Abstract
Carnitine, a ubiquitous compound in living organisms, fulfills diverse roles in energy metabolism, stress resilience, and detoxification. Its antioxidant and osmolyte traits offer relief to stressed plants. Antagonizing abscisic acid (ABA), carnitine influences ABA-responsive genes. Our study, using Arabidopsis thaliana wild-type Ler. (Landsberg erecta) and ABA-insensitive abi2-1 mutants, explored carnitine’s impact on antioxidative responses and ABI2’s role in salt-induced carnitine metabolism. The application of 5 µM carnitine has alleviated the decrease in RWC, shoot weight, and rosette diameter WT plants caused by 80 mM salt stress for 4 days. Carnitine reduced cell membrane damage and salinity effects, evidenced by decreased lipid peroxidation and H2O2. In contrast, the impaired ABI2 of abi2-1, due to deficient phosphatase activity, further exacerbated the inhibitory effect of carnitine on the enzymes of the ascorbate-glutathione cycle, consequently reducing stress mitigation. While abi2-1 mutants exhibited unchanged superoxide dismutase (SOD) activity, they demonstrated increased catalase and peroxidase activity following carnitine treatment under salt stress compared to WT plants. Conversely, wild-type WT plants treated with carnitine exhibited elevated total glutathione content under salt stress, a response not observed in abi2-1 mutants under carnitine treatment. These results underscore the crucial role of ABI2-dependent ABA signaling in regulating plant carnitine metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salt stress is one of the environmental stress factors that is highly affecting agricultural production worldwide. It adversely affects the growth and development of plants due to ion imbalance and hyperosmotic stress (Hasegawa et al. 2000). Stomatal closure is a parameter that reduces loss of water under salt stress and limiting gas exchange. As a result of that, decreased CO2 diffusion causes an increase in reactive oxygen species (ROS) production due to uncoupling of light and stromal reactions in chloroplasts, perturbation of the redox balance in mitochondria, and increased photorespiration in peroxisomes. Therefore, resulting in exceed accumulation of ROS, such as hydroxyl radical (OH•), hydrogen peroxide (H2O2), superoxide (O2•–) (Ahmad et al. 2011), and singlet oxygen (1O2). Those can cause protein oxidation, DNA damage, and lipid peroxidation in the cell membrane, or even interact with other vital components of the plant cell (Apel and Hirt 2004; Parihar et al. 2015).
Plants antioxidative defense system is a mechanism to cope with oxidative stress induced by salt stress (Gill and Tuteja 2010). Salt-stress-induced ROS accumulation is detoxified by enzymatic antioxidants, such as glutathione peroxidase (GPX), ascorbate peroxidase (APX), glutathione S-transferase (GST), catalase (CAT), superoxide dismutase (SOD), and non-enzymatic antioxidants, like glutathione (GSH), ascorbate (AsA), α-tocopherol, carotenoids, and flavonoids (Gill et al. 2011; Mittler et al. 2004). The maintenance of ion homeostasis via the salt-overly-sensitive (SOS) signaling pathway is a second mechanism in plants that copes with stress (Zhu 2000). A component of this pathway, Serine/threonine protein kinase SOS2 is activated by SOS3, a calcium-binding protein (Halfter et al. 2000). It mediates phosphorylation of the Na+/H+ antiporter SOS1, which causes extrusion of excessive Na+ from the cytosol to the apoplast (Shi et al. 2000). The loss of SOS2 in the pathway is a known entity that elicits NaCl hypersensitivity in Arabidopsis Moreover, this component does not only interact with SOS3, but it is also acknowledged that it interacts with ABA-insensitive 2 (ABI2), which is one of the protein-phosphatase-type 2Cs (PP2Cs) (Ohta et al. 2003). ABI2 negatively regulates salt tolerance by SOS2 inhibition and by that reduces SOS2 kinase activity. Therefore, ABI2 is a negative effector of SOS2 activity under stress conditions (Ohta et al. 2003). In addition, previous studies have shown that SOS2 is a negative regulator of both ABA catabolism/conjugation (or a positive regulator of ABA synthesis) and ABA signaling, thereby inhibiting the ABA response (Verslues and Bray 2006).
ABA regulates a range of developmental processes, but also a regulator of responses to environmental stresses such as salinity and drought in plants (Murata et al. 2001). In response to drought or salt-induced osmotic stress, ABA promotes stomatal closure to obtain reduced transpirational water loss in plants. ABA activates Ca2+ channels and elevates cytosolic Ca2+ in guard cells (McAinsh et al. 1990) by inducing Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space (Murata et al. 2001). Thereafter, the increased Ca2+ levels trigger slow (S-type) anion channels and inhibits K+ channels, resulting in ion release and turgor reduction that ensures stomatal closure Schroeder and Hagiwara 1989). Previous studies have revealed that ABA induces ROS production in Arabidopsis guard cells and stimulates Ca2+-permeable channels along with H2O2 (Pei et al. 2000). Thus, indicating the interactions between ABA signaling, ROS production, and Ca2+ channels. And in this ABA signaling pathway, PP2Cs act as an on–off switch (Chen et al. 2016). A positive regulator of the ABA signaling pathway, SnRK2-type protein kinase, is inhibited by low ABA levels, while excessive ABA content inhibits phosphatase activity of PP2Cs through the induction of a protein complex (Raghavendra et al. 2010; Hubbard et al. 2010) The complex consists of PYR/PYL/RCAR receptor proteins that interact with PP2Cs (Cutler et al. 2010). Following this, SnRK2 kinases in active form are released to trigger ABA-responsive genes and stomatal closure to alleviate transpirational water loss under osmotic stress induced by the stressors (Chen et al. 2016).
The ABA-mediated activation of compatible osmolyte biosynthesis, like proline and glycinebetaine (Ashraf and Foolad 2007), is a well-known defense system that counters salt stress. Osmolytes do implement the scavenging of free radicals and regulation of the redox potential, thus, perform a protective function (Charrier et al. 2012).In a previous report, another quaternary ammonium compound, carnitine, was shown to act in a manner similar to proline by providing support to conserve water and/or oxidant equilibrium (Charrier et al. 2012). Carnitine (4-N-trimethylammonium-3-hydroxybutyric acid) is present in all living organisms and is involved in many biological processes, such as hormonal regulation, lipid and energy metabolisms, and stress tolerance (Charrier et al. 2012). Its presence in plants was determined by Fraenkel (1953) for the very first time. Yet, the role of carnitine in plants is poorly documented in contrast to the vast amount of knowledge about the way it functions in mammals. Wood et al. (1992) determined the two forms of carnitine (free and acyl-carnitine) in different plant tissues. Also, the presence of carnitine acyltransferase in pea chloroplast has been revealed with a study of Mclaren et al. (1985). The function of acetyl-carnitine as a substrate in fatty acid metabolism was determined by Masterson et al. (1990). Acyl-carnitine participates in developmental processes like embryogenesis, organogenesis, and post-germination, and this aspect is associated with plants lipid metabolism (Nguyen et al. 2016). Moreover, the Na+/carnitine symporter (OCTN2 transporter) defined in mammals plays a crucial role by allowing Na+ sequestration in the vacuole (Tamai et al. 2001). Similarly, six members of the carnitine transporter (OCT) family, including OCTN2, have been specified in plants. Also, within a study, AtOCTs expression levels are increased in response to drought and salt stresses (Küfner and Koch 2008).
Various studies have determined the antioxidant properties of carnitine in yeast (Franken et al. 2008) and animals (Peluso et al. 2000; Kalaiselvia and Panneerselvam 1998; Augustyniak and Skrzydlewska 2009). Carnitine and propionylcarnitine have been shown to have protective properties against the toxic effects of O2•–, H2O2 and chelate metals, thus, inhibit lipid peroxidation in vitro (Vanella et al. 2000; Gülçin 2006). Propionylcarnitine can also act by restraining the Fenton reaction, preventing the formation of the OH.− radical from H2O2 or scavenging the OH.− radical (Reznick et al. 1992). In rodents beneath oxidative stress, Gómez-Amores et al. (2007) found that carnitine operates the activities of antioxidant enzymes glutathione reductase, glutathione peroxidase, and catalase. Similarly, in maize seedlings, carnitine was found to be responsible for reduced levels of superoxide anion and hydrogen peroxide; along with lower lipid peroxidation, protein oxidation, and beyond all those effects, it stimulates antioxidant enzymes to prevent freezing injuries (Turk et al. 2020).
In a previous study, Charrier et al. (2012) compared the effects of carnitine supply on salt stress tolerance levels of aba1-1 and abi1-1 mutants and found a plausible link between carnitine and ABA response pathways. It is known that abi1-1 is a dominant mutation in PP2C. Within this mutant, ABA does not induce ROS production because of disruption in ABA signaling upstream of ROS production. However, H2O2 activates the Ca2+ channels and promotes stomatal closure in this mutant (Murata et al. 2001). Under salt stress, both mutants responded differently to exogenous carnitine treatment. For aba1-1, the efficiency of 5 µM carnitine is less than that of 5 mM carnitine. However, it is shown that salt-stressed abi1-1 has lower responsivity toward carnitine than aba1-1 (Charrier et al. 2012). These results suggest that carnitine acts upon, or even through, the ABA signal transduction pathway. Interestingly, the same study found that while ABA has a reverse effect on the carnitine-responsive genes’ regulation, intracellular carnitine accumulation modulates ABA-responsive genes in A. thaliana Columbia (Col) ecotype. Additionally, the results of meta-analysis, which challenged the carnitine-induced transcriptome alterations in Col with various kinds of stresses, suggested a connection between carnitine and ABA signaling (Charrier et al. 2012). In the light of the facts mentioned above, we investigated how carnitine modulates antioxidative defense and whether ABI2, a distinct PP2C gene, plays a role in the homeostasis of carnitine metabolism under salt stress. Toward this goal, we selected the abi2-1 mutant as the plant material in our study since both abi1-1 and abi2-1 mutations have distinct functions in ABA signal transduction, even though both mutations disrupt ABA activation of Ca2+ channels. In contrast to abi1-1, in abi2-1 mutant, ABA does induce ROS production, yet H2O2-induced stomatal closure is still disrupted (Murata et al. 2001). These results indicate that abi2-1 mutation impairs ABA signaling downstream of ROS production, not the upstream (Murata et al. 2001). Hence, in this study, we investigated differences in the antioxidative defense of abi2-1 by means of carnitine and ABA signaling under salt stress to understand how carnitine modulates the antioxidative response and the role of ABI2 in the homeostasis of carnitine metabolism under salt stress. With the intention of enlightening these goals, we compared (i) rosette diameter and relative water content in leaves, (ii) lipid peroxidation level, H2O2 and total glutathione content, (iii) activities of antioxidant enzymes (SOD, CAT, POX, APX, GR, MDHAR, DHAR) and finally SOD isoenzymes in leaves of A. thalina abi2-1 mutants and wild type ecotype Landsberg erecta (Ler) plants under salt stress.
Materials and methods
Plant material, growth conditions, and stress application
The Arabidopsis thaliana ecotype Landsberg erecta (Ler (WT)) and ABA-insensitive mutant abi2-1 were obtained from the Arabidopsis Information Resource (TAIR, www.arabidopsis.org). The seeds were sterilized with 70% ethanol (1 min) and 4% sodium hypochlorite (NaOCl) solution (10 min). Plants were grown in a plant growth chamber (JSR, JSPC-420 C, Korea) using a hydroponic system under controlled conditions (16/8 h light/dark cycle, 23/21°C, relative humidity 60%, and light intensity 200 µmol photon m–2 s–1) with half-strength Hoagland’s solution. Preliminary studies using various concentrations of NaCl (80, 120, and 150 mM) and carnitine (5 µM and 5 mM) have shown that unlike WT plants, abi2-1 mutant plants at 120 and 150 mM NaCl have better tolerance to salt stress. Therefore, 80 mM NaCl was appropriate to determine the effects of mild stress in both genotypes. The duration of salt treatment was selected by considering the percentages of green seedlings of both genotypes that survived. Moreover, for abi2-1 mutant plants, 5 µM carnitine was more efficient than 5 mM carnitine. Therefore, after 3 weeks of growth, the plants were exposed to 80 mM NaCl for salt treatment and 5 µM L-carnitine (Sigma). Either NaCl or L-carnitine was added to Hoagland’s solution. Afterward, the plants were harvested on day 4 of treatment. Shoot fresh weights were measured using a Sartorius microbalance (Oakleigh, Victoria, Australia) immediately after harvest (within 10 s). Immediately after measurements, the rosette diameters of plants treated with L-carnitine and NaCl were measured with a ruler. Additionally, the harvested plants were frozen in liquid nitrogen and stored at − 80 °C until further enzymatic analysis.
Endogenous carnitine content
The endogenous carnitine content was determined using an L-carnitine assay kit (MAK 063; Sigma-Aldrich, USA) according to the manufacturer’s directions. An acetyl group from CoA was transferred to carnitine by an enzyme assay and then CoA forms were freed. They were processed with subsequent oxidation of a probe to obtain fluorescence (λex = 535; λem = 587 nm). Typical sensitivities of detection for this kit are 10 µM.
Relative water content (RWC)
First, five leaves from each species were harvested and their FW was determined. The leaves were floated on deionized water for 6 h under low irradiance, the turgid tissue was rapidly blotted to remove excess water, and their turgid weights (TWs) were determined. The DW was measured after drying the leaves in an oven. The relative water content (RWC) was calculated:
Determination of H2O2 content
H2O2 presence was confirmed in accordance with Cheeseman (2006) using eFOX reagent, which is broadly used throughout the literature. The modified ferrous ammonium sulfate/xylenol orange (FOX) assay was used because of its sensitivity, stability and its adaptability to a large number of samples. In this assay, 1% ethanol is added to the reagent, which increases its sensitivity to H2O2 by 50% (eFOX). This assay is also adapted to commercial H2O2 kits due to its sensitivity to H2O2 content. Extraction was done using ice-cold acetone containing 25 mM H2SO4. Samples were centrifuged for 5 min at 3000 ×g at 4 °C. For 50 µL of the supernatant, 950 µL of eFOX reagent (250 µM ferrous ammonium sulfate, 100 µM xylenol orange, 100 µM sorbitol, 1% ethanol, v/v) was used. The reaction mixtures were incubated at room temperature for 30 min, and then absorbance at 550 and 800 nm was measured. H2O2 concentrations were calculated using a standard curve prepared with known concentrations of H2O2.
Lipid peroxidation
Lipid peroxidation [reflected by the thiobarbituric acid reactive substance (TBARS) content] was measured by grinding leaf tissue (200 mg) into a fine powder in liquid nitrogen. The leaf tissue (200 mg) was homogenized in 800 µL of cold 5% (w/v) trichloroacetic acid. The homogenate was centrifuged at 12,000 ×g for 30 min and further processed based on the method described by Madhava Rao and Sresty (2000). The concentration of the TBARS was calculated using an extinction coefficient of 155 mM cm− 1.
Enzyme extractions and assays
All assays were performed at 4 °C. For protein and enzyme extractions, 0.1 g of the sample was homogenized in 500 µL of 50 mM Tris-HCl (pH 7.8) containing 0.1 mM EDTA, 0.1% (w/v) Triton-X100, 1 mM phenylmethanesulfonyl fluoride (PMSF), and polyvinylpyrrolidone (PVP; 1%, w/v). For APX activity determination, 5 mM ascorbate was added to the homogenization buffer. Samples were centrifuged at 14,000 ×g for 10 min, and supernatants were used for the determination of protein content and enzyme activities. Total soluble protein contents of the enzyme extracts were determined according to Bradford (1976) using BSA as a standard. All spectrophotometric analyses were conducted on a Shimadzu UV 1700 spectrophotometer.
NADPH oxidase (NOX) activity
NOX (EC 1.6.3.1) activity was measured according to Jiang and Zhang (2002). The assay medium contained 50 mM Tris-HCl buffer (pH 7.5), 0.5 mM sodium 3,3′-(−[(phenylamino)carbonyl]-3,4-tetrazolium)-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT), 100 µM NADPH•Na4, and 20 µg of protein. On addition of NADPH, XTT reduction followed at 470 nm. The corrections of background production were determined in the presence of 50 U SOD. Activity was calculated using the extinction coefficient 2.16 × 104 M− 1 cm− 1. One unit of NOX activity was defined as 1 nmol mL− 1 XTT oxidized min− 1.
Total SOD activity
Total SOD (EC 1.15.1.1) activity was assayed by its ability to inhibit photochemical reduction of NBT at 560 nm (Beuchamp and Fridovich 1971). The assays were carried out at 25 °C, and the reaction mixture (3 mL) contained 0.033 mM NBT, 10 mM L-methionine, 0.66 mM EDTA Na2, and 0.0033 mM riboflavin in 0.05 mM sodium phosphate buffer (pH 7.8). Riboflavin was added last, and the test tubes containing the reaction mixture were incubated for 10 min under 300 µmol m− 2s −1 irradiance at 25 °C. The reaction mixture with no enzyme developed the maximum color because of the maximum rate of reduction of NBT. A non-irradiated reaction mixture was used as the control as it did not develop color. One unit of SOD activity was defined as the amount of enzyme that inhibits 50% NBT photoreduction.
Peroxidase (POX) and cell wall peroxidase (CWPOX) activity
POX (EC1.11.1.7) and cell wall POX (CWPOX) activity was measured according to (Herzog and Fahimi 1973). For determining both activities, the same homogenates were used with different pretreatments. After enzyme extraction, centrifugation was performed at 14,000 ×g for 10 min at 4°C and supernatants were taken for POX assay, while pellets were washed in 50 mM sodium phosphate (pH 5.8) and centrifuged at 1000 ×g for 10 min at + 4°C. After centrifugation, pellets were resuspended in 1 mL dH2O and 1 M NaCl was pipetted into the tubes and the mixture stirred for 2 h. After that, the samples were centrifuged at 1000 rpm for 10 min and supernatants were used for CWPOX assay. The reaction mixture contained 3,3’-diaminobenzidine-tetra hydrochloride dihydrate solution containing 0.1% (w/v) gelatin, 150 mM Na phosphate citrate buffer (pH 4.4), and 0.6% H2O2. There was an increase in the absorbance at 465 nm for 3 min. One unit of POX activity was defined as mmol H2O2 decomposed mL− 1 min− 1.
Catalase (CAT) activity
CAT (EC 1.11.1.6) activity was measured as described by Bergmeyer (1970), by measuring the initial rate of disappearance of H2O2 at 240 nm. The reaction mixture contained 50 mM Na phosphate buffer (pH 7.0) with 0.1 mM EDTA and 3% H2O2. There was a decrease in the absorption for 3 min. One unit of CAT activity was defined as 1 mmol H2O2 oxidized mL− 1 min− 1.
Ascorbate peroxidase (APOX) activity
APX (EC 1.11.1.11) activity was estimated according to Nakano and Asada (1981). The reaction mixture contained 50 mM K phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA Na2, 0.1 mM H2O2, and 0.1 mL of enzyme extract in a final assay volume of 1 mL. The assay depends on a decrease in absorbance at 290 nm, as ascorbate is oxidized. The concentration of the oxidized ascorbate was calculated by using the extinction coefficient of 2.8 mM− 1 cm− 1. One unit of APX activity was defined as 1 mmol mL− 1 ascorbate oxidized min− 1.
Glutathione reductases (GR) activity
GR (EC 1.6.4.2) activity was measured according to Foyer and Halliwell (1976). The assay medium contained 25 mM Na phosphate buffer (pH 7.8), 0.5 mM GSSG, 0.12 mM NADPH, Na4, and 0.1 mL enzyme extract in a final assay volume of 1 mL. NADPH oxidation followed at 340 nm. Activity was calculated using the extinction coefficient of NADPH (6.2 mM− 1 cm− 1). One unit of GR was defined as 1 mmol mL− 1 GSSG reduced min− 1.
DHAR activity
DHAR activity was assessed as described by Nakano and Asada (1981) by including 2.5 mM GSH, 0.2 mM DHA, and 0.1 mM EDTA in a 50 mM K phosphate (pH 7.0) reaction mixture. There was an increase in the absorbance at 265 nm for 1 min. One unit of DHAR activity was defined as 1 nmol DHA recycled mL− 1 min− 1.
MDHAR activity
MDHAR activity was measured according to Arrigoni et al. (1981). MDHAR was observed with NADH oxidation in the presence of ascorbate oxidase (1 U) at 340 nm.
GSH and GSSG content
The content of these non-enzymatic antioxidants was determined according to Queval and Noctor (2007). The glutathione content was determined at 340 nm. The GSSG content was measured using 2-vinylpyridine derivatization after an enzyme cycling assay.
Identification of isoenzymes
Isoenzymes were identified according to Laemmli (1970). Samples containing equal amounts of protein and enzyme standards (Std) as the loading control were loaded on non-denaturing polyacrylamide gel electrophoresis (native PAGE). SOD activity was determined according to Beuchamp and Fridovich (1973). Electrophoretic separation was performed on non-denaturating polyacrylamide gels using 12% separating gel for SOD isoenzymes. The Vilber Lourmat gel imaging system was used, and analyses were performed using the BioCapt software package (Vilber Lourmat) for calculating the intensity of each isoenzyme. In densitometric analyses of SOD activities, activities of control plants were taken as 100%, and the percentage of control values for each treatment were shown. The values are average of the data from three independent gels ± SE. Within each isoenzyme, means with the same letter were not significantly different at p < 0.05 according to Tukey’s multiple-range test.
Statistical analysis
The experiments were repeated twice, and three biological replicates were used from each experiment for all analyses (n = 6). The results were expressed as the mean ± standard error of the mean. Differences between each genotype and treatment effects were identified by analysis of variance (ANOVA). The group datas and the figure letters above the bars that indicate significant differences between the control and treatment groups in each genotype, were compared by using two-way ANOVA (Genotype x Treatment), and the Tukey’s Test as the ANOVA post hoc test (p < 0.05), of the SPSS statistical analyses program (IBM SPSS Statistics 25). Possible correlations between physiological parameters, membrane damage and antioxidant enzyme activity were evaluated by principal component analysis (PCA). Each maintained PC factors were analyzed and selected based on their loading coefficients. The higher the loadings, the greater the influence of factors on a particular PC and the greater the impact.
Results
Rosette diameter
In this study, to determine the effect of carnitine on the elongation growth of Arabidopsis under salt stress, we measured the diameters of vegetative rosettes of the plants (Fig. 1). Rosette diameters decreased in both genotypes when plants were subjected to salt stress by up to 29% and 23% in WT and abi2-1 mutant plants, respectively, compared with the control group. However, compared to plants exposed solely to salt stress, carnitine treatment resulted in a significant increase in the diameters of vegetative rosettes in WT (%16) and abi2-1 (%13) mutant plants under salt stress (p < 0.05). On the other hand, Carnitine-induced alleviation in growth under salt stress was most prominently observed in the WT genotype of plants (p < 0.05).
Four-week-old Arabidopsis thaliana WT ecotype and abi2-1 mutant plants exposed to 80 mM NaCl and 5 µM carnitine for 4 days. Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on shoot length, shoot weight and endogenous carnitine content of Arabidopsis thaliana WT and ABA mutants (abi2-1). C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–g) indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
In WT plants, while salt stress decreased shoot fresh weight by 30%, salt plus carnitine treatment only reduced it by 18% compared to the control group (Fig. 1) (p < 0.05). abi2-1 mutant plants treated with carnitine showed a similar response. In abi2-1, salt stress led to a significant decrease (30%) in shoot fresh weight. However, treatment with salt plus carnitine improved this stress-induced decrease by 10% (p < 0.05) (Fig. 1). The highest shoot fresh weight among the groups subjected to salt stress was observed in WT plants treated with the combined application of carnitine and salt stress (p < 0.001).
Endogenous carnitine content
Salt stress did not affect the endogenous carnitine content in WT plants but decreased it by 18% in abi2-1 mutant plants compared with the control group. Exogenous carnitine treatment increased endogenous carnitine levels in both genotypes under normal condition. However, endogenous carnitine in WT and abi2-1 mutant plants under salt stress increased (11% and %29) on exogenous carnitine application compared with the solely salt-treated group (Fig. 1). However, under salt conditions, the endogenous carnitine levels were similar in both genotypes treated with carnitine (p ≥ 0.05).
Relative water content
In WT plants, only carnitine application increased RWC by 15%. However, salt stress decreased it by 9% compared with the control (Fig. 2). Moreover, the combination of carnitine and salt stress caused a significant increase (16.4%) in the RWC of WT plants compared with the solely salt-treated group (p < 0.05). In abi2-1 mutant plants, solely carnitine treatment increased (10%) the RWC while salt stress decreased it by 12% compared with the control group. However, carnitine treatment prevented this reduction in RWC of abi2-1 mutant plants under salt stress. Although RWC was increased similar extents with carnitine in both genotypes under salt stress, the highest RWC was observed in WT plants (p < 0.05).
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on the relative water content (RWC) of Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–e) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Lipid peroxidation and H2O2 content
The TBARS content increased by 97.5% in WT under salt stress compared with the control group (Fig. 3). However, the TBARS content decreased by 14.8% in the salt + carnitine group compared with the salt-treated groups, indicating the plausible alleviative effect of carnitine treatment (Fig. 3). On the other hand, ABI2 deficiency resulted in a 130% increase in TBARS content of abi2-1 mutants plants compared with WT under normal conditions. Salt stress, on the other hand, further increased the TBARS content of these plants by 19.7%. The carnitine treatment, however, mitigated this increase in salt-induced TBARS content by 10% in abi2-1 mutant plants. However, compared to WT, ABI2 deficiency resulted in a 47% increase in TBARS content of abi2-1 mutant plants treated with carnitine under salt stress (p < 0.001).
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on the thiobarbituric-acid-reactive substance (TBARS) of Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–f) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Salt stress enhanced the H2O2 content by 93.6% and 14% in the leaves of WT and abi2-1, respectively, compared with their control groups. However, the highest H2O2 content was observed salt-treated abi2-1 mutant plants. Because, interestingly, the absence of ABI2 also resulted in a 204.43% increase in the H2O2 content. Similar to the TBARS, salt plus carnitine treatment prevented this increase in the H2O2 content of both genotypes. Carnitine treatment decreased the H2O2 content by 38.6% and 24% in WT and abi2-1 under salt stress, respectively, compared with their salt-treated groups (Fig. 4). However, when compared with WT, the deficiency of abi2-1 has further increased the accumulation of H2O2 in plants treated with salt + carnitine (p < 0.001).
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on H2O2 of Arabidopsis thaliana WT ecotype and the abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–g) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on NADPH oxidase (NOX) of Arabidopsis thaliana WT ecotype and the abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–e) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Activities of antioxidant enzymes
NADPH oxidase (NOX) activity
NOX activity significantly increased by 45.7% in WT plants under salt stress. However, carnitine treatment under salt stress increased NOX activity by 30% compared with the salt-treated group (p < 0.05). ABI2 deficiency caused a significant decrease (38%) in abi2-1 mutant plants compared with WT plants. In abi2-1 mutant plants, salt stress enhanced NOX activity by 74% compared with the control group. However, carnitine prevented the increase in NOX activity induced by salt stress (See Fig. 5).
Superoxide dismutase (SOD) activity
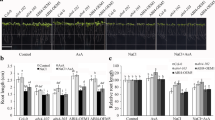
As shown in Fig. 6B, two SOD isoenzymes (MnSOD and Cu/ZnSOD) were identified in both genotypes. In both genotypes, total SOD activity was increased at similar extents (24%) with salt stress compared with the control group (Fig. 6A). Contrarily, in WT plants, salt plus carnitine treatment resulted in higher levels (41%) of SOD activity compared with the salt-treated group, in parallel with increased Mn-SOD and Cu/Zn-SOD isoenzymes. Similarly, in abi2-1 mutant plants, despite the decreased intensities of Mn-SOD, carnitine-treated plants under salt stress exhibited higher levels (20%) of SOD activity compared with the salt-treated group due to increased Cu/Zn SOD activity.
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on superoxide dismutase (SOD) (A) total activities and (B) isoenzyme patterns in Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–c) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Peroxidase (POX) activity
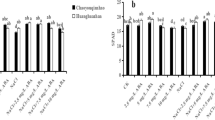
ABI2 deficiency caused a significant increase in POX activity in abi2-1 mutant plants compared with WT plants. In both genotypes, salt stress did not cause any change in POX activity compared with the control groups. Moreover, in WT plants, carnitine treatment under salt stress also did not change POX activity compared with the control group (Fig. 7). However, in abi2-1, salt plus carnitine treatment enhanced POX activity by 17% compared with the salt-treated group (p < 0.05). The highest POX activity was observed in salt plus carnitine treated-abi2-1 mutant plants.
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on (A) peroxidase (POX) and (B) cell wall peroxidase (CWPOX) activity in Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–g) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Cell wall peroxidase (CWPOX) activity
In WT plants under salt stress, CWPOX activity increased by 65.6% compared with the control group. However, it slightly increased in the salt + carnitine group compared with the salt-treated group. The deficiency of ABI2 resulted in a significant decrease in CWPOX activity. Compared to WT, the POX activity of abi2-1 decreased by 61%. On the other hand, salt stress increased the POX activity of abi2-1 plants by only 14% compared to the control group (p < 0.05). However, carnitine application to abi2-1 plants under the same conditions eleviated this increase to 24% (Fig. 7).
Catalase (CAT) activity
In WT plants, salt stress enhanced CAT activity by 76%, Salt plus carnitine treatment caused a higher increase (15%) in CAT activity than only salt stress (Fig. 8). On the other hand, the deficiency of ABI2 caused a significant increase in CAT activity under normal conditions. Similarly, also in abi2-1 mutant plants, salt stress enhanced CAT activity by 13%. Moreover, salt plus carnitine treatment slightly enhanced it by 15% compared with the control group. However, this increase in CAT activity found in the salt + carnitine group was not significant (p ≥ 0.05). Although the highest CAT activity was observed in salt-treated abi2-1 plants (salt treated- and salt plus carnitine treated-plants).
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on CAT (A) total activities and (B) isoenzyme patterns in Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–f) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
Ascorbate-glutathione cycle enzymes
APX activity increased by 93.5% and 74% in WT and abi2-1 mutant plants, respectively, under salt stress compared with their control groups. The highest APX activity was observed in abi2-1 mutant plants under salt stress compared with WT salt plus carnitine treatment decreased its activity in both genotypes compared with their salt-treated groups ((p < 0.001) Fig. 9A). However, the absence of ABI2 in abi2-1 mutant plants resulted in a greater reduction in APX activity (p < 0.001). This condition led to the APX activity of carnitine-treated abi2-1 mutants under salt stress being similar to that of WT plants.
Effect of salt stress (80 mM NaCl) and carnitine (5 µM) application on (A) ascorbate peroxidase (APOX), (B) glutathione reductase (GR), (C) monodehydroascorbate reductase (MDHAR), (D) dehydroascorbate reductase (DHAR), and (E) glutathione activities in Arabidopsis thaliana WT ecotype and abi2-1 mutant plants. C, control; S, salt stress; Car, carnitine; S + Car, salt stress + carnitine. Data were analyzed with IBM SPSS Statistics 25 using Tukey’s multiple-range test at a significance level of p < 0.05, and different letters (a–g) above the bars indicate a significant difference between treatments of both genotypes (WT ecotype and abi2-1). Data were expressed as the mean ± standard error of three independent biological replicates
The absence of ABI resulted in a 50% reduction in GR activity under normal conditions compared with WT GR activity increased by 50% and 52% in WT and abi2-1, respectively, under salt stress compared with the control groups. Moreover, carnitine treatment under salt stress resulted in higher levels of GR activities in both genotypes compared with the salt-treated group. However, the highest GR activity was observed in salt + carnitine treated- WT plants under salt stress. It was determined that under salt stress, the GR activity of carnitine-treated abi2-1 mutants was 29% lower than that of WT plants under the same conditions (p < 0.001) (Fig. 9B).
In WT plants, salt stress enhanced MDHAR activity by 98% compared with the control group. However, carnitine application reduced this increase in MDAHR activity induced by salt stress by 20%. On the other hand, ABI2 deficiency did not significantly change MDHAR activity under normal conditions (p ≥ 0.05). However, in abi2-1 mutant plants, salt stress increased MDHAR activity by 225% compared with the control group. Even, the highest MDHAR activity among all treatment groups was observed in abi2-1 mutants under salt stress. Salt plus carnitine treatment increased MDHAR activity by only 11%, compared with the control group (Fig. 9C).
In WT plants, DHAR activity increased by 167% under salt stress. In carnitine-treated WT plants, it increased by 75%, and in salt + carnitine-treated WT plants, it increased by 123% compared to the control group. However, in WT plants, carnitine treatment led to a significant decrease (16.6%) in DHAR activity under salt stress compared to the salt-treated group (p < 0.001). In abi2-1 mutant plants, DHAR activity increased by 153% under salt stress. Additionally, both carnitine and salt plus carnitine treatments enhanced its activity by 150% and 100%, respectively, compared to the control group. DHAR activity decreased by 55% in abi2-1 mutant plants, resembling the decrease observed in WT plants when both carnitine and salt stress were combined (p < 0.001) ABI2 deficiency has resulted in a 50% reduction in DHAR activity under normal conditions. Consequently, although DHAR enzyme activity increased in abi2-1 mutants under salt stress and carnitine plus salt treatments, the DHAR activity of abi2-1 was not greater than that of WT plants (Fig. 9D).
GSH/GSSG contents
To investigate the effect of carnitine on the redox pool of the cell, GSH and GSSG contents were measured in both WT and abi2-1 mutant plants. In WT plants, salt stress increased the accumulation of total GSH by 15% compared to the control group. Additionally, the GSH content was enhanced by 19.6% in the carnitine-treated group, while it increased by 27.4% in the salt + carnitine group. Consequently, the total GSH content increased by 79% in abi2-1 mutant plants under salt stress. Interestingly, the total glutathione content in the salt + carnitine group closely resembled that of the control group. Carnitine treatment alone did not alter the GSH content compared to the control group. However, the total GSH content decreased by 26% in the salt + carnitine group when compared with the salt-treated group (Fig. 9E).
Principal component analysis
PCA was utilized to examine the relationship between the activities of antioxidant enzymes and ascorbate-glutathione cycle enzymes and carnitine in both genotypes. MDHAR activity clustered separately from the activities of antioxidant enzymes and ascorbate-glutathione cycle enzymes for both genotypes, indicating a negative correlation among these features. Conversely, CAT, POX, CWPOX, NOX and GR activities exhibited a positive correlation with leaf RWC, H2O2, TBARS, GSSG and GSH contents for both genotypes. Whereas, In WT, DHAR had a negative correlation with all parameters studied, whereas in the abi2-1 mutant, Carnitine, APX, and Shoot FW showed negative correlations with all parameters studied (See Tables 1 and 2).
Discussion
Although there is limited information on the function of carnitine in plants, its effects on antioxidant enzyme activities, the nitrogen assimilation pathway, plant development, and recovery have been defined in maize and Arabidopsis under cold and salt stress (Charrier et al. 2012; Turk et al. 2020). Previous studies have demonstrated that growth impairment induced by salt and cold stresses is restored by exogenous carnitine treatment (Charrier et al. 2012; Turk et al. 2020). This alleviative effect might be related to carnitine’s osmolyte and antioxidative properties (Charrier et al. 2012). Maize plants treated with carnitine exhibited higher activities of antioxidant enzymes, such as guaiacol peroxidase, APX, CAT, GR, and SOD under cold stress (Turk et al. 2020). Moreover, carnitine treatment has been shown to induce lateral root development in Arabidopsis by antagonistic action toward ABA (Lelandais-Brière et al. 2007). This action of carnitine toward ABA could also explain the recovery of salt-stressed seedlings, as reported by Charrier et al. (2012). In addition, in the mentioned study, salt-stressed ABA-insensitive abi1-1 mutant and ABA-deficient aba1 mutant plants differentially responded to carnitine, indicating a plausible discrepancy in ABA signal transduction. Since the mutant (abi1-1), with a disruption in its ABA signaling upstream of ROS production (Murata et al. 2001), shows a remarkably low response toward carnitine (Charrier et al. 2012), we wondered whether such a disruption in ABA signaling downstream of ROS production would also affect carnitine responsiveness. Therefore, we used the ABA-insensitive abi2-1 mutant, which is defective in ABA signaling downstream of ROS production, in order to reveal how carnitine-modulates antioxidative defense under salt stress conditions and what is the role of ABI2 in the homeostasis of carnitine metabolism under salt stress. We evaluated abi2-1 mutant’s responses to carnitine for induction of salt tolerance and antioxidant enzyme activities that can be increased by carnitine in wild type (WT) plants. Evidences from the outlined studies suggests that carnitine triggers an ABI2-mediated signaling cascade, consequently initiating the process of salt tolerance in Arabidopsis.
We have demonstrated that ABA signaling orchestrates the induction of salt tolerance prompted by carnitine. In carnitine-treated wild-type plants, the development of salt tolerance occurred concurrently with the emergence of adaptive responses, pivotal for plant survival under environmental stress conditions. The reduction in relative water content (RWC) specifically correlates with diminished leaf turgor/water potential status (Ozfidan-Konakci et al. 2013; Guerfel et al. 2008) and the severity of cell membrane damage (Fig. 2) observed in control plants. The carnitine treatment to comparable plants enhances the leaf’s capacity to restore turgidity by facilitating water uptake prior to cellular disruption (Fig. 3). Previous studies have demonstrated that carnitine also behaves as a compatible solute (Charrier et al. 2012; Turk et al. 2020), decreases the osmotic potential within the cell to help to maintain adequate water absorption, stabilize membranes and/or macromolecular structures, and increase cell turgor pressure (Martin 1930; Bernstein 1961; Anjum et al. 2011). Charrier et al. (2012) reported the osmolyte property of carnitine, which might be the case in our study. Moreover, we found that the RWC decreased by salt-induced osmotic stress in abi2-1, similarly as in the wild type. However, the rate of decline was more remarkable in abi2-1 mutants. This may be related to unregulated stomatal control of the ABA-insensitive abi2-1 mutants due to disruption in ABA activation of the Ca2+ channel and H2O2-induced stomatal closure (Ohta et al. 2003; Trupkin et al. 2017; Murata et al. 2001). On the other hand, the ameliorative effect of carnitine application on the RWC of WT under salt stress is not observed in abi2-1 mutant. Thus, it is suggested that when ABI2 expression is hindered, carnitine loses its capacity to stabilize membranes, maintain adequate water absorption and initiate salt tolerance in the wild type (Fig. 2). Therefore, the responses of the ABA-insensitive abi2-1 mutant to carnitine might indicate the necessity of ABA perception.
Salt stress induces ion toxicity, oxidative stress, and osmotic stress, which limits plant development, biomass production, and survival (Lelandais-Brière et al. 2007). The inhibition of plant growth under salt stress has been reported in maize (Jiang et al. 2017), Plantago (Sekmen et al. 2007) species, and grapevines (Baby et al. 2016). Similar to these findings, in this study, a significant inhibition was observed in growth parameters, such as rosette diameter and shoot fresh weight of WT and abi2-1 exposed to salt stress. This inhibition of plant growth is generally associated with Na+ toxicity, reduced water and mineral uptake, and disturbances in cells resulting from salt stress (Isayenkov and Maathuis 2019). In this study, as compared to salt-stressed plants, increased growth parameters in carnitine-treated abi2-1 mutant and WT plants under salt stress indicates that carnitine treatment alleviates the growth inhibition and membrane lipid peroxidation induced by salt stress on elongation growth. Consistent with these findings, Charrier et al. (2012) reported a similar ameliorative effect of carnitine in abi1-1 and aba1-1 mutants of Arabidopsis thaliana, attributed to the role of carnitine in lipid metabolism, its osmolyte property, and the interaction between carnitine and the putative carnitine transporter AtOCT1 (Küfner and Koch 2008), which could regulate H+/Na+ exchange, allowing Na+ sequestration in the vacuole. Turk et al. (2020) showed that the stimulation of shoot growth in maize by carnitine treatment might be due to decreased oxidative damage under cold stress. Similarly, in this study, compared with carnitine-untreated plants, carnitine-treated plants showed much lower membrane damage, which alleviated the growth inhibition induced by salt stress, as also evidenced by decreased TBARS levels. Even in abi2-1 mutant plants, inhibition of endogenous carnitine content by salt stress increased membrane damage, while carnitine accumulation alleviated this damage (Figs. 1 and 3). These results suggest that alleviation of oxidative damage might be attributed to endogenous carnitine accumulation in abi2-1 mutant plants. We believe that carnitine may also play different roles in plant stress tolerance that might be mediated by the phosphatase ABI2.
H2O2, which is produced both under stress and normal conditions, plays a dual role in the cell. It can be a toxic molecule causing irreversible damage to the cell (Gechev et al. 2006; Tewari et al. 2006) or a secondary messenger regulating antioxidant defense (Gechev et al. 2002). Previous studies have demonstrated that H2O2 accumulation under stress conditions results in lipid peroxidation, which is an oxidative stress marker (Mittler 2002). In this study, in both genotypes, salt stress (without carnitine) induced a significantly greater increase in H2O2 accumulation. However, while exogenous application of carnitine prevented this H2O2 accumulation in both genotypes under salt stress, it reversed harmful effect of H2O2 as evidenced by the decreased lipid peroxidation level. Charrier et al. (2012) indicated that carnitine prevents H2O2-induced oxidative stress through its antioxidant and/or osmotic properties. It has also been reported that L-carnitine (active form of carnitine) and propionyl carnitine can detoxify H2O2 and superoxide anions within cells, inhibiting lipid peroxidation activities in vitro (Vanella et al. 2000; Gülçin 2006). The heightened accumulation of H2O2 in carnitine-treated abi2-1 plants, compared to WT, accentuates the predominant involvement of ABI2 in the induction of salt tolerance.
NOX, the main ROS producer during oxidative signaling, plays essential roles in signal transduction and perception in Arabidopsis (Gechev et al. 2006; Tewari et al. 2006). Jin et al. (2019) and Zhou et al. (2014) suggested that H2O2, which is produced by NOX in the apoplast, may act as a signaling molecule to trigger the antioxidant system to cope with salt stress-induced excessive ROS accumulation and membrane damage. In our study, the increased H2O2 levels observed solely in wild-type plants under salt stress may be attributed to the elevation in NOX activity. Furthermore, the augmented NOX activity observed in the same plants following carnitine application demonstrates the inductive effect of carnitine in NOX activity. Given that, carnitine fails to induce NOX activity in the absence of ABI2. The regulatory role of ABI2 in NOX activity might constitute one of the mechanisms behind the elevated H2O2 levels, particularly in wild-type plants under salt stress. Consequently, carnitine application could modulate NOX activity through ABI2, thereby impacting the levels of reactive oxygen species (such as H2O2) in plants.
Under salt stress, to cope with the excess accumulation of ROS, plants have developed a complex antioxidant defense system involving the antioxidant enzymes CAT, APX, POX, DHAR, MDHAR, GR, and SOD. Of these, DHAR, MDHAR, APX and GR comprise the ascorbate–glutathione cycle (also called the Asada–Halliwell pathway). SOD, which is found in different compartments, such as the chloroplast (Fe-SOD, Cu/Zn-SOD), mitochondria (Mn-SOD), cytosol (Cu/Zn-SOD, ) and peroxisomes (Mn-SOD), converts superoxides to H2O2. APX, POX, CWPOX, and CAT decompose H2O2 into molecular oxygen and H2O (Demiral et al. 2011). Enhancement of antioxidant enzyme activities has also been reported in different plants under salt stress (Gill and Tuteja 2010). Similar to previous studies, in this study, the activities of GR, CAT, CWPOX, APX, DHAR, MDHAR, and SOD increased in WT and abi2-1 subjected to salt stress. However, these increased activities did not prevent salt-stress-induced-cell membrane damage in both genotypes, as evidenced by increased lipid peroxidation. Under salt stress, carnitine treatment increased the activities of SOD, CAT, and CWPOX to a higher extent but not POX in wild type plants. The observed increase in carnitine-enhanced SOD activity in both carnitine- and salt + carnitine-treated groups, compared with the control group in wild type plants, may be attributed to carnitine’s role in facilitating the transport of acetyl-CoA to fatty acids for β-oxidation in mitochondria and its involvement in the tricarboxylic acid (TCA) cycle, as previously reported (Bourdin et al. 2007). Carnitine-treated abi2-1 plants also exhibited responses similar to those of wild-type plants. That is, ABA insensitivity in abi2-1 did not alter the SOD activity under salt stress following carnitine application. During ROS production process, H2O2 radicals can form in peroxisomes by β-oxidation, and superoxide (O2.−) radicals can be produced in mitochondria by respiration. The enzymes responsible for removing these radicals are Mn-SOD in mitochondria and CAT in peroxisomes. Although Cu/Zn SOD activity was determined as the highest in the salt-treated and salt + carnitine groups of both genotypes, in abi2-1 mutant plants, the Mn-SOD intensity was higher than that in WT plants. These results show that this increase in the Mn-SOD intensity in abi2-1 may be due to the formation of stress-induced superoxide radicals in mitochondrial ETC (Jithesh et al. 2006). On the other hand, ABA is known to induce isozymes, such as Cu/Zn-SOD, Fe-SOD, and Mn-SOD (Kaminaka et al. 1999), and may even down-regulate ROS-scavenging enzymes, such as SOD.
In this study, although abi2-1 is ABA insensitive, total SOD activity can still increase in plants, which might be induced by carnitine. With the comparison of the groups subjected only to salt stress, application of carnitine to abi2-1 mutant plants under salt stress resulted in the highest increase in activities of POX and CWPOX only, among all other antioxidant enzymes. Therefore, it is reasonable to conclude that carnitine protects abi2-1 mutant plants from oxidative-stress-induced H2O2 accumulation by maintaining higher activities of antioxidant enzymes (SOD, POX and CWPOX). In addition, abi2-1 mutant plants appeared more responsive to carnitine treatment than the WT plants when we compared the rates of increment in POX and CWPOX activities between the salt-treated and salt + carnitine groups for both genotypes. On the other hand, the pronounced inductive and reductive effects of ABI2 deficiency on CAT, POX, and CWPOX activities under normal conditions may indicate the sensitivities of these enzymes to ABI2. Moreover, when we compared the rates of increment in CWPOX and POX activities between the salt-treated and salt + carnitine-treated groups for both genotypes, it was observed that abi2-1 mutant plants were more responsive to carnitine treatment compared to WT plants. In this study, contrary to CWPOX and POX, CAT did not change in carnitine-treated abi2-1 mutant plants under salt stress compared with abi2-1 mutant plants solely under salt stress. These results show that carnitine does not have any effect on CAT activity in abi2-1 mutant under salt stress.
Among the ascorbate (ASC)–glutathione (GSH) cycle enzymes, APX is a very substantial H2O2 scavenger in plants, and environmental stresses enhance APX activity due to stress-related ROS production (Miller et al. 2010; Asada 2006). In this study, salt stress resulted in a significant increase in APX activity in both genotypes. Salt-stress-mediated induction of GR, MDHAR, and DHAR activities and increased GSH synthesis also support the ROS-scavenging capacity of APX in both genotypes under salt stress. However, carnitine treatment in wild-type plants under salt stress reduced the activities of ASC-GSH cycle enzymes (except GR) compared to the salt-treated group. Interestingly, impairment of phosphatase ABI2 function did not alter the inhibitory effect of carnitine on these enzymes’ activities in abi2-1 mutant plants, the protective effect of carnitine might be attributed to its own antioxidant property or increased activities of SOD, CWPOX and POX rather than its effect on ASC–GSH cycle enzymes. This was further supported by the maintenance of GSH/GSSG ratios in abi2-1 mutant plants treated with salt plus carnitine closer to that in the control group, where salt-treated plants had more oxidized glutathione pools. Evidence presented in our study indicates that the importance of ABI2 in the homeostasis of carnitine metabolism in plants.
The Principal Component Analysis (PCA) (as shown in Fig. 10) elucidated the relationship between various independent and response variables. PCA revealed negative correlations between TBARS content used as an indicator of lipid peroxidation and carnitine content, as well as APX and MDHAR activities in abi2-1. This suggests that Carnitine could enhance salt stress tolerance by increasing carnitine content and APX and MDHAR activities in abi2-1.
Conclusions
The results of this study indicate that A. thaliana wild type plants do benefit from 5 µM carnitine to improve their salt stress tolerance. The external application of carnitine also mitigates the effects of salt stress in WT by reducing H2O2 accumulation and lipid peroxidation levels. Alongside these benefits, carnitine also enhances the antioxidant activities of enzymes CAT, SOD and NOX. Conversely, abi2-1 mutants that are exposed to 80 mM NaCl stress exhibited higher sensitivity to salt stress compared to WT plants. As an addition to that outcome, abi2-1 mutants appear to be more responsive to carnitine, proven by the ratios of change in NOX, APX, MDHAR and DHAR (decreased), and POX, CWPOX, GR (increased) which have been compared within both WT and abi2-1 mutant genotypes treated with both salt and carnitine. The data regarding the antioxidant enzymes suggest that ABI2 may have a significant impact on CWPOX, POX, NOX, CAT, GR, and DHAR. Interestingly, our findings reveal that carnitine did not influence SOD activity in abi2-1 mutants under salt stress in comparison to WT plants. These findings imply that NOX-induced H2O2 production in carnitine-treated plants, might stimulate the antioxidant system by mediating ABI2 via H2O2 acting as a signaling molecule. The escalated levels of carnitine due to exogenous treatment could potentially contribute to the salt tolerance observed in abi2-1 mutants through another undefined mechanism. Therefore, conducting detailed experiments and analyses is crucial to comprehend the mechanisms and roles of carnitine in plants that lead to enhanced salt stress tolerance. Lastly, considering its significance in plants, we address the necessity for more comprehensive studies on carnitine.
References
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea(L.) Czern.& Coss.] Plants can be alleviated by salicylic acid. South Afr J Bot 77:36–44
Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6(9):2026–2032. https://doi.org/10.5897/AJAR10.027
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Arrigoni O, Dipierro S, Borraccino G (1981) Ascorbate free-radical reductase, a key enzyme of the ascorbic-acid system. FEBS Lett 125:242–244
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Env Exp Bot 59(2):206–216
Augustyniak A, Skrzydlewska E (2009) L-carnitine in the lipid and protein protection against ethanol-induced oxidative stress. Alcohol 43:217–223
Baby T, Collins C, Tyerman SD, Gilliham M (2016) Salinity negatively affects pollen tube growth and fruit set in grapevines and is not mitigated by silicon. Am J Enol Vitic 67:218–228
Bergmeyer HU (1970) Measurement of catalase activity. Biochem Z 327:255–258
Bernstein L (1961) Osmotic adjustment of plants to saline media. I. steady state. Am J Bot 48:909–918
Beuchamp C, Fridovich I (1971) Superoxide dismutase. Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beuchamp C, Fridovich I (1973) Isoenzymes of superoxide dismutase from wheat germ. Biochim Biophys Acta 317:50–64
Bourdin B, Adenier H, Perrin Y (2007) Carnitine is associated with fatty acid metabolism in plants. Plant Physiol Biochem 45:926–931
Bradford MMA (1976) Rapid and sensitive method for the quantization of microgram quan-tities of protein utilizing the principle of the protein–dye binding. Anal Biochem 72:248–254
Charrier A, Rippa S, Yu A, Nguyen PJ, Renou JP, Perrin Y (2012) The effect of carnitine on Arabidopsis development and recovery in salt stress conditions. Planta 235:123–135
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural condi-tions. J Exp Bot 57:2435–2444
Chen J, Yu F, Liu Y, Du C, Li X, Zhu S, Wang X, Lan W, Rodriguez PL, Liu X, Li D, Chen L, Luan S (2016) FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. PNAS 113(37):E5519–E5527
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Demiral T, Turkan I, Sekmen AH (2011) Signalling strategies during drought and salinity recent news. Adv Bot Res 57:293–317. https://doi.org/10.1016/B978-0-12-387692-8.00008-4
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Fraenkel G (1953) Studies on the distribution of vitamin BT (carnitine). Biol Bull 104:359–371
Franken J, Kroppenstedt S, Swiegers JH, Bauer FF (2008) Carnitine and carnitine acetyltransferases in the yeast Saccharomyces cerevisiae: a role for carnitine in stress protection. Curr Genet 53:347–360
Gechev TS, Gadjev I, Van Breusegem F, Inzé D, Dukiandjiev S, Toneva V, Minkov I (2002) Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci 59:708–714
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill SS, Khan NA, Anjum NA, Tuteja N (2011) Amelioration of cadmium stress in crop plants by nutrients management: morphological, physiological and biochemical aspects. Plant Stress 5:1–23
Gómez-Amores L, Mate A, Miguel-Carrasco JL, Jiménez L, Jos Á, Cameán AM, Revilla E, Santa-María CM, Vázquez C (2007) l-Carnitine attenuates oxidative stress in hypertensive rats. J Nutr Biochem 18:533–540
Guerfel M, Baccouri O, Boujnah D, Zarrouk M (2008) Changes in lipid composition, water relations and gas exchange in leaves of two young ‘Chemlali’ and ‘Chetoui’ olive trees in response to water stress. Plant Soil 311(1):121–129
Gülçin I (2006) Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803–811
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci 97(7):3735–3740
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Herzog V, Fahimi H (1973) Determination of the activity of peroxidase. Anal Biochem 55:554–562
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24(16):1695–1708
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jiang M, Zhang J (2002) Involvement of plasma membrane NADPH oxidase in abscisic acid-and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039
Jin X, Liu T, Xu J, Gao Z, Hu X (2019) Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol 19:48
Jithesh MN, Prashanth SR, Sıvaprakash KR, AJAY KP (2006) Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet 85(3):237–254
Kalaiselvia T, Panneerselvam C (1998) Effect of l-carnitine on the status of lipid peroxidation and antioxidants in aging rats. J Nutr Biochem 9:575–581
Kaminaka H, Morita S, Tokumoto M, Masumura T, Tanaka K (1999) Differential gene expressions of rice superoxide dismutase isoforms to oxidative and environmental stresses. Free Radic Res 31(1):219–225
Küfner I, Koch W (2008) Stress regulated members of the plant organic cation transporter family are localized to the vacuolar membrane. BMC Res Notes 1:1–10
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lelandais-Brière C, Jovanovic M, Torres GAM, Perrin Y, Lemoine R, Corre-Menguy F, Hartmann C (2007) Disruption of AtOCT1, an organic cation transporter gene, aVects root development and carnitine-related responses in Arabidopsis. Plant J 51:154–164
Madhava Rao KV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L, Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Martin JH (1930) The comparative drought resistance of sorghums and corn. Agron J 22:993–1003
Masterson C, Wood C, Thomas DR (1990) L-acetylcarnitine, a substrate for chloroplast fatty-acid synthesis. Plant Cell Environ 13:755–765
McAinsh MR, Brownlee C, Hetherington AM (1990) Abscisic acid–induced elevation of guard cell cytoplasmic Ca2+ precedes stomatal closure. Nature 343:186–188
Mclaren I, Wood C, Jalil MNH, Yong BCS, Thomas DR (1985) Carnitine acyltransferases in chloroplasts of Pisum sativum L. Planta 163:197–200
Miller G, Suzukı N, Cıftcı-Yılmaz S, Mıttler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane ca+ 2 channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2 C mutants. Plant Cell 13:2513–2523
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nguyen PJ, Rippa S, Rossez Y, Perrin Y (2016) Acylcarnitines participate in developmental processes associated to lipid metabolism in plants. Planta 243:1011–1022
Ohta M, Guo Y, Halfter U, Zhu JK (2003) A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Nati Acad Sci 100:11771–11776
Ozfidan-Konakci C, Turkan I, Sekmen AH, Seckin Dinler B (2013) Time course analysis of ABA and non-ionic osmotic stress-induced changes in water status, chlorophyll fluorescence and osmotic adjustment in Arabidopsis thaliana wild-type (Columbia) and ABA-deficient mutant (aba2). Environ Exp Bot 86:44–51
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731–734
Peluso G, Barbarisi A, Savica V, Reda E, Nicolai R, Benatti P, Calvani M (2000) Carnitine: an osmolyte that plays a metabolic role. J Cell Biochem 80:1–10
Queval G, Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Anal Biochem 363(1):58–69
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15(7):395–401
Reznick AZ, Kagan VE, Ramsey R, Tsuchiya M, Khwaja S, Serbinova EA, Packer L (1992) Antiradical effects in l-propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch Biochem Biophys 2:394–401
Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338:427–430
Sekmen AH, Turkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Plant 131:399–411
Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H + antiporter. Proc Natl Acad Sci 97(12):6896–6901
Tamai I, China K, Sai Y, Kobayashi D, Nezu J, Kawahara E, Tsuji A (2001) Na+-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim Biophys Acta 1512:273–284
Tewari RK, Kumar P, Sharma PN (2006) Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 223:1145–1153
Trupkin SA, Auge GA, Zhu JK, Sánchez RA, Botto JF (2017) Salt overly sensıtıve 2 (sos2) and interactıng partners SOS3 and Abscısıc acıd–insensıtıve 2 (abi2) promote red-light-dependent germınatıon and seedlıng deetıolatıon in Arabidopsis. Int J Plant Sci 178(6):485–493
Turk H, Erdal S, Dumlupinar R (2020) Carnitine-induced physio-biochemical and molecular alterations in maize seedlings in response to cold stress. Arch Agron Soil Sci 66:925–941
Vanella A, Russo A, Acquaviva R, Campisi A, Di Giacomo C, Sorrenti V, Barcellona ML (2000) L -propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol 16(2):99–104
Verslues P, Bray E (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57(1):201–212
Wood C, Masterson C, Thomas DR (1992) Carnitine-dependent fatty acyl transport in plant cells. In: Lambers H, Van Der Plas HLW (eds) Molecular, biochemical and physiological aspects of plant respiration. Balogh Scientific Books, Campaign, pp 195–207
Zhou J, Xia XJ, Zhou YH, Shi K, Chen ZX, Yu JQ (2014) RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J Exp Bot 65(2):595–607
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124(3):941–948
Acknowledgements
This work was supported by the Ege University Research Foundation (2014-FEN-010).
Funding
This work was financially supported by the Ege University Research Foundation (2014-FEN-010).
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gokce, A., Sekmen Cetinel, A.H. & Turkan, I. Carnitine modulates antioxidative defense in ABI2 mutant under salt stress. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01169-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01169-3