetis rhodani is tolerant against potential urban effects, such as heavy metal pollution (Rehfeldt & Söchtig, 1991). Gál et al. (2020) also found that B. rhodani is a tolerant species when studying the effect of road crossings on macroinvertebrate diversity, while Vilenica et al. (2018) noted that B. rhodani can be found on a wide range of substrates and prefers high water velocity, which is common in urban streams. In our study, B. rhodani showed a negative correlation with natural mineral substrate, but a positive correlation with concrete cover. The heptageniidaean Electrogena ujhelyii preferred natural substrate, while its abundance showed a negative correlation with concrete cover and current velocity. The body size of the final instar larvae of Baetis is smaller than those of Electrogena and small size may be advantageous in urban stream sites (Edegbene et al., 2020). Although Baetis rhodani was common in most streams, it was largely absent in the Burnót stream. Moreover, other species of the Baetis genus (B. vernus Curtis, 1834, B. muticus (Linnaeus, 1758), B. pentaphlebodes Ujhelyi, 1966) were absent in Burnót stream, while present in other streams. It is interesting to note that the invasive New Zealand mudsnail (Potamopyrgus antipodarum) was present only in this stream. Although we detected this snail with low abundance, it is known that the population of P. antipodarum can strongly fluctuate seasonally and annually in European and North American freshwaters (Extence, 1981; Moffitt & James, 2012; Moore et al., 2012; Gérard et al., 2018). As the diets of the snail and Baetis species partially overlap [both taxa graze on periphyton, but the diet of Potamopyrgus antipodarum includes organic detritus and macrophytes as well (Alonso & Castro-Díez, 2008)], interspecific competition could be the explanation. The negative effect of P. antipodarum on native grazer taxa has been demonstrated (Moore et al., 2012). On the other hand, as Burnót is the largest of our studied streams, it may support the presence of fish which can exert predatory pressure explaining the rather unique macroinvertebrate community.
We detected two gammarid species (Gammarus roeselii and G. fossarum) whose niche segregation is known (Mauchart et al., 2014). In our study, G. roeselii showed a preference for urban sites and the two species showed opposite correlations with some of the environmental variables. Mauchart et al. (2014) demonstrated that G. roeselii can adapt to degraded conditions, but G. fossarum is usually a superior competitor. In addition, Krasznai et al. (2016) found that G. roeselii prefers lower altitudes, higher conductivity, higher temperature, fine bed substrate and plant coverage, all typical characteristics of urban streams, while G. fossarum showed a preference for higher altitudes, coarse bed substrate and lower temperature, which indicate close-to-natural conditions.
Macroinvertebrate richness and diversity were positively related to natural environmental conditions both on a microhabitat and site level. In contrast, urban-related changes, such as high proportion of concrete or high current velocity, negatively affected richness and diversity. The human impact index (HII) seemed to be a good predictor for local habitat degradation and linked well to microhabitat-level degradation indicators. Considering the riparian parameters, canopy coverage was evidently a better indicator of natural conditions than the riparian condition index (RCI). The reason for this may be that RCI assessed not only trees, but also shrubs and grasses that have far less significance than trees, which have direct effects on stream fauna by providing organic matter and shading. Riparian tree cover was negatively related to the presence of shrubs and mainly grasses, which were often absent on natural stream sites where canopy cover was very high, while the deforested urban sites provided unshaded opportunities for these plants. Canopy cover was an important indicator for EPT assemblage attributes, while Baetis rhodani was negatively related to both riparian canopy cover and RCI, similar to the findings by Forio et al. (2020).
Variance partitioning showed that under natural conditions microhabitat-level environmental variables had a more decisive role in structuring macroinvertebrate assemblages than site-level parameters. In urban sections, site-level became more important and for sensitive taxa (EPT) it played a larger role than microhabitat. This finding indicates that under urban conditions not only microhabitat heterogeneity but also larger-scale effects could determine assemblage composition. Environmental factors appeared to explain a higher amount of community variation in urban compared to natural sections. The larger amount of unexplained variance in the natural sections could be due to the biotic interactions or natural stochasticity. Turrini et al. (2016) demonstrated that urbanization can decrease direct and indirect trophic interactions, such as top-down control by predators. This indicates that in urban streams environmental conditions might become more important, while biological interactions may be less prevalent in shaping local compositions. However, urban-related environmental degradation was similar in every stream, and the biotic attributes showed remarkably high variation between streams. This evidence may highlight the relevance of the local (stream) species pool in determining the biological response to urbanization. Alternatively, it could also mean that moderate differences in the degree of local habitat degradation or larger scale differences (e.g. different settlement size, closeness to Lake Balaton) may trigger distinct responses by macroinvertebrates.
Conclusions
This study indicated that low levels of urbanization can degrade stream macroinvertebrate assemblages. According to the results, low levels of urbanized conditions caused a shift from highly sensitive communities to more tolerant communities. We showed a significant effect of both microhabitat- and site-level environmental parameters on macroinvertebrate assemblages. This finding supports the notion that recovery of microhabitat heterogeneity without site-level or a larger-scale approach may not be enough for the effective rehabilitation of macroinvertebrate communities in urbanization-affected streams (Palmer et al., 2010; Verdonschot et al., 2016; Zerega et al., 2021). Conversely, stream identity had a large impact on macroinvertebrate variation, indicating that the urban degradation of assemblages is strongly dependent on the composition of individual streams. These individual responses of streams to urban stress imply that individual-site management is required in the prevention, mitigation and recovery processes and suggests that there are likely to be no general treatments to mitigate the effects of urbanization in streams. Low-order streams are essential parts of the river network, and their biota are critical for freshwater biodiversity (Meyer et al., 2007; Richardson, 2019). Our sites were not highly urbanized, similar to other studies in the region (Czeglédi et al., 2020; Gál et al., 2020), yet the negative effects of urbanization on macroinvertebrate assemblages were clear. However, further research is needed to have better knowledge of the effects of urbanization in this region, supplementing taxonomical data with functional responses.
Data availability
Data available on request from the authors.
References
Alonso, A. & P. Castro-Díez, 2008. What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614: 107–116. https://doi.org/10.1007/s10750-008-9529-3.
Al-Shami, S. A., C. S. Md Rawi, A. H. Ahmad, M. R. Madrus & K. A. L. Mutairi, 2014. Importance of regional diversity and environmental conditions on local species richness of aquatic macro-invertebrates in tropical forested streams. Journal of Tropical Ecology 30: 335–346. https://doi.org/10.1017/S0266467414000200.
Álvarez-Cabria, M., J. Barquín & J. A. Juanes, 2011. Microdistribution patterns of macroinvertebrate communities upstream and downstream of organic effluents. Water Research 45: 1501–1511. https://doi.org/10.1016/j.watres.2010.11.028.
Ambrus, A., T. Danyik, T. Kovács & P. Olajos, 2018. Magyarország szitakötőinek kézikönyve, Herman Ottó Intézet, Magyar Természettudományi Múzeum, Budapest:
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x.
AQEM Consortium, 2002. Manual for the application of the AQEM system. A comprehensive method to assess European streams using benthic macroinvertebrates, developed for the purpose of the Water Framework Directive. Version 1.0, February, 2002.
Askew, R. R., 2004. The Dragonflies of Europe,. Revised Harley Books, Colchester:
Bass, J. A. B., 1998. Last-instar larvae and pupae of the Simuliidae of Britain and Ireland. A key with brief ecological notes. Scientific Publication No. 55. Freshwater Biological Association, Ambleside.
Bauernfeind, E. & U. H. Humpesch, 2001. Die Eintagsfliegen Zentraleuropas (Insecta: Ephemeroptera): Bestimmung und Ökologie, Naturhistorisches Museum, Vienna:
Bauernfeind, E. & W. Lechthaler, 2014. Ephemeroptera – Key to Nymphs from Central Europe, Eutaxa, Vienna:
Blanchet, G. F., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632. https://doi.org/10.1890/07-0986.1.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055. https://doi.org/10.2307/1940179.
Bozóki, T., E. Á. Krasznai-Kun, A. Csercsa, G. Várbíró & P. Boda, 2018. Temporal and spatial dynamics in aquatic macroinvertebrate communities along a small urban stream. Environmental Earth Sciences 77: 1–10. https://doi.org/10.1007/s12665-018-7735-5.
Collier, K. J. & B. L. Clements, 2011. Influences of catchment and corridor imperviousness on urban stream macroinvertebrate communities at multiple spatial scales. Hydrobiologia 664: 35–50. https://doi.org/10.1007/s10750-010-0580-5.
Covich, A. P., M. A. Palmer & T. A. Crowl, 1999. The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. BioScience 49: 119–127. https://doi.org/10.2307/1313537.
Csabai, Z., 2000. Vízibogarak kishatározója I. (Coleoptera: Haliplidae, Hygrobiidae, Dytiscidae, Noteridae, Gyrinidae). Vízi Természet- és Környezetvédelem sorozat 15, Környezetgazdálkodási Intézet, Budapest:
Csabai, Z., Z. Gidó & G. Szél, 2002. Vízibogarak kishatározója II. (Coleoptera: Georissidae, Spercheidae, Hydrochidae, Helophoridae, Hydrophilidae). Vízi Természet- és Környezetvédelem sorozat 16, Környezetgazdálkodási Intézet, Budapest:
Cuffney, T. F., R. A. Brightbill, J. T. May & I. R. Waite, 2010. Responses of benthic macroinvertebrates to environmental changes associated with urbanization in nine metropolitan areas. Ecological Applications 20: 1384–1401. https://doi.org/10.1890/08-1311.1.
Cummins, K. W., 1974. Structure and function of stream ecosystems. BioScience 24: 631–641. https://doi.org/10.2307/1296676.
Cummins, K. W. & M. J. Klug, 1979. Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10: 147–172. https://doi.org/10.1146/annurev.es.10.110179.001051.
Czeglédi, I., B. Kern, R. Tóth, G. Seress & T. Erős, 2020. Impacts of urbanization on stream fish assemblages: the role of the species pool and the local environment. Frontiers in Ecology and Evolution 8: 1–10. https://doi.org/10.3389/fevo.2020.00137.
DAISIE, 2009. Handbook of Alien Species in Europe, Springer, Dordrecht:
Disney, R. H. L., 1999. British Dixidae (meniscus midges) and Thaumaleidae (trickle midges): A key with ecological notes. Scientific Publication No. 56. Freshwater Biological Association, Ambleside.
Doretto, A., J. P. Receveur, E. A. Baker, M. E. Benbow & K. T. Scribner, 2022. Nested analysis of macroinvertebrate diversity along a river continuum: identifying relevant spatial scales for stream communities. River Research and Applications 38: 334–344. https://doi.org/10.1002/rra.3889.
Duan, X., Z. Wang & S. Tian, 2008. Effect of streambed substrate on macroinvertebrate biodiversity. Frontiers of Environmental Science and Engineering in China 2: 122–128. https://doi.org/10.1007/s11783-008-0023-y.
Edegbene, A. O., F. O. Arimoro & O. N. Odume, 2020. How does urban pollution influence macroinvertebrate traits in forested riverine systems? Water 12: 3111. https://doi.org/10.3390/w12113111.
Erman, D. C. & N. A. Erman, 1984. The response of stream macroinvertebrates to substrate size and heterogeneity. Hydrobiologia 108: 75–82. https://doi.org/10.1007/BF02391635.
Erős, T., I. Czeglédi, R. Tóth & D. Schmera, 2020. Multiple stressor effects on alpha, beta and zeta diversity of riverine fish. Science of the Total Environment 748: 141407. https://doi.org/10.1016/j.scitotenv.2020.141407.
Extence, C. A., 1981. The effect of drought on benthic invertebrate communities in a lowland river. Hydrobiologia 83: 217–224. https://doi.org/10.1007/BF00008269.
Fitzgerald, E. P., W. B. Bowden, S. P. Parker & M. L. Kline, 2012. Urban impacts on streams are scale-dependent with nonlinear influences on their physical and biotic recovery in Vermont, United States. Journal of the American Water Resources Association 48: 679–697. https://doi.org/10.1111/j.1752-1688.2012.00639.x.
Forio, M. A. E., N. De Troyer, K. Lock, F. Witing, L. Baert, N. De Saeyer, G. Rîșnoveanu, C. Popescu, F. J. Burdon, B. Kupilas, N. Friberg, P. Boets, M. Volk, B. G. McKie & P. Goethals, 2020. Small patches of riparian woody vegetation enhance biodiversity of invertebrates. Water 12: 3070. https://doi.org/10.3390/w12113070.
Gabriels, W., K. Lock, N. De Pauw & P. L. M. Goethals, 2010. Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnologica 40: 199–207. https://doi.org/10.1016/j.limno.2009.10.001.
Gál, B., I. Szivák, J. Heino & D. Schmera, 2019. The effect of urbanization on freshwater macroinvertebrates – knowledge gaps and future research directions. Ecological Indicators 104: 357–364. https://doi.org/10.1016/j.ecolind.2019.05.012.
Gál, B., A. Weiperth, J. Farkas & D. Schmera, 2020. The effects of road crossings on stream macro-invertebrate diversity. Biodiversity and Conservation 29: 729–745. https://doi.org/10.1007/s10531-019-01907-4.
Gao, J., Y. Huang, Y. Zhi, J. Yao, F. Wang, W. Yang, L. Han, D. Lin, Q. He, B. Wei & K. Grieger, 2022. Assessing the impacts of urbanization on stream ecosystem functioning through investigating litter decomposition and nutrient uptake in a forest and a hyper-eutrophic urban stream. Ecological Indicators 138: 108859. https://doi.org/10.1016/j.ecolind.2022.108859.
Gérard, C., M. Hervé & R. F. Hechinger, 2018. Long-term population fluctuations of the exotic New Zealand mudsnail Potamopyrgus antipodarum and its introduced aporocotylid trematode in northwestern France. Hydrobiologia 817: 253–266. https://doi.org/10.1007/s10750-017-3406-x.
Giller, P. S. & B. Malmqvist, 1998. The Biology of Streams and Rivers, Oxford University Press, New York:
Grimm, N. B., S. H. Faeth, N. E. Golubiewski, C. L. Redman, J. Wu, X. Bai & J. M. Briggs, 2008. Global change and the ecology of cities. Science 319: 756–760. https://doi.org/10.1126/science.1150195.
Hamid, S. A. & C. S. M. Rawi, 2017. Application of aquatic insects (Ephemeroptera, Plecoptera And Trichoptera) in water quality assessment of Malaysian headwater. Tropical Life Sciences Research 28: 143–162. https://doi.org/10.21315/tlsr2017.28.2.11.
Hancock, P. J., 2002. Human impacts on the stream-groundwater exchange zone. Environmental Management 29: 763–781. https://doi.org/10.1007/s00267-001-0064-5.
Hynes, H. B. N., 1977. A key to the adults and nymphs of British stoneflies (Plecoptera) with notes on their ecology and distribution. Scientific Publication No. 17. Freshwater Biological Association, Ambleside.
Klein, R., 1979. Urbanization and stream quality impairment. Journal of the American Water Resources Association 15: 948–963. https://doi.org/10.1111/j.1752-1688.1979.tb01074.x.
Konrad, C. P. & D. B. Booth, 2005. Hydrologic changes in urban streams and their ecological significance. American Fisheries Society Symposium 2005: 157–177.
Kovács, Z., Z. J. Farkas, T. Egedy, A. C. Kondor, B. Szabó, J. Lennert, D. Baka & B. Kohán, 2019. Urban sprawl and land conversion in post-socialist cities: the case of metropolitan Budapest. Cities 92: 71–81. https://doi.org/10.1016/j.cities.2019.03.018.
Kowarik, I., 1995. On the role of alien species in urban flora and vegetation. In Pyšek, P., K. Prach, M. Rejmánek & M. Wade (eds), Plant Invasions – General Aspects and Special Problems SPB Academic Publishing, Amsterdam: 85–103. https://doi.org/10.1007/978-0-387-73412-5_20.
Krasznai, E., P. Boda, A. Csercsa, M. Ficsór & G. Várbíró, 2016. Use of self-organizing maps in modelling the distribution patterns of gammarids (Crustacea: Amphipoda). Ecological Informatics 31: 39–48. https://doi.org/10.1016/j.ecoinf.2015.11.007.
Kruskal, J. B., 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika 29: 115–129. https://doi.org/10.1007/BF02289694%0A.
Kuznetsova, A., P. B. Brockhoff, R. H. B. Christensen & S. P. Jensen, 2020. LmerTest: tests in linear mixed effects models. R package version 3.1-3.
Lau, D. C. P., K. M. Y. Leung & D. Dudgeon, 2009. Are autochthonous foods more important than allochthonous resources to benthic consumers in tropical headwater streams? Journal of the North American Benthological Society 28: 426–439. https://doi.org/10.1899/07-079.1.
Lechthaler, W., 2009. Macrozoobenthos - Key to Families of Macroinvertebrates in European Freshwaters, Eutaxa, Vienna:
Lechthaler, W. & W. Stockinger, 2005. Trichoptera – Key to Larvae from Central Europe, Eutaxa, Vienna:
Legendre, P., 2008. Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. Journal of Plant Ecology 1: 3–8. https://doi.org/10.1093/jpe/rtm001.
Leitner, P., C. Hauer, T. Ofenböck, F. Pletterbauer, A. Schmidt-Kloiber & W. Graf, 2015. Fine sediment deposition affects biodiversity and density of benthic macroinvertebrates: a case study in the freshwater pearl mussel river Waldaist (Upper Austria). Limnologica 50: 54–57. https://doi.org/10.1016/j.limno.2014.12.003.
Lenat, D. R., 1988. Water quality assessment of streams using a qualitative collection method for benthic macroinvertebrates. Journal of the North American Benthological Society 7: 222–233. https://doi.org/10.2307/1467422.
Lennert, J., 2019. Demographic scenarios for the Hungarian rural areas with consideration to the effects of climate change. Területi Statisztika 59: 498–525. https://doi.org/10.15196/TS590503.
Lennert, J., J. Z. Farkas, A. D. Kovács, A. Molnár, R. Módos, D. Baka & Z. Kovács, 2020. Measuring and predicting long-term land cover changes in the functional urban area of Budapest. Sustainability 12: 3331. https://doi.org/10.3390/SU12083331.
Lewandowski, J., S. Arnon, E. Banks, O. Batelaan, A. Betterle, T. Broecker, C. Coll, J. D. Drummond, J. G. Garcia, J. Galloway, J. Gomez-Velez, R. C. Grabowski, S. P. Herzog, R. Hinkelmann, A. Höhne, J. Hollender, M. A. Horn, A. Jaeger, S. Krause, A. L. Prats, C. Magliozzi, K. Meinikmann, B. B. Mojarrad, B. M. Mueller, I. Peralta-Maraver, A. L. Popp, M. Posselt, A. Putschew, M. Radke, M. Raza, J. Riml, A. Robertson, C. Rutere, J. L. Schaper, M. Schirmer, H. Schulz, M. Shanafield, T. Singh, A. S. Ward, P. Wolke, A. Wörman & L. Wu, 2019. Is the hyporheic zone relevant beyond the scientific community? Water 11: 2230. https://doi.org/10.3390/w11112230.
Luo, K., X. Hu, Q. He, Z. Wu, H. Cheng, Z. Hu & A. Mazumder, 2018. Impacts of rapid urbanization on the water quality and macroinvertebrate communities of streams: a case study in Liangjiang New Area, China. Science of the Total Environment 621: 1601–1614. https://doi.org/10.1016/j.scitotenv.2017.10.068.
MacArthur, R. H. & J. W. MacArthur, 1961. On bird species diversity. Ecology 42: 594–598. https://doi.org/10.2307/1932254.
Mauchart, P., C. Bereczki, A. Ortmann-Ajkai, Z. Csabai & I. Szivák, 2014. Niche segregation between two closely similar gammarids (Peracarida, Amphipoda) – native vs. naturalized non-native species. Crustaceana 87: 1296–1314. https://doi.org/10.1163/15685403-00003355.
McKinney, M. L., 2002. Urbanization, biodiversity, and conservation. BioScience 52: 883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2.
Mermillod-Blondin, F., 2011. The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. Journal of the North American Benthological Society 30: 770–778. https://doi.org/10.1899/10-121.1.
Meyer, J. L., M. J. Paul & W. K. Taulbee, 2005. Stream ecosystem function in urbanizing landscapes. The North American Benthological Society 24: 602–612. https://doi.org/10.1899/04-021.1.
Meyer, J. L., D. L. Strayer, J. B. Wallace, S. L. Eggert, G. S. Helfman & N. E. Leonard, 2007. The contribution of headwater streams to biodiversity in river networks. Journal of the American Water Resources Association 43: 86–103. https://doi.org/10.1111/j.1752-1688.2007.00008.x.
Ministry of Agriculture, 2019. Development of an Ecosystem Base Map and Data Model, Ministry of Agriculture, Budapest:
Moffitt, C. M. & C. A. James, 2012. Response of New Zealand mudsnails Potamopyrgus antipodarum to freezing and near-freezing fluctuating water temperatures. Freshwater Science 31: 1035–1041. https://doi.org/10.1899/11-160.1.
Moore, J. W., D. B. Herbst, W. N. Heady & S. M. Carlson, 2012. Stream community and ecosystem responses to the boom and bust of an invading snail. Biological Invasions 14: 2435–2446. https://doi.org/10.1007/s10530-012-0240-y.
Nery, T. & D. Schmera, 2016. The effects of top-down and bottom-up controls on macroinvertebrate assemblages in headwater streams. Hydrobiologia 763: 173–181. https://doi.org/10.1007/s10750-015-2371-5.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2019. Vegan: community ecology package. R package version 2.5-6.
Palmer, M. A., H. L. Menninger & E. Bernhardt, 2010. River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshwater Biology 55: 205–222. https://doi.org/10.1111/j.1365-2427.2009.02372.x.
Pauchard, A., M. Aguayo, E. Peña & R. Urrutia, 2006. Multiple effects of urbanization on the biodiversity of developing countries: the case of a fast-growing metropolitan area (Concepción, Chile). Biological Conservation 127: 272–281. https://doi.org/10.1016/j.biocon.2005.05.015.
Paul, M. J. & J. L. Meyer, 2001. Streams in the urban landscape. Annual Review of Ecology and the Systematics 32: 333–365. https://doi.org/10.1007/978-0-387-73412-5_12.
Peterson, B. G., P. Carl, K. Boudt, R. Bennett, J. Ulrich, E. Zivot, D. Cornilly, E. Hung, M. Lestel, K. Balkissoon, D. Wuertz, A. A. Christidis, R. D. Martin, Z. “Zenith” Zhou & J. M. Shea, 2020. Econometric Tools for Performance and Risk Analysis. R package version 2.0-4.
Podani, J., 2000. Introduction to the Exploration of Multivariate Biological Data, Backhuys, Leiden:
Pusey, B. J. & A. H. Arthington, 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54: 1–16. https://doi.org/10.1071/MF02041.
QGIS Development Team, 2019. QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org/en/site/
R Development Core Team, 2020. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Rehfeldt, G. & W. Söchtig, 1991. Heavy metal accumulation by Baetis rhodani and macrobenthic community structure in running waters of the N’ Harz Mountains (Lower Saxony/FRG) (Ephemeroptera: Baetidae). Entomologia Generalis 16: 31–37. https://doi.org/10.1127/entom.gen/16/1991/31.
Rempel, L. L., J. S. Richardson & M. C. Healey, 2000. Macroinvertebrate community structure along gradients of hydraulic and sedimentary conditions in a large gravel-bed river. Freshwater Biology 45: 57–73. https://doi.org/10.1046/j.1365-2427.2000.00617.x.
Richardson, J. S., 2019. Biological diversity in headwater streams. Water 11: 366. https://doi.org/10.3390/w11020366.
Sargac, J., R. K. Johnson, F. J. Burdon, A. Truchy, G. Rîşnoveanu, P. Goethals & B. G. McKie, 2021. Forested riparian buffers change the taxonomic and functional composition of stream invertebrate communities in agricultural catchments. Water 13: 1028. https://doi.org/10.3390/w13081028.
Savage, A. A., 1989. Adults of the British aquatic Hemiptera Heteroptera: a key with ecological notes. Scientific publications of Freshwater Biological Association 50: 173 p.
Savard, J. P. L., P. Clergeau & G. Mennechez, 2000. Biodiversity concepts and urban ecosystems. Landscape and Urban Planning 48: 131–142. https://doi.org/10.1016/S0169-2046(00)00037-2.
Schmedtje, U. & M. Colling, 1996. Ökologische Typisierung der aquatischen Makrofauna. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft, Bayerisches Landesamt für Wasserwirtschaft, München:
Schueler, T. R., L. Fraley-McNeal & K. Cappiella, 2009. Is impervious cover still important? Review of recent research. Journal of Hydrologic Engineering 14: 309–315. https://doi.org/10.1061/(asce)1084-0699(2009)14:4(309).
Simkin, R. D., K. C. Seto, R. I. McDonald & W. Jetz, 2022. Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proceedings of the National Academy of Sciences of the United States of America 119: 1–10. https://doi.org/10.1073/pnas.2117297119.
Statzner, B., 2012. Geomorphological implications of engineering bed sediments by lotic animals. Geomorphology 157–158: 49–65. https://doi.org/10.1016/j.geomorph.2011.03.022.
Stepenuck, K. F., R. L. Crunkilton, M. A. Bozek & L. Wang, 2008. Comparison of macroinvertebrate-derived stream quality metrics between snag and riffle habitats. Journal of the American Water Resources Association 44: 670–678. https://doi.org/10.1111/j.1752-1688.2002.tb05544.x.
Stoll, S., J. Kail, A. W. Lorenz, A. Sundermann & P. Haase, 2014. The importance of the regional species pool, ecological species traits and local habitat conditions for the colonization of restored river reaches by fish. PLoS ONE 9: 1–10. https://doi.org/10.1371/journal.pone.0084741.
Stubbington, R., 2012. The hyporheic zone as an invertebrate refuge: a review of variability in space, time, taxa and behaviour. Marine and Freshwater Research 63: 293–311. https://doi.org/10.1071/MF11196.
Szita, R., A. Horváth, D. Winkler, P. Kalicz, Z. Gribovszki & P. Csáki, 2019. A complex urban ecological investigation in a mid-sized Hungarian city – SITE assessment and monitoring of a liveable urban area, PART 1: water quality measurement. Journal of Environmental Management 247: 78–87. https://doi.org/10.1016/j.jenvman.2019.06.063.
Tachet, H., P. Richoux, M. Bournaud & P. Usseglio-Polatera, 2000. Invertébrés d’eau douce, systématique, biologie, écologie, CNRS Editi, París:
Taniguchi, H. & M. Tokeshi, 2004. Effects of habitat complexity on benthic assemblages in a variable environment. Freshwater Biology 49: 1164–1178. https://doi.org/10.1111/j.1365-2427.2004.01257.x.
Taylor, S. L., S. C. Roberts, C. J. Walsh & B. E. Hatt, 2004. Catchment urbanisation and increased benthic algal biomass in streams: linking mechanisms to management. Freshwater Biology 49: 835–851. https://doi.org/10.1111/j.1365-2427.2004.01225.x.
Tóth, R., I. Czeglédi, B. Kern & T. Erős, 2019. Land use effects in riverscapes: diversity and environmental drivers of stream fish communities in protected, agricultural and urban landscapes. Ecological Indicators 101: 742–748. https://doi.org/10.1016/j.ecolind.2019.01.063.
Turrini, T., D. Sanders & E. Knop, 2016. Effects of urbanization on direct and indirect interactions in a tri-trophic system. Ecological Applications 26: 664–675. https://doi.org/10.1890/14-1787.
UN DESA, 2019. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420), United Nations Department of Economic and Social Affairs, Population Division, United Nations, New York:
UN DESA, 2022. World Population Prospects 2022: Summary of Results (UN DESA/POP/2021/TR/NO. 3), United Nations Department of Economic and Social Affairs, Population Division, United Nations, New York:
Utz, R. M., R. H. Hilderbrand & R. L. Raesly, 2010. Regional differences in patterns of fish species loss with changing land use. Biological Conservation 143: 688–699. https://doi.org/10.1016/j.biocon.2009.12.006.
Verdonschot, R. C. M., J. Kail, B. G. McKie & P. F. M. Verdonschot, 2016. The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 769: 55–66. https://doi.org/10.1007/s10750-015-2575-8.
Vilenica, M., A. Brigić, M. Sartori & Z. Mihaljević, 2018. Microhabitat selection and distribution of functional feeding groups of mayfly larvae (Ephemeroptera) in lotic karst habitats. Knowledge and Management of Aquatic Ecosystems 419: 17. https://doi.org/10.1051/kmae/2018011.
Walsh, C. J., P. F. Breen, A. K. Sharpe & J. A. Sonneman, 2001. Effects of urbanization on streams of the Melbourne region, Victoria, Australia. II. Benthic diatom communities. Freshwater Biology 46: 553–565. https://doi.org/10.1046/j.1365-2427.2001.00689.x.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottingham, P. M. Groffman & R. P. Morgan, 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723. https://doi.org/10.1899/04-028.1.
Walsh, C. J., K. A. Waller, J. Gehling & R. Mac Nally, 2007. Riverine invertebrate assemblages are degraded more by catchment urbanisation than by riparian deforestation. Freshwater Biology 52: 574–587. https://doi.org/10.1111/j.1365-2427.2006.01706.x.
Wang, L., J. Lyons, P. Kanehi, R. Bannerman & E. Emmons, 2000. Watershed urbanization and changes in fish communities in southeastern Wisconsin streams. Journal of the American Water Resources Association 36: 1173–1189. https://doi.org/10.1111/j.1752-1688.2000.tb05719.x.
Wang, L., J. Lyons, P. Kanehl & R. Bannerman, 2001. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environmental Management 28: 255–266. https://doi.org/10.1007/s0026702409.
Wang, B., D. Liu, S. Liu, Y. Zhang, D. Lu & L. Wang, 2012. Impacts of urbanization on stream habitats and macroinvertebrate communities in the tributaries of Qiangtang River, China. Hydrobiologia 680: 39–51. https://doi.org/10.1007/s10750-011-0899-6.
Waringer, J. & W. Graf, 2011. Atlas of Central European Trichoptera Larvae, Erik Mauch Verlag, Dinkelscherben.
Wiederkehr, F., C. L. Wilkinson, Y. Zeng, D. C. J. Yeo, R. M. Ewers & E. J. O’Gorman, 2020. Urbanisation affects ecosystem functioning more than structure in tropical streams. Biological Conservation 249: 108634. https://doi.org/10.1016/j.biocon.2020.108634.
Williams, D. D., 1984. The hyporheic zone as a habitat for aquatic insects and associated arthropods. In Resh, V. H. & D. M. Rosenberg (eds), The Ecology of Aquatic Insects Praeger, New York: 430–455.
Williams, D. D. & H. B. N. Hynes, 1974. The occurrence of benthos deep in the substratum of a stream. Freshwater Biology 4: 233–256. https://doi.org/10.1111/j.1365-2427.1974.tb00094.x.
Willis, S. C., K. O. Winemiller & H. Lopez-Fernandez, 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142: 284–295. https://doi.org/10.1007/s00442-004-1723-z.
Zerega, A., N. E. Simões & M. J. Feio, 2021. How to improve the biological quality of urban streams? Reviewing the effect of hydromorphological alterations and rehabilitation measures on benthic invertebrates. Water 13: 2087. https://doi.org/10.3390/w13152087.
Acknowledgements
We thank Noémi Menczelesz for providing help in the fieldwork and in the identification. We are grateful for Béla Csányi, Csaba Deák, Eszter Krasznai-Kun and Arnold Móra for their extensive help during the identification process. We thank Andrew J. Hamer for his comments on the manuscript. We also thank two anonymous reviewers for their valuable and constructive comments on an earlier version of this paper.
Funding
Open access funding provided by University of Pannonia. This study was supported by the NKFI K140352 and RRF-2.3.1-21-2022-00014 (National Multidisciplinary Laboratory for Climate Change) projects. Pál Boda is financially supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO-00106-21 and by the ÚNKP-21-5, ÚNKP-22-5, New National Excellence Program of the Ministry of Innovation and Technology from the source of the National Research, Development and Innovation Fund. Judit Padisák was supported by NKFIH KKP 144068 during manuscript writing. Barbara Barta acknowledges funding through RRF-2.3.1-21-2022-00014.
Author information
Authors and Affiliations
Contributions
DS, IS, and BG did conceptualization; DS done methodology, formal analysis and investigation and funding acquisition; AB, BB, and DS provided statistical analysis; AB was involved in writing—original draft preparation; all authors provided writing—review and editing; DS and JP supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing or financial interests. Author Judit Padisák serves as an associate editor at Hydrobiologia.
Additional information
Handling Editor: Marcelo S. Moretti
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bohus, A., Gál, B., Barta, B. et al. Effects of urbanization-induced local alterations on the diversity and assemblage structure of macroinvertebrates in low-order streams. Hydrobiologia 850, 881–899 (2023). https://doi.org/10.1007/s10750-022-05130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05130-1