Abstract
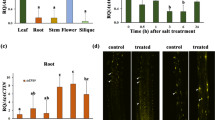
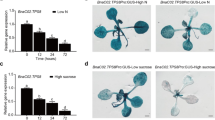
In this work, we expressed an Arabidopsis thaliana-coded protein (AKR4C9) in transgenic barley to study its enzymatic activity and to enhance the reactive aldehyde neutralizing capacity (part of the oxidative stress tolerance) of transgenic plants. Total leaf protein was extracted from transgenic plants expressing either C or N-terminally His-tagged aldo–keto reductase (AKR) enzyme and purified by affinity chromatography. The Arabidopsis-coded enzyme showed moderate activity against the synthetic reactive aldehyde, glutaraldehyde, and low but detectable enzyme activity against fructose with a low Michaelis–Menten constant (Km value). Activity of the C and the N-terminally His-tagged AKRs were found to be in the same range. Glutaraldehyde was also tested in vivo by spraying onto the leaves of the plants. The reactive aldehyde tolerance of both wild type and transgenic plants, as well as the general physiological effects of this reactive aldehyde treatment were evaluated. The growth rate was found to decrease in all (both wild type and transgenic) plants. The high AKR-expressing transgenic plants showed a lower respiratory rate, and they also showed higher fresh weight, higher chlorophyll content and photosynthetic activity, indicating a higher reactive aldehyde tolerance. Cadmium (Cd) treatment was also performed to validate this result. Cd caused strong lipid peroxidation; however, the Arabidopsis enzyme lowered the reactive aldehyde content as expected. This is the first report in which kinetic parameters of the fructose reduction by the stress inducible plant AKR enzyme are presented. Furthermore, data on the effects of a reactive aldehyde treatment on intact plants are also provided.





Similar content being viewed by others
References
Bartels D, Engelhardt K, Roncarati R, Schneider K, Rotter M, Salamini F (1991) An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J 10:1037–1043
Bartoli CG, Simontacchi M, Guiamet JJ, Montaldi E, Puntarulo S (1995) Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci 104:161–168
Browning JD, Horton JD (2004) Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114:147–152
Éva C, Csóti I, Tamás L (2008) Agrobacterium-mediated barley transformation. Acta Biol Szegediensis 52:49–51
Fodor F, Cseh E, Varga A, Záray G (1998) Lead uptake, distribution, and remobilization in cucumber. J Plant Nutr 21:1363–1373
Gavidia I, Pérez-Bermúdez P, Seitz HU (2002) Cloning and expression of two novel aldo–keto reductases from Digitalis purpurea leaves. Eur J Biochem 269:2842–2850
Jacoby RP, Taylor NL, Millar AH (2011) The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci 16:614–623
Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM (1997) Comparative anatomy of the aldo–keto reductase superfamily. Biochem J 326:625–636
Kasai K, Fukayama H, Uchida N, Mori N, Yasuda T, Oji Y, Nakamura C (1998) Salinity tolerance in Triticum aestivum–Lophopyrum elongatum amphiploid and 5E disomic addition line evaluated by NaCl effects on photosynthesis and respiration. Cereal Res Commun 26:281–288
Kovács E, Nyitrai P, Czövek P, Óvári M, Keresztes Á (2009) Investigation into the mechanism of stimulation by low-concentration stressors in barley seedlings. J Plant Phys 166:72–79
Lee T-J, Lee J-T, Moon S-K, Kim C-H, Park J-W, Kwon TK (2006) Age-related differential growth rate and response to 4-hydroxynonenal in mouse aortic smooth muscle cells. Int J Mol Med 17:29–35
Leonarduzzi G, Arkan MC, Basaga H, Chiarpotto E, Sevanian A, Poli G (2000) Lipid oxidation products in cell signalling. Free Radic Biol Med 28:1370–1378
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-(Delta Delta C(T)) method. Methods 25:402–408
Mano J, Belles-Boix E, Babiychuk E, Inzé D, Torii Y, Hiraoka E, Takimoto K, Slooten L, Asada K, Kushnir S (2005) Protection against photooxidative injury of tobacco leaves by 2-alkenal reductase. Detoxication of lipid peroxide-derived reactive carbonyls. Plant Phys 139:1773–1783
Narawongsanont R, Kabinpong S, Auiyawong B, Tantitadapitak C (2012) Cloning and characterization of AKR4C14, a rice aldo–keto reductase, from Thai Jasmine rice. Protein J 31:35–42
Noiraud N, Maurousset L, Lemoine R (2001) Transport of polyols in higher plants. Plant Phys Biochem 39:717–728
Nyitrai P, Bóka K, Gáspár L, Sárvári É, Lenti K, Keresztes Á (2003) Characterization of the stimulating effect of low-dose stressors in maize and bean seedlings. J Plant Phys 160:1175–1183
Oberschall A, Deák M, Török K, Sass L, Vass I, Kovács I, Fehér A, Dudits D, Horváth GV (2000) A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J 24:437–446
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Polle A, Schützendübel A (2004) Heavy metal signalling in plants: linking cellular and organismic responses. In: Shinozaki K, Hirt H (eds) Plant responses to abiotic stress. Springer, Berlin, pp 187–215
Porra PR, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficient and simultaneous equations for essaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Sasahara H, Izumori K (2005) Production of L-sorbitol from l-fructose by Aureobasidium pullulans LP23 isolated from soy sauce mash. J Biosci Bioeng 100:223–226
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211
Sheveleva EV, Marquez S, Chmara W, Zegeer A, Jensen RG, Bohnert HJ (1998) Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco high amounts of sorbitol lead to necrotic lesions. Plant Phys 117:831–839
Simpson PJ, Tantitadapitak C, Reed AM, Mather OC, Bunce CM, White SA, Ride JP (2009) Characterization of two novel aldo–keto reductases from Arabidopsis: expression patterns, broad substrate specificity, and an open active-site structure suggest a role in toxicant metabolism following stress. J Mol Biol 392:465–480
Solti Á, Gáspár L, Mészáros I, Szigeti Z, Lévai L, Sárvári É (2008) Impact of iron supply on the kinetics of recovery of photosynthesis in Cd-stressed poplar (Populus glauca). Ann Bot 102:771–782
Sree KB, Rajendrakumar CSV, Reddy AR (2000) Aldose reductase in rice (Oryza sativa L.): stress response and developmental specificity. Plant Sci 160:149–157
Sunkar R, Bartels D, Kirch H-H (2003) Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464
Tamás C, Kisgyörgy BN, Rakszegi M, Wilkinson MD, Yang MS, Láng L, Tamás L, Bedő Z (2009) Transgenic approach to improve wheat (Triticum aestivum L.) nutritional quality. Plant Cell Rep 28:1085–1094
Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259:508–510
Turóczy Z, Kis P, Török K, Cserháti M, Lendvai A, Dudits D, Horváth GV (2011) Overproduction of a rice aldo–keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol Biol 75:399–412
Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA (1992) Reduction of trioses by NADPH-dependent aldo–keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem 267:4364–4369
Veena Reddy VS, Sopory SK (1999) Glyoxylase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395
Wang M, Li Z, Matthews PR, Upadhyaya NM, Waterhouse PM (1998) Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. Acta Hort 461:401–408
Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. Cold Spring Harb Protoc. doi:10.1101/pdb.prot4666
Zarkovic K (2003) 4-Hydroxynonenal and neurodegenerative diseases. Mol Aspects Med 24:293–303
Zhang J, Klueva NY, Wang Z, Wu R, Ho THD, Nguyen HT (2000) Genetic engineering for abiotic stress resistance in crop plants. In Vitro Cell Dev Biol Plant 36:108–114
Acknowledgments
The authors are grateful to Gábor V. Horváth (Biological Research Centre, Szeged Hungary) for his scientific advice and for providing the vector-cloned At2g37770.2 gene. We would like to thank Wendy Harwood (JIC, Norwich, UK) for the barley transformation method. We also thank Ming-Bo Wang (CSIRO Plant Industry, Canberra) for the pWBVec8 vector. We acknowledge the valuable help of Ádám Solti (Department of Plant Physiology, ELTE) discussing the results gained on the whole plant. The project was supported by the European Union and co-financed by the European Social Fund (Grant Agreement No. TAMOP 4.2.1/B-09/1/KMR-2010-0003). László Tamás is in receipt of grants from the Hungarian Scientific Fund (OTKA T-46703 and K-67844).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Éva, C., Tóth, G., Oszvald, M. et al. Overproduction of an Arabidopsis aldo–keto reductase increases barley tolerance to oxidative and cadmium stress by an in vivo reactive aldehyde detoxification. Plant Growth Regul 74, 55–63 (2014). https://doi.org/10.1007/s10725-014-9896-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9896-x