Abstract
This study was carried out to determine the genetic relationships and variation levels among currant genotypes and cultivars in Türkiye. Ten currant cultivars and one hybrid Jostaberry cultivar, which are breeding products of Europe but are best known and have high commercial value in Türkiye, were used. One black-fruited currant cultivar from Türkiye was included. In addition, 14 currant genotypes naturally grown in Türkiye were used. In this study, 14 retrotransposon-based inter-primer binding site (iPBS) and 10 microsatellite-based simple sequence repeat (SSR) markers were used for a total of 26 currant samples. By evaluating the allele profiles obtained from PCR and capillary electrophoresis with iPBS and SSR primers, the size range, average number of alleles, total number of alleles, number of polymorphic alleles, polymorphism rate, heterozygosity, polymorphism information content, marker index and discriminating power were determined successfully. In addition, unweighted pair group method with arithmetic mean (UPGMA) dendrograms and principal coordinate analysis (PCoA) were constructed and performed. The amounts of amplification product produced using iPBS and SSR markers for currant species with different fruit colours were also compared. In this study, it was proven that the level of genetic relatedness among currant cultivars and genotypes can be determined with high accuracy by iPBS markers. Moreover, the use of iPBS markers in currants was performed for the first time in this study. On the other hand, with the preferred SSR markers, the level of genetic relatedness among currants was successfully determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currants are botanically classified in the genus Ribes L. (Grossulariaceae family). Currants are shrubs, with more than 150 described species of Ribes L. native to northern Europe, Asia, North America, South America and Northwest Africa (Brennan 1996; Hummer and Barney 2002). According to FAO-2020 data, 687,467 tons of currants were produced worldwide. Russia (436,900 tons), Poland (146,500 tons), Ukraine, the United Kingdom, Germany and France provide 94.4% of the world’s production (FAOSTAT 2022).
Brennan (2008) reported that the taxonomy and phylogeny of the genus Ribes L. have not yet been completed. The cultivated species of the genus Ribes L. were grouped into three subgenera: Coreosma (black currants), Ribesia (red currants) and Grossularia (gooseberries). According to a DNA-based molecular characterization study carried out on a total of 47 Ribes species, it was necessary to differentiate taxa at the same level into two separate subgenera: (1) black currants and red currants and (2) gooseberries (Zdunic et al. 2016). Kissling (2020) reported that the species Ribes petraeum, Ribes spicatum, Ribes rubrum, and Ribes multiflorum belonged in the European Ribes subgenus Ribesia [(Berland.) Jancz. 1907]. In Brennan (1996), while Ribes nigrum and three cultivars of this species (var. europaeum, var. scandinavicum, var. sibiricum) and Ribes ussurience, Ribes bracteousum, and Ribes petiolare were specified as black currant species, Ribes sativum, Ribes patreaum, Ribes rubrum and Ribes multiflorum were defined as red currant species. White and pink are the colour forms of Ribes rubrum (Brennan 2008; Zdunic et al. 2016). As reported in Weigend (2007), the several thousand Ribes L. cultivars can be roughly subdivided into five groups: red currants, white currants, black currants, gooseberries, and currant–gooseberry hybrids (Zdunic et al. 2016).
Onur (2006) reported wild currant species at altitudes up to 1000 m from Artvin to Çanakkale in Northern Anatolia in Türkiye and on the slopes of mountains and in wet areas such as streams at altitudes up to 3000 m in Eastern and Central Anatolia. The currants, which belonged to real grapes in the botanical sense, displayed shrubby stems according to the classification scheme based on stem characteristics. According to the information given in Kendir et al. (2019), there are eight Ribes species in the Flora of Turkey. Among these, the species R. rubrum L. was cultivated, and seven other Ribes L. species [R. petraeum Wulfen (syn. R. biebersteinii Berl. ex. DC.), R. nigrum L., R. uva-crispa L., R. alpinum L., R. orientale Desf., and R. multiflorum Kit. ex. Romer & Schultes, R. anatolica Behçet (endemic)] were found in natural habitats. R. orientale and R. alpinum have been reported as unisexual species, while R. rubrum, R. petraeum and R. multiflorum have been reported as hermaphrodite species (Chamberlain et al. 1972; Chamberlain et al. 1988; Behçet 2001). In addition, the basic chromosome number of all Ribes L. species has been reported to be x = 8 (Zielinski 1953). The cultivars were found to be diploid (2n = 2x = 16), and the mean genome size was 2C = 1.91 pg (R. petraeum Wulf., R. rubrum and R. grossularia L.) (Chiche et al. 2003; Palmieri et al. 2013).
Globally, the relationship between the consumption of wild edible fruits and human health in Türkiye has become increasingly important for consumers. Today, the demand for natural, healthy, delicious, functional foods and fruits is quite high (Ercisli 2007). Currant species were among the best examples. Edible wild fruit species, including currant species (Ribes L.), are very genetically rich. Red-fruited currants and especially the black-fruited currants have very valuable components that affect human health. These fruits are preferred for fresh consumption in the food industry as well as in the pharmacology and cosmetics industries. Therefore, these plant species have high economic value. The propagation and growth of currants are not difficult, and they are important alternative products for producers. Their commercial value can be improved further if new cultivars of better quality and greater productivity are obtained through breeding research. In Türkiye, sales of foreign breeding products such as Rovada, Red Lake, Titania, Jonkheer van Tets, Goliath and White Pearl are common. Currants are mostly preferred as hobby plant products in home gardens and are sometimes sold by some manufacturers. Although some research has been carried out on Ribes L. species in this country, these investigations have generally involved morphological and biochemical evaluations.
As elsewhere around the world, plant genetic resources in our country are at risk of decreasing or even disappearing due to environmental and other pressures in the regions where they are located. Molecular characterization for breeding research is important, as is in situ or ex situ conservation of plant genetic resources. A molecular characterization study has been planned as a preliminary study to support breeding research of currant genetic resources in Türkiye. For this purpose, iPBS and SSR marker systems were preferred. Thus, the effectiveness levels of these two marker systems for currants will be compared.
Materials and methods
Plant materials
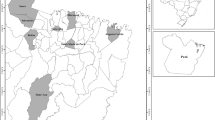
In this study, 10 currant cultivars (R. nigrum, R. rubrum) and one hybrid jostaberry cultivar (R. nigrum × nidigrolaria), which are breeding products of European countries but are the best known and have high commercial value in Türkiye, were used. One black currant cultivar from Türkiye was included. In addition to these cultivars, 14 currant genotypes naturally grown in the eastern Black Sea region and the northern parts of the Eastern Anatolian Region of Türkiye were used. The species, fruit colour, origin and pedigree information of the currant cultivars and genotypes used in the study are given in Table 1. In Fig. 1, the location information of 14 currant genotypes obtained from northeastern Türkiye is presented. Young leaf samples were taken from the currant plants, which first emerged in the spring (April–May 2022). The plant materials were transported to Atatürk University, Faculty of Agriculture, Agricultural Biotechnology Laboratory, under cold chain conditions and stored in a deep freezer (−80 °C).
DNA isolation and analysis
Genomic DNA was extracted from young leaf tissues. The DNA isolation protocol modified from Li and Quiros (2001) was used. For each sample, 0.3 g of plant material was digested with liquid nitrogen and prepared for DNA isolation. 1000 µl of DNA extraction buffer (containing 0.2% β-mercaptoethanol and 0.2 g PVP) was added to the plant material in a 2 ml tube. After the isolation steps were completed, the DNA was washed with 70% ethanol and dissolved in 100 ml of 1X TE buffer. The DNA was suspended at + 4 °C for 24 h and stored at −20 °C. The DNA quantity and purity of the samples were determined. For this purpose, optical densitometric spectrophotometer (NanoDrop, ND-1000) readings were obtained at 260 and 280 nm. The DNA concentrations were adjusted to 50 ng/µl. The DNA was stored at −20 °C. For the qualitative determination of DNA samples, electrophoresis was performed at 60 V on a 0.8% (w/v) agarose gel prepared with 1X TBE buffer.
iPBS-PCR analysis
Fourteen iPBS primers specific to retrotransposon sequences designed by Kalendar et al. (2010) were used in this study (Table 2). Gradient PCR was performed between 50 and 60 °C to determine the annealing temperatures of the iPBS primers. The PCR steps were performed in the following order: 5 min at 95 °C for predenaturation, then 1 min at 94 °C for denaturation, 1 min 5 s at 50–60 °C for annealing, 1 min at 72 °C for elongation (40 cycles) and 10 min at 72 °C for final elongation. The following PCR components were used in addition to the QIAxcel DNA Kit for all the iPBS primers: 0,01 nM primer, 8 M of Taq DNA polymerase (Amplicon), 5 µl of ultrapure water, and 1 µl of genomic DNA (50 ng/µl). Electrophoresis of the PCR products was performed using a QIAxcel DNA Kit (with a QX alignment marker and a QX DNA size marker) on a Qiagen capillary analysis system.
SSR-PCR analysis
A total of 10 SSR loci were used in the study, namely, e2-L15, g1-F04, g3-A17 (Çelenk 2015), e1-O21, g1-I02, e3-B02, g2-G12 (Palmieri et al. 2013), RJL-2, RJL-6 and RJL-11 (Brennan et al. 2002; Antonious et al. 2012) (Table 3). Gradient PCR was performed between 50 and 60 °C to determine the annealing temperatures of the SSR primers. PCR steps were performed in the following order: 3 min at 95 °C for predenaturation, then 3 s at 94 °C for denaturation, 1 min at 57 °C for annealing, 1 min at 72 °C for elongation (35 cycles) and 30 min at 72 °C for final elongation. The following PCR components were used in addition to the QIAxcel DNA Kit for all SSR primers: 0,01 nM primer, 8 M of Taq DNA polymerase (Amplicon), 5 µl of ultrapure water, and 1 µl of genomic DNA (50 ng/µl). Electrophoresis of the PCR products was performed using a QIAxcel DNA Kit (with a QX alignment marker and a QX DNA size marker) on a Qiagen capillary analysis system.
Scoring and data analysis for iPBS and SSR markers
The results of analyses at the molecular level using DNA-based markers can be evaluated using different statistical programs and methods. NTSYSpc-2.2 (Numerical Taxonomy Multivariate Analysis System), which is one of the statistical programs used in data analysis and is based on a numerical system, can be used for multivariate data (Rohlf 2000). The matrix is formed by using “1” for the presence of characters and “0” for the absence of characters in the variables. In the program, dendrogram construction and principal component analyses (2D and 3D) can be performed. Dendrograms are a representation of data divided into various levels, and vertical lines represent clusters that have come together. The positions of the lines in dendrograms indicate the distances at which the clusters come together. With cluster analysis, it is possible to group similar observations into a series of clusters based on the observed values of multiple variables for each individual. The principal component analysis (PCA) method is an orthogonal (vertical) transformation in which a set of possibly related variables are converted into a set of linearly unrelated variables called principal components (Ekinci 2020). Principal coordinate analysis (PCoA) is a multidimensional scaling method used to explore and visualize similarities or differences in data. PCoA is a generalization of PCA and involves measuring the similarity between variables. It starts with a similarity matrix or a dissimilarity matrix (distance matrix) and assigns a location for each element in a low-dimensional space as a 3D graphic (Karimizedeh et al. 2019).
The genetic similarity values among individuals were analysed using NTSYS (Rohlf 2000). The similarity coefficients and clustering analysis of plant samples were calculated and performed by using the Jaccard (1908) similarity coefficient with the help of UPGMA (unweighted pair group method with arithmetic mean) (Nei 1983). The polymorphism rate was calculated with the following formula: Polymorphism (%) = (Number of polymorphic bands/Total number of bands) × 100.
In addition, some indices of polymorphism were determined using the iMEC online tool (iMEC 2023). Among the IMEC indices, the value of heterozygosity (Havp) calculated for polymorphic markers was determined by the formula Havp = Σ Hn/np (observed heterozygosity). The polymorphism information content (PIC) was used to measure the information level of a genetic marker and was calculated with the following formula: PIC = 1—Σ pi2—Σ Σ pi2 pj2. This index differs from heterozygosity, expressing the value of a marker in detecting polymorphism based on the number of alleles and the distribution of their frequencies. The marker index (MI) was the product of the effective multiplex ratio and the mean heterozygosity for polymorphic markers and was determined by the formula MI = E Havp. The discriminating power (D) was determined by the formula D = 1—C. This index is based on the distribution of alleles in the sampled individuals and is strongly associated with the ability to distinguish among analysed samples. These indices are based on dominant and codominant DNA fingerprint markers. They allow the comparison of genetic markers and selection of the most suitable ones for a given dataset (Amiryousefi et al. 2018).
Results and discussion
There have been many investigations on currants (Ribes L.), especially in Europe. To date, the many currant cultivars that have been developed in terms of certain characteristics by selection and hybridization programs from their natural environments have been introduced and used with high commercial value (Hummer and Barney 2002). Plant genetic resources are the most important natural resource of countries. Türkiye displays rich biodiversity for many temperate- and subtropical-climate fruit species due to its geographical location and different climate and soil characteristics. However, due to interest in other fruit species (apple, cherry, almond, pistachio, olive, walnut, citrus species, etc.) with high economic value in this country, very few hybridization investigations have been carried out on currant species. The selection research on currants was mostly carried out as phenotypic and biochemical evaluations of genotypes in their natural environment.
Among the molecular characterization research carried out in currant species to date, efforts such as determining the genetic diversity levels of currant populations grown in their natural habitats, determining genetic relationships among currant cultivars that are the products of breeding research, genetic linkage mapping (Brennan et al. 2008; Pikunova et al. 2020) and genotyping by sequencing (Russell et al. 2014) can be found. In all these investigations performed at the molecular level, DNA-based RAPD (Lanham et al. 2000; Keller-Przyblkowicz et al. 2006; Huo et al. 2013; Butiuc-Keul et al. 2015; Lanham and Brennan 1999; Debnath 2015), AFLP (Lanham and Brennan 1999; Mattia et al. 2008; Brennan et al. 2008; Ipek et al. 2010), ISSR (Lanham et al. 2000; Keller-Przyblkowicz et al. 2006; Lanham and Brennan 1999; Debnath 2015; Gentili et al. 2015), SSR (Lanham and Brennan 1998; Brennan et al. 2002; Brennan et al. 2008; Sordo et al. 2008; Cavanna et al. 2009; Russell et al. 2011; Antonius et al. 2012; Palmieri et al. 2013; Gentili et al. 2015; Butiuc-Keul et al. 2015; Çelenk 2015; Mezhnina and Urbanovich 2017; Pikunova et al. 2019; Vidyaginan et al. 2021; Lacis et al. 2021), SNP (Sordo et al. 2008; Russell et al. 2011), cpSSR (Mattia et al. 2008), SCAR (Butiuc-Keul et al. 2015), and EST-SSR (Debnath 2015) marker techniques and methods were preferred, and the investigations were concluded successfully.
Results of iPBS analysis
Retrotransposons (Class-I transposons) are mobile genetic elements that can move within the genome by copying themselves through an RNA product and can be located in another location of the genome, resulting in an increase in the physical size of the genome. They cause changes in the amount of DNA in the genome and increase genetic diversity (Kalendar and Schulman 2006). Kalendar et al. (2010) developed a universal retrotransposon marker system called inter-Primer Binding Site (iPBS). In this system, retrotransposons can be found at different positions. The long tandem repeat (LTR) sequences are often located next to another retrotransposon, in which the primer binding site (PBS) sequences are often located next to the other PBS. In regions where the presence of retrotransposons is high in the genome, PBS sequences can be used because of the possibility of coexisting with other retrotransposon structures (Kuras et al. 2013; Orhan 2020). The iPBS method is an important method for characterizing and defining genetic diversity and relationships in plant species (Kalendar et al. 2010, 2011; Kalendar 2011; Kalendar and Schulman 2014; Nadeem et al. 2018).
The size ranges (SRs) of the amplification products obtained with the iPBS primers are given in Table 2. The minimum size range (18–500 bp) was obtained with the iPBS 2398 primer, while the largest size range was obtained with the iPBS 2228 (21–979 bp) and iPBS 2381 (22–979 bp) primers. The total number of alleles (Na) and the total number of polymorphic alleles (Npa) obtained from 14 iPBS markers for 26 currant samples were 4954 alleles (avg. 353.86 alleles). Therefore, the polymorphism rate (P) was determined to be 100% (Table 2). The average number of alleles (avNa) for the 26 currant samples was 13.63. This value was highest for the primers iPBS 2274 (24.58), iPBS 2373 (21.08) and iPBS 2387 (18.96).
According to the results, the mean heterozygosity (Havp) value was highest for the iPBS 2415 primer (0.277) and lowest for the iPBS 2246 (0.158) and iPBS 2376 (0.159) primers. The highest polymorphism information content (PIC) value was determined to be 0.175 for the iPBS 2246 primer, while the lowest PIC value was determined to be 0.157 for the iPBS 2415 primer (avg. 0.172). While the marker index (MI) values (avg. 0.181) were between 0.277 (iPBS 2415) and 0.158 (iPBS 2246), the discriminating power (D) values (avg. 0.182) were between 0.193 (iPBS 2373) and 0.159 (iPBS 2376) (Table 2).
According to the UPGMA dendrogram obtained with iPBS markers in our study and presented in Fig. 2, the genetic similarity among currant samples can be expressed as follows: (1) The genotypes of G13 (GC1) and G14 (GC2) (R. aureum) obtained from different geographical environments were determined to be the two most similar genotypes, with a similarity coefficient of 0.989. The hybrid jostaberry cultivar (R. nigrum × nidigrolaria) was determined to have a similar genetic relationship with these two genotypes, with a similarity coefficient value of 0.982. The black-fruited Goliath (0.969 similarity coefficient with jostaberry), G11 (EC1) (0.967 similarity coefficient with jostaberry), Titania and Wellington (R. nigrum) cultivars were determined closely related to these currant samples (mentioned above). (2) On the other hand, the samples of black-fruited (R. nigrum) [Ben Sarek, Tokat-3, G9 (ST2) and G8 (ST1)], red-fruited (R. rubrum) [Rovada, G4 (TV4), Jonker van Tets, G5 (UG1), G3 (TV3), G6 (UG2), G10 (GS2), G1 (TV1) and Rosetta] and white-fruited (R. rubrum) (White Pearl and Blanka) currants with varying similarity coefficient values were determined to be close relatives. (3) The red-fruited Red Lake cultivar together with the red-fruited genotypes G2 (TV2), G12 (EC2) and G7 (GS1) showed the lowest genetic similarity values in the dendrogram, except for the cultivars and genotypes mentioned above. The PCoA results obtained in the analysis of iPBS markers and the genetic similarities/differences among the 26 currant samples are shown in Fig. 3, and this PCoA was found to support the data obtained from the dendrogram (Fig. 2).
In this study, the amounts of amplification product produced using iPBS markers for currant species with different fruit colours were also compared (Table 4). According to the data in this table, for a total of 26 samples used in the study, the number of amplification products obtained from the red- and white-fruited cultivars and genotypes (R. rubrum) was greater than that from the black-fruited cultivars and genotypes (R. nigrum). While an average of 16.51 amplification products were detected in red-fruited currants, an average of 16.89 amplification products were detected in white-fruited currants. On the other hand, the number of amplification products in black-fruited currants, the hybrid jostaberry cultivar and golden currant genotypes (R. aureum) were 11.56, 4.50, and 3.11, respectively.
The use of retrotransposon-based iPBS markers for currant samples obtained from Türkiye was carried out for the first time in this study. iPBS markers have been successfully used in other fruit species such as grape (Guo et al. 2014), guava (Mehmood et al. 2013), and apricot (Baránek et al. 2012). According to the results of our study, the degree of genetic relatedness among currant samples can be determined with high accuracy by iPBS markers, which are DNA-based molecular markers.
Results of SSR analysis
(SSR) simple sequence repeat, microsatellite markers are composed of 2–6 repeated nucleotide groups that are distributed throughout eukaryotic genomes. Since SSRs are highly polymorphic, they provide much information about plants. In addition, the fact that they exhibit codominance and are easy to combine with PCR are among the reasons for them being highly preferred (Gupta et al. 1994; Röder et al. 1995; Yorgancılar et al. 2015). SSRs are widely distributed in plant genomes. The information based on such repeats more accurately represents the entire genome than that from other markers, as SSRs contains species-specific conserved domains. PCR is performed by developing specific primers that provide information specific to repetitive regions (Ganopoulos et al. 2011; Orhan 2020).
Table 3 shows the size ranges (SRs) of the amplification products obtained using the SSR primers in our study. The minimum size range was obtained for the RJL-11 primer (18–336 bp), while the largest size range was obtained for the e1-O21 primer (17–982 bp). While the total number of alleles (Na) and the total number of polymorphic alleles (Npa) obtained by amplification of SSR primers were 2475 alleles, the polymorphism rate (P) for currant samples was 100% (the same rate determined from iPBS markers). This high rate is thought to be related to the use of capillary electrophoresis. With the use of ten SSR primers, an average of 247.5 alleles were obtained for all the currants. Among the SSR primers with the greatest number of alleles, the g1-F04 primer produced a total of 311 alleles (avg. 11.96 alleles), and the e3-B02 primer produced a total of 300 alleles (avg. 11.54 alleles). The SSR primer that gave the fewest alleles was RJL-11 (avg. 6.62 alleles), with a total of 172 alleles. However, the average number of alleles (avNa) for the 26 currant samples ranged from 6.62 (RJL-11) to 11.96 (g1-F04) (avg. 9.52).
The mean heterozygosity (Havp) value was determined to be between 0.336 (RJL-6) and 0.213 (g3-A17) (avg. 0.243). The PIC values obtained with the SSR markers ranged from 0.207 (RJL-6) to 0.232 (g3-A17) (Table 3). In the same table, the marker index (MI) values ranged between 0.213 (g3-A17) and 0.336 (RJL-6) (avg. 0.244). The discriminating power (D) was between 0.212 (g3-A17) and 0.292 (RJL-6) (avg. 0.228).
The results obtained in our study were similar to those of many investigations using SSR markers reported in the literature. For example, Brennan et al. (2002) characterized Ribes nigrum L. samples using 11 RJL-encoded SSR markers. Among these markers, highly polymorphic results were obtained with the RJL2, RJL6 and RJL11 markers, which were also preferred in our study. With these markers, the researchers determined the allele size to be 207 bp, 291 bp and 215 bp; the number of alleles to be 16, 13 and 10; and the observed heterozygosity to be 0.555, 0.462 and 0.538, respectively. A successful genetic diversity analysis with 10 SSR markers with RJL coding was also performed by Antonious et al. (2012) for a total of 846 Ribes L. samples (400 R. nigrum, 202 R. rubrum, 242 R. uva-crispa and 2 jostaberry) from Northern Europe. Using the RJL2 marker (which was also preferred in our study), these researchers obtained alleles with sizes ranging from 197 to 238 bp, 192–252 bp and 205–279 bp for R. nigrum, R. rubrum, and R. uva-crispa, respectively. With the RJL6 marker, alleles ranging from 191 to 306 bp, 276–301 bp, and 215–308 bp in size were obtained for these species, respectively. For the same species, alleles with sizes ranging from 205 to 229 bp, 205–229 bp, and 229–263 bp were obtained with the RJL11 marker, respectively. Furthermore, Palmieri et al. (2013) reported that the observed heterozygosity and discriminating power values for 91 Ribes L. samples (85 cultivars and 6 selection products) were similar to those reported by Mezhnina and Urbanovich (2017). These researchers determined the observed heterozygosity (Ho) value to be 0.50 and the discriminating power to be 0.61 with the e3-B02 primer and the observed heterozygosity (Ho) value to be 0.84 and the discriminating power value to be 0.76 with the g2-G12 primer. In addition, heterozygosity (Ho) and discriminating power values were observed using the e1-O21 and g1-I02 primers (preferred in our study), which were determined to be 0.62 and 0.73 with the e1-O21 primer and 0.96 and 0.78 with the g1-I02 primer, respectively. On the other hand, the observed heterozygosity (Ho), effective allele number and discriminating power values obtained with the e3-B02 and g2-G12 markers (also used in our study) for the 65 Ribes L. samples by Mezhnina and Urbanovich (2017) were 0.508, 2.3 and 0.67 for the e3-B02 primer and 0.758, 8.2 and 0.95 for the g2-G12 primer, respectively. In a previous study conducted in Türkiye, Çelenk (2015) used 10 SSR markers in 5 Ribes alpinum L., 2 Ribes bieberstenii Berl. ex DC, 1 Ribes uva-crispa L. and 2 Ribes rubrum L. samples. In contrast to our study, in their study, taxonomic and phylogeographic relationships were investigated, and successful results were obtained.
In our study, according to the UPGMA dendrogram (Fig. 4) generated with SSR markers, the genetic similarity among currant cultivars and genotypes from different Ribes L. species can be expressed as follows: (1) the hybrid jostaberry cultivar (R. nigrum × nidigrolaria) and G11 (EC1) genotype (R. nigrum) were determined as the most similar samples (similarity coefficient, 0.909). The black-fruited Goliath cultivar (R. nigrum) and the cream–reddish-fruited genotype G13 (GC1) (R. aureum) were identified as two other examples with high similarity (similarity coefficient, 0.901). The G14 (GC2) genotype (R. aureum) showed similarity to these four samples and formed a subgroup (Subgroup-I) with them in the dendrogram.(2) On the other hand, the black-fruited cultivars Titania and Wellington (R. nigrum) were determined to be the two most closely related cultivars (similarity coefficient of 0.888). The red-fruited Jonker van Tets (R. rubrum) cultivar was similar to these two black-fruited cultivars and formed another subgroup with them in the dendrogram (Subgroup II).(3) The red-fruited Rovada cultivar (R. rubrum) and G6 (UG2) genotype (R. rubrum) were determined to be currants with similarity to each other (similarity coefficient, 0.865). These two cultivars were similar to Subgroup-I and Subgroup-II and formed a single group with them. On the other hand, the white-fruited cultivars White Pearl and Blanka (R. rubrum) (similarity coefficient, 0.866), which were determined to be similar to each other, were found to have high genetic similarity with this group. However, the red-fruited Red Lake cultivar (R. rubrum) and the G12 (EC2) and G1 (TV1) (R. rubrum) genotypes were also found to be closely related to this group. (4) All of the other black-fruited and red-fruited currant samples used in this study were found to have a certain genetic relationship with the group described above, with different similarity coefficient values. Furthermore, the PCoA results obtained by the analysis of SSR markers and the genetic similarities/differences among the 26 currant samples are shown in Fig. 5, and the PCoA results were found to support the data obtained from the dendrogram (Fig. 4).
As with the iPBS markers, a quantitative comparison of the amplification products obtained from the SSR primers was made for the currant samples with different fruit colours used in the study (Table 5). According to the data in this table, for the 26 samples used in this study, the number of amplification products obtained from the red- and white-fruited currant samples was greater than that from the black-fruited currant samples (avg. 9.20). While an average of 10.03 amplification products were detected in the red-fruited currant samples, an average of 10.35 amplification products were detected in the white-fruited currant samples. The numbers of amplification products of the hybrid jostaberry cultivar and golden currant genotype (R. aureum) were relatively low (avg. 7.40 and 8.10, respectively). As a matter of fact, as a result of a study conducted by Palmieri et al. (2013) using SSR markers, more amplification products were detected in red-/white-/pink-fruited currant samples (avg. 6.6) than in black-fruited samples (avg. 5.5). However, the amount of amplification product determined in our study was found to be quite high.
In addition to the results discussed above, other explanations for the results obtained with iPBS and SSR analysis in this study are given below.
In our study, according to the dendrograms obtained from both iPBS and SSR analyses (Figs. 2 and 4), the red-fruited cultivars Rovada and Jonkheer van Tets were determined to be similar, related cultivars (0.902 similarity coefficient in the iPBS dendrogram; 0.867 similarity coefficient in the SSR dendrogram). According to the information given by Hummer et al. (2018) in Table 1, the genetic similarity of Rovada (‘Fay’s Prolific’ x ‘Heinemann’s Rote Spatlese’ R. rubrum) and Jonkheer van Tets (‘Fay’s Prolific’ seedling R. rubrum) has been proven by the markers used in our study. However, in the dendrogram obtained with iPBS markers (Fig. 2), the red-fruited Red Lake cultivar was determined to be distant from the red-fruited cultivars Rovada (similarity coefficient, 0.862) and Jonkheer van Tets (similarity coefficient, 0.860). According to the dendrogram obtained with SSR markers (Fig. 4), the red-fruited Rosetta cultivar [Jonkheer Van Tests × Heinemann’s Rote Spatlese (Zdunic et al. 2016; Palmieri et al. 2013)] was genetically distant from the Rovada cultivar (similarity coefficient, 0.872) and the Jonkheer van Tets cultivar (similarity coefficient, 0.861). According to the dendrogram obtained with SSR markers (Fig. 4), the red-fruited Red Lake cultivar was also genetically distant (similarity coefficient, 0.856) from the red-fruited cultivars Rovada and Jonkheer van Tets. According to the same dendrogram, Rosetta was distantly related to the Rovada (similarity coefficient, 0.827) and Jonkheer van Tets (similarity coefficient, 0.836) cultivars.
According to the SSR marker-based dendrogram (Fig. 4), the red-fruited genotypes G12 (EC2), G1 (TV1), G6 (UG2), G4 (TV4), G2 (TV2) and G3 (TV3) from Türkiye were found to be genetically closely related to the red-fruited currant cultivars originating from Europe. The red-fruited genotypes G10 (GS2), G7 (GS1) and G5 (UG1) from Türkiye were genetically distant from European-origin cultivars. According to the iPBS dendrogram (Fig. 2), the red-fruited genotypes G4 (TV4), G5 (UG1), G3 (TV3), G6 (UG2), G10 (GS2), and G1 (TV1) from Türkiye were closely genetically related to those originating from Europe. This may be because Türkiye is intercontinental. The areas of wild fruits in Türkiye are mainly concentrated in the Mediterranean, Black Sea, Middle, Eastern, Northeast and South Anatolian regions (Ercisli 2004; Ercisli et al. 2009).
When both the iPBS and SSR dendrograms were reviewed, the black-fruited cultivars Titania, Wellington, and Goliath (European origin) and the genotype G11 (EC1) from Türkiye were found to have close genetic relationships with each other (average similarity coefficient value of 0.888). Additionally, the other black-fruited currant samples [Ben Sarek, Tokat-3, G8 (ST1) and G9 (ST2)] were found to be related to these currants to different degrees. The finding that currant forms with white and pink fruit colours are different colour forms of red-fruited currants (R. rubrum) has been reported in various investigations (Brennan 2008; Zdunic et al. 2016). The results of our study confirmed these findings. In addition, the two Ribes aureum L. genotypes G13 (GC1) and G14 (GC2) used in our study showed close genetic relationships with jostaberry (a hybrid of black currant and gooseberry described in Hummer et al. 2018). Thus, the results obtained in this study contribute to the evaluation of red-/white-/black-fruited currant cultivars and genotypes and even hybrid currant breeding lines.
Conclusion
The use of retrotransposon-based iPBS markers in currants (Ribes spp.) was carried out for the first time in this study. Here, the genetic relationships among the currant materials were determined with iPBS markers. In addition, with the preferred SSR markers used in this study, the genetic relationships among currant plant materials could be determined.
The characterization of hybridization and selection research employing plant breeding methods and rapid evaluation of gene sources are important. SSR and iPBS markers can provide highly accurate results for molecular assessments. Therefore, it is necessary to carry out more comprehensive molecular breeding research on currant species in Türkiye by utilizing the results of this preliminary study.
Data availability
All data generated or analysed during this study are included in this published article.
References
Amiryousefi A, Hyvönen J, Poczai P (2018) iMEC: Online marker efficiency calculator. Appl Plant Sci 6(6):e1159. https://doi.org/10.1002/aps3.1159
Antonius K, Karhu S, Kaldma¨e H, Lacis G, Rugenius R, Baniulis D, Sasnauskas A et al (2012) Development of the Northern European Ribes core collection based on a microsatellite (SSR) marker diversity analysis. Plant Genet Res Charac Util 10(1):70–73. https://doi.org/10.1017/S1479262111000980
Baranek M, Meszaros M, Sochorova J, ˇCechova J, Raddova J, (2012) Utility of retrotransposon-derived marker systems for differentiation of presumed clones of the apricot cultivar Velkopavlovicka. Sci Hort 14:1–6. https://doi.org/10.1016/j.scienta.2012.05.022
Barney DL (2022) Currant cultivars for the northwest & intermountain west. https://www.uidaho.edu//media/UIdahoResponsive/Files/Extension/county/Bonner/horticulture/nifrccurrantcultivars.pdf?la=en&hash=2E18F080F718BB9DB476A88FEBA931A4FEDD593A
Barney DL, Fallahi E (2009) Growing. Currants, gooseberries and jostaberries. In the Inland northwest & intermountain west http://www.extension.uidaho.edu/publishing/pdf/bul/bul0855.pdf
Behçet L (2001) A new species of Ribes L. (Grossulariaceae) from east Anatolia Turkey. Turkish J Bot 25:103–105
Brennan RM (1996) Currants and goosberries. In: Janick J, Moore JN (eds) Fruit breeding vine and small fruit crops, vol 2. Wiley, Newyork USA, pp 191–295
Brennan M (2008) Currants and gooseberries, In: Hancock JF (Ed), Temperate fruit crop breeding (Ch 6) 177−196 https://link.springer.com/chapter/10.1007/978-1-4020-6907-9_6
Brennan RM, Jorgensen L, Woodhead M, Russell J (2002) Development and characterization of SSR markers in Ribes species. Mol Eco Notes 2(3):327–330. https://doi.org/10.1046/j.1471-8286.2002.00233.x
Brennan R, Jorgensen L, Hackett C, Woodhead M et al (2008) The development of a genetic linkage map of blackcurrant (R. nigrum L.) and the identification of regions associated with key fruit quality and agronomic traits. Euphytica 161(1−2):19–34. https://doi.org/10.1007/s10681-007-9412-8
Butiuc-Keul AL, Craciunas C, Dobrota C, Clapa D (2015) Molecular characterization of new black currant cultivars bred in romania exposed to changing climate conditions. (III. International symposium on molecular markers in horticulture), Acta Hort 1100:155−159 https://www.actahort.org/books/1100/1100_24.htm
Cavanna M, Marinoni DT, Beccaro GL, Bounousa G (2009) Microsatellite-based evaluation of Ribes spp. germplasm. Genome 52(10):839–848
Çelenk AG (2015) Molecular characterization of some Ribes L. (Grossulariacea) taxa. Dissertation, University of İstanbul
Chamberlain DF (1972) Grossulariaceae. In: Flora of Turkey and the East Aegean Islands. P.H. Davis (Ed.) Edinburgh University Press, Edinburgh 4:261
Chamberlain DF (1988) Grossulariaceae. In: Flora of Turkey and the East Aegean Islands. Davis PH, Mill RR, Tan K (Ed.) Edinburgh University Press, Edinburgh 10(1):145
Chiche J, Brown SC, Leclerc J-C, Siljak-Yakovlev S (2003) Genome size, heterochromatin organisation, and ribosomal gene mapping in four species of Ribes. Can J Bot 81:1049–1057. https://doi.org/10.1139/b03-088
SC Debnath (2015) Germplasm characterization, propagation and maintenance of health-promoting wild berries and medicinal plant roseroot (Rhodiola rosea L.). International symposium on medicinal plants and natural products. Acta Hort 1098:61–69
Ekinci EH (2020) Molecular characterization of different Cucurbita pepo L. populations with SSR markers. Dissertation, University of Gebze Technical
Ercisli S (2004) A short review of the fruit germplasm resources of Turkey. Gen Res Crop Evol 51:419–435
Ercisli S (2007) Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem 104:1379–1384. https://doi.org/10.1016/j.foodchem.2007.01.053
Ercişli S, Güleryüz M, Orhan E, Ertürk Y, Karlıdağ H (2009) The use of wild edible fruits in sustainable fruit production in Turkey. 1st International Syposium on Sustainable Development (9–10 June 2009), Sarajevo 78−82 https://core.ac.uk/download/pdf/153447216.pdf
FAOSTAT (2022) Data, Currants (11 August 2022). https://www.fao.org/faostat/en/#data/QCL
Ganopoulos IV, Kazantzis K, Chatzicharisis I, Karayiannis I, Tsaftaris AS (2011) Genetic diversity, structure and fruit trait associations in Greek sweet cherry cultivars using microsatellite based (SSR/ISSR) and morpho-physiological markers. Euphytica 181:237−251 https://link.springer.com/article/10.1007/s10681-011-0416-z
Gentili R, Fenu G, Mattana E, Citterio S, De Mattia F, Bacchetta G (2015) Conservation genetics of two island endemic Ribes spp. (Grossulariaceae) of Sardinia: survival or extinction? Plant Biol 17(5):1085–1094
Guo DL, Guo MX, Hou XG, Zhang GH (2014) Molecular diversity analysis of grape cultivars based on iPBS markers. Biochem Sys Eco 52:27–32
Gupta M, Chyi YS, Romero-Severson J, Owen JL (1994) Amplification of DNA markers from evalutionarily diverse genomes using single primers of simple-sequence repeats. Theor Appl Gen 89:998–1006. https://doi.org/10.1007/BF00224530
Hatton RG (2015) Black currant cultivars—a method of classification, preliminary work carried out at the fruit experiment station, east mailing. 145−154. https://www.tandfonline.com/doi/abs/10.1080/03683621.1920.11513223?journalCode=thsb18
Hummer KE, Barney DL (2002) Crop reports, currants. Hort Techno 12(3):377−388 https://pubag.nal.usdagov/download/6280/pdf
Hummer KE, Bushakra JM, Barney DL (2018) Currant and gooseberry crop vulnerability statement https://www.ars-grin.gov/npgs/cgc_reports/Ribes_vulnerability_statement_2018nov.docx
Huo J, Yang G, Zhang Y, Li F (2013) A new strategy for identification of currant (R. nigrum L.) cultivars using RAPD markers. Gen Mol Res 12(2):2056–2067
iMEC (2023) iMEC online tool, online marker efficiency calculator https://irscope.shinyapps.io/iMEC/
Ipek A, Barut E, Gulen H, Ipek M (2010) Genetic diversity among some currants (Ribes spp) cultivars as assessed by AFLP markers. Pak J Bot 42(2):1009–1012
Jaccard P (1908) Nouvelles reserches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Kalendar R (2011) The use of retrotransposon-based molecular markers to analyze genetic diversity. Ratar Povrt Field Veg Crop Res 48:261–274
Kalendar R, Schulman AH (2006) IRAP and REMAP for retrotransposon-based genotyping and fingerprinting. Nat Protoc 1(5):2478–2484
Kalendar R, Schulman AH (2014) Transposon-based tagging IRAP, REMAP, and iPBS. Method Mol Biol book ser MIMB: 1115 https://doi.org/10.1007/978-1-62703-767-9_12
Kalendar R, Antonius K, Smykal P (2010) iPBS: auniversal method for DNA fingerprinting and retro transposonisolation. Theor Appl Genet 121:1419–1430
Kalendar R, Flavell AJ, Ellis TH, Sjakste T, Moisy C, Schulman AH (2011) Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 106(4):520–530
Karimizadeh R, Asghari A, Chinipardaz R, Sofalian O, Ghaffari A, Shahbazi K, Hosseinpour T, Ghogog H, Armion M (2019) Use of principal coordinate analysis formeasuring GE interactions in rain-fed durum wheat genotypes. J Agri Sci 25:38–46
Keller-Przybyłkowicz S, Korbin M, Gwozdedcki J (2006) RAPD and ISSR markers of black and green colour of blackcurrant (R. nigrum) fruits. J Fru Orn Plant Res 14(1):45–52
Kendir G, Köroğlu A, Özek G, Özek T, Başer KHC (2019) Glandular trichome structures and chemical composition of the volatiles of five Ribes species from Turkey. J Ess Oil Res 31(2):111–119. https://doi.org/10.1080/10412905.2018.1547226
Kissling P (2020) A taxonomic history of the European Ribesia. HAL open science https://hal.science/hal-02532032/document
Kuras A, Antonius K, Kalendar R, Kruczy D, Korbin M (2013) Application of five DNA marker techniques to distinguish between five apple (Malus × domestica Borkh.) cultivars and their sports. J Hort Sci Biotech 88(6):790–794. https://doi.org/10.1080/14620316.2013.11513040
Lacis G, Karklina K, Kota-Dombrovska I, Strautina S (2021) Evaluation of blackcurrant (R. nigrum) germplasm structure by microsatellite-based fingerprinting for the diversification of the breeding material. J Berry Res 11(3):497–510
Lanham PG, Brennan RM (1998) Characterization of the genetic resources of redcurrant (Ribes rubrum: subg. Ribesia) using anchored microsatellite markers. Theor Appl Genet 96:917–921. https://doi.org/10.1007/s001220050820.pdf
Lanham PG, Brennan RM (1999) Genetic characterization of gooseberry (Ribes grossularia subgenus Grossularia) germplasm using RAPD, ISSR and AFLP markers. J Hort Sci Biotech 74(3):361–366. https://doi.org/10.1080/14620316.1999.11511122
Lanham PG, Korycinska A, Brennan RM (2000) Genetic diversity within a secondary gene pool for R. nigrum L. revealed by RAPD and ISSR markers. J Hort Sci Biotech 75(4):371–375
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Gen 103(2–3):455–461. https://doi.org/10.1007/s001220100570
Mattia F, Grassi F, Imazio S, Labra M (2008) Chloroplast and nuclear DNA markers to characterize cultivated and spontaneous Ribes. Offi J Soci Bot Ital 142(2):204–212. https://doi.org/10.1080/11263500802150290
Mehmood A, Jaskani MJ, Ahmad S, Ahmad R (2013) Evaluation of genetic diversity in open pollinated guava by iPBS primers. Pak J Agri Sci 50(4):591–597
Mezhnina OA, Urbanovich OY (2017) Study of genetic variability of representatives of Ribes L. grown in Belarus. Cytol Genet 51(6):441–447. https://doi.org/10.3103/S0095452717060081
Nadeem MA, Nawaz MA, Shahid MQ, Dogan Y, Comertpay G, Yıldız M, Hatipoglu R, Ahmad F, Alsaleh A, Labhane N, Ozkan H, Chung G, Baloch FS (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotech Biotechnol Equip 32(2):261–285. https://doi.org/10.1080/13102818.2017.1400401
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data. J Mol Evol 19(2):153–170
Onur C (2006) From berries breeding project to symposiums, 2nd national symposium on Berries, Tokat (14–16 September 2006) pp. 8−10
Orhan E (2020) Review: some molecular breeding issues at genome level in fruit species. Int J Sci Tech Res 6(8):61–83
Palmieri L, Grando MS, Sordo M, Grisenti M, Martens S, Giongo L (2013) Establishment of molecular markers for germplasm management in a worldwide provenance Ribes spp Collection. Plant Omics 6(3):165–174
Pedersen HL (2020) Black and red currant cultivars for organic production https://www.researchgate.net/publication/266885474_Black_and_red_currant_cultivars_for_organic_production
Pikunova AV, Knyazev SD, Golyaeva OD, Bahotskaya AU, Kalinina OV (2019) Genome studies by means of DNA markers of the blackcurrant. Russ J Gen 55(9):1061–1071. https://doi.org/10.1134/S1022795419090102
Pikunova AV, Goryunovab SV, Goryunovd DV, Dolzhikovaa MA, Golyaeva OD (2020) The development of a genetic linkage map of redcurrant (R. rubrum L.) by means of SSR and SNP DNA markers. Russ J Gen 56(11):1387–1390. https://doi.org/10.1134/S1022795420100105
Röder MS, Plaschke P, Konig SU, Borner A, Sorrells ME, Tanksley SD, Ganal MW (1995) Abundance, variability and chromosomal location of microsatellites in wheat. Mol Gen Genet 246:327–333
Rohlf FJ (2000) NTSYS-PC Numerical taxonomy and multivariate analysis system version 2.01, exeter software. Setauket, New York
Russell JR, Bayer M, Booth C, Cardle L, Hackett CA, Hedley PE, Jorgensen L, Morris JA, Brennan RM (2011) Identification, utilisation and mapping of novel transcriptome-based markers from blackcurrant(R. nigrum). BMC Plant Bio 11:147. https://doi.org/10.1186/1471-2229-11-147
Russell J, Hackett C, Hedley P, Liu H, Milne L, Bayer M, Marshall D, Jorgensen L, Gordon S, Brennan R (2014) The use of genotyping by sequencing in blackcurrant (R. nigrum): developing high-resolution linkage maps in species without reference genome sequences. Mol Breed 33:835–849
Sordo M, Palmieri L, Giongo L, Grando MS (2008) Molecular characterization of currant (Ribes spp.) accessions using SNP And SSR markers. Proceedings of the 52nd italian society of agricultural genetics annual congress padova, Italy (14–17 September 2008) (Poster Abstract-F.03) ISBN 978-88-900622-8-5
Vidyagina EO, Lebedev VG, Subbotina NM, Treschevskaya EI, Lebedeva TN, Shestibratov KA (2021) The development of the genic ssr markers for analysis of genetic diversity in gooseberry cultivars. Agronomy Basel 11(6):1050
Yorgancılar M, Yakışır E, Tanur Erkoyuncu M (2015) Moleküler Markörlerin Bitki Islahında Kullanımı. J Bahri Dagdas Crop Res 4(2):1–12
Zdunić G, Šavikin K, Pljevljakušić D, Djordjević B (2016) Black (R. nigrum L.) and Red Currant (R. rubrum L.) Cultivars (Ch 5). Nut Comp Fru Culti 101−126 https://doi.org/10.1016/B978-0-124081178.00005-2
Zielinski QB (1953) Chromosome numbers and meiotic studies in Ribes. Bot Gaz 114:265–274. https://doi.org/10.1086/335768
Acknowledgements
The all molecular analyzes were performed in Atatürk University, Faculty of Agriculture, Agricultural Biotechnology Laboratory. We thanks this laboratuary coordinatorship.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research was supported by Atatürk University Scientific Research Projects Coordination Unit. BAP-FBA-2022–10433.
Author information
Authors and Affiliations
Contributions
This research was conceived by E.O. D.K. performed the experiments. EO analyzed and interpreted all data and wrote the manuscript. EO and DK edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any research with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orhan, E., Kara, D. Molecular characterization of some currants (Ribes species) from Türkiye using inter-primer binding site (iPBS) and simple sequence repeat (SSR) markers. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-01987-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-01987-4