Abstract
Context
Human-induced landscape modification, such as urbanization, creates new environments that can have adverse effects on flora and fauna, posing threats to biodiversity. Understanding how reptiles respond to urbanization is crucial, especially in light of their ongoing population declines.
Objectives
We examined the influence of landscape-scale and local-scale urbanization features on the abundance of an aquatic snake species. Our investigation focused on dice snakes (Natrix tessellata) inhabiting a lake with a heavily urbanized shoreline.
Methods
We conducted visual encounter surveys at 25 study sites during the activity period of dice snakes around Lake Balaton in Hungary. We measured both landscape-scale and local-scale variables, including urban land use cover, vegetation cover, road cover, distance of main roads and city size, emergent vegetation cover and the area of artificial rock and concrete shoreline protection structures. We analysed snake survey data using N-mixture models to estimate abundance and examine relationships with landscape-scale and local-scale variables.
Results
Urban land use cover, road cover, the proximity of main roads and the extent of artificial rock and concrete shoreline protection structures positively affected the abundance of snakes. These findings imply that urban habitats may offer new ecological opportunities for dice snakes.
Conclusions
The findings of this study indicate that both landscape-scale and local-scale human-induced landscape modifications may have a positive impact on the abundance of urban snakes. Taken together, our findings suggest that urbanization is a complex phenomenon, affecting species at different levels and with subtle effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human-induced environmental changes are globally affecting biodiversity by causing pollution, climate change and habitat modification such as urbanization (French et al. 2018; Willmott et al. 2022). The rapidly growing human population (United Nations 2019) results in the expansion of urban areas (Chen et al. 2014), generating novel environments that have the potential to negatively affect plant and animal life, posing threats to biodiversity. Species residing in urban areas may encounter challenges related to habitat fragmentation (Fardila et al. 2017), exposure to pollutants (Frixione et al. 2022), reduced vegetation (Kisvarga et al. 2023), alterations in the thermal environment (Andrade et al. 2023), changes in predation pressure and food availability (Eötvös et al. 2018), as well as the existence of novel structures and substrates (French et al. 2018). This implies that the urban environment introduces novel and intricate selective pressures and consequently has a significant effect on animal populations.
While many reptiles experience the negative effects of human-induced environmental changes, they are underrepresented in urban ecology research (Doherty et al. 2020). Ectothermic creatures demonstrate heightened sensitivity to their thermal environment, and the limited dispersal ability of reptiles makes them more vulnerable to habitat changes (Inman et al. 2023). Research on reptiles has indicated that urban anthropogenic stressors, such as noise (Mancera et al. 2017), pollution (Lettoof et al. 2020; Salvador et al. 2018), fragmentation (Tan et al. 2023) and elevated temperature (Andrade et al. 2023; Battles and Kolbe 2019; Hall and Warner 2018) can act as deterrents. For instance, road coverage is an important abiotic factor of the urban environment, presenting indirect (pollutants, impermeable surface, disturbance, fragmentation) and direct (road kills) sources of mortality (Fahrig and Rytwinski 2009; Rytwinski and Fahrig 2012). Besides these, asphalt roads and concrete pavements significantly influence the ambient temperature of urban habitats, causing urban areas to experience higher temperatures compared to nearby rural areas. This phenomenon is referred to as the urban heat island effect (Andrade et al. 2023; Hulley 2012) which is primarily linked to asphalt roads (Gong et al. 2023; Wang et al. 2021). Higher environmental temperatures resulting from the urban heat island effect can affect fitness-related traits of individuals (Batabyal and Thaker 2019; Cordero et al. 2022; Putman et al. 2019), potentially influencing survival and population sizes of many reptile species.
The majority of research investigating the impact of urbanization on reptile populations suggests a decline in population size or species richness with urbanization. For instance, a worldwide meta-analysis, incorporating 56 studies on reptiles, revealed that the average reptile abundance is one-third lower in modified habitats compared to unmodified ones (Doherty et al. 2020). Besides this, Ackley et al. (2015a) discovered that the proportion of building cover within a 200-m diameter buffer had a significantly adverse effect on both lizard diversity and abundance. Moreover, a similar pattern was observed in the case of the eastern green lizards (Lacerta viridis) in Bulgaria (Prieto-Ramírez 2023) and the skink communities across four cities of New Zealand (Woolley et al. 2023). On the contrary, many studies reveal an opposite trend as some reptile species, by adapting to novel environmental conditions, can flourish in urban habitats. For instance, the crested anoles (Anolis cristatellus) demonstrate increased tolerance to higher environmental temperatures in urban habitat when compared to their counterparts in forested areas (Campbell-Staton et al. 2020). In addition, a 13-year study of the populations of the common chuckwalla (Sauromalus ater) near the Phoenix Metropolitan region has demonstrated population stability despite the highly urbanized environment (Sullivan and Sullivan 2008; Sullivan and Williams 2010). Also, eastern long-necked turtles (Chelodina longicollis) were more abundant and grew faster in suburban areas compared to the nature reserves (Roe et al. 2011).
Exploring the factors influencing reptile populations is urgent as they are experiencing global declines (Gibbons et al. 2000). To address this, our study aimed to examine the impact of urbanization on the abundance of an aquatic snake living on a lake characterized by a highly urbanized shoreline and experiencing significant human disturbance. We examined the abundance of dice snakes (Natrix tessellata) residing in the artificial lakeside habitats of Lake Balaton, exploring correlations with various aspects of urbanization assessed at both landscape- and local-scales. In our previous study we found that urban land use cover, road cover and the proximity of main roads had a negative effect on developmental stability, while urban land use cover and the area of the artificial rock and concrete shoreline protection structures had a positive effect on the body size of the dice snakes (Mészáros et al. 2023).
We hypothesized that while different aspects of urbanization at the landscape-scale negatively impact snake populations, at the local-scale the existence of artificial rock and concrete shoreline protection structures has a beneficial effect, playing a critical role in determining their abundance. We aimed to explore the following questions: how do various land use types (urban, natural or semi-natural land cover) and settlement sizes influence snake abundance? Additionally, we sought to investigate whether road coverage and proximity to roads have adverse effects on snake abundance. Lastly, despite the overall negative impact of urbanization, do local habitat features, such as artificial rock and concrete coastline protection structures and emergent vegetation cover, positively affects snake abundance?
Given that previous findings indicated a negative correlation between urbanization and reptile abundance (Doherty et al. 2020) and developmental stability (Mészáros et al. 2023), attributed to the higher pollution rates (Tombesi et al. 2014), our first prediction was that at the landscape scale (i) urban land use cover would negatively, while; (ii) natural or semi-natural land cover would positively affect the abundance of dice snakes. We also predicted that; (iii) populations residing in larger settlements might experience heightened human disturbance due to a larger number of inhabitants and tourists resulting in decreased snake abundance. Additional to the negative effect of urban land cover and human disturbance, we also predicted that; (iv) road density; and (v) the proximity of roads would both negatively influence snake abundance due to road pollutants (Dixon et al. 2022; Yang et al. 2014) and higher risk of road mortality (Hallisey et al. 2022). Moreover, it has been shown previously that the area of road cover and the proximity of roads positively correlate with the fluctuating asymmetry of dice snakes assuming higher developmental instability (Mészáros et al. 2023). This, in turn, could indirectly impact the survival of individuals, potentially contributing to a decline in snake populations.
At the local-scale, our predictions were that, despite the adverse impacts of urbanization, (vi) the artificial rock and concrete shoreline protection structures would play a crucial role in determining the abundance of dice snakes (Conelli et al. 2011). We found in our previous study that the human-made rock-covered shoreline had a positive effect on snake body size (Mészáros et al. 2023) by presumably supporting abundant and stable food resources (Eötvös et al. 2018). Additionally, (vii) a greater cover of emergent vegetation was predicted to provide suitable habitat for sheltering, nesting and hibernation.
Materials and methods
Study area
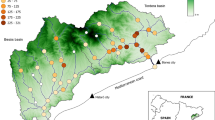
The study was conducted at Lake Balaton in Hungary, which is the largest shallow lake in Central Europe, covering a surface area of 593 km2. It has an average depth of 3.14 m, a length extending to 77.9 km, and an average width of 7.2 km (Specziár and Bíró 1998). As Lake Balaton is the main tourist attraction in Hungary and the surrounding countries, the shoreline area of the lake is experiencing intense urbanization and significant disturbance due to human activity. Over half of the shoreline, approximately 128 km out of 240 km, is artificial and shielded by riprap-covered shoreline protection structures (Karádi-Kovács et al. 2023). In addition to the extensive urbanization of the shoreline, the surrounding area of the lake is also highly fragmented by roads with high traffic density. Overall, Lake Balaton provides a suitable model system to examine the effects of urban landscapes on aquatic ecosystems.
We selected a total of 25 sites along the lake shoreline (Fig. 1 and Online Resource 1), each with a 250-m-long transect designated as the survey area. The sites included harbours, promenades and beaches, and the transects were always placed along the artificial rock and concrete shoreline protection structures. The distances between the sites were determined by the mean dispersal distances of adult dice snakes based on previous radiotelemetry studies (Conelli et al. 2011; Neumann and Mebert 2011; Velenský et al. 2011). The mean distance to the closest surveyed site was 6154 m (SD = 3235 range: 2581–16 778 m) which is much longer than the average movement distances of the species.
Snake survey
Dice snakes (Natrix tessellata) are Eurasian, non-venomous colubrid snakes (family Colubridae, subfamily Natricinae) measuring between 70 and 100 cm. They mainly feed on fishes and have a strictly aquatic lifestyle. Due to their lifestyle, they are typically found in close proximity to large, permanent, or semi-permanent waterbodies (Mebert 2011). In Lake Balaton, dice snakes are predominantly observed (sometimes in significant numbers) along the artificial rock and concrete structures of harbours, promenades, and beaches which makes it an appropriate model organism to study the response of an aquatic snake species to urbanization.
A total of six visual encounter surveys were undertaken at each site in 2022 within the activity period of dice snakes. Sites were visited twice in spring (survey 1: May 9th–May 19th; survey 2: May 20th–June 2nd), twice in summer (survey 3: July 4th–July 14th; survey 4: July 18th–July 29th) and twice in autumn (survey 5: September 9th–September 29th; survey 6: September 30th–October 14th). Transects were consistently positioned along the rock and concrete protection structures which follow a consistent design around the entire shoreline (Karádi-Kovács et al. 2023) and exhibit sparse vegetation at our designated sites. The snakes were counted in the full width of the shoreline protection structure. Snake counts were conducted along the entire width of the shoreline protection structure. In each survey, two individuals counted snakes along the transects, starting from opposite ends and maintaining a consistent walking pace towards each other for a maximum of 30 min. Only visible snakes were counted during each survey. Consistently, the same two individuals conducted all the visual surveys to minimize variation attributable to different samplers. Surveys were conducted during the day between 0900 and 1700 h, with each transect being scanned only once to avoid pseudoreplication. Water temperature was recorded in Celsius using a digital water thermometer positioned in the upper 10 cm of the water surface at the start, midpoint, and endpoint of the transect. Air temperature, also in Celsius, was measured with a shaded air thermometer at three points along the transect (beginning, middle, and end). The average of the three measurements of water (WTEMP) and air temperature ATEMP) and the Julian date (DATE) and the time of the day (TIME) of each survey was used in the further analyses as detection covariates.
Landscape-scale and local-scale covariates
To measure landscape-scale covariates (Table 1) we defined a 1000-m-long and 200-m-wide buffer zone (with 100 m on land and 100 m on water) along the lake shoreline for each site. The size of the buffer zone was determined to cover the average dispersal distance of dice snakes (Conelli et al. 2011; Neumann and Mebert 2011; Velenský et al. 2011). The URBAN, VEG and ROADS landscape-scale covariates were defined as the total area of each within the buffer zones. The urban land use cover covariate (URBAN) encompassed non-natural, human-made surface cover such as low-rise and tall buildings, paved roads, dirt roads, railways, urban green spaces with and without trees, and other paved or non-paved artificial areas. The vegetation cover covariate (VEG) represents the total area of the tall-herb vegetation of marshes and fens (mainly reeds) standing in water. Our assumption was that roads pose a high risk of mortality for dice snakes, so we additionally measured two variables related to roadkill effects (Velenský et al. 2011). The road cover covariate (ROADS) refers exclusively to the surface area covered by asphalt and paved roads serving as a measure to estimate the risk of road-kills and the effects of road pollutants. The lake is encircled by two main roads, road 71 on the north coast and road 7 on the south coast, which have the highest traffic volumes in the area. The DISTROAD covariate was determined by measuring the nearest distance (m) to the main road (road 71 or road 7) from the centre of the transects. To assess the influence of human disturbance from residents and tourists (CITY), we used the built-up area of the settlements/cities (The Land Office Portal 2023) where the transects were located.
As local-scale covariates (Table 1) we determined the area of the artificial rock and concrete shoreline protection structures (ROCKS) and estimated the percentage of emergent vegetation (EMVEG) within each transect. It has been shown in previous studies that human-made structures and vegetation cover of the shoreline are both important determinants of dice snake distribution (Conelli et al. 2011).
We used QGIS 3.28.0 and The Ecosystem Map of Hungary (Ministry of Agriculture 2019) for area calculations and distance measurements.
Statistical analyses
Count data from snake surveys was analysed by building N-mixture models to estimate the abundance and to assess relationships between the abundance of snakes and landscape and local covariates. N-mixture models are hierarchical models which allow for the estimation of species’ abundance as a function of site covariates based on repeated count surveys, while also accounting for the imperfect detection of individuals. The model estimates abundance (Ni) at site i, assuming that Ni follows a Poisson distribution with an expected abundance (λ) over all sites. Besides this, it uses repeated counts (Cij) at site i during survey j (repeated survey at the same site) to estimate individual detection probability (p) and it assumes that Cij|Ni follows a Binomial distribution (Ni, p). (Royle 2004; Royle et al. 2005). The model also enabled us to include environmental metrics as covariates; therefore both parameters (abundance and detection) could be expressed as functions of environmental covariates through a log or logit link, respectively. Previous studies have demonstrated that N-mixture models offer reliable estimates of abundance aligning with outcomes from alternative methods such as capture-mark-recaptures (Ficetola et al. 2018; Neubauer et al. 2022).
Given that reptiles are ectothermic, their detectability is mostly influenced by environmental temperature, therefore we assumed that dice snakes with aquatic habits would be more detectable in warmer air and water temperatures. Additionally, considering that detectability tends to be higher during the mating season, we assumed increased detectability in spring (at earlier dates). Based on these assumptions, we considered ATEMP, WTEMP, DATE and TIME variables which could potentially influence the detection of dice snakes. Three models were built to determine which detection covariate(s) had an important effect on the detectability of dice snakes.
The detection sub-model assumed that detection followed a binomial distribution (Cij ~ Binomial (pij, Nij) where Cij is the number of detected individuals at site i and on survey j, and pij is the detection probability of each individual at site i and on survey j) (Royle et al. 2005). Detection probability was expressed as a logit-linear function of one survey-specific covariate:
where α0 is the intercept, α1 is the covariate effect and Y is one of the detection covariates at each site i at survey j (WTEMP, ATEMP, DATE, TIME).
The three models were compared using the Watanabe-Akaike Information Criterion (WAIC) (Burnham and Anderson 2002; Gelman et al. 2014; Watanabe 2010). Models with ΔWAIC < 2 were considered to have the greatest support (McCarthy 2007). Our results showed that the model with WTEMP was the best-supported model (Table 2), so we used WTEMP as a detection covariate in the models assessing abundance.
The abundance sub-model assumed an imperfectly observed abundance where the abundance at a site (i) follows Poisson distribution (Ni ~ Poisson (λi) where λi is the expected mean abundance) (Royle et al. 2005). Overdispersion can introduce bias to parameter and abundance estimates in N-mixture models (Knape et al. 2018). To address this, we incorporated a random effect term (ε) in each model to account for overdispersion and unexplained variation in abundance resulting from repeated counts among sites (Kéry et al. 2009). The mean abundance was expressed as a log-linear function of the site covariates:
where β0 is the intercept, β1 and β2 are covariate effects and X is one of the landscape or local covariates at site i (URBAN, VEG, CITY, ROADS, DISTROAD, ROCKS, EMVEG). Given that our main hypothesis tested whether urbanisation affected snake abundance, the URBAN covariate was included in all models. Following the recommendation of a minimum ratio of ten sites to the number of estimated parameters, we incorporated a maximum of two covariates in each sub-model (Harrison et al. 2018). In order to test which landscape- and local-scale covariates influenced the abundance of dice snakes and to test our predictions we built seven different models. Then, the best-supported models were chosen based on the ΔWAIC values. Before constructing our models we standardized covariates and assessed them for collinearity, considering a cut-off for inclusion based on Pearson R < 0.7 (Dormann et al. 2013). None of the considered landscape- and local-scale covariates were strongly correlated (R < 0.53). Covariates were scaled at mean = 0 and SD = 1 to improve model convergence. Before the standardization, URBAN, VEG, CITY, ROADS, DISTROAD and ROCKS were log-transformed.
We performed all modelling using JAGS version 4.3.0 (Plummer 2017) called via the R2jags package (Su and Yajima 2015) in R (R Core Team 2021). We conducted three replicates of Markov chain Monte Carlo generating a total of 650 000 samples from the posterior distribution. We removed an initial "burn-in" phase of 50 000 samples and applied a thinning rate of 13. Convergence of the models was accepted if the Brooks-Gelman-Rubin statistic (R̂) < 1.1 (Brooks and Gelman 1998) and by visual inspection of traceplots. The mean, SD and the 95% Bayesian credible intervals (BCI) are presented for all parameters of the best supported models. Parameter estimates were considered important when the BCI did not overlap zero. However, a certain level of tolerance was allowed for a slight overlap of the BCI with zero (Cumming and Finch 2005).
Results
The detection sub-model with only WTEMP was the best supported model (WAIC = 1952.95; Table 2). There was a positive relationship between the probability of detection and water temperature (mean = 0.181; SD = 0.027; BCI: 0.127–0.234; Table 3), inferring that dice snakes were more detectable in warmer water. Subsequent models for abundance were built using WTEMP as a detection covariate.
The best-ranked abundance models included the covariates URBAN, ROADS, DISTMAINROAD and ROCKS (Table 4). There was a positive association between mean snake abundance and the URBAN covariate (mean = 0.393; SD = 0.147; BCI: 0.102–0.683; Table 5) indicating higher dice snake abundance in areas with increased urban land use cover (Fig. 2A). There was a positive influence of the ROADS covariate on mean snake abundance with a BCI slightly overlapping zero (mean = 0.288; SD = 0.161; BCI: − 0.032 to 0.605; Table 5), indicating higher snake abundance in areas with increased road cover (Fig. 2B). There was a negative relationship between mean snake abundance and the DISTMAINROAD covariate (mean = − 0.445; SD = 0.144; BCI: − 0.731 to − 0.163; Table 5) indicating greater snake abundance in close proximity to main roads (Fig. 2C). Lastly, there was a positive relationship between the area of artificial rocky and concrete shoreline protection structures (ROCKS) and the mean abundance of dice snakes (mean = 0.484; SD = 0.141; BCI 0.207–0.764; Table 5) suggesting larger snake populations where the rocky shoreline is larger (Fig. 2D).
The relationships between the mean estimates of snake abundance and landscape-scale (A–C) and local-scale (D) habitat covariates: A urban land use cover (m2) within the 1000-m buffer zone; B road cover (m2) within the 1000-m buffer zone; C the distance (m) of main roads; D the area (m2) of the artificial rock and concrete shoreline protection structures; The solid black lines represent the estimated snake abundance while the dashed black lines and the shaded areas are the error of estimated abundance, the 95% Bayesian credible intervals
Discussion
Earlier research indicates that urbanization tends to have an adverse impact on reptile populations. Nevertheless, conflicting findings demonstrate that certain species can thrive in urban environments. To understand the factors influencing reptile species in urban habitats, we conducted a study to investigate the impact of urbanization at various scales on the abundance of an aquatic snake residing in a highly urbanized lake shoreline. We found that urban land use cover, road cover, proximity of main roads and the area of the artificial rock and concrete shoreline protection structures had a positive influence on the abundance of dice snakes. Our results show that both landscape-scale urbanization and local-scale urban features exert a positive effect on the abundance of an aquatic snake, and that the highly urbanized lakeshore we studied provides suitable habitat for this reptile.
Contrary to our prediction and the results of some previous studies (Doherty et al. 2020; Prieto-Ramírez 2023; Woolley et al. 2023), we found that urban land use cover was positively correlated with the mean abundance of snakes (Fig. 2A; Table 5), implying that populations living in more urbanized habitats are more abundant. An increase in snake abundance with urbanization could be the result of multiple hypotheses that are not mutually exclusive. In urban environments reptiles may encounter additional microhabitats, such as artificially constructed rocky and metal structures, that could offer suitable locations for sheltering, basking, nesting, and hibernation (Graitson et al. 2020; Herbert et al. 2023). Moreover, the urban environment can be characterized by reduced predation pressure, leading to decreased mortality rates and increased time available for foraging and mating (Eötvös et al. 2018). Dice snakes face a wide range of predators, including various bird species (e.g. Ardea sp., Corvus sp., Bubo bubo, Accipiter sp., Buteo sp., Larus sp.) and carnivorous mammals (e.g. Lutra lutra, Mustela sp., feral cats and dogs) (Jelić and Lauš 2011; Ajtić et al 2013; Velenský et al. 2011). Regrettably, our research lacks data on predation rates across the sites, and this aspect remains unexplored in the context of Lake Balaton. This hypothesis raises an intriguing question that deserves further investigation. A third hypothesis suggests that urban areas offer richer and more consistent food resources compared to rural areas (Putman and Tippie 2020). Artificial structures, like rocky shores, can serve as ideal habitats for various fish species. Dice snakes, being aquatic creatures, predominantly prey on small fishes, such as gobies (Maier et al. 2023), which are highly abundant in harbours and close to bank reinforcement structures (Zarini et al. 2019; Czeglédi et al 2019). Moreover, dice snakes demonstrate notable dietary adaptability that corresponds to habitat characteristics (Weiperth et al. 2014). Research has demonstrated that in heavily urbanized areas, dice snakes can adjust their diet according to the prevalent prey, as seen in their exclusive consumption of invasive fish species in the Danube (Maier et al. 2023). Given the abundance of invasive fish species, such as gobies, in Lake Balaton (Czeglédi et al. 2019), it is conceivable that dice snakes predominantly target these readily available prey, leading to a diet focused primarily on invasive fish species. Besides predation pressure and abundant food resources, interspecific competitors may also have significant impact on the abundance of a species in urban areas (Møller and Díaz 2018). Another aquatic snake species, the grass snake (Natrix natrix), also occupies certain sites included in our study. However, unlike dice snakes, which primarily prey on fish, grass snakes predominantly consume amphibians (Gregory and Isaac 2004) which are predominantly found in dense reed beds and wetlands and not around the rocky shoreline. We believe that this dietary contrast and preferred habitat differences likely mitigates competition between the two species. Altogether, the presence of suitable microhabitats with reduced predation pressure and the availability of abundant, stable food resources in urban areas may create favourable habitats for dice snakes. Additionally, as a supporting observation, our previous study revealed that dice snakes residing in more urbanized areas exhibit larger body sizes (Mészáros et al. 2023), suggesting that urban habitats may offer new ecological opportunities for dice snakes.
Contrary to previous studies on the effects of roads on wildlife (Fahrig and Rytwinski 2009; Farias et al. 2022; Rytwinski and Fahrig 2012; Wagner et al. 2021), dice snake abundance was positively correlated with road cover and road proximity (Fig. 2B and C; Table 5). This result implies that dice snake populations in areas with a more intense road network and closer to the main roads are more abundant. Earlier research has demonstrated that roads can exert significant biological, physical, and chemical effects on both terrestrial and aquatic ecosystems (Dixon et al. 2022), including the introduction of pollutants (Wang et al. 2020), habitat fragmentation (Hamer et al. 2021), road mortality (Farias et al. 2022), and alterations to thermal regimes (Wang et al. 2021). The thermal conditions within human settlements undergo substantial changes compared to rural areas due to the substitution of vegetative cover with impermeable, heat-absorbing artificial surfaces like buildings, concrete pavements, parking lots and asphalt roads (Wang et al. 2021). Asphalt road cover has a notable impact on the overall temperature of urban environments (Wang et al. 2021) and serves as the primary contributor to the urban heat island effect (Andrade et al. 2023; Hulley 2012). The thermal conductivity of asphalt road cover is minimal, and as a dark impermeable surface, it has the capacity to absorb substantial amounts of solar radiation, leading to elevated ambient temperatures (Gong et al. 2023).
In ectotherms, various physiological processes show a positive correlation with body temperature within an optimal range, reaching peak performance at a preferred temperature (Angilletta 2009). Some species may avoid urban environments due to temperatures exceeding their thermal tolerances (Ackley et al. 2015b; Hall and Warner 2019). However, if urban areas align with a species' thermal tolerances, it could enhance access to preferred body temperatures, potentially reducing constraints on activity time (Gunderson and Leal 2015). Research on brown anoles (Anolis sagrei) suggests urban environments facilitate improved thermoregulation, leading to increased activity rates (Battles and Kolbe 2019). Similarly, urban Puerto Rican crested anoles (Anolis cristatellus) endure higher temperatures and exhibit enhanced heat tolerance compared to their forest counterparts (Campbell-Staton et al. 2020). This suggests that lizards can adapt to higher ambient temperatures and gain advantages from residing in an urban habitat. In our case, a potential explanation is that the elevated environmental temperature resulting from the urban heat island effect is within the optimal thermal performance range of dice snakes. This, in turn, may have a positive influence on the body temperature leading to beneficial effects on their metabolism, movement and reproduction. Nevertheless, further investigation is required to understand the impact of urban temperatures on the thermal performance of dice snakes.
At the local-scale, we observed a positive correlation between the abundance of dice snakes and the extent of artificial rock and concrete shoreline protection structures along the lake shoreline (Fig. 2D; Table 5). Supporting our finding, previous studies also showed that artificial structures, such as metal and riprap-covered structures, are widely utilized by lizard and snake species in urban habitats for basking and hiding (Herbert et al. 2023; Watchorn et al. 2023; Yu et al. 2022). For example, the density of lagartixas (Tropidurus hispidus) is elevated in urbanized areas and the primary predictor of abundance was man-made structures which provide shelter (de Andrade 2020). Besides, the density of smooth snakes (Coronella austriaca) was found to be highest in urbanized areas along railway beds and artificial rocky habitats compared to grasslands in nature reserves (Graitson et al. 2020). A plausible explanation for our result could be that rocky shorelines are associated with abundance due to the fact that these structures are favoured habitats for their prey species, including gobies and other small-sized fishes (Capula et al. 2011; Zarini et al. 2019). Furthermore, as an additional supporting observation, a prior study of ours indicated that dice snakes residing along shorelines with a greater expanse of rocks tend to have larger body sizes (Mészáros et al. 2023).
Conclusions
This study presents evidence that both landscape-scale urbanization and local-scale urban features have a positive effect on the abundance of an aquatic snake species. Besides this, in our previous research in the study area, we found that urban land use cover and the area of the artificial rock and concrete shoreline protection structures positively affected the body size of dice snakes (Mészáros et al. 2023), indicating that highly urbanized lakeshores potentially provide suitable habitat for this species. Notably, in our earlier investigation, we observed a positive correlation between urban land use cover, road cover and the proximity of roads with the level of fluctuating asymmetry (Mészáros et al. 2023). This suggests that urban land use cover and roads have an adverse effect on the developmental stability of dice snakes, likely due to the presence of pollutants. Further investigation is necessary to identify the pollutants that have a negative impact on snake development and evaluate their potential long-term adverse effects on survival and reproduction. Finally, our findings suggest that urbanization is a multifaceted phenomenon, affecting species at various levels and exerting subtle effects.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ackley JW, Angilletta MJ, DeNardo D, Wu BSJ, Wu JG (2015a) Urban heat island mitigation strategies and lizard thermal ecology: landscaping can quadruple potential activity time in an arid city. Urban Ecosyst 18(4):1447–1459. https://doi.org/10.1007/s11252-015-0460-x
Ackley JW, Wu JG, Angilletta MJ, Myint SW, Sullivan B (2015b) Rich lizards: how affluence and land cover influence the diversity and abundance of desert reptiles persisting in an urban landscape. Biol Conserv 182:87–92. https://doi.org/10.1016/j.biocon.2014.11.009
Ajtić R, Tomović L, Sterijovski B, Crnobrnja-Isailović J, Djordjević S, Djurakić M, Golubović A, Simović A, Arsovski D, Andjelković M, Krstić M, Šukalo G, Gvozdenović S, Aïdam A, Michel CL, Ballouard JM, Bonnet X (2013) Unexpected life history traits in a very dense population of dice snakes. Zool Anz 252(3):350–358. https://doi.org/10.1016/j.jcz.2012.10.001
Andrade C, Fonseca A, Santos JA (2023) Climate change trends for the urban heat island intensities in two major Portuguese cities. Sustainability 15(5):20. https://doi.org/10.3390/su15053970
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, New York
Batabyal A, Thaker M (2019) Social coping styles of lizards are reactive and not proactive in urban areas. Gen Comp Endocrinol 270:67–74. https://doi.org/10.1016/j.ygcen.2018.10.007
Battles AC, Kolbe JJ (2019) Miami heat: Urban heat islands influence the thermal suitability of habitats for ectotherms. Glob Change Biol 25(2):562–576. https://doi.org/10.1111/gcb.14509
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7(4):434–455. https://doi.org/10.2307/1390675
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach. Springer-Verlag, New York
Campbell-Staton SC, Winchell KM, Rochette NC, Fredette J, Maayan I, Schweizer RM, Catchen J (2020) Parallel selection on thermal physiology facilitates repeated adaptation of city lizards to urban heat islands. Nat Ecol Evol 4(4):652–658. https://doi.org/10.1038/s41559-020-1131-8
Capula M, Filippi E, Rugiero L, Luiselli L (2011) Dietary, thermal and reproductive ecology of Natrix tessellata in central Italy: a synthesis. Mertensiella 18:147–153
Chen M, Zhang H, Liu W, Zhang W (2014) The global pattern of urbanization and economic growth: evidence from the last three decades. PLoS ONE 9(8):e103799. https://doi.org/10.1371/journal.pone.0103799
Conelli AE, Nembrini M, Mebert K (2011) Different habitat use of dice snakes, Natrix tessellata, among three populations in Canton Ticino, Switzerland—a radiotelemetry study. Mertensiella 18:100–106
Cordero GA, Birk K, Ruane S, Dinkelacker SA, Janzen FJ (2022) Effects of the egg incubation environment on turtle carapace development. Evol Dev. https://doi.org/10.1111/ede.12425
Cumming G, Finch S (2005) Inference by eye - Confidence intervals and how to read pictures of data. Am Psychol 60(2):170–180. https://doi.org/10.1037/0003-066x.60.2.170
Czeglédi I, Preiszner B, Vitál Z, Kern B, Boross N, Specziár A, Takács P, Erős T (2019) Habitat use of invasive monkey goby (Neogobius fluviatilis) and pumpkinseed (Lepomis gibbosus) in Lake Balaton (Hungary): a comparison of electrofishing and fyke netting. Hydrobiologia 846(1):147–158. https://doi.org/10.1007/s10750-019-04060-9
de Andrade AC (2020) Metropolitan lizards? Urbanization gradient and the density of lagartixas (Tropidurus hispidus) in a tropical city. Ecol Evol 10(4):1740–1750. https://doi.org/10.1002/ece3.5518
Dixon HJ, Elmarsafy M, Hannan N, Gao VV, Wright C, Khan L, Gray DK (2022) The effects of roadways on lakes and ponds: a systematic review and assessment of knowledge gaps. Environ Rev 30(4):501–523. https://doi.org/10.1139/er-2022-0022
Doherty TS, Balouch S, Bell K, Burns TJ, Feldman A, Fist C, Garvey TF, Jessop TS, Meiri S, Driscoll DA (2020) Reptile responses to anthropogenic habitat modification: a global meta-analysis. Glob Ecol Biogeogr 29(7):1265–1279. https://doi.org/10.1111/geb.13091
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1):27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Eötvös CB, Magura T, Lovei GL (2018) A meta-analysis indicates reduced predation pressure with increasing urbanization. Landsc Urban Plan 180:54–59. https://doi.org/10.1016/j.landurbplan.2018.08.010
Fahrig L, Rytwinski T (2009) Effects of roads on animal abundance: an empirical review and synthesis. Ecol Soc 14(1):21
Fardila D, Kelly LT, Moore JL, McCarthy MA (2017) A systematic review reveals changes in where and how we have studied habitat loss and fragmentation over 20 years. Biol Conserv 212:130–138. https://doi.org/10.1016/j.biocon.2017.04.031
Farias W, Napoli MF, Dodonov P, Forti LR (2022) Watch out for the car! Almost a thousand amphibians and reptiles ran over by cars at a single location during one reproductive season in Bahia state. Brazil Biodiversity 23(3–4):129–137. https://doi.org/10.1080/14888386.2022.2140710
Ficetola GF, Barzaghi B, Melotto A, Muraro M, Lunghi E, Canedoli C, Lo Parrino E, Nanni V, Silva-Rocha I, Urso A, Carretero MA, Salvi D, Scali S, Scari G, Pennati R, Andreone F, Manenti R (2018) N-mixture models reliably estimate the abundance of small vertebrates. Sci Rep 8:10357. https://doi.org/10.1038/s41598-018-28432-8
French SS, Webb AC, Hudson SB, Virgin EE (2018) Town and country reptiles: a review of reptilian responses to urbanization. Integr Comp Biol 58(5):948–966. https://doi.org/10.1093/icb/icy052
Frixione MG, D’Amico V, Adami MA, Bertellotti M (2022) Urbanity as a source of genotoxicity in the synanthropic Kelp Gull (Larus dominicanus). Sci Total Environ 850:157958. https://doi.org/10.1016/j.scitotenv.2022.157958
Gelman A, Hwang J, Vehtari A (2014) Understanding predictive information criteria for Bayesian models. Stat Comput 24(6):997–1016. https://doi.org/10.1007/s11222-013-9416-2
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, Deja Vu Amphibians. Bioscience 50(8):653–666. https://doi.org/10.1641/0006-3568(2000)050[0653:Tgdord]2.0.Co;2
Gong X, Liu QT, Lv Y, Chen SC, Wu SP, Ying H (2023) A systematic review on the strategies of reducing asphalt pavement temperature. Case Stud Constr Mater 18:e01852. https://doi.org/10.1016/j.cscm.2023.e01852
Graitson E, Ursenbacher S, Lourdais O (2020) Snake conservation in anthropized landscapes: considering artificial habitats and questioning management of semi-natural habitats. Eur J Wildlife Res 66:39. https://doi.org/10.1007/s10344-020-01373-2
Gregory PT, Isaac LA (2004) Food habits of the Grass snake in southeastern England: is Natrix natrix a generalist predator? J Herpetol 38:88–95
Gunderson AR, Leal M (2015) Patterns of thermal constraint on ectotherm activity. Am Nat 185(5):653–664. https://doi.org/10.1086/680849
Hall JM, Warner DA (2018) Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J Exp Biol 221(14):jeb181552. https://doi.org/10.1242/jeb.181552
Hall JM, Warner DA (2019) Thermal tolerance in the urban heat island: thermal sensitivity varies ontogenetically and differs between embryos of two sympatric ectotherms. J Exp Biol 222(19):jeb210708. https://doi.org/10.1242/jeb.210708
Hallisey N, Buchanan SW, Gerber BD, Corcoran LS, Karraker NE (2022) Estimating road mortality hotspots while accounting for imperfect detection: a case study with amphibians and reptiles. Land 11(5):739. https://doi.org/10.3390/land11050739
Hamer AJ, Barta B, Bohus A, Gal B, Schmera D (2021) Roads reduce amphibian abundance in ponds across a fragmented landscape. Glob Ecol Conserv 28:e01663. https://doi.org/10.1016/j.gecco.2021.e01663
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CE, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. https://doi.org/10.7717/peerj.4794
Herbert SM, Knox C, Clarke D, Bell TP (2023) Use of constructed rock piles by lizards in a grassland habitat in Otago, New Zealand. N Z J Ecol 47(1):3543. https://doi.org/10.20417/nzjecol.47.3543
Hulley M (2012) The urban heat island effect: causes and potential solutions. In: Zeman F (ed) Metropolitan sustainability, understanding and improving the urban environment. Woodhead Publishing, Cambridge, pp 79–98
Inman RD, Esque TC, Nussear KE (2023) Dispersal limitations increase vulnerability under climate change for reptiles and amphibians in the southwestern United States. J Wildl Manage 87(1):e22317. https://doi.org/10.1002/jwmg.22317
Jelić D, Lauš B (2011) The gull Larus cachinnans (Pallas, 1811) as a natural predator of Natrix tessellata (Laurenti, 1768). Mertensiella 18:451–452
Karádi-Kovács K, Boda P, Csabai Z, Deák C, Móra A, Szivák I, Schmera D (2023) Negligible native and significant alien colonization of artificial shoreline by macroinvertebrates in a large shallow lake (Lake Balaton, Hungary). Hydrobiologia 80(8):1837–1848. https://doi.org/10.1007/s10750-023-05186-7
Kéry M, Dorazio RM, Soldaat L, van Strien A, Zuiderwijk A, Royle JA (2009) Trend estimation in populations with imperfect detection. J Appl Ecol 46(6):1163–1172. https://doi.org/10.1111/j.1365-2664.2009.01724.x
Kisvarga S, Horotán K, Wani MA, Orlóci L (2023) Plant responses to global climate change and urbanization: implications for sustainable urban landscapes. Horticulturae 9(9):1051. https://doi.org/10.3390/horticulturae9091051
Knape J, Arlt D, Barraquand F, Berg Å, Chevalier M, Pärt T, Ruete A, Zmihorski M (2018) Sensitivity of binomial N-mixture models to overdispersion: the importance of assessing model fit. Methods Ecol Evol 9(10):2102–2114. https://doi.org/10.1111/2041-210x.13062
Lettoof DC, Bateman PW, Aubret F, Gagnon MM (2020) The broad-scale analysis of metals, trace elements, organochlorine pesticides and polycyclic aromatic hydrocarbons in wetlands along an urban gradient, and the use of a high trophic snake as a bioindicator. Arch Environ Contam Toxicol 78(4):631–645. https://doi.org/10.1007/s00244-020-00724-z
Maier ARM, Telcean IC, Cadar AM, Dumbrava AR, Ferenti S, Cupsa D (2023) Nothing else eaten! The fish diet of Natrix tessellata in the Danube Gorge, Romania. Herpetol Conserv Bio 18(1):30–37
Mancera KF, Murray P, Lisle A, Dupont C, Faucheux F, Phillips CJC (2017) The effects of acute exposure to mining machinery noise on the behaviour of eastern blue-tongued lizards (Tiliqua scincoides). Anim Welf 26(1):11–24. https://doi.org/10.7120/09627286.26.1.011
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, Cambridge
Mebert K (2011) The dice snake, Natrix tessellata: biology, distribution and conservation of a Palaearctic species. Deutsche Gesellschaft für Herpetologie und Terrarienkunde, Rheinbach
Mészáros B, Bürgés J, Tamás M, Gál B, Bohus A, Schmera D (2023) Effects of the urban environment on the developmental stability, size and body condition of dice snakes (Natrix tessellata) living in artificial lakeside habitats. Ecol Indic 156:111117. https://doi.org/10.1016/j.ecolind.2023.111117
Ministry of Agriculture (2019) The ecosystem map of Hungary ministry of agriculture. Ministry of Agriculture, Budapest. https://doi.org/10.34811/OSZ.ALAPTERKEP
Møller AP, Díaz M (2018) Niche segregation, competition, and urbanization. Curr Zool. 64(2):145–152. https://doi.org/10.1093/cz/zox025
Neubauer G, Wolska A, Rowinski P, Wesolowski T (2022) N-mixture models estimate abundance reliably: a field test on marsh tit using time-for-space substitution. Ornithol Appl 124(1):duab054. https://doi.org/10.34811/OSZ.ALAPTERKEP10.1093/ornithapp/duab054
Neumann C, Mebert K (2011) Migration behavior of endangered dice snakes (Natrix tessellata) at the River Nahe, Germany. Mertensiella 18:39–48
Prieto-Ramírez AM (2023) Effects of landscape structure and patch characteristics on the density of central populations of the eastern green lizard Lacerta viridis. Ecol Evol 13(8):e10419. https://doi.org/10.1002/ece3.10419
Putman BJ, Tippie ZA (2020) Big city living: a global meta-analysis reveals positive impact of urbanization on body size in lizards. Front Ecol Evol 8:580745. https://doi.org/10.3389/fevo.2020.580745
Putman BJ, Gasca M, Blumstein DT, Pauly GB (2019) Downsizing for downtown: limb lengths, toe lengths, and scale counts decrease with urbanization in western fence lizards (Sceloporus occidentalis). Urban Ecosyst 22(6):1071–1081. https://doi.org/10.1007/s11252-019-00889-z
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roe JH, Rees M, Georges A (2011) Suburbs: dangers or drought refugia for freshwater turtle populations? J Wildl Manage 75(7):1544–1552. https://doi.org/10.1002/jwmg.219
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60(1):108–115. https://doi.org/10.1111/j.0006-341X.2004.00142.x
Royle JA, Nichols JD, Kéry M (2005) Modelling occurrence and abundance of species when detection is imperfect. Oikos 110(2):353–359. https://doi.org/10.1111/j.0030-1299.2005.13534.x
Rytwinski T, Fahrig L (2012) Do species life history traits explain population responses to roads? A meta-analysis. Biol Conserv 147(1):87–98. https://doi.org/10.1016/j.biocon.2011.11.023
Salvador RMV, Pim F, Nalini HA, de Abreu AT, Pimentel EF, de Cerqueira LO, Ferreira PD, Endringer DC (2018) Tropidurus torquatus (Squamata: Tropiduridae) as a bioindicator of heavy metal (aluminum and zinc) pollution in Vila Velha, Brazil. Environ Sci Pollut Res 25(2):1210–1219. https://doi.org/10.1007/s11356-017-0427-y
Specziár A, Bíró P (1998) Spatial distribution and short-term changes of benthic macrofauna in Lake Balaton (Hungary). Hydrobiologia 389:203–216
Sullivan BK, Sullivan KO (2008) Common chuckwalla (Sauromalus ater) populations in the Phoenix metropolitan area: stability in urban preserves. Herpetol Conserv Bio 3(2):149–154
Sullivan BK, Williams RE (2010) Common chuckwallas (Sauromalus ater) in urban preserves: do food plants or crevice retreats influence abundance. Herpetol Conserv Bio 5(1):102–110
Tan WC, Herrel A, Rödder D (2023) A global analysis of habitat fragmentation research in reptiles and amphibians: what have we done so far? Biodivers Conserv 32(2):439–468. https://doi.org/10.1007/s10531-022-02530-6
Tombesi N, Pozo K, Harner T (2014) Persistent organic pollutants (POPs) in the atmosphere of agricultural and urban areas in the Province of Buenos Aires in Argentina using PUF disk passive air samplers. Atmos Pollut Res 5(2):170–178. https://doi.org/10.5094/apr.2014.021
United Nations DoEaSA, Population Division (2019) World urbanization prospects: the 2018 revision (ST/ESA/SER.A/420). United Nations, New York (eISBN: 978-92-1-004314-4)
Velenský M, Velenský P, Mebert K (2011) Ecology and ethology of dice snakes (Natrix tessellata) in the city district Troja, Prague. Mertensiella 18:157–176
Wagner RB, Brune CR, Popescu VD (2021) Snakes on a lane: road type and edge habitat predict hotspots of snake road mortality. J Nat Conserv 61:125978. https://doi.org/10.1016/j.jnc.2021.125978
Wang ZY, Shen QS, Hua P, Jiang SS, Li RF, Li YB, Fan GD, Zhang J, Krebs P (2020) Characterizing the anthropogenic-induced trace elements in an urban aquatic environment: a source apportionment and risk assessment with uncertainty consideration. J Environ Manage 275:111288. https://doi.org/10.1016/j.jenvman.2020.111288
Wang C, Wang Z-H, Kaloush KE, Shacat J (2021) Cool pavements for urban heat island mitigation: a synthetic review. Renew Sust Energ Rev 146:111171. https://doi.org/10.1016/j.rser.2021.111171
Watanabe S (2010) Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. J Mach Learn Res 11:3571–3594
Watchorn DJ, Duncan TA, Cowan MA (2023) The risks and rewards of using artificial habitat structures for wildlife conservation. Austral Ecol 48(6):1207–1222. https://doi.org/10.1111/aec.13376
Weiperth A, Gaebele T, Potyó I, Puky M (2014) A global overview on the diet of the dice snake (Natrix tessellata) from a geographical perspective: foraging in atypical habitats and feeding spectrum widening helps colonisation and survival under suboptimal conditions for a piscivorous snake. Zool Stud 53:42. https://doi.org/10.1186/s40555-014-0042-2
Willmott NJ, Wong BBM, Lowe EC, McNamara KB, Jones TM (2022) Wildlife exploitation of anthropogenic change: interactions and consequences. Q Rev Biol 97(1):15–35. https://doi.org/10.1086/718748
Woolley CK, Hartley S, Innes JG, Shanahan DF, van Heezik Y, Wilson DJ, Nelson NJ (2023) Conservation of skinks in New Zealand cities. Urban Ecosyst 26(5):1493–1508. https://doi.org/10.1007/s11252-023-01398-w
Yang J, Meng XZ, Duan YP, Liu LZ, Chen L, Cheng HF (2014) Spatial distributions and sources of heavy metals in sediment from public park in Shanghai, the Yangtze River Delta. Appl Geochem 44:54–60. https://doi.org/10.1016/j.apgeochem.2013.08.007
Yu X, Wu NC, Ge LY, Li LS, Zhang ZW, Lei J (2022) Artificial shelters provide suitable thermal habitat for a cold-blooded animal. Sci Rep-Uk 12:5879. https://doi.org/10.1038/s41598-022-09950-y
Zarini S, Abdoli A, Kiabi BH (2019) The effects of riprap in enhancing the abundance and coexistence of Gobiidae along the southern Caspian Sea coast. J Gt Lakes Res 45(2):317–323. https://doi.org/10.1016/j.jglr.2018.12.001
Plummer M (2017) JAGS Version 4.3.0 User manual. https://sourceforge.net/projects/mcmc-jags/files/Manuals/
Su Y, Yajima M (2015) Package 'R2jags': Using R to run 'JAGS'. Version 05–7. http://CRAN.R-project.org/package=R2jags
The Land Office Portal (2023) Településkereső. Ministry of Rural Development Department of Land Administration and Geoinformation, Budapest. Available from https://www.foldhivatal.hu/content/view/89/126/. Accessed 4 Apr 2023
Acknowledgements
We are grateful to the many people who helped in the field work, in particular: Attila Bohus, Ildikó Szivák and Ingrid Győri. We adhered to relevant international, national, and/or institutional guidelines regarding the care and use of animals. All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Experimentation Scientific Ethical Committee (license number: VE/21/00184-9/2022) and Hungarian Nature Conservation laws (license number: VE/30/00658-9/2022).
Funding
Open access funding provided by HUN-REN Balaton Limnological Research Institute. The research presented in this article was supported by the Széchenyi Plan Plus program (RRF 2.3.1–21-2022-00008 project) and the Sustainable Development and Technologies National Programme of the Hungarian Academy of Sciences (FFT NP FTA). AJH was supported by the RRF-2.3.1-21-2022-369-00014 project and OTKA K142296.
Author information
Authors and Affiliations
Contributions
Boglárka Mészáros, Andrew J. Hamer and Dénes Schmera participated in the conceptualization and designed the methodology. Andrew J. Hamer and Dénes Schmera supervised the study. Material preparation, field sampling and data collection were performed by Boglárka Mészáros, József Bürgés, Blanka Gál and Mónika Tamás. Boglárka Mészáros and AJH performed the data analysis and visualization. MB wrote the original draft. Andrew J. Hamer, Dénes Schmera, Blanka Gál and József Bürgés reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mészáros, B., Bürgés, J., Tamás, M. et al. Urban oasis? Abundant dice snake (Natrix tessellata) populations along artificial lakeside habitats in urban landscapes. Landsc Ecol 39, 124 (2024). https://doi.org/10.1007/s10980-024-01917-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01917-w