Abstract
Little is known about the insular diversity and its determinants in the freshwater invertebrates in comparison to land animals. Our goal is to obtain global-scale information on the insular diversity in Cyclopidae, test its relationships with the geographical variables in different types of islands, and compare the patterns to those observed in other organisms. In total 291 species and subspecies were reported in the 35 islands included in our analyses. The total and endemic species richness have strong positive correlations with surface area and maximum elevation of the islands; regression slopes are larger in the oceanic than in continental islands. Small-island effects occur in the relationships between the endemic species richness and area and elevation. Distance from mainland has negative correlations with the total and endemic species richness in the oceanic islands. Compositional similarity (in contrast to species richness) is determined by the geographic variables to only a minor extent, while space has stronger impact. The relationships found in Cyclopidae generally fit those observed in other predominantly terrestrial organisms, yet some characteristics (negative intercepts in the area–species and elevation–species relationships; large area and high elevation thresholds below which no endemic species occurs) are suggested to be specific to fresh waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Island biogeography has inspired a few recent articles on the determinants of the diversity patterns in habitat islands (spring fauna and lake plankton) and arctic islands in the freshwater Copepoda and Cladocera (Fattorini et al., 2016; Novichkova & Azovsky, 2017; Gooriah & Chase, 2020), yet a global overview of the diversity patterns of the true island fauna is lacking for the freshwater microcrustaceans. Freshwater organisms and microcrustaceans in particular are still poorly represented in island biogeographic meta-analyses. Recent papers on the island species-area relationships analyzing more than 600 datasets included only few freshwater taxa and not a single one representing aquatic crustaceans (Triantis et al., 2012; Matthews et al., 2016). This may be due to the incomplete knowledge of the taxonomy and fragmentary information on the geographical distribution in these small-sized invertebrates (< 1 or few mm). Also, the widespread belief of the prevalent cosmopolitan distribution of the freshwater zooplankton organisms lessened their attractiveness for the zoogeographical investigations. Current knowledge of the taxonomy and biogeography of the Copepoda and Cladocera indicate that species with cosmopolitan distribution are the exceptions rather than the rule (e.g., Reid, 1997/1998; Boxshall & Defaye, 2008; Forró et al., 2008; Hołyńska, 2011; Korovchinsky, 2013). One of those exceptions is the cyclopine Thermocyclops decipiens (Kiefer, 1929), for which the term “fugitive species” (good disperser yet competitively inferior and usually rare) was coined by G. Evelyn Hutchinson (1951).
The Cyclopidae (~ 1100 species) are distributed worldwide, and occurs ovewhelmingly in continental waters, in both surface and subterranean realms (Hołyńska, 2011 updated by the authors). Cyclopids are sexually reproducing crustaceans, and storage of sperm in the female seminal receptacle enables the production of viable eggs sometimes for several weeks or even months (Næss & Nilssen, 1991; Maier, 1995; Phong et al., 2008). In contrast to other freshwater crustaceans such as the diaptomid copepods and branchiopod crustaceans which possess resting eggs (dormant embryos), cyclopids diapause in the larval or adult (also as ovigerous female) stages (Næss & Nilssen, 1991; Dahms, 1995; Bruno et al., 2001; Frisch & Threlkeld, 2005; Pinel-Alloul & Alekseev, 2019). These traits, along with the rapid ontogenetic development in some species facilitate rapid colonization of water bodies (Frisch & Green, 2007; Frisch et al., 2012). Little is known about the mode of dispersal other than via hydrological connections, although indirect evidence suggests birds or insects acting as dispersal vectors (Frisch & Green, 2007; Frisch et al., 2012). On the other hand, there have been numerous records of cyclopids living in semiterrestrial environments (leaf litter, mosses, wet soil) (Reid, 2001; Watiroyram et al., 2012), which suggest an important role of high moisture habitats in the dispersal of this group.
Concerning the age of the Cyclopidae, the only fossil (Apocyclops californicus Holynska et al., 2016) is known from the Miocene Barstow Formation (16 mya) in California, yet the current distribution patterns found in many genera suggest a much older (early Mesozoic or Paleozoic) origin of the family (Boxshall & Jaume, 2000; Hołyńska et al., 2016). Phylogenetic reconstructions applying nearly complete taxon sampling are still few, which hampers understanding of both the origin and diversity patterns of the insular fauna (but see Hołyńska, 2006; Hołyńska & Stoch, 2012).
Although there are a few factors that make interpretation of the insular diversity patterns in the Cyclopidae a challenging task, we believe that the currently available data deserve a global-scale overview of the cyclopid species richness on islands. Our principal question is whether the relationships between the geographic variables and species richness (with a special focus on the species-area curves) found in this freshwater group deviate in any aspect from the general trends documented mainly in land organisms. Based on published works on 35 islands occurring in the Atlantic, Indian, and Pacific Oceans and Mediterranean Sea, we tested the impact of the geographical variables on the overall and endemic species richness, and compared these relationships between different types of islands (oceanic vs continental, and tropical vs temperate). In addition, we analysed the compositional similarity of the insular cyclopid faunas, while noting that some clusters revealed here may not necessarily represent biogeographical entities, as we could only include relatively few islands in the analysis. We also examine the impact of the geographic variables and ‘space’ (geographic distance between islands) on the compositional similarity.
Material and methods
Data collection
Geographical information on the 35 islands included in the analyses (Table 1; Fig. 1) has been collected from different sources: island surface—Global Islands Explorer of US Geological Survey https://rmgsc.cr.usgs.gov/gie/; latitude centre and maximum elevation—Wikipedia crosschecked by Island Directory http://islands.unep.ch/; (shortest) distance from continent and distance between islands were measured using Google Maps.
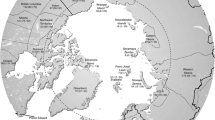
Geographical location of the islands included in this study. 1, Iceland; 2, Ireland; 3, Great Britain; 4, Hallands Vaderö; 5, Bornholm; 6, Öland; 7, Sardinia; 8, Sicily; 9, Crete; 10, Flores (Azores); 11–14, Florida Keys (Long Key, Duck Key, Big Pine Key and Cudjoe Key, respectively; see Fig. 1b); 15, Cuba; 16, Hispaniola; 17, Puerto Rico; 18, Socotra; 19, Madagascar; 20, Sri Lanka; 21, Sumatra; 22, Java; 23, Borneo; 24, Mindanao; 25, Luzon; 26, Kyushu; 27, Honshu; 28, Hokkaido; 29, New Caledonia; 30, Viti Levu (Fiji); 31, South Island (New Zealand); 32–33, Hawaii (Oahu and Molokai, respectively; see inset map); 34, Wallis; 35, Easter Island. Equator is indicated by solid line. Arctic Circle, Tropic of Cancer and Tropic of Capricorn are indicated by dotted lines
Based on recent land connections (or their lack) and the climatic characters, the islands were a priori classified as continental vs oceanic and tropical vs temperate islands. Periodic contact of the islands with continents influences both species richness and speciation processes. Temperate/tropical categorization of the islands is justified by the hypothesis that higher environmental energy and climatic stability in the tropics may generate processes that are different from those in the temperate zone (higher rate of speciation and lower extinction rate vs lower rate of speciation and higher extinction rate) (Pyron & Wiens, 2013). “Continental” island refers to islands that either lie on the continental shelf (e.g., Great Britain) or are relatively close to the mainland (e.g., Honshu, Sardinia) and supposedly experienced indirect (via other islands) or direct connection with the continent during the Pleistocene (Millien-Parra & Jaeger, 1999; Tojo et al., 2017; Hupało et al., 2021; Schmitt et al., 2021). All the other islands, which are either permanently isolated (e.g., Hawaiian Islands) or had only old (Miocene or older) contact with a continent (e.g., Socotra, Madagascar, New Caledonia, New Zealand) are grouped as “oceanic” islands.
The present analysis included only those islands in which regional-scale faunistic studies have been conducted by copepod specialists, and/or a checklist existed. Data on the geographical distribution of the species were principally collected from the literature (catalogues, checklists, fauna monographs and ecological studies), though in a few cases the original data have been revised by us (the species-island matrix, literature sources and taxonomic notes are provided in Online Resources 1 and 2, respectively). The various methods applied in those publications did not allow us to estimate the sampling effort on different islands in a quantitative manner. Nonetheless, we may assume that cyclopid fauna is better understood in the northern temperate islands than in other regions. Putative alien species were not excluded from the analysis. There are only few taxa, the Asian Mesocyclops pehpeiensis Hu, 1943 in Cuba, and the Holarctic A. robustus (G.O. Sars, 1863) in New Zealand perhaps, in which the morphological, biogeographical (widely disjunct populations; restricted occurrence in the new territory) and phylogenetic data support alien status (Karanovic, 2005; Díaz et al., 2006). Concerning the validity of data in the species/islands matrix, seven species and subspecies recorded on the 35 islands may be synonymous with other taxa or are nomina dubia. Doubtful occurrence records constitute ~ 11% (83) of all (751) records (see Online Resources 1 and 2). Distribution of the doubtful records is uneven among different types of islands: 6% (26 of 412 records) and 17% (57 of 339 records) in the temperate and tropical islands, respectively; and 9% (48 of 508 records) and 14% (35 of 243 records) in the continental and oceanic islands, respectively.
Nearly half (38) of the doubtful records pertain to four species [Eucyclops serrulatus (Fischer, 1851), Microcyclops rubellus (Lilljeborg, 1901), Microcyclops varicans (G.O. Sars, 1863), and Paracyclops fimbriatus (Fischer, 1853)], originally described from Europe, and regarded as cosmopolitan during most of the twentieth century. Recent revisions (Karaytug, 1999; Alekseev & Defaye, 2011; Mirabdullayev & Defaye, in press) found more restricted species ranges in these cyclopids, and we may assume that most (all?) tropical records in the four species refer to other taxa. As a consequence, the total number of species occurring in the islands may be underestimated rather than overestimated. We did not exclude the dubious occurrence (grey cells) records from the analyses, because it could not only result in loss of information, but might also distort the results (see uneven rate of the doubtful records among different types of islands above).
Incomplete knowledge of the geographical distribution in some species may inflate the data on endemic species richness. On the other hand, more research on the high altitude and cryptic, semiterrestrial habitats (swamps, leaf litter, groundwater) likely reveal new endemic taxa, counterbalancing the former effect. Despite the uncertainties about the taxonomic position and geographical distribution of some species, the amount of data reported here prompted us to explore the diversity patterns and to test the effects of the geographical variables on the cyclopid species richness and faunal similarity.
Analyses
The non-parametric Mann–Whitney U test was used to test the differences in the species richness and geographic variables between the continental and oceanic and between the temperate and tropical islands. Relationships between the cyclopid diversity and geographic variables were analysed by linear regression in different types (continental vs oceanic, temperate vs tropical) of islands. The geographic and diversity variables were log10 transformed. Normality was rejected by the Shapiro–Wilk test in some variables; therefore the non-parametric Spearman's rank correlation was used to test the relationships between the cyclopid diversity (overall and endemic species richness) and islands characteristics (surface area, highest elevation, latitude and distance from continent). Redundancy among the explanatory variables was checked with the variance inflation factor (VIF) (XLSTAT, Addinsoft 2021). VIF analysis is a diagnostic tool used to identify uninformative constraints among the primary predictors (area, maximum elevation, latitude and shortest distance from continent). The VIF values were lower than 1.7 in the raw data and lower than 4.7 for the log10 transformed data, indicating that the variables in question are moderately correlated (Chatterjee & Hadi, 2006).
Redundancy analysis (RDA) combining multivariate linear regression with principal components analysis was used to explore the effects of the primary predictors on the diversity variables in islands. Response data (species richness and endemism) had a gradient less than 2.5 SD units, indicating that linear regression, a limitation of RDA, is appropriate (ter Braak & Šmilauer, 2012). RDA ordination analysis was performed using the software package CANOCO v. 5. Compositional (dis)similarity of the cyclopid fauna was calculated using Jaccard’s, and Simpson’s indices, and Jaccard and Sørensen binary distance formulas.
Sørensen distance(i,j) = \(\sqrt{1-2c/(A+B)},\)
where A, and B are the number of species in the islands “i” and “j” compared, c equals the number of species shared between the two islands, and min(A,B) equals the number of species in the island with smaller fauna. Todeschini et al. (2012) found the Simpson index to perform worse in the similarity analyses than the Jaccard index, yet the Simpson formula has sometimes been used in the regional comparisons of the copepod fauna (Chertoprud et al., 2011). Also, Kreft and Jetz (2010) recommended applying the “beta-sim” dissimilarity index (1—Simpson similarity) in the biogeographical regionalization analyses. Based on the Jaccard and Simpson coefficients, the UPGMA (unweighted pair group method with arithmetic mean) dendrograms were generated in MVSP software. Apart from UPGMA, we also used non-metric multidimensional scaling (NMDS) that produced an ordination of the islands, based on Jaccard’s binary distance of the cyclopid composition.
The correlation between the inter-island distance and compositional similarity was calculated using the Mantel test with p-values computed using 10,000 Monte Carlo simulations in XLSTAT (Addinsoft). Relationships between the environmental variables and compositional similarity (Sørensen binary distance) of the cyclopid fauna were tested by distance-based redundancy analysis (db-RDA). As db-RDA reported all environmental variables to be significant, we applied the principal coordinates of neighbourhood matrix method (PCNM) and variance partitioning to detect the most reliable predictors of the compositional similarity, taking both environmental variables and space (distance based on the geographic coordinates) into account. PCNM and dbRDA were performed in CANOCO v. 5 (Borcard et al., 1992; Legendre & Anderson, 1999; ter Braak & Šmilauer, 2012).
Results
Relationships between the insular species richness and geographical variables
The Cyclopidae possessed a substantial diversity on the 35 islands included in our study, representing 27% (291 of 1083) of the species and subspecies and half (36 of 71) of the genera so far known in the world. Considering the species rich genera only (Table 2), in half of them the proportion of species occurring on islands oscillates around the value that we obtained for the whole insular fauna (21–30% vs 27). Merely 9 species or subspecies (3%!) appear as cosmopolitan, i.e. reported from both the eastern and western hemispheres and from both the temperate and tropical regions: Acanthocyclops vernalis (Fischer, 1853) (11 isl.), Diacyclops bisetosus (Rehberg, 1880) (9 isl.), Ectocyclops phaleratus (Koch, 1838) (15 isl.), Macrocyclops albidus albidus (Jurine, 1820) (20 isl.), Microcyclops rubellus (14 isl.), Microcyclops varicans varicans (21 isl.), Paracyclops chiltoni (G.M.Thomson, 1883) (14 isl.), Paracyclops fimbriatus fimbriatus (20 isl.), Tropocyclops prasinus prasinus (Fischer, 1860) (15 isl.). True cosmopolitans are likely to be even fewer, as taxonomic revisions of Microcyclops varicans and Paracyclops fimbriatus revealed restricted geographical distribution in these species (Karaytug, 1999; Mirabdullayev & Defaye in press). The majority of the species (181 of 291) have been recorded in only one of the 35 islands (Fig. 2). Based on our current knowledge of the global distribution of the species (Dussart & Defaye, 2006; supplemented with our data) 29% (83 species and subspecies) of the insular taxa are endemic to a single island. The Japanese island, Honshu, has the richest fauna (69 spp.) followed by Madagascar (53 spp.) and Great Britain (50 spp.); the rate of endemism is 22% and 26% in Honshu and Madagascar, respectively, while it is 6% in Great Britain.
Recent land connections to continents or their lack (continental vs oceanic island), and climatic characteristics of the islands (tropical vs temperate) were hypothesized here as significant factors having large influences on the relationships between the geographical variables and insular diversity in the Cyclopidae.
The cyclopid species richness (total and endemic) and geographical features of the islands were compared between the continental and oceanic and between the temperate and tropical islands (Online Resource 3). The species richness is significantly greater in the continental islands than in oceanic islands (P = 0.015), while the number of island endemics does not differ between continental and oceanic islands. On the other hand, except for the distance from continent, none of the island characteristics (surface area, altitude and latitude) differ significantly between these two groups. In the tropical vs temperate comparisons, except for the trivial difference in the latitude neither the island characteristics (surface area, elevation, shortest distance from continent) nor the cyclopid diversity (total and endemic species richness) differs between the tropical and temperate groups. These results suggest that insular species richness in the Cyclopidae is affected by the recent land connections with mainland (vs permanent or old isolation) rather than latitudinal positions of the islands.
RDA ordination plot (Fig. 3a) shows separation of the continental and oceanic islands on right side of the biplot (small islands), and lack of separation of the large continental and oceanic islands (left side of the biplot) with respect to their diversity responses to the geographic variables. Tropical vs temperate islands do not constitute distinct groups for either the large or small islands. The explanatory variables account for 71.2% of the total variation (adjusted explained variation is 67.3%) in the diversity variables. Based on simple term effects, area and maximum elevation explained 50.0% and 26.3% of the total variation, respectively (P = 0.002). Considering conditional (partial) effect (the effect of maximum elevation on the area was controlled), area and distance explain 50.0% and 20.0% of the variation, respectively, in the diversity variables (P = 0.002). The total and endemic species richness increased with increasing area and maximum elevation. Distance from continent negatively correlated with total species richness, while no correlation was indicated between the latitude and total species richness (see right angle between vectors in Fig. 3a).
Insular diversity of the Cyclopidae in relation to the geographic variables. a Ordination biplot depicting the effect of log10 transformed geographic variables on the log10 transformed total and endemic species richness. Acute and obtuse angles of the vectors indicate positive and negative correlations, respectively; right angle of vectors indicates no correlation. Vector projection of each vector on the two RDA axes reveals how much weight they have on the RDA vectors. For the island codes see Table 1. Green dots—continental islands, brown dots—oceanic islands, blue letters—temperate islands, red letters—tropical islands. b Contour plot of species richness (non-logarithmized) within the ordination space of the first two axes of RDA, based on a fitted loess model. Scaling was added to present relation between the number of species and log based area values
According to the fitted loess model plotted on RDA axes (Fig. 3b), the area and maximum elevation contributed mostly to the total number of species, whereas distance from continent was related to decrease of species richness (residual SE = 4.16; R2loess[%] = 90.6, i.e. the variance in the loess fitted values is ~ 91% the size of the total variance in the species richness data). Geographic variables account for 24.4% of the variation in (non-logarithmized) species richness (adjusted explained variation is 14.3%).
Relationships between the species richness and geographical variables were analysed in five classes of the islands (“all”, “continental”, “oceanic”, “tropical” and “temperate”) (for Spearman correlations and regressions see Online Resource 4a and Fig. 4a, respectively). Surface area significantly positively correlated with species richness in each class of the islands. Maximum elevation likewise significantly positively correlated with insular species richness in all but one group (temperate islands). Oceanic islands show a steeper slope than any other group of islands when the species richness is regressed on the surface area and maximum elevation (Fig. 4a). The trend lines also depict another particular feature of the oceanic islands: there exists a minimum surface area (~ 11 km2) and highest elevation (~ 157 m) below which there is little chance that any cyclopid would occur. By contrast, in a very small shelf island near Sweden, Hallands Vaderö (3 km2, 24 m a.s.l.) for instance, 12 species have been recorded. As for the temperate vs tropical comparisons, the species richness grows with the island area at the same rate (slope, 0.2) in the temperate and tropical groups, while the intercept value is slightly higher in temperate islands.
Relationships between the cyclopid diversity (a total species richness; b endemic species richness) and geographical variables. Regression formulas and trend lines are given for the significant relationships, unless the variance of the residuals is uneven (see the relationships between total/endemic species richness and latitude), or the linear relationship is questionable (relationship of distance from mainland and total species richness in the “continental” and “all” islands; for explanation see the text), or obviously non-linear (relationships between the area, maximum elevation and endemic species richness). Beige boxes show the area and maximum elevation ranges, within which no endemic species occurs, regardless of the area size and maximum elevation (small-island effect). Black—all islands, green—continental islands, brown—oceanic islands, blue—temperate islands, red—tropical islands
Latitude had a significant (weak) negative correlation with species richness in the tropical islands only (Online Resource 4a). Apparently large variation in species richness at latitudes greater than 10 degrees (Fig. 4a) can be explained by the large range of the surface area of the islands included here, which in turn affects the diversity data. Shortest distance of the islands from continent had significant inverse relationships with the species richness in “all islands” and “oceanic islands” groups, yet a significant though weak positive correlation was found in the continental islands (Online Resource 4a). This unexpected result is due to the fact that more remote continental islands tend to be also larger in area in our data set, and can sustain more species. The correlation between distance and surface area is positive and significant in the continental islands (Spearman’s r = 0.59; P = 0.01), while no significant correlation was found between the distance and area in the “oceanic” and “all” islands groups. The partial correlation between the distance and species richness with the area factored out is weak and negative (r = − 0.28) and non-significant (t = − 1.14; P = 0.27) in the continental islands. This result is consistent with our intuitive prediction about near-mainland systems. On the other hand, when the partial correlation between the area and species richness with distance was factored out, there still remained a high positive (0.91) and significant (t = 8.44; P < 0.001) correlation, which indicates that distance has no effect on the species-area relationship in the continental islands.
The relationships between the number of endemic species and geographical variables were likewise analysed separately in the five groups of islands (Online Resource 4b, Fig. 4b). Both surface area and maximum elevation had significant positive correlations with the number of island endemic species in each group of the islands, yet the scatterplots (Fig. 4b) unambiguously depict non-linear relationships between these variables and endemic diversity. Islands below a threshold area (~ 3620 km2, Socotra) or max. elevation (~ 1340 m, Puerto Rico) do not support any endemic species regardless of their size (small-island effect). Above these thresholds, endemic species richness grows faster with increasing area and max elevation, than total species richness (Fig. 4a, b). Similarly, in the patterns observed in overall species richness, latitude had a significant inverse yet weak relationship with endemic diversity in tropical islands (Online Resource 4.b). Distance from mainland had a significant (P < 0.05) negative correlation with the endemic species in the oceanic island only.
Compositional similarity of the insular fauna and its determinants in Cyclopidae
Compositional similarity of the island faunas was analysed using two different similarity indices (Fig. 5). The Simpson similarity index, unlike the Jaccard index, measures the nestedness of the faunas: the similarity between islands is 1.0, when one of the faunas compared is fully nested by the other fauna. For example, all species occurring in Ireland are also present in Great Britain; therefore while Jaccard (0.74) similarity index shows some distance between Great Britain (with larger fauna) and Ireland, the Simpson similarity is the maximum 1.0.
Similarity coefficients dendrograms using UPGMA method, and based on two different similarity indices. For the codes of the names of the islands see Table 1
Relatively few islands could be included in the analyses, and as a consequence the relationships revealed by clustering may only inform us about the approximate biogeographic patterns. There is only one large cluster shared by both dendrograms: all the temperate islands (incl. South Island in New Zealand) form one group, with the exception of Flores Azores (4 species) and Easter Island (1 species). Also, the two Philippine islands (Luzon and Mindanao) and the Florida Keys (Duck, Long, Cudjoe and Big Pine) grouped together in both dendrograms. On the other hand, there are many groups in which the Jaccard and Simpson dendrograms differ from each other. The cluster of the Greater Sunda Islands, absent in Simpson, was recovered by the Jaccard dendrogram. The faunas of Java and Sumatra are closer to each other, while that of Borneo is more distant. The Simpson dendrogram also differs from Jaccard in the relationships to the Hawaiian Islands. The association of the two Hawaiian islands, supporting only a few widely distributed species (Oahu, 3 spp.; Molokai 4 spp.), with disparate regions (Oahu to Borneo; Molokai to Hispaniola) are the results of the nestedness effect in the Simpson dendrogram. The NMDS ordination (Fig. 6), based on the Jaccard distance, confirmed all main clusters recovered by the Jaccard dendrogram. Yet, while Sri Lanka and the Philippine islands constitute one cluster in the Jaccard dendrogram, Sri Lanka is closer to the Greater Sundas in the NMDS plot. The effect of the inter-island distance on the compositional similarity of the faunas was measured using the Mantel test, applying two different similarity indices in three classes (“all”, “oceanic” and “continental”) of the islands (Fig. 7). A significant inverse relationship was found in each group of the islands regardless of the similarity index, yet the correlation was stronger in the continental islands, than in the oceanic group. The correlations were apparently weaker if the faunas were compared by the Simpson index.
Non-metric multidimensional scaling (NMDS) ordination plot of Jaccard’s binary distance for the cyclopid composition (stress criterion value = 0.10). For the codes of the names of the islands see Table 1
Distance-based redundancy analysis showed that geographic variables of the islands (area, maximum elevation, latitude and shortest distance from the continent; all variables were log transformed) account for 24.2% of variation in the compositional dissimilarity (Online Resource 5). All four explanatory variables were significant (P = 0.002). Area, maximum elevation, latitude and shortest distance from continent explain 6.3%, 6.9%, 6.7% and 5.8% of the variance, respectively. Variation partitioning based on PCNM analysis revealed that these environmental variables alone explain 11.4% of the explained variation in the compositional dissimilarity, while space (geographic distance) is responsible for 67.5%, and the shared effect is 21.1% (Fig. 8, Online Resource 6). Climate alone is responsible for 9.2% of the explained variation in copepod composition, space explains 68.2%, while the shared effect is 22.6%. Considering the continental/oceanic type of islands vs space, 6.4% of the explained variation is related to the type of island; 84.8% is related to space, and 8.8% is the shared effect.
Results of PCNM and variation partitioning. PCNM fractions of variation explained by the primary predictor variables versus space. “a” and “b” represent the variations in cyclopid composition explained by three groups of the primary predictors, and space (geographic distance) respectively, “c” indicates the shared effect. All tested fractions in configurations a + b + c, a + c, and b + c were significant (P = or < 0.004)
Discussion
The cyclopid genera differ highly in their preference for island occurrence. Mesocyclops and Thermocyclops have relatively high insular diversity, while other genera such as Cryptocyclops, Neocyclops, Speocyclops and Acanthocyclops show less propensity to disperse and/or diversify in islands (Table 2). Except for Cryptocylops, a significant portion of the species in these genera occur in the groundwaters, and as such they are considered to be poor dispersers (Galassi, 2001; Galassi et al., 2009; Fattorini et al., 2016). Other genera (Halicyclops and Diacyclops) that also show large diversity in the groundwaters, however, are well represented on islands which suggests that low diversification rate rather than low dispersal ability is responsible for the low insular diversity in Neocyclops, Speocyclops and Acanthocyclops.
Single-island endemic species and subspecies (83) constitute ~ 29% of all insular cyclopids. About two-thirds of the endemics live in special/cryptic environments: groundwater, 43; mosses, 6; associates of polychaetes and crustacea other than copepods, 3; bromeliads (phytotelmata), 1 (see Online Resource 1). Cryptic habitats are sampled perhaps at a lower rate than typical surface water bodies, which may affect our knowledge of the geographic distribution in some species. Nonetheless the current observations, i.e. dominance of the subterranean and associated way of life among the endemic species, suggest that majority of endemics are poor dispersers and/or habitat specialists.
Concerning the overall species richness in particular islands, Honshu ranked first. The large species richness (69) and relatively high rate of endemism (0.22), in comparison to islands of similar or even larger area, may due to a few factors. First, Honshu is part of a 3000-km-long chain of islands extending in a north–south direction, and is connected to both the Palearctic and Oriental (subtropical) regions. Second, the fauna of the Japanese islands (incl. Honshu) have a long history that is independent from mainland Asia, beginning at Middle-Late Miocene. On the other hand, marine regressions could facilitate faunal exchange between Honshu and the Asian continent via Hokkaido and Sakhalin, or across a narrow sea channel between Korea and Honshu during the Pleistocene (Millien-Parra & Jaeger, 1999; Karanovic et al., 2013; Tojo et al., 2017). Third, with the exception of some high mountain ranges, Honshu remained non-glaciated during the Pleistocene (Sawagaki et al., 2004). Fourth, taxonomic studies on the freshwater copepods have a long tradition in the Japanese islands (Online Resource 2), and Honshu belongs to the more explored islands. Comparison with the fauna of Great Britain (50 spp.; rate of endemism, 0.06), the best explored island with an area similar in size to Honshu, nevertheless suggests that factors other than sampling effort have had more influence on the cyclopid diversity in Honshu.
Geographic determinants of the insular diversity in Cyclopidae
Both the climate (temperate vs tropical islands) and recent contact with mainland vs permanent or old isolation (continental vs oceanic islands) have been hypothesized here to affect the relationships between the geographic variables and cyclopid species richness in islands.
Our data do not support a significant impact of latitude on the insular diversity in the Cyclopidae. The total and endemic species richness did not differ between the tropical and temperate islands (Online Resource 3b). Latitudinal position of the islands did not correlate significantly with the total and endemic species richness, with the exception of a weak inverse relationship in the tropical islands (Online Resource 4). Also, the linear regressions of the species-area relationships have the same slope in tropical and temperate islands, though the intercept is somewhat larger in the temperate islands. It is difficult to estimate the extent to which uneven sampling effort (likely greater in the northern temperate than in the tropical islands) could influence the results. Nevertheless the similar species richness here observed in the tropical and temperate islands is consistent with the global diversity pattern found in the Cyclopidae (Reid, 1994; Hołyńska, 2011). The family as a whole has ca. 530 and 510 (sub)species in the temperate (northern and southern combined) and tropical regions, respectively. Two (Halicyclopinae and Eucyclopinae) of the three subfamilies living in the continental waters have a tropical diversity peak, yet the largest subfamily (Cyclopinae) comprising ca. 2/3 of all cyclopid species is more diversified in the temperate zone (Hołyńska, 2011). The lack of an apparent difference in the cyclopid diversity between the tropical and temperate islands cannot, however, be extrapolated to the polar vs temperate islands comparisons. Regional analyses of the freshwater copepod and cladoceran fauna in Canada (Pinel-Alloul et al., 2013) and Norway (Hessen et al., 2007), as well as a global-scale overview of the distribution in Cyclopidae (Hołyńska, 2011) show a major reduction in the species richness towards the polar regions, and we expect this same trend to hold true for the insular fauna.
By contrast, the continental and oceanic islands differed not only in the species richness (Online Resource 3a), but also in the parameters of the regression lines (Fig. 4a). These two types of islands especially are clearly separate from each other if we consider the overall species-maximum elevation relationship: the slope of the regression line is distinctly steeper in the oceanic islands (Fig. 4a). The difference in the species richness between continental and oceanic islands is more conspicuous at the bottom left end of the regression lines (small surface area or elevation) (Fig. 4a). The greater overall species richness in the continental islands can be explained by both the Pleistocene land connections with mainland (source area), and the relatively small distance from the continent which acts as a permeable barrier in many taxa.
As regards the relationships of the parameters of the species-area curves in the continental and oceanic islands, our results fit the general trends observed in other organisms (Triantis et al., 2012; Matthews et al., 2016): a higher positive slope (z value) appears in the oceanic islands, while the intercept (logc value) is larger in continental islands.
Interestingly, in some aspects the species-area curves obtained for the freshwater Cyclopidae deviate from those observed in overwhelmingly terrestrial organisms (Triantis et al., 2012). First, the slope (z) and intercept values (logc) found in a large meta-analysis by Triantis et al. (2012) are distinctly larger than that obtained in the Cyclopidae: means (± standard error) of the z values for the continental and oceanic islands were 0.30 ± 0.008 (cyclopids: 0.186), and 0.379 ± 0.017 (cyclopids: 0.326), respectively; means (± standard error) of the logc values for the continental and oceanic islands were 0.966 ± 0.049 (cyclopids: 0.65) and 0.616 ± 0.058 (cyclopids: − 0.34), respectively. Large area scale (area ratio of the largest and smallest islands larger than five orders of magnitude) of the data set presented here may contribute to the decrease in the z values in the Cyclopidae. An inverse relationship between the slopes of the species-area curves and surface area ratios of the largest and smallest islands have been documented by Triantis et al. (2012) and Matthews et al. (2019). The relatively low z and logc values in the freshwater taxa might also be related to their lower diversification (global species richness) in comparison to many terrestrial groups, which correlates with the number of insular species in these taxa. Meta-analyses suggest that the total species richness in an archipelago positively correlates with both logc and z, and especially strongly affects the slope of the species-area curve (Matthews et al., 2019). Second, the negative logc observed in Cyclopidae seems to be rare in the datasets (> 600) analysed by Triantis et al. (2012). We speculate that the negative logc value is related to the highly limited availability or lack of permanent fresh water bodies in smaller oceanic islands that are still available for terrestrial organisms. To test this hypothesis we need data on various (invertebrate and vertebrate) taxa whose life cycle depends on freshwater environments.
The small-island effect (SIE) was apparent in both the endemic species-area and endemic species-max. elevation relationships: below a threshold area and elevation, the surface area and max elevation had no effect on the endemic species richness (which remained 0). SIE was not observed when we regressed the total species richness on area or elevation, except for the oceanic islands in which the negative intercept values of the regression lines generated the area and elevation thresholds. In a similar pattern, SIE is absent for total species richness yet present in the endemic species richness—area relationship in the vascular plants of the Aegean archipelago (Kallimanis et al., 2010). As far as the total species richness is concerned, the size of the threshold (or breakpoint) area/elevation depends on the dispersal abilities of the organisms and isolation of the islands: the threshold area is smaller for the good dispersers, and larger in more isolated archipelagos (MacArthur & Wilson, 2001; Schrader et al., 2020). Lack of SIE for the overall species-area curve in the continental islands in Cyclopidae (Fig. 4a) is consistent with this generalization. In the small oceanic islands the lack of permanent freshwater sources and isolation likely increase extinction and decrease colonization rates, resulting in the absence of cyclopids. The large threshold area (~ 3620 km2) and relatively high threshold elevation (~ 1340 m) for island endemics indicate that both emergence of new species and the survival of ancient relict lineages in Cyclopidae might be associated with a significant geographical heterogeneity in the islands. The small sample size did not allow testing if the threshold values differed between the continental and oceanic islands, yet the area diagram in Fig. 4b at least seems to suggest that the threshold area might be larger in the continental than in the oceanic islands (and larger than the values mentioned above for all islands). A greater threshold area for the endemic species diversity in the continental islands might be explained by gene flow from the mainland populations, decelerating the island speciation processes (Heaney, 2000; Whittaker et al., 2008). Triantis et al. (2008) compared the minimum area required to have at least one single-island endemic species among various vertebrate, invertebrate and plant taxa. In most of these groups the threshold areas were orders of magnitude smaller than what we observed in the Cyclopidae. It was only the West Indian orchids in which an estimate of the critical area (1961.5 km2) somewhat approached the value for the Cyclopidae. The authors explained this relatively high value by the good dispersal capacity of the orchids (“dust-like and wind dispersed seeds”). Yet, beyond gene flow, taxon-specific speciation processes, and island age, the limited availability of the permanent freshwater habitats is assumed to be the major constraint for speciation in the Cyclopidae.
The biogeographic literature does not seem to support any general trend in the relationship between the number of endemic species and distance from mainland. A positive correlation was hypothesized by Heaney (2000) and Whittaker et al. (2008), while a weak negative or non-significant relationship has been found in vascular plants (Aegean archipelago) and birds (West Indies, Wallacea) (Kallimanis et al., 2010; Dalsgaard et al., 2014). The strong inverse relationship between distance from mainland and cyclopid endemism in the oceanic islands might be explained if we assume that the ancestors of the endemic species were similar to their extant descendants in being habitat specialists with poor dispersal capacities. As a consequence, the gene flow from the source area might have only a marginal effect on insular speciation in the oceanic islands, while isolation could act as significant constraint for colonization of the islands and adaptive radiations in these crustaceans.
Compositional similarity of the insular fauna and its determinants in the Cyclopidae
Based on the available geographic occurrence data, the Jaccard dendrogram fits the currently used biogeographic regionalisation systems (e.g., Boxshall & Defaye, 2008) better than the Simpson dendrogram: the Greater Sundas, Hawaiian islands and Caribbean islands forming separate clusters in the Jaccard dendrogram, have not been recovered by the Simpson dendrogram. The cluster of the temperate islands, including all European (except for the Flores Island in the Azores) and Japanese islands, plus South Island in New Zealand, was the single large group shared by the Jaccard and Simpson dendrograms. In a biogeographic regionalization based mainly on the freshwater fishes (Bănărescu, 1991), all the Japanese islands as well as Sakhalin are parts of the East Asian subregion of the Sino-Indian region, including also the South Asian (Indian subcontinent, Indochina except for Vietnam, Malay Peninsula, and Greater Sundas) and High Asian (Tibet, western China and part of Central Asia) subregions. A higher faunal similarity between the Japanese and European islands than between the Japanese and Sino-Indian islands (e.g., Sri Lanka and Greater Sundas), however, is in line with the classic Sclater-Huxley-Udvardy regionalization (Udvardy, 1983; Boxshall & Defaye 2008), which considers Kyushu, Honshu and Hokkaido as parts of the Palearctic region. The closer relationship of the Sri Lankan fauna to the Greater Sundas rather than to the Philippines (Fig. 5 cf. Fig. 6), is consistent with that both Sri Lanka and the Greater Sundas lying on the Asian shelf had land connection with continental Asia during the Pleistocene, while Mindanao and Luzon remained isolated from the continent (Voris, 2000; Sathiamurthy & Voris, 2006). As to the relatedness of the South Island (New Zealand) fauna to the Palearctic islands, such a grouping may be an artefact, rather than a natural biogeographic entity. Nine of the 14 species reported from South Island are shared with Great Britain: some of these taxa can be genuine cosmopolitan (Paracyclops chiltoni and perhaps Diacyclops bisetosus), while others [Acanthocyclops robustus (G.O. Sars, 1863), Acanthocyclops vernalis, Diacyclops bicuspidatus (Claus, 1857), Eucyclops serrulatus s. str., Halicyclops magniceps (Lilljeborg, 1853), Macrocyclops albidus s. str. Microcyclops varicans s. str.] might either be introduced by the European settlers to the island, or in fact refer to other species. A taxonomic revision of the New Zealand Cyclopidae is still not available, yet some studies on the copepods of cryptic environments (subterranean waters, Nothofagus litter) revealed a considerable potential of these habitats to harbour a unique native fauna (Karanovic, 2005). Five of the 14 species occurring on South Island are known as endemic to New Zealand: two of them [Metacyclops monacanthus (Kiefer, 1928), Goniocyclops silvestris Harding, 1958] have the closest relatives in Australia (Karanovic et al., 2011), while two other species (Zealandcyclops) seem to show morphological affinities to some Diacyclops in Lake Bajkal and Itocyclops in Eastern Asia and North America (Karanovic, 2005). More research, especially on the groundwater fauna, is needed to better understand the biogeographic history of Cyclopidae in New Zealand, an old Gondwanan microcontinent with major extinction of the biota occurring during Oligocene submergence of the islands (Goldberg et al., 2008).
We found significant inverse relationships between the compositional similarity and inter-island distance in both the continental and oceanic islands, with a stronger correlation in the continental islands (Fig. 7). Different strengths of the relationships can be explained by greater “connectedness” of the continental islands sampled here, in comparison to the oceanic islands. With the exception of the four Florida Keys islands, all the continental islands are located on or near the Eurasian shelves, and were directly (or indirectly) connected to the mainland during the Pleistocene. Also, East Asia extending from the Russian Far East to Vietnam served as a wide dispersal corridor for both temperate-Palearctic and tropical-Oriental organisms during Late Neogene and Quaternary climatic oscillations (Corlett, 2014; Qian et al., 2017). The geographic variables major determinants of the total and endemic species richness on islands explain only minor portion of the variance in faunal dissimilarity, relative to ‘space’ (distance between islands) (Fig. 8). Yet even including both the geographic and space variables, a significant part (~ 90%) of the variation in cyclopid composition remains unexplained (Online Resource 6), which suggests that historical factors (island age and origin, land connections, catastrophic events such as glaciation or submergence of island) may have much stronger impact on the species composition.
Conclusions
The relationships between the geographic variables and the cyclopid species richness and faunal similarity greatly differ in the continental and oceanic islands. In several aspects these patterns are similar to those richly documented in the terrestrial organisms. On the other hand, the relatively large size of the islands (in terms of both surface area and max. elevation) supporting at least one endemic species, and the negative intercept values of the species–area and species–elevation regression lines in the oceanic islands, might be a characteristic of freshwater organisms. To verify this hypothesis data on the insular diversity in a wide range of the freshwater taxa are needed. Area and maximum elevation were considered here as the proxies for habitat heterogeneity, yet future studies on the determinants of insular species richness could consider the diversity of land cover and “active surface” of lake and rivers on the islands. Species composition, in contrast to species richness, has been affected by the geographic variables to only a minor extent, while space (distance between islands) is a more important determinant of the faunal (dis)similarity. Nevertheless, the most serious limitation in interpreting island biogeography in the freshwater copepods is our still fragmentary knowledge about the insular fauna. Dominant habitat types of the endemic cyclopid species indicate that cryptic environments (subterranean waters, semiterrestrial biotopes) harbour a significant portion of the native fauna and may provide clue to the history of the islands.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files [Online Resource 1 to 6].
References
Alekseev, V. R. & D. Defaye, 2011. Taxonomic differentiation and world geographical distribution of the Eucyclops serrulatus group (Copepoda, Cyclopidae, Eucyclopinae). In Defaye, D., J. C. von Vaupel Klein & E. Suárez Morales (eds), Studies on Freshwater Copepoda: A Volume in Honour of Bernard Dussart, Crustaceana Monographs, Vol. 16. Koninklijke Brill NV, Leiden: 41–72. https://doi.org/10.1163/9789004188280_003.
Bănărescu, P., 1991. Zoogeography of Fresh Waters 2, Distribution and Dispersal of Freshwater Animals in North America and Eurasia. AULA-Verlag, Wiesbaden: 524–1091.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055. https://doi.org/10.2307/1940179.
Boxshall, G. A. & D. Defaye, 2008. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 595: 195–207. https://doi.org/10.1007/s10750-007-9014-4.
Boxshall, G. A. & D. Jaume, 2000. Making waves: the repeated colonization of fresh water by copepod crustaceans. In Rossiter, A. & H. Kawanabe (eds), Ancient Lakes: Biodiversity, Ecology and Evolution, Advances in Ecological Research 31. Academic Press, London: 61–79. https://doi.org/10.1016/S0065-2504(00)31007-8.
Bruno, M. C., W. F. Loftus, J. W. Reid & S. A. Perry, 2001. Diapause in copepods (Crustacea) from ephemeral habitats with different hydroperiods in Everglades National Park (Florida, U.S.A.). Hydrobiologia 453/454: 295–308. https://doi.org/10.1023/A:1013161210836
Chatterjee, S. & A. S. Hadi, 2006. Regression Analysis by Example. Wiley, Hoboken.
Chertoprud, E. S., H. Gheerardyn & S. Gomez, 2011. Harpacticoida (Crustacea: Copepoda) of the South China Sea: faunistic and biogeographical analysis. Hydrobiologia 666: 45–57. https://doi.org/10.1007/s10750-010-0228-5.
Corlett, R. T., 2014. The Ecology of Tropical East Asia, 2nd ed. Oxford University Press, Oxford.
Dahms, H.-U., 1995. Dormancy in the Copepoda – and overview. Hydrobiologia 306: 199–211. https://doi.org/10.1007/BF00017691.
Dalsgaard, B., D. W. Carstensen, J. Fjeldså, P. K. Maruyama, C. Rahbek, B. Sandel, J. Sonne, J.-C. Svenning, Z. Wang & W. J. Sutherland, 2014. Determinants of bird species richness, endemism, and island network roles in Wallacea and the West Indies: is geography sufficient or does current and historical climate matter? Ecology and Evolution 20: 4019–4031. https://doi.org/10.1002/ece3.1276.
Díaz, Z. M., J. W. Reid, I. C. Guerra & I. V. Ramos, 2006. A new record of Mesocyclops pehpeiensis Hu, 1943 (Copepoda: Cyclopoida) for Cuba. Journal of Vector Ecology 31: 193–195. https://doi.org/10.3376/1081-1710(2006)31[193:ANROMP]2.0.CO;2.
Dussart, B. & D. Defaye, 2006. World Directory of Crustacea Copepoda of Inland Waters II − Cyclopiformes. Backhuys Publishers, Leiden.
Fattorini, S., P. A. V. Borges, B. Fiasca & D. M. P. Galassi, 2016. Trapped in the web of water: groundwater-fed springs are island-like ecosystems for the meiofauna. Ecology and Evolution 6: 8389–8401. https://doi.org/10.1002/ece3.2535.
Forró, L., N. M. Korovchinsky, A. A. Kotov & A. Petrusek, 2008. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595: 177–184. https://doi.org/10.1007/s10750-007-9013-5.
Frisch, D. & A. J. Green, 2007. Copepods come in first: rapid colonization of new temporary ponds. Fundamental and Applied Limnology 168: 289–297. https://doi.org/10.1127/1863-9135/2007/0168-0289.
Frisch, D. & S. T. Threlkeld, 2005. Flood-mediated dispersal versus hatching: early recolonisation strategies of copepods in floodplain ponds. Freshwater Biology 50: 323–330. https://doi.org/10.1111/j.1365-2427.2004.01321.x.
Frisch, D., K. Cottenie, A. Badosa & A. J. Green, 2012. Strong spatial influence on colonization rates in a pioneer zooplankton metacommunity. PLoS ONE 7: e40205. https://doi.org/10.1371/journal.pone.0040205.
Galassi, D. M. P., 2001. Groundwater copepods: diversity patterns over ecological and evolutionary scales. Hydrobiologia 453(454): 227–253. https://doi.org/10.1023/A:1013100924948.
Galassi, D. M. P., R. Huys & J. W. Reid, 2009. Diversity, ecology and evolution of groundwater copepods. Freshwater Biology 54: 691–708. https://doi.org/10.1111/j.1365-2427.2009.02185.x.
Goldberg, J., S. A. Trewick & A. M. Paterson, 2008. Evolution of New Zealand’s terrestrial fauna: a review of molecular evidence. Philosophical Transactions of the Royal Society B 363: 3319–3334. https://doi.org/10.1098/rstb.2008.0114.
Gooriah, L. D. & J. M. Chase, 2020. Sampling effects drive the species–area relationship in lake zooplankton. Oikos 129: 124–132. https://doi.org/10.1111/oik.06057.
Heaney, L. R., 2000. Dynamic disequilibrium: a long-term, large-scale perspective on the equilibrium model of island biogeography. Global Ecology and Biogeography 9: 59–74.
Hessen, D. O., V. Bakkestuen & B. Walseng, 2007. Energy input and zooplankton species richness. Ecography 30: 749–758. https://doi.org/10.1111/j.2007.0906-7590.05259.x.
Hołyńska, M., 2006. Phylogeny of Mesocyclops (Copepoda: Cyclopidae) inferred from morphological characters. Zoological Journal of the Linnean Society 147: 1–70. https://doi.org/10.1111/j.1096-3642.2006.00231.x.
Hołyńska, M., 2011. Latitudinal gradients in diversity of the freshwater copepod family Cyclopidae (Copepoda, Cyclopoida). In Defaye, D., J. C. von Vaupel-Klein & E. Suárez-Morales (eds), Studies on Freshwater Copepoda: A Volume in Honour of Bernard Dussart, Crustaceana Monographs, Vol. 16. Koninklijke Brill NV, Leiden: 245–269. https://doi.org/10.1163/9789004188280_012.
Hołyńska, M. & F. Stoch, 2012. Mesocyclops (Crustacea, Copepoda, Cyclopidae) in the South Pacific islands. Zoologischer Anzeiger 251: 237–252. https://doi.org/10.1016/J.JCZ.2011.09.004.
Hołyńska, M., L. Leggitt & A. A. Kotov, 2016. Miocene cyclopid copepod from a saline paleolake in Mojave, California. Acta Palaeontologica Polonica 61: 345–361. https://doi.org/10.4202/app.00137.2014.
Hupało, K., F. Stoch, I. Karaouzas, A. Wysocka, T. Rewicz, T. Mamos & M. Grabowski, 2021. Freshwater malacostraca of the Mediterranean islands - diversity, origin, and conservation perspectives. In Tadashi, K. & C. D. Rogers (eds), Recent Advances in Freshwater Crustacean Biodiversity and Conservation. Taylor & Francis Group, Boca Raton: 139–219.
Hutchinson, G. E., 1951. Copepodology for the ornithologist. Ecology 32: 571–577.
Kallimanis, A. S., E. Bergmeier, M. Panitsa, K. Georghiou, P. Delipetrou & P. Dimopoulos, 2010. Biogeographical determinants for total and endemic species richness in a continental archipelago. Biodiversity and Conservation 19: 1225–1235. https://doi.org/10.1007/s10531-009-9748-6.
Karanovic, T., 2005. Two new genera and three new species of subterranean cyclopoids (Crustacea, Copepoda) from New Zealand, with redescription of Goniocyclops silvestris Harding, 1958. Contributions to Zoology 74: 223–254. https://doi.org/10.1163/18759866-0740304002.
Karanovic, T., S. M. Eberhard & A. Murdoch, 2011. A cladistic analysis and taxonomic revision of Australian Metacyclops and Goniocyclops, with description of four new species and three genera (Copepoda, Cyclopoida). Crustaceana 84: 1–67. https://doi.org/10.1163/001121610X546698.
Karanovic, T., M. J. Grygier & W. Lee, 2013. Endemism of subterranean Diacyclops in Korea and Japan, with descriptions of seven new species of the languidoides-group and redescription of D. brevifurcus Ishida, 2006 and D. suoensis Ito, 1954 (Crustacea, Copepoda, Cyclopoida). Zookeys 267: 1–76. https://doi.org/10.3897/zookeys.267.3935.
Karaytug, S., 1999. Copepoda: Cyclopoida Genera Paracyclops, Ochridacyclops and key to the Eucyclopinae. In Dumont, H. J. F. (ed), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 14. Backhuys, Leiden.
Korovchinsky, N. M., 2013. Cladocera (Crustacea: Branchiopoda) of South East Asia: history of exploration, taxon richness and notes on zoogeography. Journal of Limnology 72(s2): 109–124. https://doi.org/10.4081/jlimnol.2013.s2.e7.
Kreft, H. & W. Jetz, 2010. A framework for delineating biogeographical regions based on species distributions. Journal of Biogeography 37: 2029–2053. https://doi.org/10.1111/j.1365-2699.2010.02375.x.
Legendre, P. & M. J. Anderson, 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69: 1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2.
MacArthur, R. H. & E. O. Wilson, 2001. The theory of Island Biogeography − With a New Preface by Edward O. Princeton University Press, Princeton and Oxford Wilson.
Maier, G., 1995. Mating frequency and interspecific matings in some freshwater cyclopoid copepods. Oecologia 101: 245–250. https://doi.org/10.1007/BF00317290.
Matthews, T. J., F. Guilhaumon, K. A. Triantis, M. K. Borregaard & R. J. Whittaker, 2016. On the form of species–area relationships in habitat islands and true islands. Global Ecology and Biogeography 25: 847–858. https://doi.org/10.1111/geb.12269.
Matthews, T. J., F. Rigal, K. A. Triantis & R. J. Whittaker, 2019. A global model of island species–area relationships. PNAS 16: 12337–12342. https://doi.org/10.1073/pnas.1818190116.
Millien-Parra, V. & J.-J. Jaeger, 1999. Island biogeography of the Japanese terrestrial mammal assemblages: an example of a relict fauna. Journal of Biogeography 26: 959–972.https://doi.org/10.1046/j.1365-2699.1999.00346.x.
Mirabdullayev, I. & D. Defaye, in press. A redescription of Microcyclops varicans (G.O. Sars, 1863) (Crustacea, Copepoda, Cyclopidae). Invertebrate Zoology.
Næss, T. & J. P. Nilssen, 1991. Diapausing fertilized adults – A new pattern of copepod life cycle. Oecologia 86: 368–371. https://doi.org/10.1007/BF00317602.
Novichkova, A. A. & A. I. Azovsky, 2017. Factors affecting regional diversity and distribution of freshwater microcrustaceans (Cladocera, Copepoda) at high latitudes. Polar Biology 40: 185–198. https://doi.org/10.1007/s00300-016-1943-9.
Phong, T. V., N. Tuno, H. Kawada & M. Takagi, 2008. Comparative evaluation of fecundity and survivorship of six copepod (Copepoda: Cyclopidae) species, in relation to selection of candidate biological control agents against Aedes aegypti. Journal of the American Mosquito Control Association 24: 61–69. https://doi.org/10.2987/5672.1.
Pinel-Alloul, B. & V. R. Alekseev, 2019. The role of abiotic and biotic interactions in summer diapause of cyclopoids: conceptual model and field validation in southern Quebec boreal lakes. In Alekseev, V. R. & B. Pinel-Alloul (eds), Dormancy in Aquatic Organisms: Theory, Human Use and Modeling, Monographiae Biologicae 92. Springer Nature Switzerland AG, Cham: 221–246. https://doi.org/10.1007/978-3-030-21213-1.
Pinel-Alloul, B., A. André, P. Legendre, J. A. Cardille, K. Patalas & A. Salki, 2013. Large-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Global Ecology and Biogeography 22: 784–795. https://doi.org/10.1111/geb.12041.
Pyron, R. A. & J. J. Wiens, 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proceedings of the Royal Society B 280: 20131622. https://doi.org/10.1098/rspb.2013.1622.
Qian, H., Y. Jin & R. E. Ricklefs, 2017. Phylogenetic diversity anomaly in angiosperms between eastern Asia and eastern North America. Proceedings of the National Academy of Sciences of the United States of America 114: 11452–11457. https://doi.org/10.1073/pnas.1703985114.
Reid, J. W., 1994. Latitudinal diversity patterns of continental benthic copepod species assemblages in the Americas. Hydrobiologia 292(293): 341–349.
Reid, J. W., 1997. How “cosmopolitan” are the continental cyclopoid copepods? Comparison of the North American and Eurasian faunas, with description of Acanthocyclops parasensitivus sp. n. (Copepoda: Cyclopoida) from the U.S.A. Zoologischer Anzeiger 236: 109–118.
Reid, J. W., 2001. A human challenge: discovering and understanding continental copepod habitats. Hydrobiologia 453(454): 201–226.
Sathiamurthy, E. & H. K. Voris, 2006. Maps of Holocene sea level transgression and submerged lakes on the Sunda Shelf. The Natural History Journal of Chulalongkorn University Suppl. 2: 1–44.
Sawagaki, T., T. Aoki, H. Hasegawa, S. Iwasaki, S. Iwata & K. Hirakawa, 2004. Late Quaternary glaciations in Japan. In Ehlers, J. & P. L. Gibbard (eds), Quaternary Glaciations - Extent and Chronology, Part III. Elsevier B.V, Amsterdam: 217–225. https://doi.org/10.1016/s1571-0866(04)80127-1.
Schmitt, T., U. Fritz, M. Delfino, W. Ulrich & J. C. Habel, 2021. Biogeography of Italy revisited: genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Frontiers in Zoology 18: 34. https://doi.org/10.1186/s12983-021-00418-9.
Schrader, J., C. König, K. A. Triantis, P. Trigas, H. Kreft & P. Weigelt, 2020. Species–area relationships on small islands differ among plant growth forms. Global Ecology and Biogeography 29: 814–829. https://doi.org/10.1111/geb.13056.
ter Braak, C. J. F. & P. Šmilauer, 2012. Canoco Reference Manual and User’s Guide: Software for Ordination (version 5.0). Microcomputer Power, Ithaca.
Todeschini, R., V. Consonni, H. Xiang, J. Holliday, M. Buscema & P. Willett, 2012. Similarity coefficients for binary chemoinformatics data: overview and extended comparison using simulated and real data sets. Journal of Chemical Information and Modeling 52: 2884–2901. https://doi.org/10.1021/ci300261r.
Tojo, K., K. Sekiné, M. Takenaka, Y. Isaka, S. Komaki, T. Suzuki & S. D. Schoville, 2017. Species diversity of insects in Japan: their origins and diversification processes. Entomological Science 20: 357–381. https://doi.org/10.1111/ens.12261.
Triantis, K. A., M. Mylonas & R. J. Whittaker, 2008. Evolutionary species−area curves as revealed by single-island endemics: insights for the inter-provincial species−area relationship. Ecography 31: 401–407. https://doi.org/10.1111/j.2007.0906-7590.05323.x.
Triantis, K. A., F. Guilhaumon & R. J. Whittaker, 2012. The island species–area relationship: biology and statistics. Journal of Biogeography 39: 215–231. https://doi.org/10.1111/j.1365-2699.2011.02652.x.
Udvardy, M., 1983. Dinamikus állatföldrajz – A szárazföldi állatok elterjedése [Dynamic Zoogeography with Special Reference to Land Animals]. Tankönyvkiadó, Budapest.
Voris, H. K., 2000. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography 27: 1153–1167. https://doi.org/10.1046/j.1365-2699.2000.00489.x.
Watiroyram, S., A. Brancelj & L.-O. Sanoamuang, 2012. A new Bryocyclops Kiefer (Crustacea: Copepoda: Cyclopoida) from karstic caves in Thailand. The Raffles Bulletin of Zoology 60: 11–21. https://doi.org/10.5281/zenodo.4509245.
Whittaker, R. J., K. A. Triantis & R. J. Ladle, 2008. A general dynamic theory of oceanic island biogeography. Journal of Biogeography 35: 977–994. https://doi.org/10.1111/j.1365-2699.2008.01892.x.
Acknowledgements
The authors are very grateful to Dr. Grace A. Wyngaard (James Madison University, USA) for her critical reading, helpful suggestions and English editing of the manuscript. We appreciate the reviewers’ suggestions and insightful comments on the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
MH and ŁS designed the research; MH collected the data; ŁS performed the statistical analyses, MH & ŁS together analyzed the results and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: Bernadette Pinel-Alloul
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Hołynska and Ł. Sługocki are joint first author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hołyńska, M., Sługocki, Ł. Freshwater microcrustaceans (Copepoda: Cyclopidae) on islands: a review. Hydrobiologia 850, 183–201 (2023). https://doi.org/10.1007/s10750-022-05053-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05053-x