Abstract
Increased fluorine pollution represents a serious limitation for the productivity of important crops such as beans. The present study was conducted to detect antagonistic/synergistic ion mobility during seed germination in the presence of F contamination (KF and NaF). NaCl was used as a benchmark. The results showed that germination of Jesca, an African (Tanzania) bean variety, significantly dropped with high F levels (10% KF and 3% NaF). High F levels reduced Jesca growth and decreased root and shoot biomass (by 50% and 95% with KF and NaF, respectively). NaF 200 mg kg−1 had the most depressive effect on the seedling stage. Elevated F levels negatively affected seedling health, revealing toxicity symptoms such as chlorophyll degradation and low photosynthetic activities that degraded after a threshold level of 80 mg kg−1. In addition, an inhibitory effect of F on the mineral status of the seedlings, especially on the Ca content, was observed. An opposite trend of endogenous Ca response to NaCl stress was observed. Indeed, while endogenous Ca content increased with increasing NaCl concentration, it decreased when the F level increased. Therefore, tolerance to F at the germination and seedling stages might be used as a criterion for selecting F-tolerant bean varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoride (F) is one of the major global contaminants that is drawing increasing attention due to its long long-term persistence in air, soil, and water even at low levels (Weerasooriyagedara et al. 2020). F is contained in several rocks and minerals that gradually leach out by natural weathering and precipitation (Loganathan et al. 2013; Vithanage et al. 2014). As a consequence, the availability of F in the environment is destined to increase over time, inducing its entry into the food chain (He et al. 2021). Furthermore, F can pollute the environment through man-made industrial processes such as coal combustion and water or waste coming from various industrial processes, including steel manufacture, primary aluminum, copper and nickel production, phosphate ore processing, phosphate fertilizer production and use, glass, brick and ceramic manufacturing, and glue and adhesive production (Kimambo et al. 2019). Mineral and industrial effluents mainly increase F in the aquatic environment. In addition, volcanic activity, as well as coal combustion, contributes to F pollution, generating gas residues that induce F contamination at the airborne level (He et al. 2021). Although the presence of volcanic bedrock and arid or semiarid climatic conditions, as well as Ca2+-deficient Na–HCO3-type groundwater, are factors promoting F contamination and accumulation (Raj and Shaji 2017; Kumar et al. 2019), F availability in soils is influenced by soil pH, which acts in fluorine adsorption by soil minerals (Samal et al. 2015).
Low levels of F, since it is considered an essential micronutrient, have a positive effect on human health, reducing the risk of dental caries and inducing strengthened bones and teeth (Yu and Yang 2020). Conversely, exposure to high levels of F can cause several health problems called fluorosis, which represents a serious issue in many countries worldwide (Singh et al. 2018; Yang et al. 2020). High F concentrations have been found in groundwaters of a belt extending from Syria through Jordan, Egypt, Libya, Algeria, Morocco, and the Rift Valley. Another contaminated area is a belt extending from Turkey through Iraq, Iran and Afghanistan to India, northern Thailand and China (World Health Organization 1984). In particular, the East African Rift valley (Ethiopia, Kenya, Tanzania) is a naturally F-rich zone due to its geophysical and geochemical characteristics (Davies 2008). Overall, fluorosis symptoms are currently prevalent in more than 40 countries with a wide range of degrees, and they induce serious public health concerns (Ren et al. 2022).
F is adsorbed by the soil, and plant uptake is usually very low (Álvarez-Ayuso et al. 2011; Ropelewska et al. 2016). However, in soils polluted with F and/or under low pH, F availability and solubility are increased, and plants may take up it in excess, leading to plant damage or excess F in the human or animal diet (Álvarez-Ayuso et al. 2011), causing severe problems for agriculture and human health.
Excess F levels in soil or solution culture affect plant germination, root and shoot growth, chlorosis, leaf tip burn, leaf necrosis and reduction in grain yield (Datta et al. 2012; Dey et al. 2012; Maitra et al. 2016). High levels of F inside plant cells have negative effects on physiological cycles, nutrient mobility and water usage and reveal toxicity symptoms, such as chlorophyll degradation, low seedling establishment, growth rate and photosynthetic activities, high reactive oxygen species (ROS) generation and consequently membrane damage (Panda 2015; Yadu et al. 2016). Several studies have been conducted on the effect of salinity based on NaCl on the germination and emergence of different plant species, while only a few data are available for F-contaminated seeds in this stage of development. The effect of F on germination has been only partially explored in a few species, such as rice (Oryza sativa L.) (Chakrabarti and Patra 2015); gram seed (Cicer arietinum) (Datta et al. 2012), tomato (Solanum lycopersicum) (Singh et al. 2012), wheat (Triticum aestivum) (Kumar Aske and Iqbal 2014), maize (Zea mays), soybeans (Glycine max), sorghum, (Sorghum vulgare) (Fina et al. 2016) and bean (Cyamopsis tetragonoloba) (Sabal et al. 2006; Chahine et al. 2022).
Common bean (Phaseolus vulgaris L.) is a grain legume extensively grown and consumed all over the world and represents one of the main food sources in Africa (Binagwa et al. 2018). In Africa, beans are shifting from a traditional subsistence to a market-oriented crop, playing the main role in the livelihoods of smallholder farmers in Tanzania and Kenya as a food security crop and source of income (Binagwa et al. 2018).
Seed germination is one of the most fundamental and vital phases in the growth cycle of a crop. Salinity hampers germination and seedling growth due to the lower osmotic potential of germination media (Khan and Weber 2008). In addition, it causes toxicity, which affects the activities of enzymes of nucleic acid metabolism (Gomes-Filho et al. 2008), alters protein metabolism (Dantas et al. 2007), interrupts hormonal balance (Khan and Rizvi 1994), and reduces the utilization of seed reserves (Othman et al. 2006).
The soluble fluorine fraction in soil is absorbed by roots and transported in plants. Although F contamination causes severe limitations in plant yield, a complete exploration of morphological, mineral, and metabolic profile responses in plants has only been partially explored.
To the best of our knowledge, this study represents one of the first explorations of the impact of two sources of F (KF and NaF) on the mineral status at germination and seedling growth in an African local bean variety (Jesca). We hypothesized that the mineral nutrition of seedlings and the production of photosynthetic pigments were influenced by the F concentration and that a possible antagonistic or synergistic effect of ions on seed germination in relation to the kind of salt-containing F could exist.
The main aims of this research were: (a) to assess the effect of F on the germination and seedling growth of an African local bean variety (Jesca); (b) to compare the response of the plant to two sources of F (NaF and KF) at the germination stage; and (c) to analyze the effect of different sources of F salt and sodium chloride treatments on the morphological, mineral nutrition and photosynthetic pigments of germinated Jesca seeds.
Materials and methods
Plant material and experimental conditions
Mature seeds of Phaseolus vulgaris L. var. Jesca, commonly grown in Tanzania (Laizer and Mbwambo 2022), were used for a germination experiment. Seeds were provided by Nelson Mandela University of Science and Technology, Tanzania. Three salt sources (sodium fluoride, NaF; potassium fluoride, KF; and sodium chloride, NaCl) were added directly to the silica sand (substrate). The germination test was conducted in plastic containers (58 × 72 mm filled with approximately 90 g of washed sand). Overall, the experimental design consisted of four treatments and four levels as follows:
Treatments:
-
Control (0): sand without salt
-
Sand + NaF
-
Sand + KF
-
Sand + NaCl
Level of each salt:
-
0
-
15 mg kg−1
-
80 mg kg−1
-
200 mg kg−1
NaF, KF and NaCl were added directly to the substrate according to the level indicated. The correct amount of salt was verified (before seed planting) using an ion-selective electrode (ORION 4 star) (F) and inductively coupled plasma (ICP) atomic emission spectrometry (PerkinElmer) (Na and K).
Each treatment × level was indicated with salt type and level (i.e., NaF15; NaF80; NaF200). The choice of the salt concentration levels explored in the experiment was based on the range of soil F contents observed in F-rich sites of the East African Rift Valley in Tanzania, as reported by Rizzu et al. (2020). Two seeds per container were placed, and for each treatment*level combination, 15 replicates were used. The experiment was carried out in a growth chamber (24 ± 2 °C, 18 h photoperiod, with an average irradiance of 1500 lx), and it was repeated twice. Two milliliters of tap water were used every 2 days for irrigation.
Germination and growth measurement
Jesca seeds were considered germinated when the radicle reached a length of at least 2 mm. Germination was monitored daily and recorded for 14 days. The percentage of germinated seeds (%G) was measured as follows:
At the end of the experiment, at the stage of two full leaves (first leaf pair unfolded) (BBCH scale 12) (Weber and Bleiholder 1990; Feller et al. 1995), the shoot length (SL) and root length (RL) were measured using ImageJ software (Image processing and analysis in Java). Root length (RL) was estimated using GIA ROOTS software (Galkovskyi et al. 2012). Five replicates from each treatment*level combination were stocked at − 80 °C for chlorophyll, carotenoid, and total phenol analysis.
The remaining ten plants were oven-dried at 65 °C until a constant weight was reached and were used for root and shoot dry weight (DW) determinations.
Determination of chlorophyll content, total carotenoids and phenols
Total chlorophyll (Tot Chl), chlorophyll a (Chl a), chlorophyll b (Chl b), total carotenoids (Tot carot) and phenols (Tot phenol) were determined by extraction using 99.9% methanol as the solvent (0.1 mL of methanol for mg of fresh weight). Samples were incubated with the solvent for 48 h at − 20 °C, and the solution was replaced after 24 h. Quantitative chlorophyll and carotenoid determinations were carried out immediately after extraction. Absorbance readings were performed at 665.2 and 652.4 nm for chlorophyll pigments and 470 nm for total carotenoids. Total chlorophyll and carotenoid contents were calculated using Lichtenthaler and Buschmann (2001) methods. Total phenol was determined by directly measuring the extracts at 320 nm (Maggini et al. 2018). Chlorophylls, total carotenoids and phenols are expressed as µg g−1 of leaf fresh weight (FW).
Determination of F and cation contents in Jesca seedlings
Dried samples of roots and shoots were powdered, and 150 mg was digested with 2 mL of nitric acid (65%), 3 mL of hydrogen peroxide (30%) and 5 mL of deionized water in a microwave digestion unit (Milestone Ethos Easy, Milestone s.r.l, Sorisole (BG), Italy) (Rocha et al. 2013). Then, the samples were placed in a closed vessel in a refrigerated bath (− 30 °C) for 30 min to avoid losses of F in the form of hydrogen fluoride (HF) (Rizzu et al. 2020). Neutralization with aqueous sodium hydroxide (NaOH, 8 M) was carried out in vessels. The extractant solution was mixed with 10% (v/v) total ionic strength adjustment buffer “TISAB III” solution. The mixture was analyzed by an ion-selective electrode (ORION 4 star). The digestion was applied at least in triplicate to each of the samples analyzed. The amounts of F in roots and shoots are expressed in mg kg−1 DW.
Concentrations of Na, K, Ca, and Mg were analyzed by inductively coupled plasma (ICP) atomic emission spectrometry (PerkinElmer, Norwalk, USA) after perchloric acid digestion (Maggio et al. 2000). The amounts of Na, K, Ca, Mg in roots and shoots are expressed in mg g−1 DW.
Statistical analysis
A matrix made of all variables was obtained as the difference between each observed value minus the average of the control treatment. A 3 × 3 factorial, unbalanced (nr. replicates were dependent on treatment) analysis of variance was performed using the generalized linear model function (GLM) in the RStudio application of R software (version 3.5.1) (R Core Team 2018). Factors were the type of salt (KF, NaCl, NaF) and salt levels (15, 80 and 200 mg kg−1). When significant differences were observed (P ≤ 0.05), means were compared by orthogonal contrasts (P ≤ 0.05). All data are presented as the average ± standard error.
Results
Germination percentage and seedling growth
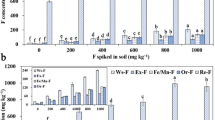
The results of % G are shown in Fig. 1. Jesca was tolerant to salinity caused by NaCl, since seeds were able to germinate under all levels, showing 94% %G under NaCl200. A significant reduction in % G was observed using F (P < 0.001). Jesca seeds expressed their sensitivity to F starting from 80 mg kg−1 by significantly reducing the % G of 7% and 17% under KF and NaF, respectively. Under the highest F level (200 mg kg−1), germination was deeply compromised: only 10% (KF) and 3% (NaF) of seeds germinated.
To assess the effects of salt stress on Jesca seedling growth, both shoot and root lengths were recorded. Shoot and root length significantly decreased in the presence of NaF and KF (Table 1; Supplementary Fig. 1). Moreover, the application of F at the highest level in both forms severely reduced shoot length (− 45% and − 36% under KF200 and NaF200, respectively, compared to the control) (P = 0.002 and P < 0.001, respectively). Similarly, a significant decrease was observed in root length (− 51% and − 92% under KF200 and NaF200, respectively, compared to the control). Conversely, the addition of NaCl to the substrate did not impact seedling growth (Table 1). As predicted, as a consequence of seedling growth reduction under F salts, a significant decrease in shoot and root DW was also recorded (Table 1). Major effects were detected using the 200 mg kg−1 level in shoot DW (− 87% and − 88% under KF200 and NaF200, respectively) and root DW (85% and − 99% under KF200 and NaF200, respectively). In addition, NaCl 200 mg kg−1 impacted only the root dry weight, with a significant reduction in Jesca biomass of 48% compared to the control.
F content in shoot and root tissues
A significant increase in F uptake was recorded for Jesca grown under increasing levels of KF and NaF (Fig. 2). Overall treatment and level and treatment × level were statistically significant in both plant tissues (Supplementary Table 1). F uptake was calculated from DW of organs, and it ranged from a minimum of 2.40 mg g−1 to a maximum of 112.84 mg g−1 (shoot control and shoot NaF200, respectively). The shoots and roots showed different F uptake, and in general, higher F uptake was detected under NaF treatments than under KF treatments. At the highest F level (200 mg kg−1), regardless the salt source used, shoots showed more efficient uptake of F than roots (Fig. 2b). As a result, Jesca accumulated 47.63 mg kg−1 and 75.70 mg kg−1 F in roots and shoots, respectively, when treated with KF, whereas under NaF treatment, Jesca accumulated 73.64 mg kg−1 and 112.84 mg kg−1 F in roots and shoots, respectively.
Total F in shoots (S) (A) and roots (R) (B). Two different sources of F (KF; NaF) and four levels of F for each treatment (0, 15, 80, 200 mg kg−1) were applied. The average value ± standard error of three replicates is presented in the figure. Different letters indicate significant differences based on levels within each salt treatment and the control
Photosynthetic pigments, total carotenoids and phenols
Leaf chlorophylls (total chlorophyll, Tot Chl; chlorophyll a, Chl a; chlorophyll b, Chl b), total phenols (Tot phenols) and carotenoids (Tot carot) were estimated for all treatments in fresh tissue. A significant reduction in chlorophylls and carotenoids with increasing F levels was observed under KF (Table 2).
Indeed, Chl a, Chl b and Tot Chl reached the minimum value under KF200 (69%, 7% and 62%, respectively) compared to the control. A similar trend was observed under NaF200, where a significant reduction in Chl a and Tot Chl was recorded (59% and 45%, respectively), while an increase in Chl b (74%) was revealed. Nevertheless, NaCl treatment at 200 mg kg−1 induced a smooth increase in the content of all chlorophyll pigments compared to the control (Chl a, + 4%; Chl b, + 143%; and Tot Chl, + 20%). In addition, in contrast to the F salt treatment, at the lowest NaCl level, minor contents of Chl a, Chl b and Tot Chl were observed.
Tot carot decreased by 56% and 72% under KF200 and NaF200, respectively. Conversely, under NaCl stress, Tot carot increased significantly with increasing salt level (P < 0.001). Tot phenol significantly decreased when F was applied at KF200, reaching the minimum value of 5.81 µg g−1 (plant FW).
Micronutrient content
The mobility of Na, Ca, K and Mg was explored. Mineral data were not detected for seedlings cultivated under NaF200 due to the scarcity of plant material available because of severe damage induced by salt stress. Significance of the effects of salts, levels and their interaction on mineral content (F, Na, Ca) in Jesca shoot and root is shown in Supplementary Table 1. All mineral contents are expressed as mg g−1 of plant organ DW.
The Na content significantly varied with the seedling organ (root and shoot) and salt used (Fig. 3). Obviously, under NaF and NaCl treatments, higher Na levels were found in roots and shoots than in the control or under KF treatments. In particular, in roots, a higher content of Na was found under NaF compared to NaCl treatments. Under NaF80, which represents the highest Na level monitored, roots accumulate more than five times more Na than the control, achieving a maximum value of 28.65 mg g−1. Under NaCl200, minor accumulation of Na compared to NaF80 was found, and roots reached the maximum value of 14.03 mg g−1.
Increase in Na concentrations in roots (A) and shoots (B) of bean treated with KF, NaF, and NaCl at 0, 15, 80 and 200 mg kg−1. The average value ± standard error of three replicates is presented in the figure. Different letters indicate significant differences based on levels within each salt treatment and the control
The Na content in shoots was approximately ten times lower than that in roots (Fig. 3). In general, the Na content showed a similar trend in both seedling organs. In addition, although the Na content detected in shoots was low, consistent accumulation was observed in plants treated with NaCl, reaching a maximum value of 2.3 mg g−1. Regarding the Ca content in roots and shoots, two opposite trends were recorded based on the salt used: F or NaCl (Fig. 4). A significant decrease in Ca content in roots was observed in F-treated plants by − 38% and − 44% under KF80 and NaF80 treatments, respectively. At the highest level of KF (200 mg kg−1), Ca in roots decreased by 86% compared to the control. A similar trend was observed in shoots. Ca in shoots decreased by 28% and 13% under KF80 and NaF80, respectively. Overall, under F treatments, roots presented higher content of Ca compared to shoot, ranging from a minimum of 1.36 mg g−1, under KF200 treatment, to a maximum of 4.67, under control treatment in the absence of salts. In the presence of NaCl, a decrease of 10% in Ca in roots was observed (NaCl15 and NaCl80). Furthermore, an increase in Ca in roots was detected in NaCl200, reaching the maximum value of 5.20 mg g−1 (Fig. 4). However, the Ca in shoots showed a constant increase with NaCl treatments, reaching a maximum value of 6.20 mg g−1 (Fig. 4).
Ca, K and Mg content in roots (R) and shoots (S) treated with KF, NaF, and NaCl 0, 15, 80 and 200 mg kg−1 (A–C respectively). The average value ± standard error of three replicates is presented in the figure. Different letters indicate significant differences based on levels within each salt treatment and the control
Finally, the K and Mg contents were also evaluated. The effect of the salt, level and the interaction between salt × level was also explored (Supplementary Table 1). As expected, K in roots increased with increasing KF level from 9.19 mg g−1 (control) to 40.32 mg g−1 (KF200). In the presence of NaF and NaCl, a slight decrease in K content was observed among the control (9.19 mg g−1), NaF80 (4.25 mg g−1) and NaCl200 (3.56 mg g−1) (Fig. 4). In shoots, the K content presented a slight increase from 27.78 mg g−1 (control) to 30.04 mg g−1 (NaF80) and 29.33 mg g−1 (NaCl200). Mg trend was also explored. In general, little variation in Mg content was found in roots and shoots. At KF200 and NaCl200, roots presented a slight increase in Mg concentration. Under NaF80, a similar Mg content was found with the control. In shoots, Mg increased in all treatments except for KF200, where its content reached a minimum (2.07 mg g−1) compared to the control.
Discussion
In agricultural lands contaminated by fluoride, bean cultivation has to face both germination and seedling development in hard conditions, as confirmed by our results with Jesca. Indeed, a consistent inhibition of germination was recorded for both NaF and KF when the highest F level (200 mg kg−1) was taken into account. Similar results regarding the effect of F on seed germination and seedling development were already reported by Chahine et al. (2022) for different bean varieties, Sreedevi and Damodharam (2011) for chickpeas, and Saini et al. (2013) and Dulska et al. (2019) for small shrubs such as Prosopis juliflora, Colobanthus apetalus, and Colobanthus quitensis.
Seedling exposure to F induced a general reduction in seedling growth, with a significant decrease in shoot and root length (Table 1), as well as their weight, as already reported for lettuce (Wang et al. 2022). Moreover, the F treatments had a more consistent effect on the DW of both shoot and root portions than NaCl.
Therefore, the decrease in shoot and root DW in Jesca under salt stress should be related to the changes in the allocation of assimilates between roots and shoots (Mondal 2017; Sabal et al. 2006). The reduction in root growth, therefore, could be the result of the F effect on phytin (Ram et al. 2014). It is well known that during germination, phytin is broken down by the activity of the enzyme phytase to supply seedlings with inorganic phosphate. F inhibits phytase enzyme, mineral nutrition, and amylase activity, thus delaying seedling development (Panda 2015; Yadu et al. 2016). A similar trend was found in other seeds exposed to F, such as rice (Chakrabarti and Patra 2015; Chakrabarti et al. 2012; Mondal 2017), wheat (Gautam and Bhardwaj 2010; Tak and Asthir 2017), tomato, wheat, mustard and cluster bean (Sabal et al. 2006). In all these experiments, a reduction in roots and shoots with increasing NaF levels was found.
Furthermore, Kabata-Pendias (2010) suggested that the fraction of F translocated in leaves depends on the Mg2+ and Ca2+ concentrations in the medium. Considering the role of Ca and Mg in F uptake and tissue accumulation, the content of these two anions in Jesca seedlings was also explored (Fig. 4).
Our results showed a lower Ca content in root seedlings as a consequence of F exposure. These data could be due to the sequestration of F in root vacuoles (Banerjee and Roychoudhury 2019). The compartmentalized F was probably immobilized by cations such as Al3+, Fe3+, Ca2+, and Mg2+ and/or by organic compounds. The formation of such complexes reduced the Ca2+ content in roots, which disturbed membrane stability and signal transduction. This hypothesis will justify the response of plants exposed to NaCl, in which the root content of Ca did not change. Comparing the Ca content in the shoots of plants under different F treatments, Jesca showed the lowest content of Ca under KF (regardless of the level), which represents the less toxic F salt used. The mechanisms by which F is toxic are thought to involve inhibition of enzymes and interference with membrane permeability through precipitation with Ca2+ (Stevens et al. 1998). If the Ca content in plants is still high, the plants would be more tolerant of F exposure. Changes in membrane permeability could overcome the barrier to F uptake in the root cortex and increase the F concentration in plants. Some explanations could be proposed justifying the Ca response to F compared to NaCl: (i) Ca might change the properties of the cell wall (Ruan et al. 2004), (ii) Ca might bind F to form the CaF2 complex, which interferes with membrane permeability (Cai et al. 2014), and (iii) a Ca2+-dependent signaling mechanism might be inhibited by F uptake (Zhang et al. 2015).
The toxic action of F is also thought to involve the inactivation of Mg2+ at its sites of physiological activity. Ca is intensely competitive with Mg, and the binding sites on the root plasma membrane appear to have less affinity for the highly hydrated Mg2+ than for Ca2+ (Marschner 1995). Overall the impact of F in mineral nutrition of plant is only partially, Reddy and Kaur 2008, Li and Ni 2009, Panda 2015). Our investigation revealed that the Mg concentration was higher in the shoot than in the root, and the Mg uptake in the shoot decreased with the increase of F level, whereas in the root, an opposite trend was observed. Higher F levels (i.e. KF200, NaF80) increased the Mg uptake in shoots and decreased it in roots, revealing two opposite trends. One of the hypotheses is that F may disturb the ion uptake, thereby altering the selectivity and permeability of cell membranes.
Potassium (K) in roots decreased as overall NaF and NaCl levels increased. This could be attributed to the increase in Na in substrate solutions, which inhibits the uptake of K by interfering with K ion channels in the plasma membrane of the root and competes with K for binding sites (Ghassemi-Golezani and Farhangi-Abriz 2019; Zouari et al. 2017). The increase in Na and K in plant shoots can be directly tied to the increasing NaCl–KF and NaF levels of the substrate. Similar results were found by Carter et al. (2005) for Limonium perezii, in which the levels of Na and Cl− in plant tissues were directly influenced by the NaCl concentration of the substrate solutions. The amount of Na in plants exposed to NaF was higher than that found in plants exposed to NaCl, which could be related to the mobility of Na in the presence of F compared to Cl−.
Furthermore, the increased Na in the shoots under F stress might also be related to a possible decrease in Na exclusion. It is well known that many plants have a Na exclusion mechanism through a Na+/H+ antiport, such as salt overly sensitive 1 (SOS1), exchanging cytoplasmic Na+ with external H+ (Li et al. 2010; Munns and Tester 2008). In contrast, in the case of KF treatment, K is preferentially transported against the Na concentration gradient. As a result, K levels in the Jesca leaves increased with increasing KF-salinity. The higher germination percentage of seeds treated with KF than of those treated with NaF can be related to the less toxicity effect of K compared to Na (Massa et al. 2009; Massa and Melito 2019). Furthermore, the substitution of K by Na may lead to nutritional imbalance.
The chlorophyll and total carotenoid contents of leaves showed different behaviors based on the salt used. Photosynthesis was found to be affected by F toxicity above a threshold level (80 mg kg−1). As reported by Sahariya et al. (2021), chloroplasts are one of the primary targets of F. Several studies have reported a negative effect of F on photosynthetic pigments such as anthocyanins, chlorophyll-a, chlorophyll-b and carotenoids (Gadi et al. 2021). Under the highest F level, plant tissue net photosynthesis was reduced, suggesting that F may interfere with pigment biosynthesis, which is a primary symptom of F-induced chlorosis (Mondal 2017; Baunthiyal and Ranghar 2014).
However, based on chlorophyll content, Jesca was shown to be a salt-tolerant genotype to NaCl salinity since its chlorophyll content under medium and high NaCl levels showed comparable values to the control. These data suggested that Jesca presents, as previously observed in other African varieties, a protection system against oxidative damage caused by salt treatment (NaCl), which might negatively affect photosynthesis (Yasar et al. 2008).
In our study, we observed two opposite trends of carotenoids in response to NaCl and F (NaF and KF). In the first case, an increase in carotenoid content was observed as a consequence of salinity stress associated with NaCl. Carotenoids, which include carotenes and their derived molecules, have several functions that range from a direct role in photosynthesis to their involvement in oxidative stress defense mechanisms (Gill and Tuteja 2010). Several studies have demonstrated that most salt-resistant plants present an enhanced production of carotenoids (Juan et al. 2005; Coesel et al. 2008). In our study, the total carotenoid content increased with increasing levels of NaCl, suggesting its role in Jesca protection against NaCl salt stress. However, in the presence of F, a dramatic decrease in carotenoids was detected under both sources of F. Carotenoids are important antioxidants that protect photosynthesis, avoiding the production of singlet oxygen by quenching the excited state of chlorophyll (Chakrabarti and Patra 2015).
Salt stress often creates both ionic and osmotic stress in plants, resulting in the accumulation or decrease of specific secondary metabolites in plants. In this study, for instance, although the reduced growth of bean seedlings as consequence of F stress was detected under NaF200, unlike under NaCl and KF treatments, there was an increase in the concentration of phenolic compounds.
In conclusion, F deeply impacts Jesca germination, seedling growth, and pigment and chlorophyll contents, with a stronger effect associated with NaF than with KF. Indeed, alteration of the mineral (Na, Mg, K, Ca) status of the seedlings was observed. The endogenous Ca content showed two opposite trends in response to F and NaCl stress: while endogenous Ca increased with increasing NaCl, it decreased when the F level increased. Future studies should explore the dynamics of F in soils and uptake by crops grown on F-affected soils.
References
Álvarez-Ayuso E, Giménez A, Ballesteros JC (2011) Fluoride accumulation by plants grown in acid soils amended with flue gas desulphurisation gypsum. J Hazard Mater 192:1659–1666
Banerjee A, Roychoudhury A (2019) Fluorine: a biohazardous agent for plants and phytoremediation strategies for its removal from the environment. Biol Plant 63:104–112
Baunthiyal M, Ranghar S (2014) Physiological and biochemical responses of plants under fluoride stress: an overview. Fluoride 47(4):287–293
Binagwa PH, Magdalena W, Michael K, Zakayo E, Mbiu J, Msaky J, Mdachi M, Kasubiri F, Kisamo A, Nestory SM, Rubyogo JC (2018) Selian Agricultural Research Institute (SARI) released seven (7) improved common bean (Phaseolus vulgaris) varieties January 2018
Cai HM, Peng CY, Chen J, Hou RY, Gao HJ, Wan XC (2014) X-ray photoelectron spectroscopy surface analysis of fluoride stress in tea (Camellia sinensis (L.) O. Kuntze) leaves. J Fluor Chem 158:11–15
Carter CT, Grieve CM, Poss JA (2005) Salinity effects on emergence, survival, and ion accumulation of Limonium perezii. J Plant Nutr 28:1243–1257
Chahine S, Melito S, Giannini V, Roggero PP, Seddaiu G (2022) Endogenous calcium mediates seedling growth and fluoride stress tolerance in four bean genotypes. Ital J Agron 17(3):2073–2082
Chakrabarti S, Patra P (2015) Biochemical and antioxidant responses of paddy (Oryza sativa L.) to fluoride stress. Fluoride 48:56–61
Chakrabarti S, Patra PK, Mandal B, Mahato D (2012) Effect of sodium fluoride on germination, seedling growth, and biochemistry of Bengal gram (Cicer arieninum). Fluoride 45:257–262
Coesel SN, Baumgartner AC, Teles LM, Ramos AA, Henriques NM, Cancela L, Varela JCS (2008) Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar Biotechnol 10:602–611
Dantas BF, De Sá RL, Aragão CA (2007) Germination, initial growth and cotyledon protein content of bean cultivars under salinity stress. Rev Bras Sementes 29:106–110
Datta J, Maitra A, Mondal N, Banerjee A (2012) Studies on the impact of fluoride toxicity on germination and seedling growth of gram seed (Cicer arietinum L. cv. Anuradha). J Stress Physiol Biochem 8:194–202
Davies TC (2008) Environmental health impacts of East African rift volcanism. Environ Geochem Health 30:325–338
Dey U, Mondal NK, Das K, Datta JK (2012) Dual effects of fluoride and calcium on the uptake of fluoride, growth physiology, pigmentation, and biochemistry of Bengal gram seedlings (Cicer arietinum L.). Fluoride 45(4):389–393
Dulska J, Wasilewski J, Androsiuk P, Kellmann-Sopyła W, Głowacka K, Górecki Chwedorzewska K, Giełwanowska I (2019) The effect of sodium fluoride on seeds germination and morphophysiological changes in the seedlings of the Antarctic species Colobanthus quitensis (Kunth) Bartl. and the Subantarctic species Colobanthus apetalus (Labill.) Druce. Pol Polar Res 40(3):255–272
Feller C, Bleiholder H, Buhr L, Hack H, Hess M, Klose R, Meier U, Stauβ R, van den Boom T, Weber E (1995) Phanologische entwicklungsstadien von gemusepflanzen II. fruchtgemuse und hulsenfruchte. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 47(9):217–232
Fina BL, Lupo M, Dri N, Lombarte M, Rigalli A (2016) Comparison of fluoride effects on germination and growth of Zea mays, Glycine max and Sorghum vulgare. J Sci Food Agric 96:3679–3687
Gadi BR, Kumar R, Goswami B, Rankawat R, Rao SR (2021) Recent developments in understanding fluoride accumulation, toxicity, and tolerance mechanisms in plants: an overview. J Soil Sci Plant Nutr 21:209–228
Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang S, Harer J, Benfey PN, Weitz JS (2012) GiA roots: software for the high throughput analysis of plant root system architecture. BMC Plant Biol 12:116
Gautam R, Bhardwaj N (2010) Bioaccumulation of fluoride in different plant parts of Hordeum vulgare (Barley) var. RD-2683 from irrigation water. Fluoride 43:57–60
Ghassemi-Golezani K, Farhangi-Abriz S (2019) Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus tinctorius L.) seedlings. Chemosphere 223:406–415
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gomes-Filho E, Lima CRFM, Costa JH, Da Silva ACM, Da Guia Silva Lima M, De Lacerda CF, Prisco JT (2008) Cowpea ribonuclease: properties and effect of NaCl-salinity on its activation during seed germination and seedling establishment. Plant Cell Rep 27:147–157
He L, Tu C, He S, Long J, Sun Y, Sun Y, Lin C (2021) Fluorine enrichment of vegetables and soil around an abandoned aluminium plant and its risk to human health. Environ Geochem Health 43:1137–1154
Juan M, Rivero RM, Romero L, Ruiz JM (2005) Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ Exp Bot 54(3):193–201
Kabata-Pendias A (2010) Trace elements in plants. In: Kabata-Pendias A (ed) Trace elements in soils and plants, 4th edn. CRC Press (Taylor and Francis Group), Boca Raton
Khan MA, Rizvi Y (1994) Effect of salinity, temperature, and growth regulators on the germination and early seedling growth of Atriplex griffithii var. stocksii. Can J Bot 72:475–479
Khan MA, Weber DJ (2008) Ecophysiology of high salinity tolerant plants, ecophysiology of high salinity tolerant plants. Springer, Elmira College, 1 Park Place, Elmira, Abstract
Kimambo V, Bhattacharya P, Mtalo F, Mtamba J, Ahmad A (2019) Fluoride occurrence in groundwater systems at global scale and status of defluorination—state of the art. Groundw Sustain Dev 9:100223
Kumar S, Singh R, Venkatesh AS, Udayabhanu G, Sahoo PR (2019) Medical geological assessment of fluoride contaminated groundwater in parts of Indo-Gangetic Alluvial plains. Sci Rep 9(1):1–16
Kumar Aske D, Iqbal S (2014) Laboratory study of fluoride toxicity on wheat (Triticum aestivum Var.lok-1). Sci Res Rep 4(2):159–162
Laizer HC, Mbwambo FE (2022) Effectiveness of Sphaeranthus suaveolens (Forssk) DC powder in the control of bean bruchid (Acanthoscelides obtectus) (Coleoptera: Chrysomelidae) in stored common bean (Phaseolus vulgaris L.) seeds. J Nat Pest Res 2:100016
Li C, Ni D (2009) Effect of fluoride on chemical constituents of tea leaves. Fluoride 42(3):237
Li R, Shi F, Fukuda K, Yang Y (2010) Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci Plant Nutr 56:725–733
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Curr Protoc Food Anal Chem 1(1):F4-3
Loganathan P, Vigneswaran S, Kandasamy J, Naidu R (2013) Defluoridation of drinking water using adsorption processes. J Hazard Mater 248:1–19
Maggini R, Benvenuti S, Leoni F, Pardossi A (2018) Terracrepolo (Reichardia picroides (L.) Roth.): wild food or new horticultural crop? Sci Hortic 240:224–231
Maggio A, Reddy MP, Joly RJ (2000) Leaf gas exchange and solute accumulation in the halophyte Salvadora persica grown at moderate salinity. Environ Exp Bot 44:31–38
Maitra A, Kumar Datta J, Mondal NK (2016) Bioremediation through the use of indigenous natural resources vis-a-vis its impact on morphology, metabolism, yield, soil health and soil biodiversity of paddy field under fluoride toxicity. Int J Environ Agric Res 2:111–133
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, Cambridge
Massa D, Melito S (2019) Signaling molecules in ecophysiological response mechanisms of salt-stressed plants. In: Khan MIR, Reddy PS, Ferrante A, Khan NA (eds) Plant signaling molecules: role and regulation under stressful environments. Woodhead Publishing, Sawston, pp 1–18
Massa D, Mattson NS, Lieth HJ (2009) Effects of saline root environment (NaCl) on nitrate and potassium uptake kinetics for rose plants: a Michaelis-Menten modelling approach. Plant Soil 318:101–115
Mondal NK (2017) Effect of fluoride on photosynthesis, growth and accumulation of four widely cultivated rice (Oryza sativa L.) varieties in India. Ecotoxicol Environ Saf 144:36–44
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Othman Y, Al-Karaki G, Al-Tawaha AR, Al-Horani A (2006) Variation in germination and ion uptake in barley genotypes under salinity conditions. World J Agric Sci 2(1):11–15
Panda D (2015) Fluoride toxicity stress: physiological and biochemical consequences on plants. Int J Bioresour Environ Agric Sci 1:70–84
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Front 8:117–124
Ram A, Verma P, Gadi BR (2014) Effect of fluoride and salicylic acid on seedling growth and biochemical parameters of watermelon (Citrullus lanatus). Fluoride 47:49–55
Reddy MP, Kaur M (2008) Sodium fluoride induced growth and metabolic changes in Salicornia brachiata Roxb. Water Air Soil Pollut 188:171–179
Ren C, Li H, Zhang CY, Song XC (2022) Effects of chronic fluorosis on the brain. Ecotoxicol Environ Saf 244:114021
Rizzu M, Tanda A, Canu L, Masawe K, Mtei K, Deroma MA, Roggero PP, Seddaiu G (2020) Fluoride uptake and translocation in food crops grown in fluoride-rich soils. J Sci Food Agric. https://doi.org/10.1002/jsfa.10601
Rocha RA, Rojas D, Clemente MJ, Ruiz A, Devesa V, Vélez D (2013) Quantification of fluoride in food by microwave acid digestion and fluoride ion-selective electrode. J Agric Food Chem 61:10708–10713
Ropelewska E, Dziejowski J, Zapotoczny P (2016) Changes in the microbial activity and thermal properties of soil treated with sodium fluoride. Appl Soil Ecol 98:159–165
Ruan JY, Ma LF, Shi YZ, Han WY (2004) The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.). Ann Bot 93(1):97–105
Sabal D, Khan TL, Saxena R (2006) Effect of sodium fluoride on cluster bean (Cyamopsis tetragonoloba) seed germination and seedling growth. Fluoride 39(3):228
Sahariya A, Bharadwaj C, Emmanuel I, Alam A (2021) Fluoride toxicity in soil and plants: an overview. Asian J Adv Res 8:26–34
Saini P, Khan S, Baunthiyal M, Sharma V (2013) Effects of fluoride on germination, early growth and antioxidant enzyme activities of legume plant species Prosopis juliflora. J Environ Biol 34:205–209
Samal AC, Bhattacharya P, Mallick A, Ali MM, Pyne J, Santra SC (2015) A study to investigate fluoride contamination and fluoride exposure dose assessment in lateritic zones of West Bengal, India. Environ Sci Pollut Res 22:6220–6229
Singh J, Sastry EVD, Singh V (2012) Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol Mol Biol Plants 18:45–50
Singh G, Kumari B, Sinam G, Kumar N, Mallick S (2018) Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective—a review. Environ Pollut 239:95–108
Sreedevi R, Damodharam T (2011) Exterminate consequence of NaF on seed germination and some morphological changes of major pulse crop Cicer aritinum L. cv. Anuradha (Bengal gram). Asian J Plant Sci Res 3:38–41
Stevens DP, Mclaughlin MJ, Alston AM (1998) Phytotoxicity of the fluoride ion and its uptake from solution culture by Avena sativa and Lycopersicon esculentum. Plant Soil 200(2):119–129
Tak Y, Asthir B (2017) Fluoride-Induced changes in the antioxidant defences system in two contrasting cultivars of Triticum aestivum L. Fluoride 50(3):324–333
Vithanage M, Rajapaksha AU, Bootharaju MS, Pradeep T (2014) Surface complexation of fluoride at the activated nano-gibbsite water interface. Colloids Surf A 462:124–130
Wang M, Liu L, Chen D, Hamid Y, Shan A, Chen Z, Yu S, Feng Y, Yang X (2022) Fluorine in 20 vegetable species and 25 lettuce cultivars grown on a contaminated field adjacent to a brick kiln. Environ Geochem Health 45(5):1655–1667
Weber E, Bleiholder H (1990) Explanations of the BBCH decimal codes for the growth stages of maize, rape, faba beans, sunflowers and peas-with illustrations. Gesunde Pflanzen 42(9):308–321
Weerasooriyagedara M, Ashiq A, Rajapaksha AU, Wanigathunge RP, Agarwal T, Magana-Arachchi D, Vithanage M (2020) Phytoremediation of fluoride from the environmental matrices: a review on its application strategies. Groundw Sustain Dev 10:100349
World Health Organization (1984) Fluorine and fluorides. World Health Organization, Geneva
Yadu B, Chandrakar V, Keshavkant S (2016) Responses of plants to fluoride: an overview of oxidative stress and defense mechanisms. Fluoride 49:293–302
Yang JY, Wang M, Lu J, Yang K, Wang KP, Liu M, Luo H, Pang L, Wang B (2020) Fluorine in the environment in an endemic fluorosis area in Southwest, China. Environ Res 184:109300
Yasar F, Ellialtioglu S, Yildiz K (2008) Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russ J Plant Phys 55:782–786
Yu YQ, Yang JY (2020) Health risk assessment of fluorine in fertilizers from a fluorine contaminated region based on the oral bioaccessibility determined by biomimetic whole digestion-plasma in-vitro method (BWDPM). J Hazard Mater 383:121124
Zhang XC, Gao HJ, Wu HH, Yang TY, Zhang ZZ, Mao JD, Wan XC (2015) Ca2+ and CaM are involved in Al3+ pretreatment-promoted fluoride accumulation in tea plants (Camellia sinesis L.). Plant Phys Biochem 96:288–295
Zouari M, Elloumi N, Bellassoued K, Ben Ahmed C, Krayem M, Delmail D, Elfeki A, Ben Rouina B, Ben Abdallah F, Labrousse P (2017) Enzymatic antioxidant responses and mineral status in roots and leaves of olive plants subjected to fluoride stress. S Afr J Bot 111:44–49
Acknowledgements
The authors would like to thank the pedology laboratory team of the University of Sassari, especially Mario Deroma and Linda Canu, for technical support.
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement. Part of this work was supported by EU H2020 FLOWERED project 690378 “de Fluoridation technologies for improving quality of WatEr and agRo—animal products along the East African Rift Valley in the context of aDaptation to climate change”.
Author information
Authors and Affiliations
Contributions
SC—data collection. SC, SM, VG—manuscript drafting, editing, revising and figure plotting. GS—funding acquisition, statistical analysis support, manuscript draft editing. PPR—conceptualization, manuscript draft editing.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Communicated by Zsófia Bánfalvi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chahine, S., Melito, S., Giannini, V. et al. Fluoride stress affects seed germination and seedling growth by altering the morpho-physiology of an African local bean variety. Plant Growth Regul 102, 339–350 (2024). https://doi.org/10.1007/s10725-023-01064-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01064-3