Abstract
Background
Abscisic acid (ABA) plays an important role in plant abiotic stress responses, and ABA INSENSITIVE 4 (ABI4) is a pivotal transcription factor in the ABA signaling pathway. In Arabidopsis, ABI4 negatively regulates salt tolerance; however, the mechanism through which ABI4 regulates plant salt tolerance is poorly understood. Our previous study showed that ABI4 directly binds to the promoter of the VITAMIN C DEFECTIVE 2 (VTC2) gene, inhibiting the transcription of VTC2 and ascorbic acid (AsA) biosynthesis.
Results
In the present study, we found that treatment with exogenous AsA could alleviate salt stress sensitivity of ABI4-overexpressing transgenic plants. The decreased AsA content and increased reactive oxygen species (ROS) levels in ABI4-overexpressing seedlings under salt treatment indicated that AsA-promoted ROS scavenging was related to ABI4-mediated salt tolerance. Gene expression analysis showed that ABI4 was induced at the early stage of salt stress, giving rise to reduced VTC2 expression. Accordingly, the abundance of the VTC2 protein decreased under the same salt stress conditions, and was absent in the ABI4 loss-of-function mutants, suggesting that the transcriptional inhibition of ABI4 on VTC2 resulted in the attenuation of VTC2 function. In addition, other encoding genes in the AsA biosynthesis and recycling pathways showed different responses to salt stress, demonstrating that AsA homeostasis is complicated under salinity stress.
Conclusions
This study elucidates the negative modulation of ABI4 in salt stress tolerance through the regulation of AsA biosynthesis and ROS accumulation in plants.
Similar content being viewed by others
Background
Ascorbic acid (AsA) plays an important role in plant growth and development [1, 2]. It is an efficient non-enzymatic antioxidant that scavenges reactive oxygen species (ROS), and not only regulates growth and development, but also modulates stress responses [3,4,5,6]. The biosynthesis of AsA and its regulatory mechanisms in plants have garnered increasing attention [7,8,9,10]. Its biosynthesis in plant leaves is regulated by light and dark [11], and it shows a circadian rhythm and responds to seasonal changes [12, 13]. The AsA content is also affected by temperature [14]. In addition, the transcriptional expression of the genes involved in AsA biosynthesis are regulated by phytohormones or secondary metabolites [15]. The L-galactose pathway is the dominant pathway of AsA biosynthesis in Arabidopsis. The homologous genes VITAMIN DEFECTIVE 2 (VTC2) and VTC5 encode the key GDP-L-galactose phosphorylase in this pathway, and VTC2 plays a leading role [16,17,18]. Jasmonic acids (JAs) promote AsA biosynthesis through inducing the expression of VTC2 [15, 19]. In addition to the de novo synthesis of AsA, AsA recycling also affects the AsA level [17, 20].
Salt stress limits plant growth and development, and plants have evolved a variety of adaptive mechanisms to deal with it. A large amount of ROS are produced in cells under salinity stress, which makes the antioxidant capacity of AsA more important [6, 21,22,23]. Previous research found that the content of H2O2 in the AsA deficient mutant vtc1 significantly increased under salt stress [24]. The zinc-finger protein SlZF3 promoted the accumulation of AsA and enhanced plant salt stress tolerance [25]. Many studies have also reported that the exogenous supply of AsA can improve the resistance to salt stress in various plants such as corn, rice, and wheat [26,27,28], indicating that AsA has a positive role in salt tolerance in plants.
Abscisic acid (ABA) is known as the plant stress hormone [29, 30]. High salinity and drought dramatically increase ABA levels, which in turn induce the expression of many genes involved in stress responses [31]. Abscisic acid INSENSITIVE 4 (ABI4) functions as an important transcription factor downstream of the ABA signaling pathway [32]. The mutant abi4 was first isolated from a screening for ABA-insensitive mutants during seed germination [33], and ABI4 has a higher transcript expression in seeds, but a lower expression at the seedling stage [34]. The ABA-deficient mutants aba1, aba2, and aba3 show a readily-wilting phenotype under salt or drought stress, but the abi4 mutant exhibits salt stress resistance. Plants overexpressing ABI4 had increased salt sensitivity, because ABI4 down-regulated the Na+ transporter HKT1;1 expression, indicating that a plant’s salt tolerance is related to its ability to reduce sodium accumulation in the aerial parts [35, 36]. The chloroplast development gene AtDPG1 is involved in the salt stress response through ABI4 [37]. Therefore, ABI4 contributes to salt stress responses; however, the mechanism by which ABI4 regulates salt tolerance still needs further research.
We previously showed that ABI4 directly binds to the promoter of VTC2, inhibiting the transcription expression of VTC2, and then alleviating AsA biosynthesis [10]. ABI4 directly combines the key genes involved in ROS production and scavenging to modulate ROS metabolism during seed germination under salinity stress [38]. We found that AsA partially recovered the salt stress sensitivity of ABI4-overexpressing plants, which had lower AsA content and more ROS accumulation. Salt stress initially inhibited the expression of VTC2 via promoting ABI4 expression. Thus, ABI4-VTC2 coordinately regulates the biosynthesis of AsA under salt stress. It was revealed that the molecular mechanism of the ABI4 response to salt stress was through the regulation of AsA biosynthesis and ROS accumulation in Arabidopsis.
Results
Ascorbic acid contributes to ABI4-regulated salt stress sensitivity
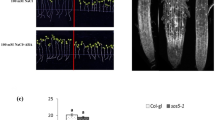
Ascorbic acid has an important effect on the scavenging of the accumulated ROS under salt stress to enhance the tolerance of plants [6]. It was demonstrated that abi4 mutants exhibit increased salt stress resistance [35]. We previously showed that ABI4 inhibits AsA biosynthesis [10], so we further analyzed the role of AsA in the ABI4-mediated response to salt stress by supplying exogenous AsA. 3-day-old seedlings of Col-0, two recessive knockout alleles of ABI4 (abi4–102 and abi4–103) and two ABI4-overexpressing lines with C-terminal truncated peptide lines (OEM1 and OEM5) [39] were transferred to 1/2 MS medium containing 40 μmol AsA only or 100 mM NaCl supplied with or without AsA. The root length of the seedlings was observed after they were cultured for another four days. The different genotypes exhibited a little increased root length with AsA treatment only in abi4–103 and different root length reductions under the salt stress treatment (Fig. 1a). The statistical analysis indicated that, compared to Col-0 under normal growth conditions, the root lengths of the abi4 mutants were shorter. The root length of abi4–103 and abi4–102 were less inhibited under salt stress compared with Col-0, while ABI4-OEM1 and ABI4-OEM5 displayed the same trend as the Col-0 seedlings. Additionally, supplementation with AsA under salt stress partially recovered the root length in Col-0 and ABI4-OEM, but this effect was absent in the abi4 mutants, possibly due to low concentrations of exogenous AsA (Fig. 1b and c). These results demonstrated that AsA could partially recover the ABI4-mediated salt inhibition on root growth.
Ascorbic acid (AsA) alleviates the inhibitory effect of salt stress on root growth in ABI4-overexpressing seedling lines. a Seedling growth phenotype of the Col-0, abi4–102, abi4–103 and ABI4-overexpressing lines under 100 mM NaCl treatment with or without 40 μM AsA. 3-day-old seedlings were transferred to 1/2 MS medium containing salt with or without AsA and grown for another four days. Bar = 1.4 cm. b The root length of Col-0, abi4–102, abi4–103 and ABI4-overexpressing lines under 100 mM NaCl treatment with or without 40 μM AsA. Values are means ± SD (n = 30). c The relative root length of Col-0, abi4–102, abi4–103 and ABI4-overexpressing lines under 100 mM NaCl treatment with or without 40 μM AsA compared with those under control conditions. Values are means ± SD n = 3. The root length of all seedlings under control conditions were normalized as 100. Different letters indicate statistically significant differences among the indicated data (P < 0.05, ANOVA with Tukey’s test)
High salinity stress causes leaves to whiten and even die in Arabidopsis [21] We observed the seedling survival rate under 150 mM NaCl with or without supplementation of AsA. The occurrence of albinism in the abi4 mutant seedlings was reduced, while it was higher in the OEM transgenic lines under the 150 mM NaCl treatment (Fig. 2a). Supplementation with AsA significantly enhanced the tolerance of all genotypes to salt stress. The survival rates of OEM1 and OEM5 were about 18 and 42% under NaCl treatment, respectively, while the survival rates increased up to about 75 and 69% with exogenous AsA, which were significantly improved than the Col-0 seedlings (Fig. 2b). Supplementation with AsA also improved the survival rate of the abi4 mutants to high salinity stress. These results indicated that the ABI4-inhibited AsA synthesis mediated the salt stress sensitivity of the abi4 mutants and ABI4-OEM seedlings.
Ascorbic acid (AsA) improves the high salinity tolerance of ABI4-overexpressing lines. a The survival phenotype of different ABI4 genotypes after 150 mM NaCl treatment (with or without 40 μM AsA). 3-day-old seedlings were transferred to 1/2 MS medium containing salt with or without exogenous AsA and grown for another four days. Bar =1.4 cm. b The survival rate of different ABI4 genotypes after 150 mM NaCl treatment (with or without 40 μM AsA). Values are means ± SD (n = 3). Statistically significant differences are indicated by different letters (P < 0.05, ANOVA with Tukey’s test)
ABI4 regulates ascorbic acid biosynthesis and reactive oxygen species scavenging under salt stress
Ascorbic acid plays an important role in scavenging ROS, which significantly improves plants’ tolerance to stress [40,41,42]. We previously found that abi4 accumulated less ROS through ABI4 negatively regulating AsA synthesis [10]. We measured the AsA and ROS contents in 7-day-old seedlings under salt stress in the current study. The results showed that the AsA contents under the salt treatment were higher in the abi4–103 mutants and lower in the ABI4-OEM1 and ABI4-OEM5 seedlings than in Col-0 (Fig. 3a). We then compared the ROS contents in the ABI4 knockout mutants and the OEM transgenic plants under normal conditions and the NaCl treatment with or without AsA. Based on staining with diaminobenzidine (DAB) or nitroblue tetrazolium (NBT), H2O2 and O2− accumulated more in the leaves of OEM1 and OEM5 than Col-0 and abi4 mutants under both conditions. In contrast, the H2O2 and O2− contents in the abi4–103 leaves were significantly lower than in the Col-0 plants (Fig. 3b). The quantitative analysis showed that the level of H2O2 increased significantly with the salt stress treatment in all genotypes except the abi4 mutants, and the ABI4-OEM seedlings accumulated more H2O2 than the Col-0 under the salt stress treatment (Fig. 3c). The content of O2− was lower in the abi4–103 mutants than that in the Col-0 and ABI4-OEM plants under normal and salt stress conditions, and with the addition of exogenous AsA under salt stress treatment, O2− accumulation was obviously decreased in the ABI4-OEM plants (Fig. 3d). These results indicated that the AsA levels in ABI4 mutants and overexpressing plants contribute to the ROS accumulations under the salt stress treatment.
ABI4 confers to ascorbic acid (AsA) biosynthesis and reactive oxygen species (ROS) accumulation under salt stress. a AsA contents in Col-0, abi4–102, abi4–103 and ABI4-overexpressing lines under the 100 mM NaCl treatment. b Diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining of ROS in Col-0, abi4–102, abi4–103 and ABI4-overexpressing seedlings with or without the salt treatment. Measurements of c H2O2 and d O2− in Col-0, abi4–102, abi4–103 and ABI4-overexpressing lines with or without the salt treatment. 7-day-old seedlings were treated with 100 mM NaCl (with or without 40 μM AsA) for 24 h. Values in a, c and d are means ± SD (n = 3). Statistically significant differences are indicated by different letters (P < 0.05, ANOVA with Tukey’s test). FW, fresh weight
Salt stress negatively regulates VTC2 expression through inducing ABI4 expression
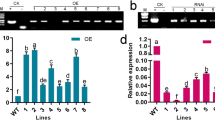
It was demonstrated that salt stress induced ABI4 expression in Arabidopsis seedling shoots [36]. Our previous research indicated that ABI4 inhibits VTC2 expression [10]. Therefore, it is necessary to further detect the gene expression of VTC2 under salt stress. The expression of ABI4 was quickly and significantly induced with 2 h until to 24 h of salt treatment, as measured by a quantitative polymerase chain reaction (qPCR) analysis (Fig. 4a). The VTC2 expression was obviously inhibited at the beginning of 4 h of salt stress in Col-0 (Fig. 4b), while the inhibition was impaired in abi4–103 mutants (Fig. 4c) and slightly increased in ABI4-OEM1 (Fig. 4d), suggesting that the inhibited VTC2 transcription at the early stage of salt stress is partially ABI4 dependent.
Effect of salt stress on the expression of ABI4 and VTC2. The expressions of ABI4 in Col-0 a, VTC2 in Col-0 b, VTC2 in abi4–103 c, VTC2 in ABI4-OEM1 d seedlings under 200 mM NaCl were analyzed by qPCR. After normalizing to the internal control ACTIN, the transcript levels of ABI4 and VTC2 at the indicated treatment times were compared with that at 0 h, which was normalized as “1”. Error bars represent the SD from three biological replicates. Statistically significant differences are indicated by star symbol (P < 0.05, Mann-Whitney U test). e Comparison of VTC2 protein levels in Col-0 and abi4 seedlings. The numbers under the bands indicate the relative light intensity of bands, the detected band in pVTC2::VTC2-GFP/Col-0 without NaCl treatment was normalized as “1”. Total proteins were extracted from pVTC2::VTC2-GFP/Col-0 and pVTC2::VTC2-GFP/abi4–103 seedlings under 100 mM NaCl treatment for 24 h and detected using anti-GFP antibodies. The detection of ACTIN was used as loading control
The VTC2 protein levels in the Col-0 and abi4 mutants under the salt stress treatment were analyzed to verify the downregulated VTC2 expression related to the enzymatic function of VTC2. We detected the VTC2-GFP fusion protein driven by the VTC2 promoter in the background of the Col-0 and abi4–103 mutant treated with or without NaCl by using anti-GFP antibodies. We found that the VTC2-GFP protein accumulated more in abi4–103 than in Col-0, which was decreased by salt stress in the Col-0 background, but less change occurred in the abi4–103 mutant background (Fig. 4a). These results implied that the ABI4 transcriptional suppression of VTC2 conferred to decreased AsA contents and accumulated ROS at an early stage of salt stress.
We also detected the expression of other genes involved in AsA synthesis and recycling under salt stress. The transcription levels of VTC1 and VTC5 significantly increased with 1 h of salt treatment (Fig. 5a and c). The other key genes involved in the L-gal pathway of AsA synthesis were downregulated by salt (Fig. 5b, d, and e). In the first 24 h of salt stress, the genes encoding ascorbic acid peroxidase (APX) were upregulated by salt stress except for APX3 and APX4 (Fig. 5F-5K). The results showed that the decreased AsA contents were related to AsA biosynthesis and recycling at the early stages of salt stress.
Effect of salt stress on the expression of genes involved in the L-gal pathway of ascorbic acid (AsA) synthesis and encoding ascorbic acid peroxidase (APX). The gene expressions of VTC1, VTC4, VTC5, GaIDH, GLDH, APX1, APX2, APX3, APX4, APX5, and APX6 in Col-0 seedlings under 200 mM NaCl were analyzed by qPCR. After normalizing to the internal control ACTIN, the transcript levels at indicated treatment times were compared with that at 0 h, which was normalized as “1”. Error bars represent the SD from three biological replicates. Statistically significant differences are indicated by star symbol (P < 0.05, Mann-Whitney U test). 7-day-old seedlings were treated with 200 mM NaCl for 24 h
ABI4 modulates salt tolerance in coordination with VTC2
According to the ABI4 and VTC2 gene expression responses to salt stress, we measured the salt tolerance of abi4 vtc2 double mutants to confirm their coordinative work. The double mutants displayed shorter root length compared to Col-0 under normal conditions, and the root growth inhibition in abi4 vtc2 mutants was more than that in abi4–103 and less than that in vtc2 under the salt treatment (Fig. 6a, b, and c). Further statistical analysis indicated that the root lengths of the abi4–103, vtc2 and abi4 vtc2 mutants were same under NaCl treatment, which is recovered partially with the addition of exogenous AsA (Fig. 6b). The AsA contents in the vtc2 and abi4 vtc2 mutants under the salt stress treatment were much less than in Col-0 (Fig. 6d), and the ROS accumulation in abi4 vtc2 was higher than in abi4 under salt stress (Fig. 6e and f). These results demonstrated that VTC2 was downstream of ABI4 in modulating AsA biosynthesis and ROS accumulation under salinity stress.
VTC2 functions downstream of ABI4 in modulating seedling growth under salt stress through modifying ascorbic acid (AsA) biosynthesis and reactive oxygen species accumulation. a Seedling growth phenotype of abi4–103, vtc2 and abi4 vtc2 mutants under 100 mM NaCl treatment with or without 40 μM AsA. Bar = 1.4 cm. b Root length of abi4–103, vtc2 and abi4 vtc2 mutants in (A). c Relative values of root length in (B). The root length of Col-0 and all mutants under normal growth conditions was normalized as “1”. d Contents of AsA in Col-0, abi4–103, vtc2 and abi4 vtc2 under the salt stress treatment. e H2O2 and f O2− in Col-0, abi4–103, vtc2 and abi4 vtc2 under the salt stress treatment with or without 40 μM AsA. 7-day-old seedlings were treated with 100 mM NaCl (with or without 40 μM AsA) for 24 h. Values are means ± SD (n = 3). Statistically significant differences are indicated by different letters (P < 0.05, ANOVA with Tukey’s test). FW, fresh weight
Discussion
It was reported that ABI4 regulated plant salt tolerance and the abi4 mutant exhibited salt stress resistance, which was related to less sodium accumulation in plant shoots [35]. However, based on our previous study showing that ABI4 mediates the cross-talk of ethylene and ABA in AsA biosynthesis [10], it is unclear whether increased AsA contents confer to the enhanced salt tolerance of the abi4 mutants. Salinity damage to plants is caused in part by salt-induced ROS [43]. Whether the mechanism of how ABI4 regulates plant salt tolerance is related to oxidative stress has not been reported. In the current study, we uncovered the modulation of ABI4-mediated AsA biosynthesis in the salt tolerance of Arabidopsis. Salt stress-induced ABI4 expression caused decreased VTC2 transcription, leading to a reduction of AsA biosynthesis and increased ROS contents during the early stage of stress, which gave rise to retarded seedling growth. Therefore, AsA contributed to the ABI4-mediated salt stress sensitivity.
The accumulated ROS in plants has an important effect on plant growth and development [6, 44, 45]. If the ROS produced during the early stage of stress is not scavenged in time, it will cause severe cell damage. Studies have shown that reduced ROS scavenging ability can significantly impair the tolerance of plants to salt stress [46]. The abi4–103 mutants accumulated more AsA in vivo, which can reduce the damage of ROS, while the ABI4-OEM plants were more sensitive to salt due to decreased AsA contents. Further evidence of ABI4 regulating AsA in scavenging ROS and contributing to salt tolerance was observed in the recovery of salt tolerance through the addition of AsA.
A key enzyme in the AsA synthesis pathway in plants is VTC2, and its activity has an important effect on AsA synthesis [9, 47]. The activity of VTC2 in vivo can be regulated in transcription and post-transcription levels [16]. Combining the suppressed VTC2 expression and decreased VTC2 protein level at the early stage of salt stress, the abundance of VTC2 protein was more in the abi4 mutants than in Col-0, suggesting that the enzymatic activity of VTC2 was enhanced in abi4 mutants and helped to improve AsA biosynthesis under salinity stress. The contents of AsA and ROS in the abi4 mutants were in accordance with its salt tolerance, demonstrating that the modulation of ABI4 in AsA biosynthesis played an important role in salt stress responses. It makes sense that AsA biosynthesis was downregulated during the early stage of salt stress, which gave rise to ROS accumulation and retarded the growth of the seedlings. With the accumulation of ROS under salinity stress, the genes encoding the last two steps of AsA biosynthesis, GalDH and GLDH, were induced at 24 h of the salt stress treatment to promote AsA biosynthesis and scavenge ROS. Thus, the enzymes involved in AsA biosynthesis may have complicated regulation modes in response to salinity stress.
Meanwhile, we also detected the transcription levels of the APX genes in the AsA recycling pathway, which are pivotal in keeping ROS homeostasis [48]. APXs in Arabidopsis are classified on the basis of their sub-cellular localization, three cytosolic (APX1, APX2, APX6), three microsomal (APX3, APX4, APX5) and two chloroplastic types (stromal sAPX, thylakoid tAPX) isoforms [49]. In this study, most APX genes had no significant changes in the first 2 h of salt stress except for APX2. The genes APX1, APX2, APX5, and APX6 were induced at 12 h or 24 h of the salt stress treatment, while APX3 and APX4 were downregulated at 24 h of the treatment, which suggested that the reduced AsA contents at early stage of salt stress have direct impact on the peroxisome-localized APX3 [50] and chloroplast-localized APX4 [51], and the simultaneous ROS accumulation could be eliminated by cytosolic APXs firstly. These results are consistent with the previous conclusions that the APXs localized in different organelles have distinct functions [52]. These results showed that the AsA-mediated elimination of ROS under salt stress still requires further study.
Due to the negative regulation of ABI4 on VTC2, the similarity of the salt sensitivity of the abi4 vtc2 double mutant with vtc2 indicated that VTC2 is downstream of ABI4 in regulating salt tolerance. The response of ABI4 expression to salt stress resulted in decreased VTC2 expression, causing reduced AsA biosynthesis and increased ROS accumulation. The increased salt tolerance of the ABI4 loss-of-function mutants was closely related to the higher level of AsA.
Conclusions
In conclusion, our findings offer insights into how ABI4 modulates salt-inhibited seedling growth in coordination with VTC2. The expression of ABI4 was promoted when plants were exposed to salt stress, and then ABI4 bound to the promoter of VTC2 to suppress its expression. Therefore, the reduced expression of VTC2 led to reduced AsA production and increased ROS accumulation, ultimately inhibiting seedling growth (Fig. 7). Under normal conditions, the lower expression levels of ABI4 gave rise to higher VTC2 expression and AsA contents for scavenging ROS. Under salinity stress, ABI4 was induced and suppressed VTC2 expression. The decreased expression of VTC2 reduced AsA production during the early stage of salt stress; thus, more ROS accumulated in the plants. The high level of ROS would cause seedling growth inhibition or death. Thus, the synergistic regulation of the ABI4-VTC2 module at the early stage of salt stress led to the accumulation of ROS and the inhibition of seedling growth.
ABI4 and VTC2 coordinately regulate salinity-inhibited seedling growth through ascorbic acid (AsA) scavenging reactive oxygen species (ROS). Under normal conditions, the lower levels of the expression of ABI4 give rise to higher levels of VTC2 expression and AsA contents to scavenge ROS. Under salinity stress, ABI4 is induced and suppresses VTC2 expression. The decreased expression of VTC2 causes reduced AsA production at the early stage of salt stress; thus, more ROS is accumulated in plants. High levels of ROS can cause seedling growth inhibition or death
Methods
Plant material and growth conditions
Arabidopsis thaliana ecotype Columbia (Col-0) was used for all experiments in this study. The abi4–103(CS3838), abi4–102 (CS3837) were obtained from the Arabidopsis Biological Resource Center. The vtc2 mutant, ABI4-OEM1, ABI4-OEM5 and pVTC2::VTC2-GFP transgenic plants have been previously described [10, 40, 53]. The plants were grown on 1/2 MS medium [54] containing 0.4% (w/v) phytagel under a 16-h-white light /8-h-dark cycle at 22 °C.
Genetic manipulation
The double mutant abi4 vtc2 was generated by crossing abi4–103 with vtc2, and the F2 progeny from the crosses were subsequently screened by sequencing. Information about the primers used is summarized in Supplemental Table 1.
Salt stress assays
The 3-day-old seedlings were transferred to the 1/2 MS medium containing 100 mM or 150 mM NaCl supplied with or without 40 μM AsA. Photos were taken after four days of the salt treatment, followed by a statistical analysis of the root length and survival rates. For the gene expression detection, the 7-day-old seedlings were transferred to clean filter paper and treated with 1/2 MS liquid culture with 200 mM NaCl.
Measurement with ascorbic acid contents
For the standard curve, 0.175 g ascorbic acid was transferred into a 1.5 ml centrifuge tube and 1 ml of 6% perchloric acid (HClO4) was added to prepare the 1 mM AsA solution. The AsA mother liquor was diluted with 6% HClO4 to 0.1 μM, 0.2 μM, 0.4 μM, 0.6 μM, 0.8 μM, and 1 μM AsA standard solutions. Then, each 200 μl standard sample was transferred into a 2 ml centrifuge tube with 1800 μl 0.2 M sodium butyrate buffer (pH =12.7). The absorption value of each sample was measured at A265.
The measurements of the AsA contents were conducted as previously described [28]. The 7-day-old seedlings were treated with 100 mM NaCl for 24 h, then about 0.1 g samples were added into a 2 ml centrifuge tube and frozen in liquid nitrogen, crushed into a powder with plant crusher, had 1 ml 6% HClO4 added and were mixed well on ice for 5 min. The samples were centrifuged at 12,000 rpm for 10 min. Then, 200 μl of the supernatant was transferred to a new tube containing 1800 μl 0.2 M sodium succinate buffer (pH = 12.7). The OD1 (Optical Density 1) was measured at 265 nm by a spectrophotometer. Another 200 μl of the supernatant was added into another tube containing 1800 μl 0.2 M sodium succinate buffer (pH = 12.7) and 4 U ascorbic acid oxidase (Sigma). After being mixed and left at room temperature in the dark for 20 min, the OD2 was measured at 265 nm. Meanwhile, another 200 μl of the supernatant was added into a tube containing 1800 μl 0.2 M sodium succinate buffer (pH = 12.7) and 60 μl 1 M dithiothreitol (DTT). After being mixed and left at room temperature (25 °C) in the dark for 30 min, the OD3 was measured at 265 nm by a spectrophotometer. The concentration of the reduced or oxidized form of AsA was calculated by the value of OD1-OD2 and OD3-OD1 according to the standard curve, respectively. The sum of the two was the total AsA concentration of each sample.
Reactive oxygen species staining
The seedlings treated with water (control) or salt (NaCl) were placed in tubes with 2 ml of the DAB staining solution (including 1 mg/ml DAB; 50 mM NaAc-HAc, pH = 3.8) for H2O2 or the NBT solution (including 1 mg/ml NBT; 25 mM Hepes, pH = 7.6) for superoxide, followed by vacuuming for 10 min. These samples were incubated for about 15 min to several hours (based on the staining degree) in the dark at 37 °C, and then the plants were transferred into 75% (v/v) ethanol to remove the chlorophyll.
Reactive oxygen species measurement
The 7-day-old seedlings were transferred to clean filter paper and treated with 1/2 MS liquid culture with or without 100 mM NaCl for 24 h. The 0.1 g samples were frozen in liquid nitrogen, then the H2O2 and superoxide content was determined according to the H2O2 measuring kit (Solarbio) and superoxide measuring kit (Solarbio) protocols, respectively.
RNA extraction and reverse transcription-quantitative polymerase chain reaction analysis
The total RNA of the seedlings was extracted using a Plant RNA Isolation Mini Kit (CWBIO). The RNA reverse transcription was performed using the HiScript II QRT Super mix for the qPCR (Vazyme Biotech), and the qPCR was performed using the SYBR Green Master Mix (Vazyme Biotech) and the iQ5 system (Bio-Rad). Three biological samples were analyzed with three separate technical replicates. ACTIN (AT3G18780) was used as a reference gene for normalization. The relative gene expressions were calculated using the 2inu − ΔΔct method [55]. Three biological replicates were analyzed with three separate technical replicates. Error bars represent the SD from three biological replicates. The primers used for the RT-qPCR are listed in Supplemental Table 1.
Western blotting
The seven-old-day seedlings were transferred to clean filter paper and treated with 1/2 MS liquid culture with or without 100 mM NaCl for 24 h. The total proteins were extracted, followed by 12% SDS/PAGE gel electrophoresis. The protein was transferred into a PVDF membrane (BioRad) by wet-tank transfer and detected using anti-GFP antibodies (Abmart). The antibody against ACTIN (Abmart) was used as the loading control. Quantitative analysis was performed by ImageJ software.
Statistical analysis
The statistical data were analyzed with one-way ANOVAs (Tukey’s test, P < 0.05) and Mann- Whitney U test (p < 0.05) in SPSS16.0 (Polar Engineering and Consulting, http://www.winwrap.com). Different letters and star symbol were used to indicate statistically significant differences.
Availability of data and materials
All the data and materials that are required to reproduce these findings can be shared by contacting the corresponding author, wangjuan@caas.cn (J.W.).
Abbreviations
- ABA:
-
Abscisic acid
- AsA:
-
L-Ascorbic acid
- APX:
-
Ascorbate peroxidase
- DAB:
-
Diaminobenzidine
- GFP:
-
Geen fluorescent protein
- MS:
-
Murashige and skoog
- NBT:
-
Nitroblue tetrazolium
- OE:
-
Over-expression
- qPCR:
-
Quantitative polymerase chain reaction
- ROS:
-
Reactive oxygen species
References
Davey MW, Van Montagu M, Inzé D, Sanmartin M, Kanellis A, Smirnoff N. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80:825–70. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:73.0.CO;2-6.
Smirnoff N, Wheeler GL. Ascorbic acid in plants: Biosynthesis and function. Crit Rev Biochem Mol Biol. 2000;35:291–314. https://doi.org/10.1098/rstb.2000.0706.
Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–9. https://doi.org/10.1038/30728.
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–51. https://doi.org/10.1105/tpc.010538.
Barth C, Gouzd ZA, Steele HP, Imperio RM. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot. 2010;61:379–94. https://doi.org/10.1093/jxb/erj198.
Akram NA, Shafifiq F, Ashraf M. Ascorbic acid: a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front.Plant Sci. 2017;8:613. https://doi.org/10.3389/fpls.2017.00613.
De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61:209–19. https://doi.org/10.1007/s00018-003-3203-8.
Gallie DR. L-Ascorbic acid: a multifunctional molecule supporting plantgrowth and development. Scientifica (Cairo). 2013:795–964. https://doi.org/10.1155/2013/795964.
Bulley S, Laing W. The regulation of ascorbate biosynthesis. Curr Opin Plant Biol. 2016;33:15–22. https://doi.org/10.1016/j.pbi.2016.04.010.
Tabata K, Takaoka T, Esaka M. Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry. 2002;61:631–5. https://doi.org/10.1016/s0031-9422(02)9422(02)00367-9.
Yu YW, Wang J, Li SH, Kakan X, Zhou Y, Miao YC, Wang FF, Qin H, Huang RF. Ascorbic acid integrates the antagonistic modulation of ethylene and abscisic acid in the accumulation of reactive oxygen species. Plant Physiol. 2019;4:1861–75. https://doi.org/10.1104/pp.18.01250.
Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell. 2003;15:1212–26. https://doi.org/10.1105/tpc.009464.
Grace SC, Logan BA. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996; 112: 1631–1640. doi: 10.1104/pp.112.4.1631.
Richardson AC. High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ. 2004;27:423–35. https://doi.org/10.1111/j.1365-3040.2003.01161.x.
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, et al. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stresstolerance in Arabidopsis. Plant J. 2005;44:653–68. https://doi.org/10.1111/j.1365-313X.2005.02560.x.
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–89. https://doi.org/10.1111/j.1365-313X.2007.03266.x.
Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Biol. 2001; 52: 437–467. doi: 10.1146/annurev.a.plant.52.1.437
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007;58:2661–71. https://doi.org/10.1093/jxb/erm124.
Bartoli CG, Yu J, Gómez F, Fernandez L, McIntosh, Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot. 2006; 57: 1621–1631. doi: 10.1093/jxb/erl005
Chen Z, Young TE, Ling J, Chang SC, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA. 2003;100:3525–30. https://doi.org/10.1073/pnas.063517100.
Zhang ZJ, Wang J, Zhang R, Huang RF. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012;71:273–87. https://doi.org/10.1111/j.1365-313X.2012.04996.x.
Wang J, Yu YW, Zhang ZJ, Quan RD, Zhang HW, Ma LG, Deng XW, Huang RF. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013;25:625–36. https://doi.org/10.1105/tpc.112.106880.
Qin H, Wang YY, Wang J, Liu H, Zhao H, Deng ZA, Zhang ZL, Huang RF, et al. Knocking down the expression of GMPase gene OsVTC1–1 decreases salt tolerance of rice at seedling and reproductive stages. PloS One. 2016;11:e0168650. https://doi.org/10.1371/journal.pone.0168650.
Huang C, He W, Guo J, Chang X, Su P, Zhang L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005; 56: 3041-3049. doi: 10.1093/jxb/eri301
Li Y, Chu ZN, Luo JY, Zhou YH, Cai YJ, Lu YE, et al. The C2H2 zinc-fifinger protein SIZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol J. 2018;16:1201–13. https://doi.org/10.1111/pbi.12863.
Athar HUR., Khan A, Ashraf M. Inducing salt tollerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr. 2009; 32: 1799–1817. doi: 10.1080/01904160903242334
Terzi R, Kalaycioglu E, Demiralay M, Saglam A, Kadioglu A. Exogenous ascorbic acid mitigates accumulation of abscisic acid, proline and polyamine under osmotic stress in maize leaves. Acta Physiol Plant. 2015; 37: 1–9.doi: 10.1 007/s11738–015–015-1792-0.
Wang YY, Zhao H, Qin H, Li ZX, Liu H, Wang J, et al. The synthesis of ascorbic acid in rice roots plays an important role in the salt tolerance of rice by scavenging ROS. Int J Mol Sci. 2018;19:33–47. https://doi.org/10.1007/s11738-015-015-1792-0.
Vishwakarma KC, Updayay N, Kumar N, Yadav G, Singh J, Mishra RK, et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci. 2017;8:161. https://doi.org/10.3389/fpls.2017.00161.
Yin C, Zhao H, Ma B, Chen S, Zhang J. Diverse roles of ethylene in regulating agronomic traits in rice. Front. Plant Sci. 2017;8:1676. 10.3 389/fpls.2017.01676.
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011;124:509–25. https://doi.org/10.1007/s10265-011-0412-3.
Wind JJ, Peviani A, Snel B, Hanson J, Smeekens SC. ABI4: versatile activator and repressor. Trends Plant Sci. 2013;18:125–32. https://doi.org/10.1016/j.tplants.2012.10.004.
Finkelstein RR. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 1994;5:765–71. https://doi.org/10.1046/j.1365-313X.1994.5060765.x.
Finkelstein RR, Gampala S, Rock C. Abscisic acid signaling in seeds and seedling. Plant Cell. 2002;14:S15–45. https://doi.org/10.1105/tpc.010441.
Shkolnik-Inbar D, Adler G, Bar-Zvi D. ABI4 downregulates expression of the sodium transporter HKT1,1 in Arabidopsis roots and affects salt tolerance. Plant J. 2013;73:993–1001005. https://doi.org/10.1111/tpj.12091.
Li P, Huang J, Yu S, Li Y, Sun P, Wu C, Zheng C. Arabidopsis YL1/BPG2 is involved in seedling shoot response to salt stress through ABI4. Sci. Rep. 2016;6:30163. https://doi.org/10.1038/srep30163.
Yi J, Zhao DM, Chu JF, Yan JJ, Liu JS, Wu MJ, et al. AtDPG1 is involved in the salt stress response of Arabidopsis seedling through ABI4. Plant Sci. 2019;287:110–80. https://doi.org/10.1016/j.plantsci.2019.110180.
Luo XF, Dai YJ, Zheng C, Yang YZ, Chen W, Wang QC, et al. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2020;11. https://doi.org/10.1111/nph.16921.
Dong ZJ, Yu Y, Li SH, Wang J, Tang S, Huang RF. Abscisic acid antagonizes ethylene production through the ABI4-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis. Mol Plant. 2016;9:126–35. https://doi.org/10.1016/j.molp.2015.09.007.
Kocsy G, Tari I, Vanková R, Zechmann B, Gulyás Z, Poór P, Galiba G. Redox control of plant growth and development. Plant Sci. 2013;211:77–91. https://doi.org/10.1016/j.Plantsci.2013.07.004.
Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and combination. Plant J. 2017;90:856–67. https://doi.org/10.1111/tpj.13299.
Shabala S, Wu H, Bose J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015;241:109–19. https://doi.org/10.1016/j.plantsci.2015.10.003.
Inzé D, Van Montague M. Oxidative stress in plants. Curr Opin Biotech. 1995;6:153–8. https://doi.org/10.1016/0958-1669(95)80024-7.
Foyer CH, Noctor G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011;155:2–18. https://doi.org/10.1104/pp.110.167569.
Venkatesh J, Park SW. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot Stud. 2014;55:38. https://doi.org/10.1186/1999-3110-55-38.
Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-defificient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–4. https://doi.org/10.1073/pnas.93.18.9970.
Linster CL, Clarke SG. L-Ascorbate biosynthesis in higher plants: The role of VTC2. Trends Plant Sci. 2008;13:567–73. https://doi.org/10.1016/j.tplants.2008.08.005.
Tao C, Jin X, Zhu L, Xie Q, Wang X, Li H. Genome-wide investigation and expression profiling of APX gene family in Gossypium hirsutum provide new insights in redox homeostasis maintenance during different fiber development stages. Mol Gen Gen. 2018;293:685–97. https://doi.org/10.1007/s00438-017-1413-2.
Panchuk II, Zentgraf U, Volkov RA. Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta. 2005;222:926–32. https://doi.org/10.1007/s00425-005-0028-8.
Shen G, Kuppu S, Venkataramani S, Wang J, Yan J, Qiu X, Zhang H. ANKYRIN REPEAT-CONTAINING PROTEIN 2A is an essential molecular chaperone for peroxisomal membrane-bound ASCORBATE PEROXIDASE3 in Arabidopsis. Plant Cell. 2010;22:811–31. https://doi.org/10.1105/tpc.109.065979.
Wang YY, Hecker AG, Hauser BA. The APX4 locus regulates seed vigor and seedling growth in Arabidopsis thaliana. Planta. 2014;239:909–19. https://doi.org/10.1007/s00425-014-2025-2.
Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M. Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta. 2006;224:300–14. https://doi.org/10.1007/s00425-005-0214-8.
Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S. The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of Jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell. 2011;23:3319–34. https://doi.org/10.1105/tpc.111.090100.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962; 15: 473–497. doi: 10.111/j 1399-3054.1962.tb08052.x
Chang J, XuID Z, Li M, Yang M, Qin H, Yang J, Wu S. Spatiotemporal cytoskeleton organizations determine morphogenesis of multicellular. PLoS Genet. 2019;10:e1008438. https://doi.org/10.1371/journal.pgen.1008438.
Acknowledgments
We greatly appreciate Dr. Christine H. Foyer from the University of Leeds for kindly providing the vtc2 mutants. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research was funded by the National Natural Science Foundation of China (31670280), the Fundamental Research Funds for Central Non-profit Scientific Institution (1610392020004), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
J.W. and R.H. conceived the project. X. K, Y.Y., L.S. and X. L. performed the assays. X.K., J.W. and R.H. analyzed the data. X.K and J.W. wrote the manuscript. J.W. and R.H. reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplemental Table 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kakan, X., Yu, Y., Li, S. et al. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol 21, 112 (2021). https://doi.org/10.1186/s12870-021-02882-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-021-02882-1