Abstract
We used data from whole-lake studies to assess how changes in food quantity (phytoplankton biomass) and quality (phytoplankton community composition, seston C:P and N:P) with N fertilization affect zooplankton biomass, community composition and C:N:P stoichiometry, and their N:P recycling ratio along a gradient in lake DOC concentrations. We found that despite major differences in phytoplankton biomass with DOC (unimodal distributions, especially with N fertilization), no major differences in zooplankton biomass were detectable. Instead, phytoplankton to zooplankton biomass ratios were high, especially at intermediate DOC and after N fertilization, implying low trophic transfer efficiencies. An explanation for the observed low phytoplankton resource use, and biomass responses in zooplankton, was dominance of colony forming chlorophytes of reduced edibility at intermediate lake DOC, combined with reduced phytoplankton mineral quality (enhanced seston N:P) with N fertilization. N fertilization, however, increased zooplankton N:P recycling ratios, with largest impact at low DOC where phytoplankton benefitted from light sufficiently to cause enhanced seston N:P. Our results suggest that although N enrichment and increased phytoplankton biomass do not necessarily increase zooplankton biomass, bottom-up effects may still impact zooplankton and their N:P recycling ratio through promotion of phytoplankton species of low edibility and altered mineral quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many northern oligotrophic lakes in the boreal and arctic zones are undergoing large-scale changes in biogeochemistry (Solomon et al., 2015; Creed et al., 2018). This includes enhanced loadings of terrestrial colored dissolved organic carbon (DOC) (lake browning) (Monteith et al., 2007; de Whit et al., 2016; Finstad et al., 2016) and decreased or increased loadings of nitrogen (N) and phosphorus (P) related to browning (Jones, 1992), forestry or agricultural activities (Kreutzweiser et al., 2013; Sponseller et al., 2014), and changes in climate and atmospheric N deposition (Eimers et al., 2009; Isles et al., 2018). Together, alterations in colored DOC and nutrient concentrations may have profound effects on the basal production of lakes (Jansson et al., 2003; Solomon et al., 2015; Deininger et al., 2017a, b). Yet, the knowledge of the combined impact of colored DOC and nutrient across wide gradients in lake DOC on higher trophic levels, e.g., zooplankton, is much more limited (Patoine et al., 2000, 2002; Kelly et al., 2016; Deininger et al., 2017c; Hessen et al., 2017).
Zooplankton has a key role in lakes by serving as a trophic link between basal trophic levels and fish (Jansson et al., 2007), controlling phytoplankton biomass and affecting turnover of nutrients through nutrient recycling (Sterner & Hessen, 1994). Their biomass development depends on the quantity and quality of their food resources (Persson et al., 2007; Müller-Navarra, 2008; Brett et al., 2009), and fish predation (Hessen et al., 1995). The diet of zooplankton contains a mixture of phytoplankton, bacteria and detritus of different mineral and biochemical composition (Müller-Navarra, 2008; Persson et al., 2007; Brett et al., 2009; Taipale et al., 2016; Wenzel et al., 2021). Phytoplankton are considered as high-quality food for zooplankton (Brett et al., 2009, 2017; Taipale et al., 2013; Wenzel et al., 2021) depending on community composition and fatty acid composition (Taipale et al., 2013). The relationships between phytoplankton (biomass and production) and lake DOC appears to be unimodal because of the influence of DOC on light availability (negative effect) and nutrient availability (positive effect) (Kelly et al., 2018; Bergström & Karlsson, 2019; Vasconcelos et al., 2019), with the magnitude of the phytoplankton peak determined by the DOC:nutrient stoichiometry (Kelly et al., 2018). No clear relationships between zooplankton and DOC, and the influencing role of phytoplankton have to our knowledge been described over wide gradients in lakes DOC (e.g., Patoine et al., 2000, 2002; Planas et al., 2000; Deininger et al., 2017a, b, c).
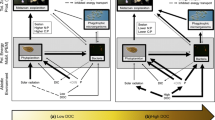
Specifically, although responses in phytoplankton biomass with DOC and N enrichment are largely known on whole-lake scale (Bergström & Karlsson, 2019), questions remain regarding to what extent changes in phytoplankton biomass and community composition, and seston C:N:P stoichiometry, impact zooplankton biomass and community composition, C:N:P stoichiometry, and the N:P recycling ratio of zooplankton. Modelling suggests that zooplankton biomass express similar unimodal distributions with DOC as phytoplankton (Vasconcelos et al., 2019). However, to test these model results, empirical data have hitherto been largely lacking (see Kelly et al., 2014; Vasconcelos et al., 2019). Based on the general coupling between zooplankton and phytoplankton biomass (McCauley & Kalff, 1981), it seems reasonable to assume that zooplankton biomass is also unimodally distributed with DOC during natural and N enriched conditions (Fig. 1A). However, this relationship could be modified by changes in food quality with increasing lake DOC, going in hand with increased nutrient concentrations and reduced light availability.
Conceptual model illustrating trajectories in (A) zooplankton biomass, B seston C:P and N:P and C the N:P recycling ratio of zooplankton along a gradient in colored dissolved organic carbon (DOC) without (thick brown line), and with nitrogen (+ N) fertilization (dotted blue line). Hypothesized models for small northern lakes: A zooplankton biomass is unimodally distributed with DOC during reference (moderate hump shape) and N fertilized (strong hump shape) conditions following the unimodal distribution in phytoplankton, where mineral food quality and phytoplankton community composition increase in importance in constraining zooplankton biomass when phytoplankton biomass increase; B seston C:P and N:P decline with increased DOC as caused by reduced light:nutrient ratio, with higher ratios after fertilization and reduced DOC:N ratio; C during reference conditions, the N:P recycling ratio of zooplankton increase with increased DOC (after Bergström et al. 2018). During N fertilized conditions, the N:P recycling ratio of zooplankton increases and declines with increasing lake DOC. Net change in N:P recycling ratio of zooplankton with N fertilization are highest in clear water lakes (high light, high seston N:P) and lowest in humic lakes (low light, low seston N:P)
According to ecological theory, amplified nutrient imbalances between phytoplankton and zooplankton will not only reduce growth (Sterner & Hessen, 1994; Müller-Navarra et al., 2000), but also impact the N:P recycling ratio of zooplankton (Sterner & Hessen, 1994; Hassett et al., 1997). A previous study (Bergström et al., 2018) has shown that the N:P recycling ratio of zooplankton is low in arctic low DOC lakes (< 16 molar ratio) and increases slightly with increased colored DOC (ca. 18 in boreal high DOC lakes). This observation has been attributed partly to shifts in zooplankton community, from a dominance of calanoid copepods with high somatic N:P, to a dominance of cladocerans and cyclopoids copepods of low somatic N:P. A similar shift in community composition with increased DOC has also been shown in Canadian boreal shield lakes following forest harvesting and logging (Patoine et al., 2000, 2002). However, to what extent the N:P recycling ratio of zooplankton change with N fertilization has not yet been assessed at a whole-lake scale. In contrast to phytoplankton, zooplankton have been considered relatively more homeostatic (Andersen & Hessen, 1991). Yet, the degree of homeostasis and variation in N:P stoichiometry can vary between species and taxonomic groups of zooplankton (Hood & Sterner, 2010; Bergström et al., 2018), being influenced by size distributions (Elser et al., 1988), temperature (Bullejos et al., 2014), growth rates (Elser et al., 2000; Vrede et al., 2002), reproduction modes (Ventura & Catalan, 2005), and ontogeny (Villar-Argaiz et al., 2002). Yet, since phytoplankton are highly plastic (Sterner & Elser, 2002), they are likely to respond stronger to N enrichment than do zooplankton (Sterner & Hessen, 1994), with expected declining trends in their C:N:P stoichiometry with increasing lake DOC (Sterner et al., 1997; Diehl, 2007; see Fig. 1B). The N:P recycling ratio of zooplankton are therefore likely to change the most with N enrichment in clear water lakes where light conditions are favorable for promoting enhanced seston N:P (and C:P) (Deininger et al., 2017c, Fig. 1C). If N fertilization causes major changes in zooplankton community composition, as well as their N:P stoichiometry, the N:P recycling ratio of zooplankton may be affected in addition.
In this study, we used data from natural and fertilized small northern-latitude lakes in both boreal and arctic regions to assess how changes in food quantity (phytoplankton biomass) and quality (phytoplankton community composition, seston C:P and N:P) with N fertilization, propagate to affect zooplankton biomass, community composition, C:N:P stoichiometry, and specifically the N:P recycling ratio of zooplankton along a gradient in lake DOC concentrations. We predicted that:
-
1.
Zooplankton biomass is (a) unimodally distributed with DOC during reference conditions (moderate hump shape) and after N fertilization (strong hump shape), following the unimodal distribution in phytoplankton (see Bergström & Karlsson, 2019); (b) when phytoplankton biomass increases following N fertilization mineral food quality and/or phytoplankton community composition increase in importance in constraining zooplankton biomass (see Deininger et al., 2017a).
-
2.
Zooplankton community composition shifts from dominance of calanoid copepods (high N:P) to dominance of cladocerans (low N:P) with increasing lake DOC (see Patoine et al., 2000, 2002; Bergström et al., 2018).
-
3.
Zooplankton C:N:P ratio of specific species is neither affected by DOC, nor N fertilization, given consumer homeostasis. Rather, changes in the overall zooplankton stoichiometry (C:N:P) will reflect changes in community composition.
-
4.
The N:P recycling ratio of zooplankton during reference conditions increase with increasing lake DOC (see Bergström et al., 2018). During N fertilized conditions, the N:P recycling ratio of zooplankton increase with increased seston N:P and declines with increasing lake DOC. Net changes in N:P recycling ratio of zooplankton following N fertilization are highest in clear water lakes (high light, high seston N:P) and lowest in humic lakes (low light, low seston N:P) (see Deininger et al., 2017c).
Methods
Site description
Data was compiled from a total of 14 small lakes (lake area 0.01–0.1 km2) in northern boreal (n = 8) and arctic Sweden (n = 6), of which 5 lakes were subject to whole-lake inorganic nutrient enrichment (Table S1, 3 in boreal, 2 in arctic). Lakes are small, shallow, vary in watercolor, DOC and nutrient content, and differ in food web structures (Table S1). The study regions have low atmospheric N deposition (Isles et al., 2018), and except from forestry in the boreal region, anthropogenic influences on the lakes are low. Lake catchments consist mainly of open mires and coniferous forests (boreal) or mountain birch (Betula pubescens) (sub-alpine arctic). Boreal lakes usually stratify from mid-May until mid to late September, whereas arctic lakes stratify from mid-June until early September or remain unstratified over summer.
Whole lake fertilization experiments
The whole–lake experiments are explained in detail in the original publications (summarized in Bergström & Karlsson, 2019). We used data from three pairs of boreal experimental lakes representing three levels in DOC (one reference and one N fertilized lake in each lake pair). The experiment had one reference year (2011; all lakes) and two impact years (2012, 2013, N fertilization in 3 of 6 lakes). N in the form of dissolved potassium nitrate (KNO3, in 2012) and concentrated nitric acid (16 M HNO3 in 2013) were used. The whole water column was fertilized once during ice cover (late March 2012; early April 2013), and then biweekly from the onset of stratification (late May/early June) until late August. In 2013, fertilization was performed weekly in the high DOC N lake, as adjusted to its’ shorter water residence time. The areal artificial N loading to the lakes were: Fisklösan: 1 g N m−2 year−1; Lapptjärn: 1.1 g N m−2 year−1 and Nedre Björntjärn: 1.8 g N m−2 year−1 (Deininger et al., 2017a).
These whole-lake fertilization experiments were complemented with data from similar ones conducted in arctic lakes in the 1970s in the Kuokkel area, close to Abisko (Holmgren, 1983; Persson, 1984). Here, one lake served as a reference (Stugsjön 1971–1974) and two as enrichment lakes (Magnusjaure + N, Hymenjaure + P). For Magnusjaure, 1973 was a reference year, and 1974 was an N fertilization year. For Hymenjaure, 1971 was a reference year, and 1972–1974 were P fertilization years. The fertilization procedure was similar among lakes—that is lakes were fertilized equally across the lake surfaces on a weekly basis from early June until late August/early September. Ammonium-nitrate was used for N and phosphoric acid (H3PO4) was used for P fertilization. The areal artificial nutrient loadings were: Magnusjaure: 3.86 g N m−2 (1974); Hymenjaure: 0.094 g P m−2, 0.246 g P m−2, 0.312 g P m−2 (1972, 1973, 1974) (Holmgren, 1983). We decided to include the P-fertilization experiment in this study since our previous assessments have shown that phytoplankton in low DOC lakes are more prone to be dual-limited by N and P (Bergström & Karlsson, 2019).
Lake sampling and analysis of chemical and biological parameters
We used seasonal means for physical, chemical, and biological parameters that were measured during the open water season (early June–mid-September). Data include published and unpublished data on physical–chemical lake variables, phytoplankton biomass and community composition, seston C:N:P stoichiometry, zooplankton biomass, community composition and C:N:P stoichiometry from Jansson (1975), Holmgren (1983), Persson (1984), Bergström et al. (2015, 2018, unpublished data), Deininger et al. (2017a, b, c), and Bergström & Karlsson (2019). Composite samples for chemical and biological parameters were taken from the mid epilimnion (stratified condition) or the whole water column (un-stratified lakes). Subsamples from the composite samples were analyzed for water chemistry, phytoplankton biomass and community composition, and seston C:N:P. Samples for zooplankton biomass were taken by vertical net hauling (100 µm mesh net) from the deepest point of the lake (starting 1 m above the lake bottom). Zooplankton samples for C:N:P analyses followed the same procedure as for biomass, with the exception that several hauls (100 µm mesh net) were performed in order to collect sufficient amounts of zooplankton for C:N:P analyses. After being collected, zooplankton bulk samples were transferred to plastic bottles and then left standing cold and dark over night (12–24 h) for gut evacuation before sorting (see below).
Lake water chemistry was analyzed for dissolved organic carbon (DOC), dissolved inorganic nitrogen (DIN; NO2−-N, NO3−-N, and NH4+-N), total nitrogen (TN) and total phosphorous (TP). For detailed analytical procedures see Holmgren (1983) and Bergström et al. (2015). For lakes Stugsjön, Hymenjaure and Magnusjaure the DOC concentrations were estimated using linear regression analysis between TN and DOC (n = 22; R2 = 0.71; P < 0.001) using data from Jansson et al. (2010) and Bergström et al. (2015). Photosynthetic active radiation (PAR) profiles were measured using handheld probes (LI- 193 Spherical Quantum Sensor/LI- COR Biosciences). The vertical light extinction coefficient (kd) was calculated as the slope of the linear regression of the natural logarithm of PAR versus depth. For the reference year for lakes Stugsjön, Magnusjaure and Hymenjaure, the kd was estimated using linear regression analysis between DOC and kd (n = 16; R2 = 0.95; P < 0.001) using data from Karlsson et al. (2001), Bergström et al. (2015) and Deininger et al. (2017a). The dimensionless estimate for light availability in the mixed layer (Im) was estimated following Karlsson et al. (2009).
Phytoplankton biomass samples were preserved with Lugol’s iodine and counted using inverted phase contrast microscopy. Phytoplankton were identified to genus level and species level where possible. Bio-volumes were calculated using biometry measurements and geometrical formulas and transformed to biomass (μg L−1 wet weight) by assuming a density of 1 g cm−3 (Holmgren, 1983; Deininger et al., 2017a), and then converted to biomass C equivalents by assuming a C content of 22% for cyanophytes, 16% for chlorophytes, and 11% for other phytoplankton following Blomqvist et al. (1995). For lakes SA2, SA3, and SA4, phytoplankton biomass was estimated from Chl-a data from Bergström et al. (2015) using the conversion factor of 50 µg C L−1 per µg Chl-a (Ahlgren, 1983).
Seston C:N:P stoichiometry was determined by filtering known volumes of prescreened epilimnion water (filtered through a 50 µm net) onto pre combusted (550 °C, 4 h) and acid washed (1.2 M HCl) GF/F filters. Seston C and N content were measured using a Costech ECS 4010 elemental analyzer (Costech International S. P. A.). Analyses for seston P were made according to Swedish standard (ISO 15681–1, rev 4) using the molybdate blue method after hydrolysis with persulphate using a FIAstar 5000 (FOSS Inc.) following Bergström et al. (2015). Seston C:N:P stoichiomtery was only measured in lakes Stugsjön, Magnusjaure and Hymenjaure in 1974 (Jansson, 1975).
Zooplankton biomass samples were preserved with Lugol’s iodine, and taxa were identified and counted using inverted microscopy. Length–weight regressions were used to estimate zooplankton biomass in dry weight (DW) (Bottrell et al., 1976). For detailed description see (Persson, 1984; Bergström et al., 2015; Deininger et al., 2017b). For C:N:P stoichiomtery, zooplankton were picked from fresh samples, sorted and pooled into groups of cladocerans, calanoid and cyclopoid copepods, respectively, and then analyzed for C, N and P content. For analyses of C and N, samples from each group were freeze dried, homogenized, and transferred into tin capsules and weighed. Samples were analyzed using a continuous-flow isotope ratio mass spectrometer at UC Davis, US. For P, each zooplankton group was transferred onto pre-weighed and acid-washed (1.2 M HCl) GF/C filters, dried over night (60 °C), and then weighed again (Mettler Toledo MT5; resolution ± 1 µg) to determine dry mass. The P content was analyzed according to Swedish standard (ISO 15681–1, rev 4) using a FIAstar 5000 (FOSS Inc.), as described above. Zooplankton C:N:P stoichiometry was not measured in lakes Stugsjön, Magnusjaure and Hymenjaure.
Elemental imbalances and N:P recycling ratio of zooplankton
We used the ecological stoichiometric models by Sterner (1990) and Hassett et al. (1997) to estimate the elemental N:P imbalances between resources (seston) and consumers (zooplankton) and the N:P recycling ratio of zooplankton (all expressed in molar) during reference and fertilized conditions. We assumed that body mass stoichiometry applies for estimating the N:P recycling ratio of zooplankton on this scale of the food chain, even though evidence comparing organism groups on a larger scale points out that differences in N:P recycling ratios and excretion rates are related to body mass (Allgeier et al., 2015). In the ecological stoichiometric models, a positive nutrient imbalance (N:Pseston > N:Pconsumer) indicates that zooplankton are feeding on resources with suboptimal P content relative to their nutrient demand, and the N:P recycling ratio of zooplankton is calculated as: [(N:P of seston − N:P of zooplankton) × accumulation efficiency (AE)]/(1 − AE). A negative imbalance (N:Pseston < N:Pconsumer) indicates that zooplankton are feeding on resources with suboptimal N content relative to their nutrient demand. The N:P recycling ratio of zooplankton is then calculated as: [N:P of seston × (1 − AE)]/[1 − (AE × N:P of seston/N:P of zooplankton)] (Hassett et al. 1997). The N:P recycling ratio of zooplankton were estimated by assuming an identical AE of 0.75 for both N and P (Sterner, 1990). The N:P elemental imbalances and recycling ratios were calculated for all zooplankton combined (weighted means), using the respective measured N:P stoichiometry of calanoid/cyclopoid copepods and cladocerans multiplied by their relative proportions of the total zooplankton biomass (as in Bergström et al., 2015, 2018), except lakes Stugsjön, Magnusjaure and Hymenjaure (arctic lakes) where zooplankton C:N:P stoichiomtery was not measured. For these lakes, we assumed zooplankton N:P to be similar to the arctic lakes SA2-SA3 dominated by calanoid copepods (Stugsjön and Magnusjaure), and to the boreal lake Övre Björntjärn with similar proportions of cladocerans (for Hymeanjaure) (“Results” section).
Statistical analysis
We analyzed seasonal means for physico‐chemical (DOC, DIN, TN, TP, kd) and biological data (phytoplankton biomass, proportions of dominate phytoplankton groups in phytoplankton biomass, seston C:N:P stoichiometry, zooplankton biomass, community composition and C:N:P stoichiometry, N:P imbalance and zooplankton N:P recycling ratio) for the different lakes and years. Lake water DIN:TP, seston and zooplankton C:N:P stoichiometry was log10-transformed for normal-distribution approximation and/or variance homoscedasticity (Isles, 2020).
Pearson correlation, linear regression and nonlinear regression (Peak Gaussian 3 parameter) analyses were used to test the following: (1) if zooplankton biomass was unimodally distributed with DOC and phytoplankton biomass during reference (moderate hump shape) and N fertilized conditions (strong hump shape) (for prediction 1a); (2) if mineral food quality and phytoplankton community composition increased in importance in constraining zooplankton biomass when phytoplankton biomass increased (for prediction 1b); (3) if zooplankton community composition was related to DOC during reference and N fertilized conditions (for prediction 2); (4) if zooplankton C:N:P ratio of specific species was affected by DOC, or N fertilization (for prediction 3); and (5) if the N:P recycling ratio of zooplankton increased with increased DOC during reference conditions, and declined with increased DOC during N fertilized conditions with highest net changes in N:P recycling ratio of zooplankton with N fertilization in clear water lakes (high light, high seston N:P) and lowest in humic lakes (low light, low seston N:P) (for prediction 4).
Pearson correlation and regression analyses were conducted using Sigma Plot 14.0. Model selection (between Linear, Sigmoid and Gaussian (3 parameter)) (only for assessing relationships between DOC and phytoplankton biomass, zooplankton biomass, and phytoplankton:zooplankton biomass ratios) was done using the Akaike information criterion (AICc). Gaussian and Sigmoid models were assessed using automated curve fitting (Dynamic Fit Wizard) with 200 fits (Supplementary Table S2). Since whole-lake experiments, for both logistic and economic reasons, are few albeit potentially very valuable because of their realism, only limited data is available. Therefore, the statistical power is low and there is a relatively large risk of committing type II errors—to accept an erroneous null hypothesis, when making a statistical analysis (Carpenter, 1989). To decrease the risk of type II error we chose α = 0.10 although this inevitably increases the risk of committing type I errors—to reject a correct null hypothesis. For clarity and for making a critical discussion of results possible, we present all P values, regardless of whether they are significant at α = 0.10 or not.
Results
Lake water chemistry
The study lakes varied from clear water lakes to brown and humic lakes with DOC ranging between 3.8 and 22.8 mg C L−1, all with low or moderate phosphorus (2.9–20.6 µg P L−1) and DIN (3–24 µg N L−1) concentrations (Table S1). N fertilization enhanced DIN concentrations in all N lakes compared to their reference conditions (for Magnusjaure, Fisklösan, Lapptjärn and Nedre Björntjärn). In Hymenjaure, P fertilization increased TP concentrations three times relative to the reference condition before fertilization (Table S1). For reference- and treatment lakes during reference years, the kd tended to increase with DOC (R = 0.94, P < 0.05) and light conditions (Im) became poorer with increased DOC concentrations. TN and TP concentrations tended to increase with increased DOC concentrations (R = 0.94 and 0.89, respectively; P < 0.001 for both).
Phytoplankton biomass and community composition
The biomass of phytoplankton was low and ranged between 20 and 192 µg C L−1 during reference conditions and was somewhat higher in the boreal lakes compared to in the Arctic lakes. After fertilization, the biomass increased especially in the N-fertilized lakes (3.0–5.1 times increase) compared to in the P-fertilized lake (1.8 times increase) (Table 1). As previously shown with 11 of the 14 lakes included here (Bergström & Karlsson, 2019), phytoplankton biomass showed a unimodal relationship with DOC during reference and fertilized conditions (nonlinear regressions, Peak Gaussian 3 parameter: R2 = 0.71, F3,17 = 21.3, P < 0.001, and R2 = 0.65, F3,7 = 6.3, P = 0.03), with peaks in biomass at DOC between 11 and 12 mg C L−1, and with higher biomass following single P (only at low DOC) and single N fertilization (all DOC levels) (Figs. 2A and S1A; Table S2).
Phytoplankton biomass (PhyBM) against a dissolved organic carbon (DOC) concentrations, and b proportions of chlorophytes in phytoplankton biomass, and c log10seston C:P. Black circles denote reference conditions, open white and grey circles denote conditions after whole-lake fertilization with nitrogen (N) or phosphorus (P) alone, respectively. For model statistics see Results
The biomass of phytoplankton was dominated by the group chrysophytes (47% of total, Table 1; Fig. S1E), and especially so in the Arctic lakes (60%). In the Arctic lakes dinophytes was the second dominant group (29%), but in the boreal lakes chlorophytes composed a larger share of the biomass (25%). In N-fertilized lakes there was no change in the dominating group of phytoplankton, and the biomass increases were primarily caused by species that were already present and dominant before fertilization. These were for the N fertilization lakes (ordered with increased lake DOC): chrysophytes (Uroglena americana) in Magnusjaure; large colony forming chlorophytes in Fisklösan; colony forming flagellated chrysophytes in Lapptjärn; and cryptophytes in Nedre Björntjärn (Holmgren, 1983; Deininger et al., 2017a; Table 1; Fig. S1). In contrast, P fertilization in Lake Hymenjaure induced a massive change in phytoplankton community composition with a shift from a dominance of chrysophytes towards a dominance of chlorophytes (78%), primarily small coccoid green algae (classified today as raphidophytes) (Table 1; Figs. 2B and S1; Holmgren, 1983).
During reference and N fertilized conditions, proportions of chlorophytes were unimodally distributed with DOC (nonlinear regressions, Peak Gaussian 3 parameter: R2 = 0.60, F3,15 = 35.0, P < 0.0001, and R2 = 0.87, F3,7 = 33.2, P = 0.003). During reference conditions proportions in phytoplankton biomass of chrysophytes and diatoms were poorly related (R = − 0.286, − 0.251; P = 0.302, 0.431, respectively), whereas the proportions of dinophytes and cryptophytes were negatively (R = − 0.649, P = 0.009) and positively (R = 0.931, P < 0.001) related to DOC, respectively (Fig. S1).
Seston C and C:N:P stoichiometry
Seston averaged 360 µg C L−1 during reference conditions and 566 µg C L−1 following fertilization, with particularly high concentrations in Fisklösan and Lapptjärn after N-fertilization (Table 1). During reference conditions, seston C was related to phytoplankton biomass (R = 0.41; P = 0.09), and this relationship became stronger with fertilization (R = 0.75; p = 0.03) (Table 1). Large proportions of detritus and bacteria C in seston were found at low and at high DOC, where phytoplankton biomass only contributed to ca 20% and 10% of seston C, respectively. With fertilization, phytoplankton biomass proportions in seston C increased to between 30 and 55%.
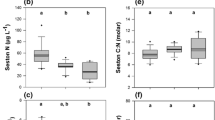
During reference conditions, seston C:P and N:P were not related to phytoplankton biomass (R = − 0.02; P = 0.93; R = 0.21; P = 0.4). Increased phytoplankton biomass with N fertilization contributed to an increase in seston C:P (Fig. 2C) and N:P (Table 1), and both became positively related to phytoplankton biomass (C:P, R = 0.80; P = 0.03; N:P, R = 0.83; P = 0.02). Linear regression explained changes in seston C:P and N:P with DOC during reference conditions (log10seston C:P: R2 = 0.33, F2,16 = 7.9, P = 0.01; log10seston N:P: R2 = 0.17, F2,16 = 3.3, P = 0.09; Fig. 3A, B). Seston C:P declined linearly with increased DOC also after N fertilization (log10seston C:P R2 = 0.58, F2,5 = 6.8, P = 0.05), with most pronounced increases in low (< 10 mg C L−1) compared to in high DOC lakes. (Fig. 3A, Table 1). For seston N:P, the linear regression model was not statistically significant after N fertilization (log10seston N:P R2 = 0.27, F2,5 = 1.8, P = 0.23) (Fig. 3B). In Hymenjaure, P fertilization lowered seston C:P and N:P to 83 (1.92) and 12 (1.08) (Fig. 3A, B).
a log10seston C:P, b log10seston N:P and log10predicted N:P recycling ratio of zooplankton along gradients in dissolved organic carbon (DOC) concentration during reference conditions (black circles) and after whole-lake fertilization with nitrogen (N; open white circles) or phosphorus (P; grey circle) alone. For each panel, solid (control lakes) or dotted (treatment lakes) lines, represents modeled linear relationships. For model statistics see Results
Zooplankton biomass
Zooplankton biomass ranged between 18 and 84 µg DW l−1 during reference conditions. In the N-fertilized lakes there was no large response in biomass, but in the P-fertilized lake the biomass increased 2.9 times (to 155 µg DW l−1). In both reference lakes and fertilized lakes calanoid copepods dominated the biomass over cyclopoid copepods and cladocerans, with one exception for the boreal lake Stortjärn where calanoids were absent (Table 2). In the lake Hymenjaure the cladocerans dominated both before and, even more so, after P-fertilization. Arctic lakes tended to have higher proportions of calanoid copepods (71% during reference conditions) compared to the boreal lakes (40% during reference conditions) that also had higher proportions of especially cyclopoid copepods (Table 2; Fig. S2).
Biomass of zooplankton tended to be negatively related to phytoplankton biomass during reference, but not during fertilized conditions (Table 3; Fig. 4A). Zooplankton biomass was neither related to DOC and did not show any unimodal distributions with DOC during reference or fertilized conditions (Fig. 4C; Table S2). When assessing each zooplankton group, calanoid copepods were negatively related to DOC both during reference and fertilized conditions, whereas cyclopoid copepods and cladocerans were positively related to DOC but only during reference conditions (Table 3; Fig. S2). Calanoid copepods and cladocerans were further negatively related to phytoplankton biomass during reference conditions (Table 3; Fig. S3). As a result of the low variability in zooplankton biomass (Fig. 4A), the phytoplankton to zooplankton biomass ratio expressed unimodal distributions with lake DOC during both reference and fertilized conditions (nonlinear regressions, Peak Gaussian 3 parameter, reference: R2 = 0.60, F3,17 = 12.5, P = 0.001; fertilized: R2 = 0.62, F3,5 = 4.1, P = 0.09; Table S2; Fig. 4B) with around 30 times higher phytoplankton than zooplankton biomass after fertilization at the peak (DOC ca 10 mg C L−1).
Zooplankton biomass (ZooBM) against a phytoplankton biomass, and b phytoplankton to zooplankton biomass ratios (PhyBM:ZooBM) against dissolved organic carbon (DOC) concentration, and c zooplankton biomass (ZooBM) against dissolved organic carbon (DOC) concentration, and d zooplankton biomass (ZooBM) against log10seston C:P. Black circles denote reference conditions, open white and grey circles denote conditions after whole-lake fertilization with nitrogen (N) or phosphorus (P) alone, respectively. For model statistics and relationships see Results and Tables 3 and S3
During reference conditions, zooplankton biomass tended to be positively related to proportions of cryptophytes in phytoplankton biomass. For each taxonomic group, biomass of calanoids was positively related to proportions of chrysophytes and dinophytes; biomass of cyclopoids was negatively related to proportions of dinophytes, and biomass of cladocerans was positively related to proportions of chrysophytes and cryptophytes and negatively related to proportions of chlorophytes (Table 3). These relationships between different groups of zooplankton and phytoplankton, thus, very much followed their relative distribution with lake DOC (see Table 3; Figs. S1-S2). Considering mineral quality, only cladoceran biomass tended to be negatively related to seston C:P during reference conditions (Table 3; Fig. S4).
With fertilization (+ N, + P), these relationships to some extent changed or became intensified (Table 3). Zooplankton and cladoceran biomass became negatively related to seston C:P (Fig. 4D; Table 3) and positively related to proportions of chlorophytes (Table 3), that is strongly driven by the responses in zooplankton and cladoceran biomass in Hymenjaure which was fertilized with P (Table 3; Figs. 4D and S4). With N fertilization, zooplankton biomass became positively related to proportions of dinophytes which to a large extent was caused by changes in calanoids becoming increasingly more positively related to proportions of dinophytes following N fertilization (Table 3).
N:P imbalances and zooplankton N:P recycling ratios
There were some differences in somatic C, N and P content among zooplankton taxa, with generally higher N content among copepods, and higher P content among cyclopoids and cladocerans (Table S3). These differences contributed to higher N:P ratios in calanoids than in cyclopoids and cladocerans (see Table S3; Bergström et al., 2018), which together with the differences in zooplankton community composition (Table 2), contributed to a declining trend in zooplankton N:P (based on weighted means) with increasing DOC during reference conditions (Linear regression; R2 = 0.34, F2, 15 = 8.2, P = 0.01). There was no effect of treatment (fertilized/un-fertilized) or DOC × treatment interaction (ANCOVA with DOC as covariate; whole model R2 = 0.33, F4, 24 = 3.5, P = 0.03, effect of DOC P = 0.02, effect of treatment P = 0.37, effect of DOC × treatment P = 0.72) on zooplankton N:P. The variation in zooplankton N:P with N fertilization was therefore lower than in seston N:P (Tables 1 and S4).
As previously shown (Bergström et al., 2018) increased N:P imbalances (Table S4) contributed to an increased N:P recycling ratio of zooplankton with increased DOC (Fig. 3C; linear regression, log10N:P-re: R2 = 0.19, F2,16 = 3.8, P = 0.07). With N fertilization, the N:P imbalance (Table S4) and the N:P recycling ratio of zooplankton increased, especially in clear lakes (Fig. 3C) (DOC < 10 mg C L−1, N:P imbalance and N:P recycling ratio increased from − 10 to 0–50 (Table S4) and from 8 to 40–160 (0.9 to 1.6–2.2 in Fig. 3C). At high DOC (≥ 15 mg C L−1), the N:P imbalance and the N:P recycling ratio of zooplankton changed little with N fertilization (N:P imbalance from ca 0 to 10 (Table S4); N:P recycling ratio from 20–25 to 35; or from 1.3–1.4 to 1.5 in Fig. 3C). The N:P recycling ratio of zooplankton did not significantly decline with increased DOC with N fertilization (linear regression, log10N:P-re: R2 = 0.27, F2,5 = 1.9, P = 0.23).With P fertilization in lake Hymenjaure, the N:P imbalance became more negative (−18) compared to reference conditions (−9) (Table S4), and the zooplankton N:P recycling ratio declined to 4 (to 0.6 in Fig. 3C).
Discussion
Bottom-up effects on zooplankton biomass
We found that, despite significant changes in phytoplankton biomass, the zooplankton biomass did not show the predicted hump shape responses with DOC and fertilization (lack of support for prediction 1a). Instead, phytoplankton and zooplankton biomass were negatively related (Table 3). The low biomass development of zooplankton compared to that of phytoplankton during reference and fertilization conditions, being especially pronounced around the phytoplankton biomass peak at intermediate DOC especially after fertilization (Fig. 4B), implies low transfer efficiencies of C and an accumulation of phytoplankton not being preyed upon or transferred to zooplankton (Persson, 1984; Deininger et al., 2017a, b, c).
We interpret the poor phytoplankton resource use of zooplankton being related to the phytoplankton community composition and the reduction in phytoplankton mineral quality following N fertilization. During reference condition the phytoplankton group that contributed the most to the unimodal distribution in phytoplankton biomass with DOC, and therefore also to the negative trend between phytoplankton and zooplankton biomass (specifically the calanoids and cladocerans) were chlorophytes (Table 3; Figs. 2B, 4A, S1A and C). Chlorophytes are highly plastic (Sterner & Elser, 2002), of intermediate food quality when considering their fatty acid composition (Taipale et al., 2013), and are more prone to change morphology when being nutrient stressed compared to other species, that is by increasing their cell wall thickness (Lürling & Van Donk., 1997; Van Donk et al., 1997), or forming colonies to avoid predation (Deininger et al., 2017a). Since N fertilization did not affect phytoplankton community composition and biomass increases were caused by dominant species present before fertilization (Table 1, Fig. S1; Holmgren, 1983; Deininger et al., 2017a), the species that primarily contributed to the peak in phytoplankton biomass also after N fertilization were chlorophytes. Thus, an explanation for the observed low phytoplankton resource use, as well as biomass responses in zooplankton could be the dominance of phytoplankton species with reduced edibility, especially at intermediate lake DOC where the observed colony forming chlorophytes dominated (in support of prediction 1b). It is also possible that allochthonous detritus particles through physical inference further reduced the capability of zooplankton to utilize the phytoplankton (Wenzel et al., 2021).
An additional constraint hindering positive zooplankton biomass responses to increased food availability with N fertilization is stoichiometric fold quality of phytoplankton. Increased phytoplankton biomass with N fertilization caused enhanced seston C:P, with a declining trend with increasing lake DOC. In clear water lakes, seston C:P reached levels > 400 (logged > 2.6), e.g., well above threshold elemental ratios for P-limitation in Daphnia (200–300; Sterner & Elser, 2002) and for some species of calanoids copepods (Eudiaptomus > 300; Kibby, 1971; Hessen & Lyche, 1991). However, the only group of zooplankton that were negatively related to seston C:P were the cladocerans (Table 3) that can use the mixed seston diets (Taipale et al., 2016). This relationship became stronger with fertilization, as did the relationship for total zooplankton biomass. Growth rates in cladocerans, e.g., Daphnia, correlate with seston C:P (DeMott & Gulati, 1999; Vrede et al., 2004)—although they are also influenced by PUFA (Müller-Navarra, 1995). Mineral quality should also play a greater role in constraining cladocerans development when the food concentration increases (Sterner, 1990; Persson et al., 2007; Deininger et al., 2017c), in line to what we found (in support of prediction 1b). The reducing impact of N fertilization on phytoplankton mineral quality, especially to that of chlorophytes being dominant around the peak (Figs. 2 and 3; Table 1), was therefore superimposed on phytoplankton community composition enhancing an already low trophic transfer efficiency that was present before fertilization (Fig. 4B).
Bottom-up effects on zooplankton community composition
Zooplankton community composition varied with lake DOC, and both communities and their N:P stoichiometry were largely unaffected by N fertilization (Tables 1–2 and S3; Figs. S1-S2) (in support of prediction 2 and 3). The shift in zooplankton communities with increasing DOC (and increasing nutrient content; Table S1), from dominance of calanoids (of high somatic N:P) towards higher proportions of cladocerans (and to some extent also cyclopoids; both of low somatic N:P), might be related to the high somatic P requirement of cladocerans (Table S3; Andersen & Hessen, 1991) making the more P- and DOC-rich lakes a more suitable habitats for cladocerans in contrast to the less P requiring calanoids copepods. Other studies have also found declining trends of calanoid copepods with increased nutrient and DOC concentrations (Pace, 1986; Patoine et al., 2000). It could also be that the capability among cladocerans of using mixed diets (Taipale et al., 2016), and the raptorial feeding mode of cyclopoids allowing them to use large food particles like microplankton (Pace, 1986; Berggren et al., 2015), enables them to better cope in the high DOC lakes where phytoplankton biomass was low and close to the estimated threshold for metabolic maintenance (50 µg C L−1; Lampert, 1977; Andersen, 1997; Sterner, 1997) (Table 1), than do calanoid copepods feeding primarily on phytoplankton (Berggren et al., 2015). Stable isotope analyses from these regions also show a high degree of allochthony for cladocerans and cyclopoid copepods, but not for calanoids copepods (Berggren et al., 2015; Deininger et al., 2017b).
Interestingly, we also found that different zooplankton groups were related to different classes of phytoplankton (Table 3). We suggest these relationships being caused by different fatty acid (FA) composition among different phytoplankton groups (Taipale et al., 2013). Specifically, calanoid copepods tended to be related to proportions of dinoflagellates which are known to be rich of DHA (Peltomaa et al., 2019; Hikihara et al., 2020), e.g., the most important FA for copepods (Napolitano, 1999; Taipale et al., 2013). In contrast, cladocerans were related to proportions of cryptophytes, known to be rich in EPA (Taipale et al., 2013), the most important FA for support growth and reproduction in Daphnia (Napolitano, 1999; Ravet & Brett, 2006). With fertilization (+ N, + P), cladocerans instead became related to proportions of chlorophytes, a change largely driven by the increased biomass of raphidophytes in the fertilized P lake (Hymenjaure) (Figs. S1-S2), also known of being rich in essential FA for zooplankton (Taipale et al., 2013). Thus, P fertilization in lake Hymenjaure did not only promote increased phytoplankton biomass of enhanced mineral (reduced seston C:P; Fig. 3A), but also most likely enhanced the biochemical (FA) quality of phytoplankton, and this combined improved food quality did indeed promote the highest increase in cladocerans and in total zooplankton biomass (Table 2; Fig. 4D).
In all, we found that N fertilization led to considerably less changes in zooplankton biomass compared to phytoplankton biomass across our studied DOC gradient (Figs. 2A and 4B-C). Thus, although zooplankton and phytoplankton biomass may indeed be coupled (McCauley & Kalff, 1981), unimodal distributions in zooplankton biomass with DOC and N fertilization may be unrealistic to be found among lakes due to the structuring impact of DOC on lake specific phytoplankton and zooplankton community composition.
N:P imbalances and zooplankton N:P recycling ratios
In line with our prediction 4, N fertilization had little effect on zooplankton community composition or their N:P stoichiometry (Tables 2 and S3), but increased the N:P recycling ratio of zooplankton, especially in clear lakes, where phytoplankton benefitted from light sufficiently to cause enhanced seston N:P (Fig. 3). In the DOC rich and dark lakes, the N:P recycling ratio changed little with fertilization. The increase in N:P imbalance (and N:P recycling ratio) with N fertilization in low DOC lakes (increased from negative to positive values) suggest that conditions changed for zooplankton that is that they moved from feeding on phytoplankton of suboptimal N to suboptimal P content. In high DOC lakes the slight increase in seston N:P moved the food resources from being equal and matching the N:P requirements of zooplankton (N:P imbalance close to 0) to slightly above. Combined, this implies that N fertilization and raised seston N:P and zooplankton N:P recycling ratio could in theory promote shifts from N- to more P-limited conditions for phytoplankton in clear low DOC lakes but not in dark high DOC lakes (see Hessen, 2013). Yet, the extent changes in zooplankton N:P recycling ratios might impact phytoplankton nutrient limitation regimes depend on the magnitude in zooplankton N:P recycling rates relative to other nutrient supplies, such as external loadings (Bergström et al., 2015) and internal sediment nutrient release (Levine and Schindler, 1992). The N:P recycling rates of zooplankton increases with increasing body size (Allgeier et al., 2015), biomass (Elser et al., 1988), and temperature (Allen & Gillooly, 2009). Since primarily large specimens were picked, differences in body size among zooplankton taxa should be of lesser importance compared to differences in total zooplankton biomass among our study lakes. As zooplankton communities (impacting somatic N:P), and biomass did not change with N fertilization, impacts of enhanced zooplankton N:P recycling rates following N fertilization on phytoplankton nutrient limitation regimes might be modest and is likely to be quantitatively most important during summer in connection to low external nutrient loadings (Bergström et al., 2015), and high lake water temperatures (Alcaraz et al., 2013).
Role of trophic cascade effects
Our findings might have been affected by presence and absence of fish e.g., via trophic cascades (Carpenter et al., 1985). Thus, it is in theory possible that zooplankton biomass might have been higher following N fertilization in the high DOC lakes if they have been fishless. Yet, in the fishless low DOC lakes, zooplankton biomass did not increase with increased phytoplankton biomass with N fertilization (see Magnusjaure vs. Fisklösan), and zooplankton biomass was also similar in the low DOC reference lakes with fish present (SA lakes) or absent (Stugsjön, Magnusjaure and Hymenjaure). Zooplankton biomass has also been shown to be poorly correlated to fish predation especially in oligotrophic lakes (Hessen et al., 1995). Community responses can further be obscured by eco- and physiological adaptations (Hessen et al., 1995) and by food quality controls being superimposed on food quantity especially following fertilization (Sterner & Hessen, 1994).
Conclusion
To conclude, our results suggest that although N enrichment and increased phytoplankton biomass do not necessarily increase zooplankton biomass, bottom-up effects may still affect zooplankton development and their nutrient recycling ratio. This by changing phytoplankton food quality through stimulating phytoplankton species of reduced edibility or altering phytoplankton mineral quality especially in clear lakes where light conditions are sufficient to enhance seston N:P. Our study showcases that for detecting bottom-up effects (here: N and DOC), the traditional usage of biomass or abundance as indicators of effects may not provide sufficient information on neither ecological effects, nor involved biogeochemical processes. We suggest the inclusion of additional producer and especially consumer traits for ecological effect evaluation (such as edibility, stoichiometry, nutrient recycling; not solely biomasses or productivity), as these parameters may provide highly insightful knowledge on involved processes and ecological feedback mechanisms.
Data availability
Data are available from the authors upon reasonable request.
References
Ahlgren, G., 1983. Comparison of methods for estimation of phytoplankton carbon. Archiv für Hydrobiologie 98: 489–508.
Alcaraz, M., R. Almeda, E. Saiz, A. Calbet, D. M. Duarte, S. Augusti, R. Santiago & A. Alonso, 2013. Effects of temperature on the metabolic stoichiometry of Arctic zooplankton. Biogeosciences 10: 689–697.
Allen, A. P. & J. F. Gillooly, 2009. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecology Letters, 12: 369–384.
Allgeier, J.E., S. J. Wenger, A. D. Rosemond, D. E. Schindler & C. A. Layman, 2015. Metabolic theory and taxonomic identity predict nutrient recycling in a diverse food web. Proceedings of the National Academy of Sciences of the United States 111 (20): E2640–E2647.
Andersen, T., 1997. Herbivores as sources and sinks for nutrients. Ecological Series 129. Springer, New York.
Andersen, T. & D. O. Hessen, 1991. Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnology and Oceanography 36: 807–814.
Berggren, M., A-K. Bergström & J. Karlsson, 2015. Intraspecific autochthonous and allochthonous resource use by zooplankton in a humic lake during the transitions between winter, summer and fall. PLoS One 10: 1-14.
Bergström, A-K. & J. Karlsson, 2019. Light and nutrient control phytoplankton biomass responses to global change in northern lakes. Global Change Biology 25: 2021-2029.
Bergström, A-K., D. Karlsson, J. Karlsson & T. Vrede, 2015. N-limited consumer growth and low nutrient regeneration N:P ratios in lakes with low N deposition. Ecosphere 6: Article 9.
Bergström, A-K., J. Karlsson, D. Karlsson & T. Vrede, 2018. Contrasting plankton stoichiometry and nutrient regeneration in northern arctic and boreal lakes. Aquatic Sciences 80: 24.
Blomqvist, P., R. T. Bell, H. Olofsson, U. Stensdotter & K. Vrede, 1995. Plankton and water chemistry in lake Njupfatet before and after liming. Canadian Journal of Fisheries and Aquatic Sciences 52: 551-565.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzig, A. Hillbrichtilkowska, H. Kurasawa, P. Larsson & T. Weglenska, 1976. Review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Brett, M.T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences of the United States 106: 21197–21201.
Brett, M.T., S:E. Bunn, S. Chandra, A. W. E. Galloway, , F. Guo, M. J. Kainz, P. Kankaala, D. C. P. Lau, T. P. Moulton, M. E. Power, J. B. Rasmussen, S. J. Taipale, J. H. Thorp & J. D. Wehr, 2017. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshwater Biology 62:833–853.
Bullejos, F. J., P. Carillo, E. Gorokhova, J. M. Medina-Sanchez & M. Villar-Argaiz, 2014. Nucleic acid content in crustacean zooplankton: bridging metabolic and stoichiometric predictions. Plos One 9(1): e86493.
Carpenter, S. R., 1989. Replication and treatment strength in whole-lake experiments. Ecology 70: 453-463.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading Trophic Interactions and Lake Productivity. BioScience Vol. 35 No. 10. 634-639.
Creed, I. F., A-K. Bergström, C. G. Trick, N. N. Grimm, D. O. Hessen, … & G. A. Weyhenmeyer, 2018. Global change-driven effects on dissolved organic matter composition: Implications for food webs of northern lakes. Global Change Biology 24: 3692-3714.
Deininger, A., C. L. Faithfull & A-K. Bergström, 2017a. Phytoplankton response to whole lake inorganic N fertilization along a gradient in dissolved organic carbon. Ecology 98: 982-994.
Deininger, A., C. L. Faithfull, J. Karlsson, M. Klaus & A-K. Bergström, 2017b. Pelagic food web response to whole lake N fertilization. Limnology and Oceanography 62: 1498-1511.
Deininger, A., C. L. Faithfull & A-K. Bergström, 2017c. Nitrogen effects on the pelagic food web are modified by dissolved organic carbon. Oecologia 184: 901-916.
DeMott, W. R. & R. D. Gulati, 1999. Phosphorus limitation in Daphnia: Evidence from a long term study of three hypereutrophic Dutch lakes. Limnology and Oceanography 44: 1557–1564.
Diehl, S., 2007. Paradoxes of Enrichment: Effects of Increased Light versus Nutrient Supply on Pelagic Producer-Grazer Systems. American Naturalist 169: 173-191.
de Whit, H. A., S. Valinia, G. A. Weyhenmeyer, M. N. Futter, P. Kortelainen, K. Austnes, … & J. Vuorenmaa, 2016. Current browning of surface waters will be further promoted by wetter climate. Environmental Science and Technology Letters 3: 430-435.
Eimers, M. C., S. A. Watmough, A. M. Paterson, P. J. Dillon PJ & H. Yao, 2009. Long-term declines in phosphorus export from forested catchments in south-central Ontario. Canadian Journal of Fisheries and Aquatic Sciences 66: 1682–1692.
Elser, J.J., M. M. Elser, N. A. MacKay & C. R. Carpenter, 1988. Zooplankton-mediated transitions between N- and P-limited algal growth. Limnology and Oceanography 33: 1–14.
Elser, J. J., W. J. O’Brien, D. R. Dobberfuhl & T. E. Dowling, 2000. The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. Journal of Evolutionary Biology 13: 845-853.
Finstad, A. G., T. Andersen, S. Larsen, K. Tominaga, S. Blumentrath, H. A. de Wit, H. Tømmervik & D. O. Hessen, 2016. From greening to browning: catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Scientific Reports 6: 31944.
Hassett, R. P., B. Cardinale, L. B. Stabler & J. J. Elser, 1997 Ecological stoichiometry of N and P in pelagic ecosystems: comparison of lakes and oceans with emphasis on the zooplankton-phytoplankton interaction. Limnology and Oceanography 42: 648–662.
Hessen, D. O., 2013. Inorganic nitrogen deposition and its impacts on N:P ratios and lake productivity. Water 5: 327–341.
Hessen, D. O. & A. Lyche, 1991. Interspecific and intraspecific variations in zooplankton elemental composition. Archiv für Hydrobiologie 121: 343-353.
Hessen, D. O., B. A. Faafeng & T. Andersen, 1995. Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Canadian Journal of Fisheries and Aquatic Sciences 52: 433-742.
Hessen, D. O., J. P. Håll, J.-E. Thrane & T. Andersen, 2017. Coupling dissolved organic carbon, CO2 and productivity in boreal lakes. Freshwater Biology 62: 945–953.
Hikihara, R., Y. Yamasaki, T. Shikata, N. Nakayama, S. Sakamoto, S. Kato, H. Hatate & R. Tanaka, 2020. Analysis of Phytosterol, Fatty Acid, and Carotenoid Composition of 19 Microalgae and 6 Bivalve Species. Journal of Aquatic Food Product Technology 29: 461–479.
Holmgren, S., 1983. Phytoplankton biomass and algal composition in natural, fertilized and polluted subarctic lakes. Acta Universitatis Upsaliensis 674, Uppsala University, Uppsala.
Hood, J. M. & R. W. Sterner, 2010. Diet Mixing: Do Animals Integrate Growth or Resources across Temporal Heterogeneity? American Naturalist 176: 651- 663.
Isles, P. D. F., 2020. The misuse of ratios in ecological stoichiometry. Ecology 101 (11): e03153.
Isles, P. D. F., I. F. Creed & A-K. Bergström, 2018. Recent synchronous declines in DIN:TP in Swedish lakes. Global Biogeochemical Cycles 32: 208-225.
Jansson, M., 1975. Kol, kväve och fosfor i partikulärt material. In: Experiment med gödsling av sjöar i Kuokkelområdet. Kuokkelprojektets rapport Nr. 4. Limnologiska Institutionen, Uppsala Universitet, Uppsala (in Swedish).
Jansson, M., J. Karlsson & P. Blomqvist, 2003. Allochthonous organic carbon decreases pelagic energy mobilization in lakes. Limnology and Oceanography 48: 1711–1716.
Jansson, M., L. Persson, A. M. De Roos, R. I. Jones & L. J. Tranvik, 2007. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends in Ecology and Evolution 22: 316-322.
Jansson, M., A. Jonsson, A. Andersson & J. Karlsson, 2010. Biomass and structure of planktonic communities along an air temperature gradient in subarctic Sweden. Freshwater Biology 55: 691-700.
Jones, R. I., 1992. The influence of humic substances on lacustrine planktonic food webs. Hydrobiologia 229: 73-91.
Karlsson, J., A. Jonsson & M. Jansson, 2001. Bacterioplankton production in lakes along an altitude gradient in the subarctic north of Sweden. Microbial Ecology 42: 372-383.
Karlsson, J., P. Byström, J. Ask, P. Ask, L. Persson & M. Jansson, 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460: 506-509.
Kelly, P. T., C. T. Solomon, B. C. Weidel & S. E. Jones, 2014. Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology 95(5): 1236–1242.
Kelly, P. T., C. T. Solomon, J. A. Zwart & S. E. Jones, 2016. Experimental whole-lake increase of dissolved organic carbon concentration produces unexpected increase in crustacean zooplankton density. Global Change Biology 22: 2766–2775.
Kelly, P.T., C. T. Solomon, J. A. Zwart & S. E. Jones, 2018. A framework for understanding variation in pelagic gross primary production of lake ecosystems. Ecosystems 21: 1364-1376.
Kibby, H. V., 1971. Energetics and population dynamics of Diaptomus gracilis. Ecological Monographs 41: 311-&.
Kreutzweiser, D., F. Beall, K. Webster, D. Thompson & I. Creed, 2013. Impacts and prognosis of natural resource development on aquatic biodiversity in Canada’s boreal zone. Environmental Reviews 21:227–259.
Lampert, W., 1977. Studies on the carbon balance in Daphnia pulex de Geer as related to environmental conditions. I–IV Archiv für Hydrobiologie 48: 287–368.
Levine, S. N. & D. W. Schindler, 1992. Modification of the N:P ratio in lakes by in situ processes. Limnology and Oceanography 37: 917-935.
Lürling, M. & E. Van Donk, 1997. Life history consequences for Daphnia pulex feeding on nutrient-limited phytoplankton. Freshwater Biology 38: 693–709.
McCauley, E. & J. J. Kalff, 1981. Empirical relationships between phytoplankton and zooplankton biomass in lakes. Canadian Journal of Fisheries and Aquatic Sciences 38: 458–463.
Monteith, D. T., J. L. Stoddard, C. D. Evans, H. A. de Wit, M Forsius, T. Høga, … & J. Vesely, 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537-540.
Müller-Navarra, D. C., 1995. Biochemical versus mineral limitation in Daphnia. Limnology and Oceanography 40: 1209-1214.
Müller-Navarra, D. C., 2008. Food web paradigms: the biochemical view on trophic interactions. International Review of Hydrobiology 93(4–5):489–505.
Müller-Navarra, D. C., M. T. Brett, A. M. Liston & C. R. Goldman, 2000. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 406: 74-77.
Napolitano, G. E., 1999. Fatty acids as trophic and chemical markers in freshwater ecosystems. In: Arts, M. T., & B. C. (eds), Lipids in freshwater ecosystems. Springer, New York, pp 21–44.
Pace , M. L., 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnology and Oceanography 31: 45-55.
Patoine, A., B. Pinel-Alloul, E. E. Prepas & R. Carignan, 2000. Do logging and forest fires influence zooplankton biomass in Canadian Boreal Shield lakes? Canadian Journal of Fisheries and Aquatic Sciences 57: 155–164.
Patoine, A., B. Pinel-Alloul & E. E. Prepas, 2002. Effects of catchment perturbations by logging and wildfires on zooplankton species richness and composition in Boreal Shield lakes. Freshwater Biology 47: 1996–2014.
Peltomaa, E., H. Hällfors & S. J. Taipale, 2019. Comparison of Diatoms and Dinoflagellates from Different Habitats as Sources of PUFAs. Marine Drugs 17: 233.
Planas, D., M. Desrosiers, S-R. Groulx, S. Paquet & R. Carignan, 2000. Pelagic and benthic algal responses in eastern Canadian Boreal Shield lakes following harvesting and wildfires. Canadian Journal of Fisheries and Aquatic Sciences 57: 136–145.
Persson, G., 1984. Zooplankton studies within the lake fertilization experiments of the Kuokkel area, northern Sweden. Acta Universitatis Upsaliensis 737, Uppsala University, Uppsala.
Persson, J., M. T. Brett, T. Vrede & J. L Ravet, 2007. Food quantity and quality regulation of trophic transfer between primary producers and a keystone grazer (Daphnia) in pelagic freshwater food webs. Oikos 116: 1152–1163.
Ravet, J. L. & M. T. Brett, 2006. Phytoplankton essential fatty acid and phosphorus content constraints on Daphnia somatic growth and reproduction. Limnology and Oceanography 51(5): 2438–2452.
Solomon, C. T., S. E. Jones, B. C Weidel, I. Buffam, M. L. Fork, J. Karlsson, S. Larsen, J. T. Lennon, J. S. Read, S. Sadro & J. E. Saros, 2015. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems 18: 376–389.
Sponseller, R. A., J. Temnerud, K. Bishop & H. Laudon, 2014. Patterns and drivers of riverine nitrogen (N) across alpine, subarctic, and boreal Sweden. Biogeochemistry 120:105–120.
Sterner, R. W., 1990. The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. American Naturalist 136: 209–229.
Sterner, R. W., 1997. Modeling interactions of food quality and quantity in homeostatic consumers. Freshwater Biology 38: 473–481.
Sterner, R. W. & D. O. Hessen, 1994. Algal nutrient limitation and the nutrition of aquatic herbivores. Annual Review of Ecology and Systematics 25: 1-29.
Sterner, R. W. & J. J. Elser, 2002. Ecological Stoichiometry. Princeton University Press, Princeton, New Jersey, USA.
Sterner, R. W., J. J. Elser, E. J. Fee, S. J. Guildford & T. H. Chrzanowski, 1997. The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. American Naturalist 150: 663–684.
Taipale, S., U. Strandberg, E. Peltomaa, A. W. E. Galloway, A. Ojala & M. T. Brett, 2013. Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquatic Microbial Ecology 71: 165–178.
Taipale, S., W. E. Aaron, S. L. Aalto, K. K. Kahilainen, U. Strandberg U & P. Kankaala, 2016. Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Scientific reports 6:30897.
Van Donk, E., M. Lürling & D. O. Hessen, 1997. Altered cell wall morphology in nutrient-deficient phytoplankton and its impact on grazers. Limnology and Oceanography 42: 357–364.
Vasconcelos, F. R., S, Diehl, P. Rodríguez, P. Hedström, J. Karlsson & P. Byström, 2019. Bottom‐up and top‐down effects of browning and warming on shallow lake food webs. Global Change Biology 25: 504–521.
Ventura, M. & J. Catalan, 2005. Reproduction as one of the main causes of temporal variability in the elemental composition of zooplankton. Limnology and Oceanography 50: 2043–2056.
Vrede, T., J. Persson & A. Aronsen, 2002. The influence of food quality (P:C ratio) on RNA:DNA ratio and somatic growth rate of Daphnia. Limnology and Oceanography 47: 487-494.
Vrede, T., D. R. Dobberfuhl, A. L. M. Kooijman & J. J. Elser, 2004. Fundamental connections among organisms C:N:P stoichiometry, macromolecular composition, and growth. Ecology 85: 1217–1229.
Villar-Argaiz, M., J. M. Medina-Sanchez & P. Carrillo, 2002. Linking life history strategies and ontogeny in crustacean zooplankton: implications for homeostasis. Ecology 83: 1899–1914.
Wenzel, A., T. Vrede, M. Jansson & A-K. Bergström, 2021. Daphnia performance on diets containing different combinations of high-quality algae, heterotrophic bacteria, and allochthonous particulate organic matter. Freshwater Biology 66, 157–168.
Acknowledgements
This study was funded in part by the Swedish Research council’s VR (dnr:621-2010-4675), Formas (dnr: 215-2010-922) and the foundation Oscar and Lili Lamms Minne and Knut and Alice Wallenberg Foundation (dnr: 2016.0083).
Funding
Open access funding provided by Umea University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editors: Bernadette Pinel-Alloul & Andy Dzialowsky
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergström, AK., Deininger, A., Jonsson, A. et al. Effects of nitrogen enrichment on zooplankton biomass and N:P recycling ratios across a DOC gradient in northern-latitude lakes. Hydrobiologia 848, 4991–5010 (2021). https://doi.org/10.1007/s10750-021-04689-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04689-5