Abstract
Knowledge of genetic diversity within crop species and the nature of their breeding systems are critical for crop improvement. These determine the appropriate species specific breeding methodologies to deploy. Genetic diversity analysis is an ongoing process in the breeding programmes of ‘major crops’, which is used to direct or re-direct breeding objectives (especially selection of parental lines). In this regard, the importance of such information in ‘underutilised’ or ‘minor’ crop species, which largely exist as landraces with little information about their genetic diversity and breeding systems, becomes very important. One such important underutilized crop species which could contribute positively to global food security is Bambara groundnut (Vigna subterranea (L.) Verdc.). We present here an overview of the past two decades of genetic diversity analysis of Bambara groundnut landraces. Various genetic diversity analyses of the available germplasm for the crop using phenotypic descriptors and molecular marker technologies have been reported. Generally, most of these studies lack adequate representation of the available global germplasm. For those studies that involved relatively a large germplasm collections (above 100; sampled from different agro-ecologies) the marker density employed in these analyses has been so far relatively low. Specifically, for breeding systems, high genetic diversity and low heterozygosity have been reported across the germplasm analysed in this highly cleistogamous species. In terms of population structure, the West African and the Southern African accessions appear as distinct clusters. This raises the possibility of the southern African region a secondary centre of domestication or diversity for the crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant breeding is defined as the art of crop improvement (Acquaah 2007). Similar to the practice of an art, the availability of requisite tools and resources as well as the creative act of their combination to achieve identified objectives is critical. Adequate variability within the germplasm of the species is the most important resource needed to achieve breeding objectives in crop improvement programmes (Hawkes 1991; Rao and Hodgkin 2002). Moreover, adequate knowledge of variability within germplasm collections (and the breeding systems itself) has important implications for conservation, management and future utility of germplasm resources (Rao and Hodgkin 2002).
Molecular markers have emerged as power tools in the assessment of genetic variability relative to the conventional approach of using phenotypic descriptors because the former are independent of environmental factors (Agarwal et al. 2008). The use of molecular markers to assess genetic diversity in landraces, breeding lines, varieties, wild relatives and mutant populations to aid breeding decisions in major crops such as rice (Oryza sativa L.) (Zhang et al. 2011; Choudhary et al. 2013), maize (Zea mays L.) (Lu et al. 2011; Wen et al. 2012), soya bean (Glycine max (L.) Merr.) (He et al. 2012) and cowpea (Vigna unguiculata (L.) Walp.) (Huynh et al. 2013), is an important on-going component of their crop improvement. The significance of ‘underutilized’ crop species (also known as ‘minor’, ‘orphan’ or ‘neglected’) for global food security is gradually gaining the attention of the international research community and food security policy organizations (Jaenicke and Höschle-Zeledon 2006; Will 2008; Massawe et al. 2016). Underutilized species exist mostly as landraces and wild collections due to decades of neglect by the scientific community (Williams and Haq 2002; Massawe et al. 2005; Will 2008). To fully harness their potential, knowledge of their breeding systems and genetic diversity within germplasm is important. One such important underutilized legume is Bambara groundnut (Vigna subterranea (L.) Verdc.).
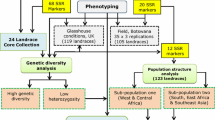
Bambara groundnut is a leguminous crop of African origin and mostly grown with limited inputs by farmers in the semi-arid tropics (Basu et al. 2007b; Massawe et al. 2002, 2007). The desirable agronomic traits of Bambara groundnut such as yield potential (Berchie et al. 2010; BAMFOOD 2012), nutritional composition (Brough and Azam-Ali 1992; Brough et al. 1993) drought tolerance and adaptations to marginal soils (Collinson et al. 1996; Mwale et al. 2007a, b), N-fixation and soil fertility improvement (Kishinevsky et al. 1996; Dakora 1998; Herridge and Rose 2000; Herridge et al. 2008; Mohale et al. 2013) have been documented. The crop largely exists as landraces with few varieties developed through controlled breeding (Massawe et al. 2002; Basu et al. 2007b; Massawe et al. 2007). Various landraces and wild type accessions of the crop are held in trust on behalf of the International community by a number of organizations (Table 1). The ability to develop improved genotypes with desired phenotypic traits that meet the demand of various actors (especially farmers) could be very useful. A conceptual representation of the global germplasm of Bambara groundnut and its utility for crop improvement is presented (Fig. 1).
The importance of using molecular technology to understand breeding systems and to assess genetic diversity within available germplasm collections of underutilized species to enable their effective utilization in breeding programme has been emphasized (Mayes et al. 2011). Specifically for Bambara groundnut, the use of molecular markers to assess the level of inter- and intra-landrace polymorphism in collections of the crop has been noted as critical to enable their effective utilization in future breeding programmes (Massawe et al. 2005, 2007). Various approaches have been used to assess genetic diversity within the available germplasm of Bambara groundnut; phenotypic descriptors (Goli 1995; Olukolu et al. 2012; Aliyu and Massawe 2013; Molosiwa et al. 2015), biochemical markers (Pasquet et al. 1999) molecular markers (Massawe et al. 2002, 2003a, b; Stadler 2009; Olukolu et al. 2012; Aliyu and Massawe 2013; Molosiwa et al. 2015). A number of breeding objectives have been reported in Bambara groundnut (Massawe et al. 2005, 2007; Aliyu et al. 2015). In this regards, matching identified breeding objectives with reported diversity analysis to guide important breeding decisions (particularly selection of parental lines) becomes important. ‘Next’ generation breeding populations [such as multi-parent advanced generation inter-cross (MAGIC)] have appeared as strategic approaches to plant breeding in recent times (Nordborg and Weigel 2008; Kover et al. 2009; Jannink et al. 2010; Bandillo et al. 2013). Specifically for Bambara groundnut, the importance of these ‘next’ generation breeding germplasm resources in achieving the identified breeding objectives within an integrated breeding framework has been highlighted (Aliyu et al. 2015). We aim to present a review of the past two decades of diversity analysis reports on Bambara groundnut global germplasm with particular emphasis on its implications for current and future crop improvement programmes.
The need for a specific molecular ‘tool box’ for Bambara groundnut
Molecular markers have appeared as a power tools to aid and speed up plant breeding (Collard et al. 2005; Collard and Mackill 2008; Kesawat and Das 2009). The recent advances in the next generation sequencing platforms have further strengthened our understanding of the genetics of important phenotypic traits both in the context of forward and in reverse genetics (Alonso and Ecker 2006; Rosenberg et al. 2010). This has increased the efficiency and accuracy with which QTL and underlying candidate genes can be localised using high density markers and novel second/next generation germplasm resources. Against this backdrop, genomic assisted breeding is now a routine practice in the breeding programmes of some major crops. However, for most underutilized crop species, the lack of availability of large numbers of species-specific molecular markers is the main hindrance to applying genomic assisted breeding in their crop improvement programmes (Mayes et al. 2011).
Specifically for Bambara groundnut, some species-specific genomic resources have been reported; 10 SSR markers (Basu et al. 2007c), 143 SSR markers (Beena et al. 2013), 75 SSR markers (Mayes et al. 2013; Molosiwa et al. 2015), 201 DArT Array markers (Stadler 2009; Olukolu et al. 2012; Molosiwa et al. 2015), XSpecies (Affymetrix microarray) (Chai et al. 2013). Through the African orphan crop consortium (http://africanorphancrops.org/), efforts are underway for sequencing of Bambara groundnut to produce a genome sequence draft, alongside an effort to re-sequence 100 inbred lines of Bambara groundnut. For this, the following points are worth emphasizing. (1) Specifically for the re-sequencing exercise, the ability to use a representative sample that captures (as far as possible) the available global germplasm diversity of the crop would be important for downstream gene annotation and functional genomic analysis. Moreover, ensuring that the inbred lines that have been sequenced are generally available to researchers and through germplasm banks is an important step to begin to embed genomics into breeding programmes. However, the lack of efficient characterization of the global germplasm remains a key challenge. (2) Specifically for molecular markers, while those developed now represents a good start, in terms of numbers, these are small set to enable efficient genome analysis. To fully deploy genomic assisted breeding in Bambara groundnut crop improvement programmes, more species-specific marker system with substantial wider genomic coverage will have to be developed.
Overview of qualitative and quantitative phenotypic diversity in Bambara groundnut germplasm
Research focusing on inter and intra-landrace variation for phenotypic descriptors (either as an independent study or as part of a broader characterization of germplasm) has been extensively reported in Bambara groundnut (Goli 1995; Karikari and Tabona 2004; Ntundu et al. 2006; Ouedraogo et al. 2008; Abu and Buah 2011; Olukolu et al. 2012; Touré et al. 2012; Aliyu and Massawe 2013; Shego et al. 2013; Molosiwa et al. 2015). While most of these reports have been largely country specific, a few have a global germplasm diversity perspective (including landraces from the major agro-ecologies across Africa and Southeast Asia; Goli 1995; Olukolu et al. 2012; Molosiwa 2012; Molosiwa et al. 2015). The standard descriptors for Bambara groundnut [International Plant Genetic Resources Institute/International Institute of Tropical Agriculture/The International Bambara groundnut Network (IPGRI/IITA/BAMNET 2000)] have been used as guidelines in these phenotypic characterization exercises. Qualitatively, the guidelines capture the following indexes; growth habit, terminal leaflet shape, colour of fully expanded terminal leaflet, stem hairiness, photoperiodic reaction, dark pigmentation on wings and banner, pod shape, pod colour, pod texture, seed shape and seed colour/pattern. Across the various reports, the dominance of specific phenotypic traits among the collections of germplasm analysed have been highlighted (Table 2).
To the best of our knowledge, no report involving relatively a larger landrace characterization has included photoperiodic reaction, stem hairiness and Pigmentation on wings and banner in the analysis of quanlitative traits in Bambara groundnut. Specifically for Photoperiodic reaction, this could be because a high throughput phenotyping protocol does not exist. Moreover, a highly controlled environment is needed for such phenotyping in most ecologies (especially in the tropical regions where natural day length variation is limited). However, studies focusing on photo-thermal response mechanisms in Bambara groundnut have been reported (Linnemann 1991; Linnemann et al. 1995; Brink 1997, 1999; Brink et al. 2000; Jørgensen et al. 2009; Kendabie et al. 2014). The opportunity to use molecular markers linked to QTLs associated with photo-thermal response could be useful in future germplasm characterization. While Aliyu and Massawe (2013) included Petiole Pigmentation (relative simple and easy to score phenotypic marker) in their analysis, it is not listed in the standard descriptor for Bambara groundnut (IPGRI/IITA/BAMNET 2000).
A number of quantitative phenotypic traits have been listed in the standard descriptor set (IPGRI/IITA/BAMNET 2000) for Bambara groundnut and some have been extensively reported (Ntundu et al. 2006; Ouedraogo et al. 2008; Abu and Buah 2011; Olukolu et al. 2012; Jonah et al. 2012; Aliyu and Massawe 2013; Shego et al. 2013; Molosiwa et al. 2015). Olukolu et al. (2012) reported floral quantitative data, some components of which were not listed in the IPGRI/IITA/BAMNET (2000) descriptors (namely, banner/flag petal length, wing length, gap between the banner and wing tips, ratio of banner to wing lengths, peduncle length, and pedicel length). In general, floral descriptors (both quantitative and qualitative) are poorly captured during germplasm characterization in the various reports. Studies focused on the proximate analysis of Bambara groundnut seed have often been reported (Brough et al. 1993; Ojimelukwe 1998; Omoikhoje 2008; Sirivongpaisal 2008; Nti 2009; Abdulsalami and Sheriff 2010; Mahala and Mohammed 2010; Mazahib et al. 2013). However, to the best of our knowledge, no single characterization exercise on Bambara groundnut germplasm has reported on the full nutrient composition of seed (fat, protein and carbohydrate content or any mineral and nutrient variation) of a relatively large landrace collections.
Comparative analysis of quantitative phenotypic diversity studies in Bambara groundnut germplasm and its implication for crop improvement programmes
We performed a comparative analysis using mean values of phenotypic traits reported in selected reports during Bambara groundnut characterization exercises. Two main criteria for selection were; reports that used comparatively greater genotypes (≥100 cumulatively in their analysis) and/or their choice of landraces have a broader geographical origin and included genotypes from major agro-ecologies where Bambara groundnut is grown. Based on these criteria, four reports (Goli 1995; Ntundu et al. 2006; Olukolu et al. 2012; Molosiwa 2012) were selected (Table 3). Comparatively, these studies also reported more phenotypic descriptors in their analysis. In our comparative analysis, where a particular report involved different data set (population samples) based on location of evaluation (Molosiwa 2012), or different data set based on categorization of landraces/accessions according to known geographical origin (Olukolu et al. 2012), the mean values of each of these dataset (samples or sub-populations) were included as a separate replicates. Data was subjected to statistical analysis in Genstat (17th edition, VSN International). Analysis of variance (ANOVA) using the generalized linear regression model was used to test for significant differences of phenotypic traits across the different reports. Correlations between individual phenotypic traits were explored, Shannon–Weaver diversity index was calculated, while Principal Component Analysis (PCA) was used to deduce the relative importance or contribution of the individual phenotypic traits to the total phenotypic variability.
From the linear regression model results, only three phenotypic traits (shelling percentage, peduncle length and number of seeds per pod) showed statistically significant linear relationship across the different mean values or datasets (Table 4). Coefficient of variation ranged from 1.2 % (for seed length) to 33.3 % (for 100 seed weight) across the phenotypic traits analysed (Table 4). The Shannon–Weaver diversity index ranged from 1.604 for shell thickness to −2.079 for seed length across the phenotypic traits (Table 4). The PCA based on a correlation matrix revealed that 56.13, 33.29 and 10.53 % of the total variation is accounted for by PC1, PC2 and PC3 respectively. In PC1, the first ten traits with the highest vector loading values in descending order are; seed width, terminal leaflet width, days to 50 % flowering, canopy width, petiole length, seed length, shell thickness, internode length, days to maturity and plant height. For PC2, the first ten traits with the highest vector loading values in descending order of importance are; number of leaves per plant, pod length, 100 seed weight, days to maturity, seed length, internode length, number of pods per plant, terminal leaflet width, days to 50 % flowering and number of stems per plant. While in PC3 the following ten traits in descending order had higher vector loading values; number of stems per plant, pod length, days to 50 % flowering, petiole length, number of seeds per pod, peduncle length, canopy width, internode length, shelling percentage and days to maturity. The results of the correlation analysis revealed some interesting relationships across the various phenotypic traits (Table 5).
The diversity index (Shannon–Weaver) recorded in our comparative analysis (1.604–2.079) is comparatively higher than those reported by the individual studies (Olukolu et al. 2012; Molosiwa 2012). It is worth noting that except for the report of Ntundu et al. (2006) where landraces mostly consisting of Tanzanian origin were used, the three other reports (Goli 1995; Olukolu et al. 2012; Molosiwa 2012) had either all or a substantial part of the landraces used in their study being a subset of the collection held at IITA. Against this backdrop, it may be reasonable to assume that the phenotypic diversity in our comparative analysis should not depart significantly from those reported earlier (Goli 1995; Olukolu et al. 2012; Molosiwa 2012). The possible explanations to this departure could be; (1) either the individual reports analysed substantially different samples of the same IITA collection, (2) there is significant intra-landrace variability (3) or there is a large environmental influence in the trait expression. The first two scenarios points to the likely existence of substantial phenotypic variability in Bambara groundnut germplasm. Worthy of emphasis is the high variability of 100 seed weight (CV of 33.3 %) in our comparative analysis which suggest a positive outlook for achieving the breeding objective of improving grain yield. However, it should be kept in mind that yield is a complex trait which may have significant environmental components, suggesting poor heritability. The result of our PCA shows that most of the twenty phenotypic traits in our comparative analysis have importance with respect to germplasm characterization and diversity analysis in Bambara groundnut. Phenotypic traits related to growth and development in Bambara groundnut have significant variation and to different extents account for variability among landraces (Ntundu et al. 2006; Olukolu et al. 2012; Aliyu and Massawe 2013; Molosiwa et al. 2015). Worthy of emphasis in the present analysis is the fact that consistently, ‘primary’ yield component traits such as; seeds per pod, pods per plant, and 100 seed weight and ‘secondary’ yield component traits such as; seed length, seed width, shelling percentage and shell thickness as well as various vegetative indexes (days to 50 % flowering, days to maturity, internode length, petiole length, number of leaves per plant) ranked high in all the three PCs.
The results of the correlation analysis provides context for integrated plant breeding (with a particular emphasis on crop ideotype development, analysis of photosynthetic efficiency via resource capture and utilization). These correlation models have direct/indirect implications for achieving breeding objectives. There is a strong negative correlation among the following phenotypic traits; canopy width and 100 seed weight, plant height and 100 seed weight, seed per plant and canopy width, pods per plant and canopy width, 100 seed weight and terminal leaflet length. This could give an indication of significant competition for photosynthetic assimilate between vegetative growth and reproductive sinks (pod initiation, and pod filling). Juxtaposing the above correlation models with the reports that Bambara groundnut is largely indeterminate with respect to growth and development (Linnemann 1993; Brink 1999; Mwale et al. 2007a) puts a spotlight on certain critical implications for photosynthetic efficiency, source-sink relationships, (partitioning of photosynthate), harvest index and the overall objective of grain yield improvement in an integrated breeding framework. There is strong positive linear correlation among the following phenotypic traits; days to 50 % flowering and seed width, days to 50 % flowering and seed length, days to 50 % flowering and days to maturity, days to maturity and seed length among others. This gives an indication that yield components could be a direct linear function of days to physiological maturity. This could imply that the objective of shortening physiological maturity in Bambara groundnut may happen with a certain level of trade-off with grain yield. However, the apparent negative relationship between seed per plant and days to maturity in the current analysis makes this inconclusive. In general, the direct linear relationship of physiological maturity and grain yield which necessitate a trade-off between the two objectives in breeding programmes is reported (Ndjeunga et al. 2008; Dugje et al. 2009a, b; Ndjeunga et al. 2010).
The following points are worth emphasizing. (1) There seems to be high level of phenotypic diversity in the Bambara groundnut germplasm. (2) Specifically, on the correlation models and its implications for crop improvement programmes, a much larger data sets need to be generated to help make conclusive predictions. (3) Within the context of an integrated breeding framework in general, the issue of indeterminacy, source-sink relationships (competition for assimilate between vegetative and reproductive parts), trade-offs between grain yield and time to physiological maturity, are among some of the important ‘competing’ breeding decisions/objectives. (4) A trait such as photoperiodic response (included in the standard descriptors) is highly unrealistic to phenotype under field conditions during germplasm characterization because highly controlled conditions are needed. However, there is no reason why simple and easy to phenotype traits such as petiole colour cannot be included in the standard descriptors. In this regard, the standard descriptors for Bambara groundnut (IPGRI/IITA/BAMNET 2000) need to be revised based on current knowledge to make it more relevant for phenotyping and aid effective scientific communication.
Genetic diversity, population structure analysis and its implication for crop improvement programmes
Molecular genetic diversity analyses have been used to aid breeding decisions and germplasm conservation agenda in crop species (Choudhary et al. 2013; Huynh et al. 2013). Specifically for Bambara groundnut, various molecular analyses of diversity have been reported to either argument or validate the various reports based on phenotypic descriptors (Table 6; Pasquet et al. 1999; Massawe et al. 2003a, 2002; Olukolu et al. 2012; Aliyu and Massawe 2013; Mayes et al. 2013; Molosiwa et al. 2015). The earliest report of diversity analysis at the molecular/cellular level in Bambara groundnut includes the report of Pasquet et al. (1999). In an analysis of 79 domesticated landraces of Bambara groundnut and 29 wild relatives at 41 isozyme loci, Pasquet et al. (1999) concluded that the wild relative is the true progenitor of the former based on the high level of genetic similarities. Pasquet et al. (1999) confirmed the report of Howell (1990) that the overall level of isozyme diversity in Bambara groundnut is low. Despite the low heterozygosity among both wild and domesticated landraces, intra population genetic diversity among the domesticated landraces is high and this may be due to the autogamous breeding system of the crop (Pasquet et al. 1999). In summary, reports on genetic diversity analysis using molecular markers include; RAPD (Amadou et al. 2001; Massawe et al. 2003a; Rungnoi et al. 2012), AFLP (Massawe et al. 2002; Singrϋn and Schenkel 2003; Ntundu et al. 2004). Microsatellite (Olukolu et al. 2012; Somta et al. 2011; Aliyu and Massawe 2013; Molosiwa et al. 2015), DArT Array markers (Olukolu et al. 2012; Stadler 2009; Molosiwa et al. 2015). In all these analyses, a high level of allelic diversity (both inter and intra) have been highlighted. In most of these reports, the clustering of landraces/genotypes based on known geographical location of origin have been reported (Amadou et al. 2001; Massawe et al. 2002; Singrϋn and Schenkel 2003; Ntundu et al. 2004). For example, the Central African accession and the East African accession were grouped separately but each mergers with the West Africa accessions (Rungnoi et al. 2012). Structure analysis revealed two main clusters with all the landraces belonging to subpopulation one while only thirteen landraces belongs to the subpopulation two (Rungnoi et al. 2012). Based on these findings Rungnoi et al. (2012) concluded that all 363 landraces analysed may belong to the same population structure and that West Africa (including the Cameroon/Nigeria area) is the centre of diversity/domestication of Bambara groundnut. The crop may have reached Southeast Asia (Thailand) from West Africa via East Africa (Rungnoi et al. 2012). In generic terms, the West-Central African accession and the South-Eastern African accession are always clustered separately during molecular analysis (Stadler 2009; Somta et al. 2011; Rungnoi et al. 2012; Molosiwa et al. 2015). The opinion that genotypes from West African have high gene diversity has been expressed (Somta et al. 2011; Molosiwa 2012).
For crop improvement programmes with an integrated breeding objective(s) the following points are worth emphasizing. (1) There is evidence for high levels of allelic diversity in Bambara groundnut germplasm across genotypes (inter and intra landrace) and across geographical origin (Stadler 2009; Somta et al. 2011; Rungnoi et al. 2012; Molosiwa 2012). The power of heterosis/hybrid vigour could be harnessed in breeding programmes for variety development. (2) The high level of intra-landrace allelic diversity reported gives an indication that farmer selection criteria/pressure may have resulted in collections of landraces exhibiting uniformity for certain agronomic traits (at the phenotypic level) albeit genetically different. This may have been necessitated by the desire for a certain level of uniformity for the purpose of synchronization of farm operations within the various cropping system in which Bambara groundnut is cultivated. (3) Based on the various reports indicating high allelic diversity, low observed heterozygosity and high inbreeding coefficient, a single landrace could be considered as an ‘unselected cultivar/variety’ (Molosiwa et al. 2015). This could lead to ‘short circuiting’ of breeding with just few rounds of single seed/plant selection, enough to purify landraces for variety development (Mayes et al. 2013; Aliyu et al. 2015; Molosiwa et al. 2015). This present an opportunity to explore various short to medium term strategies for variety development in breeding programmes (Fig. 2; Aliyu et al. 2015).
Population structure and centres of crop domestication
The unanimity on Africa as the geographical origin of Bambara groundnut has been extensively reported (Dalziel 1937; Hepper 1963; Begemann 1988; Goli 1995; Basu et al. 2007a, b, c). Despite this unanimity, the specific place of domestication has sometimes been a subject of disagreement (Basu et al. 2007a, b, c). The generally acceptable common name of V. subterranea as ‘Bambara groundnut’ is linguistically linked to the Bambara tribe (a derivative of the Mandé group of languages/people) whose descendants now live mainly in modern day Mali. The Vavilov (1926) concept of crops domestication is based on the theory that the centre of domestication is tightly linked to the location where diverse relatives including ancestral relatives (its immediate wild relatives) are found. In recent times, the opinion that centre of domestication and centre of diversity could be mutually exclusive has gained some level of acceptance (Harlan 1971). From a breeding perspective, centres of diversity are of more of ‘economic values’ than centres of domestication. Areas such as Sudan have been suggested in the past as putative centres of domestication of Bambara groundnut (Jacques-Fe’lix 1946, 1950). Wild relatives of V. subterranea are reported to be localized around the Jos Plateau and Yola in Nigeria, to Garoua in Cameroon and probably beyond (Dalziel 1937; Hepper 1963; Harlan 1977; Goli 1995; Basu et al. 2007a, b, c). Generally, this region has been accepted as a putative centre of domestication of V. subterranea (Dalziel 1937; Hepper 1963; Harlan 1977; Goli 1995; Basu et al. 2007a, b, c).
The diversity analysis reports on Bambara groundnut generally all point to the West African accessions (including those from Jos Plateau and Yola in Nigeria, to Garoua in Cameroon) as more diverse hence a likely geographical location where domestication of the crop may have occurred (Hepper 1963; Begemann 1988; Pasquet et al. 1999; Olukolu et al. 2012). In contrast, Somta et al. (2011) reported that gene diversity was higher in accessions from Burkina Faso than Cameroon/Nigeria. Somta et al. (2011) asserted that there has been no report of wild relatives of Bambara groundnut found anywhere in Burkina Faso. However, Somta et al. (2011) cited the report of Albert et al. (2000) of rare possible archaeological evidence of the crop at Oursi site in Burkina Faso (dating back 1800 before present) and hypothesized that areas around Burkina Faso may be the more accurate place of domestication of Bambara groundnut. Generally, conclusions that the East African accessions are derivatives of the West African accession (based on cluster patterns) have been reported (Olukolu et al. 2012; Somta et al. 2011). Rungnoi et al. (2012) confirmed West Africa as the centre of domestication of Bambara groundnut with the hypothesis that the crop may have reached Southeast Asia (Thailand) from West Africa via East Africa.
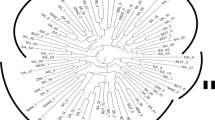
In recent analyses of relatively larger numbers of genotypes and relatively with higher marker density; Stadler 2009 (635 DArT markers on 342 individual landrace accessions) and Molosiwa et al. 2015 (68 SSR and 201 DArT markers on 123 individual landrace accessions) both reported two major sub-populations based on marker fingerprinting patterns (Fig. 3). These two sub-populations are West and Central African cluster (hereafter considered as sub-population one) and South and East African cluster (hereafter considered as sub-population two). Together, these two reports (Stadler 2009; Molosiwa et al. 2015) are making a strong case for the likely existence of two distinct gene pools in Bambara groundnut.
SSR marker fingerprinting of Bambara groundnut landraces (Adopted from Molosiwa et al. 2015)
Generally, West Africa (specifically the Jos Plateau and Yola in Nigeria, to Garoua in Cameroon) has been strongly proposed as the main putative centre of domestication of Bambara groundnut (Dalziel 1937; Hepper 1963). A call for re-evaluation of this theory/hypothesis based on emerging new genetic data (Stadler 2009; Molosiwa et al. 2015) could be prudent and worthwhile. In this regards, proposals for the following hypothesis would not be farfetched; (1) West Africa remains the ‘sole centre’ of domestication of Bambara groundnut and that the Southern African accessions are direct derivative of this single domestication event and were introduced to their present locations via various forms of economic and socio-cultural historical scenarios. However, complex interactions of selection pressures (environment factors, farmer preferences and natural mutations) may have significantly altered the gene pool of the introduced Bambara groundnut from their original West African ancestors. (2) That the Southern African region could potentially be a secondary centre of domestication for the crop. These two domestication events may have occurred concurrently or over different time and space in a multi-cultural and multi-regional fashion.
The effect of selection pressure based on the first hypothesis is a complex scenario that might need detailed long term empirical evidence to prove. The few current reports do not seem to point to significant differences in phenotypic traits preferences across geographical regions which might significantly impact on selection pressure (Abu and Buah 2011; BAMFOOD 2012; Pungulani et al. 2012). However, the occurrence of such differences some time in history is an issue that may need to be investigated further. In general, the effects of variables in the first hypothesis (environment, farmer preferences and natural mutations) and their effect on selection pressure and gene pool organisation of Bambara groundnut germplasm are beyond the scope of this review.
The scenario in the second hypothesis has to be viewed within both inter and intra genera context in a comparative analysis fashion. Advances in genomics have changed our understanding of how genomes are organized and it is opening paradigms of new hypothesis on crop domestications (Gepts 2014). Generally, for most crops species, but more specifically cereals, new genetic data are revealing that contrary to the long held view of the uniquely geographically localized nature of domestication; rather most of these events occurred simultaneously in a multi-regional fashion (Brown et al. 2009). Specifically, for Bambara groundnut domestication, a comparative analysis with other native African Legume, most appropriately cowpea, will present a contextual framework. Recently, new genetic data on cowpea (Huynh et al. 2013) contradicted previously held hypothesis on domestication of the crop. Huynh et al. (2013) in their analysis point to two distinct gene pools of Western and Eastern African sub-populations (each more closely related to the wild relatives natives in the respective regions). Contrary to the general belief that cowpea and sorghum occupy the same agro-ecology and often intercropped historically; therefore it may have followed single domestication event of Westward to Europe (via Middle East), Huynh et al. (2013) concluded that two divergent domestication events namely Western and Eastern Africa may have occurred.
Conclusion
Our conceptual framework of genetic diversity and population structure of global Bambara groundnut germplasm and its utility in the context of crop improvement is presented (Figs. 1, 2). In general, research on underutilized species lack consistency and coordination often times leading to duplication of elementary level data. While this may be the case for some of the diversity studies conducted in Bambara groundnut, the following reports (Goli 1995; Singrϋn and Schenkel 2003; Olukolu et al. 2012; BAMFOOD 2012; Molosiwa et al. 2015; Stadler 2009) have some level of importance. In conclusion, the following points are worth re-emphasizing. (1) While the level of phenotypic diversity in Bambara groundnut is clearly reasonable, there is evidence for substantial diversity at the allelic level to support improvement programmes. (2) Based on this new emerging data (Stadler 2009; Molosiwa et al. 2015) a call for re-evaluation of the Bambara groundnut current domestication theory/hypothesis will be worthwhile. The possibility that the Southern African region might constitute a divergent and simultaneous or different time-spaced domestication event (aside West Africa) needs further examination. (3) The standard descriptor for Bambara groundnut (IPGRI/IITA/BAMNET 2000) needs to be revised based on the current knowledge of the crop to aid effective scientific communication.
References
Abdulsalami MS, Sheriff HB (2010) Effect of processing on the proximate composition and mineral content of Bambara groundnut (Voandezeia Subterranean). Bayero J Pure Appl Sci 3:188–190
Abu HB, Buah JSS (2011) Characterization of bambara groundnut landraces and their evaluation by farmers in the Upper West Region of Ghana. J Dev Sustain Agric 6:64–74
Acquaah G (2007) Principles of plant genetics and breeding, 1st edn. Blackwell Publishing Ltd, Oxford
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep 27:617–631. doi:10.1007/s00299-008-0507-z
Albert KD, Hallier M, Kahlheber S, Pelzer C (2000) Montée et abandon des collines d’occupation de’âge du fer au nord du Burkina Faso. Ber SFB 268(14):335–351
Aliyu S, Massawe FJ (2013) Microsatellites based marker molecular analysis of Ghanaian Bambara groundnut (Vigna subterranea (L.) Verdc.) landraces alongside morphological characterization. Genet Resour Crop Evol 60:777–787. doi:10.1007/s10722-012-9874-y
Aliyu S, Massawe F, Mayes S (2015) Beyond landraces: developing improved germplasm resources for underutilized species—a case for Bambara groundnut. Biotechnol Genet Eng Rev. doi:10.1080/02648725.2014.992625
Alonso JM, Ecker JR (2006) Moving forward in reverse: genetic technologies to enable genome-wide phenomic screens in Arabidopsis. Nat Rev Genet 7:524–536. doi:10.1038/nrg1893
Amadou HI, Bebeli PJ, Kaltsikes PJ (2001) Genetic diversity in Bambara groundnut (Vigna subterranea L.) germplasm revealed by RAPD markers. Genome 44:995–999. doi:10.1139/gen-44-6-995
BAMFOOD: INCO-DC (2012) International cooperation with developing Countries CONTRACT NUMBER: ICA4-CT-2000-30002 Increasing the productivity of Bambara groundnut (Vigna subterranea (L.) Verdc.) for sustainable food production in semi-arid Africa. The University of Nottingham, Sutton Bunington, UK
Bandillo N, Raghavan C, Muyco PA et al (2013) Multi-parent advanced generation inter-cross (MAGIC) populations in rice: progress and potential for genetics research and breeding. Rice 6:11. doi:10.1186/1939-8433-6-11
Basu S, Mayes S, Davey M et al (2007a) Inheritance of “domestication” traits in bambara groundnut (Vigna subterranea (L.) Verdc.). Euphytica 157:59–68. doi:10.1007/s10681-007-9396-4
Basu S, Roberts JA, Azam-Ali SN, Mayes S (2007b) Bambara groundnut. In: Kole C (ed) Genome mapping and molecular breeding in plants. Pulses, Sugars and Tuber Crops, vol 3. Springer, Berlin
Basu S, Roberts JA, Azam-Ali SN, Mays S (2007c) Development of microsatellite markers for bambara groundnut (Vigna subterranea L. Verdc.)—an underutilized African legume crop species. Mol Ecol Notes 7:1326–1328. doi:10.1111/j.1471-8286.2007.01870.x
Beena R, Sheshshayee MS, Madhura JN, Prasad TG, Udayakumar M (2013) Developing of SSR markers and genetic variability in physiological traits in Bambara groundnut (Vigna subterranea L. Verdc.). In: Prospect in bioscience: addressing the issues. Springer India
Begemann F (1988) Ecogeographic differentiation of bambara groundnut (Vigna subterranea) in the collection of the international institute of tropical agriculture (IITA). PhD Thesis, Technical University of Munich, Germany
Berchie J, Sarkodie-Addo J, Adu-Dappah H et al (2010) Yield evaluation of early maturity bambara groundnut (Vigna subterranea L. Verdc) landraces at the CSIR-Crops Research Institute, Fumesua-Kumasi, Ghana. J Agron 9:175–179
Brink M (1997) Rates of progress towards flowering and podding in Bambara groundnut (Vigna subterranea) as a function of temperature and photoperiod. Ann Bot 80:505–513. doi:10.1006/anbo.1997.0479
Brink M (1999) Development, growth and dry matter partitioning in Bambara groundnut (Vigna subterranea) as influenced by photoperiod and shading. J Agric Sci Cambridge 133:159–166
Brink M, Sibuga KP, Tarimo AJP, Ramolemana GM (2000) Quantifying photothermal influences on reproductive development in bambara groundnut (Vigna subterranea): models and their validation. F Crop Res 66:1–14
Brough SH, Azam-Ali SN (1992) The effect of soil moisture on the proximate composition of Bambara groundnut (Vigna subtarranea (L.) Verdc.). J Sci Food Agric 60:197–203
Brough SH, Azam-Ali SN, Taylor AJ (1993) The potential of Bambara groundnut (Vigna subterranea) in vegetable milk production and basic protein functionality systems. Food Chem 47:277–283
Brown TA, Jones MK, Powell W, Allaby RG (2009) The complex origins of domesticated crops in the fertile crescent. Trends Ecol Evol 24:103–109. doi:10.1016/j.tree.2008.09.008
Chai HH, Lai H, Guo H et al (2013) Developing XSpecies approaches for genomics and transcriptomics—using resources developed in major species for research in Bambara. In: Massawe F, Mayes S, Alderson P (eds) 2nd international symposium on underutilized species: crops for the future-Beyond food security. Acta Horticulture 979, Kuala Lumpur, pp 773–778
Choudhary G, Ranjitkumar N, Surapaneni M, Deborah AD, Anuradha G, Siddiq EA, Vemireddy LR (2013) Molecular genetic diversity of major Indian rice cultivars over decadal periods. PLoS ONE. doi:10.1371/journal.pone.0066197
Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 363:557–572. doi:10.1098/rstb.2007.2170
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142:169–196. doi:10.1007/s10681-005-1681-5
Collinson S, Azam-Ali SN, Chavula K, Hodson D (1996) Growth development and yield of Bambara groundnut (Vigna subterranea) in response to soil moisture. J Agric Sci Cambridge 126:307–318
Dakora FD (1998) Nodule function in symbiotic Bambara groundnut (Vigna subterranea L.) and Kersting’ s Bean (Macrotyloma geocarpum L.) is tolerant of nitrate in the. Ann Bot 82:687–690
Dalziel JM (1937) Voandzeia Thou. In: The useful plants of west tropical Africa: Crown Agents, London. pp 269–271
Doku EV, Karikari SK (1970) Fruit development in Bambarra groundnut (Voandzeia subterranea). Ann Bot 34:951–957
Dugje IY, Omoigui LO, Ekeleme F, Bandyopadhyay R, Kumar PL, Kamara AY (2009a) Farmers’ guide to soybean production in Northern Nigeria. International Institute of Tropical Agriculture, Ibadan, p 21
Dugje IY, Omoigui LO, Ekeleme F, Kamara AY, Ajeigbe H (2009) Farmers’ Guide to Cowpea Production in West Africa. International Institute of tropical Agriculture (IITA). Ibadan, Nigeria. p 20
Genstat statistical package, 17th edition. VSN International, 5 The Waterhouse, Waterhouse Street, Hemel Hempstead, Hertfordshire HP1 1ES, UK
Gepts P (2014) The contribution of genetic and genomic approaches to plant domestication studies. Curr Opin Plant Biol 18C:51–59. doi:10.1016/j.pbi.2014.02.001
Goli AE (1995) Characterization and evaluation of IITA’s Bambara groundnut collection. In: Begemann, JH, Mushonga J. (eds.) In: Proceedings of the workshop on conservation and improvement of Bambara groundnut (Vigna subtarranea (L.) Verdc.). Zimbabwe: Internatinal Plant Genetic Resources Institute (IPGRI)
Harlan JR (1971) Agricultural origins: centers and noncenters. Science 174(4008):468–474
Harlan JR (1977) The origin of cereals agriculture in the Old World. In: Reed A (eds) Origin of agriculture: mouton, The Hague, Netherland. pp 357–383
Hawkes JG (1991) The importance of genetic resources in plant breeding. Biol J Linn Soc 43:3–10. doi:10.1111/j.1095-8312.1991.tb00578.x
He S, Wang Y, Volis S et al (2012) Genetic diversity and population structure: implications for conservation of wild soybean (Glycine soja Sieb. et Zucc.) based on nuclear and chloroplast microsatellite variation. Int J Mol Sci 13:12608–12628. doi:10.3390/ijms131012608
Hepper FN (1963) The Bambara groundnut (Voandzeia subterranea) and Kersting’s groundnut (Kerstingiella geocarpa) wild in West Africa. Kew Bull 16:395–407
Herridge D, Rose I (2000) Breeding for enhanced nitrogen fixation in crop legumes. F Crop Res 65:229–248
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18. doi:10.1007/s11104-008-9668-3
Howell JA (1990) Variation and evolution of Bambara groundnut (Vigna subterranea (L.) Verdc.; Fabaceae). PhD Thesis, Miami University, OH, USA
Huynh B-L, Close TJ, Roberts PA et al (2013) Gene pools and the genetic architecture of domesticated cowpea. Plant Genome. doi:10.3835/plantgenome2013.03.0005
IPGRI/IITA/BAMNET (2000) Descriptors for Bambara groundnut (Vigna subterranea). International plant genetic resources Institute, Rome, Italy; International Institute of Tropical Agriculture, Ibadan, Nigeria; The International Bambara groundnut Network, Germany
Jacques-Fe’lix H (1946) Remarques sur l’origine et la ge´ocarpie du Voandzeia subterranea Thou (Pap.). [Observations on the origin and geocarpy of Voandzeia subterranea Thou (Pap.)]. Bull Soc Bot France 93(9):360–362
Jacques-Fe’lix H (1950) Pour une Enquete sur le Voandzou (Voandzeia subterranean) Thou. L’Agronomie Tropicale 5(1–2):62–73
Jaenicke H, Höschle-Zeledon I (eds) (2006) Strategic Framework for Underutilized Plant Species Research and Development, with Special Reference to Asia and the Pacific, and to Sub-Saharan Africa. International Centre for Underutilised Crops, Colombo, Sri Laka and Global Facilitation Unit for underutilized Species, Rome, Italy, Rome, Italy
Jannink J-L, Lorenz AJ, Iwata H (2010) Genomic selection in plant breeding: from theory to practice. Brief Funct Genomics 9:166–177. doi:10.1093/bfgp/elq001
Jonah PM, Aliyu B, Kadams AM, Jibung GG (2012) Variation in pod yield characters and heritability estimates in some accessions of Bambara groundnut (Vigna subterranea (L.) Verdc. Glob Res J Agric Biol Sci 3:360–369
Jørgensen ST, Aubanton M, Harmonic C et al (2009) Identification of photoperiod neutral lines of Bambara groundnut (Vigna subterranea) from Tanzania. IOP Conf Ser Earth Environ Sci. doi:10.1088/1755-1307/6/7/372023
Karikari SK, Tabona TT (2004) Constitutive traits and selective indices of Bambara groundnut (Vigna subterranea (L.) Verdc.) landraces for drought tolerance under Botswana conditions. Phys Chem Earth 29:1029–1034. doi:10.1016/j.pce.2004.08.002
Kendabie P (2014) Unravelling photoperiod effects on pod-set and pod filling in Bambara groundnut [Vigna subterranea (L.) Verdc.]-A drought tolerant African legume with potential to contribute to global food security. PhD Thesis. The University of Nottingham, UK
Kesawat MS, Das BK (2009) Molecular markers: it’s application in crop improvement. J Crop Sci Biotechnol 12:169–181
Kishinevsky BD, Zur M, Friedman Y et al (1996) Variation in nitrogen fixation and yield in landraces of Bambara groundnut (Vigna subterranea L.). F Crop Res 48:57–64. doi:10.1016/0378-4290(96)00037-8
Kover PX, Valdar W, Trakalo J et al (2009) A multiparent advanced generation inter-cross to fine-map quantitative traits in Arabidopsis thaliana. PLoS Genet 5:e1000551. doi:10.1371/journal.pgen.1000551
Linnemann AR (1991) Preliminary observations on photoperiod regulation of phenological development in bambara groundnut (Vigna subterranea). F Crop Res 26:295–304. doi:10.1016/0378-4290(91)90006-H
Linnemann AR (1993) Phenological development of Bambara groundnut (Vigna subterranea) at constant exposure to photoperiod of 10 to 16 h. Ann Bot 71:445–452
Linnemann AR, Westphal E, Wessel M (1995) Photoperiod regulation of development and growth in Bambara groundnut (Vigna subterranea). Field Crop Res 40:39–47
Lu Y, Shah T, Hao Z et al (2011) Comparative SNP and haplotype analysis reveals a higher genetic diversity and rapider LD decay in tropical than temperate germplasm in maize. PLoS ONE 6:e24861. doi:10.1371/journal.pone.0024861
Mahala AG, Mohammed AAA (2010) Nutritive evaluation of bambara groundnut (Vigna subterranean) pods, seeds and hull as animal feeds. J Appl Sci Res 6:383–386
Massawe FJ, Dickinson M, Roberts JA, Azam-Ali SN (2002) Genetic diversity in Bambara groundnut (Vigna subterranea (L.) Verdc.) landraces revealed by AFLP markers. Genome 45:1175–1180
Massawe FJ, Roberts JA, Davey MR (2003a) Genetic diversity in Bambara groundnut (Vigna subterranea (L.) Verdc.) Landraces assessed by Random Amplified Polymorphic DNA (RAPD) markers. Genet Resour Crop Evol 50:737–741
Massawe FJ, Schenkel W, Basu S, Temba EM (2003b) Artificial hybridization in bambara groundnut (Vigna subterranea (L.) Verdc.). In: International Bambara groundnut symposium. 8 = 12th August, Botswana College of Agriculture, Botswana, pp 195–211
Massawe FJ, Mwale SS, Roberts JA (2005) Breeding in bambara groundnut (Vigna subterranea (L.) Verdc.): strategic considerations. Afr J Biotechnol 4:463–471
Massawe FJ, Mwale SS, Azam-Ali SN, Roberts JA (2007) Towards genetic improvement of bambara groundnut [Vigna subterranea (L.) Verdc.]. In: Ochatt S, Jain SM (eds) Breeding of neglected and under-utilized crops: spices and herbs. Science Publishers, p 468
Massawe F, Mayes S, Cheng A (2016) Crop diversity: an unexploited treasure trove for food security. Trends Plant. In press. doi:10.1016/j.tplants.2016.02.006
Mayes S, Massawe FJ, Alderson PG et al (2011) The potential for underutilized crops to improve security of food production. J Exp Bot 63:1075–1079. doi:10.1093/jxb/err396
Mayes S, Basu SM, Molosiwa O et al (2013) Molecular analysis of Bambara Groundnut, an underutilized African Legume Crop as Part of the BAMLINK Project—What Lessons Can We Learn? In: Massawe F, Mayes S, Alderson P (eds) 2nd international symposium on underutilized species: crops for the future-Beyond food security. Acta Horticulture 979, Kuala Lumpur, pp 451–458
Mazahib A, Nuha M, Salawa I, Babiker E (2013) Some nutritional attributes of bambara groundnut as influenced by domestic processing. Int Food Res J 20:1165–1171
Mohale KC, Belane AK, Dakora FD (2013) Symbiotic N nutrition, C assimilation, and plant water use efficiency in Bambara groundnut (Vigna subterranea L. Verdc.) grown in farmers’ fields in South Africa, measured using 15 N and 13C natural abundance. Biol Fertil Soils 50:307–319. doi:10.1007/s00374-013-0841-3
Molosiwa OO (2012) Genetic diversity and population structure analysis of Bambara groundnut [Vigna subterranea (L.) Verdc.] landraces using morpho-agronomic and SSR markers. PhD Thesis, The University of Nottingham, UK
Molosiwa OO, Aliyu S, Stadler F et al (2015) SSR marker development, genetic diversity and population structure analysis of Bambara groundnut [Vigna subterranea (L.) Verdc.] landraces. Genet Resour Crop Evol 62:1225–1243. doi:10.1007/s10722-015-0226-6
Mwale SS, Azam-Ali SN, Massawe FJ (2007a) Growth and development of Bambara groundnut (Vigna subterranea) in response to soil moisture- 1. Dray matter and yield. Eur J Agron 26:345–353. doi:10.1016/j.eja.2006.09.007
Mwale SS, Azam-Ali SN, Massawe FJ (2007b) Growth and development of Bambara groundnut (Vigna subterranea) in response to soil moisture-2. Resource capture and conversion. Eur J Agron 26:354–362. doi:10.1016/j.eja.2006.12.008
Ndjeunga J, Ntare BR, Waliyar F et al (2008) Early adoption of modern groundnut varieties in West Africa. Working Paper Series no. 24. Sahelian Center, BP 12404 Niamey, Niger: International Crops Research Institute for the Semi-Arid Tropics. p 62
Ndjeunga J, Ntare BR, Abdoulaye A et al (2010) Farmer preferences for groundnut traits and varieties in West Africa: Cases of Mali, Niger and Nigeria. Working Paper Series no. 27. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. p 32
Nordborg M, Weigel D (2008) Next-generation genetics in plants. Nature 456:720–723
Nti CA (2009) Effects of bambara groundnut (Vigna subterranea) variety and processing on the quality and consumer appeal for its products. Int J Food Sci Technol 44:2234–2242. doi:10.1111/j.1365-2621.2009.02064.x
Ntundu WH, Bach IC, Christiansen JL, Andersen SB (2004) Analysis of genetic diversity in bambara groundnut [Vigna subterranea (L.) Verdc.] landraces using amplified fragment length polymorphism (AFLP) markers. Afr J Biotechnol 3:220–225
Ntundu WH, Shillah SA, Marandu WYF, Christiansen JL (2006) Morphological diversity of Bambara groundnut [Vigna subterranea (L.) Verdc.] landraces in Tanzania. Genet Resour Crop Evol 53:367–378. doi:10.1007/s10722-004-0580-2
Ojimelukwe PC (1998) Cooking characteristics of four cultivars of Bambara groundnuts seeds and starch isolate. J Food Biochem 23:109–117
Olukolu BA, Mayes S, Stadler F et al (2012) Genetic diversity in Bambara groundnut (Vigna subterranea (L.) Verdc.) As revealed by phenotypic descriptors and DArT marker analysis. Genet Resour Crop Evol 59:347–358. doi:10.1007/s10722-011-9686-5
Omoikhoje SO (2008) Assessment of the nutritive value of Bambara groundnut as influenced by cooking time. Livest Res Rural Dev 20:1–5
Ouedraogo M, Ouedraogo JT, Tignere JB et al (2008) Characterization and evaluation of accessions of Bambara groundnut (Vigna subterranea (L.) Verdcourt) from Burkina Faso. Sci Nat 5:191–197
Pasquet RS, Schwedes S, Gepts P (1999) Isozyme diversity in Bambara groundnut. Crop Sci 39:1228–1236
Pungulani L, Kadyampakeni D, Nsapato L, Kachapila M (2012) Selection of high yielding and farmers’ preferred genotypes of bambara nut (Vigna subterranea (L.) Verdc.) in Malawi. Am J Plant Sci 3:1802–1808. doi:10.4236/ajps.2012.312A221
Rao VR, Hodgkin T (2002) Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult 68:1–19
Rassel A (1960) Le voandzou Voandzeia subterranea Thou. et sa culture au Kwango. Bull. Agric. du Congo Belge et du Ruanda-Urundi 51:1–26
Rosenberg NA, Huang L, Jewett EM et al (2010) Genome-wide association studies in diverse populations. Nat Rev Genet 11:356–366. doi:10.1038/nrg2760
Rungnoi O, Suwanprasert J, Somta P, Srinives P (2012) Molecular genetic diversity of Bambara groundnut (Vigna subterranea L. Verdc.) revealed by RAPD and ISSR marker analysis. SABRAO J Breed Genet 44:87–101
Shego A, Jansen van Rensburg WS, Adebola PO (2013) Assessment of genetic variability in bambara groundnut (Vigna subterranea (L.) Verdc.) using morphological quantitative traits. Acad J Agric Res 1:45–51
Singrϋn C, Schenkel W (2003) Fingerprinting of Bambara groundnut germplasm with molecular marker, In: Proceedings of the International Bambara groundnut Symposium. International Cooperation with Developing Countries: Botswana college of Agriculture, Botswana 8–12 August, 2003, Gaborone, Botswana pp 161–170
Sirivongpaisal P (2008) Structure and functional properties of starch and flour from bambarra groundnut. Songklanakari J Sci Technol 30:51–56
Somta P, Chankaew S, Rungnoi O, Srinives P (2011) Genetic diversity of the Bambara groundnut (Vigna subterranea (L.) Verdc.) as assessed by SSR markers. Genome 54:898–910. doi:10.1139/G11-056
Stadler F (2009) Analysis of differential gene expression under water-deficit stress and genetic diversity in Bambara groundnut [Vigna subterranea (L.) Verdc.] using novel high-throughput technologies. PhD Thesis, Technische Universität München, Germany
Suwanprasert J, Toojinda T, Srinives P, Chanprame S (2006) Hybridization technique for Bambara Groundnut. Breed Sci 56:125–129
Touré Y, Koné M, Tanoh HK, Koné D (2012) Agromorphological and phenological variability of 10 Bambara Groundnut [Vigna subterranea (L.) Verdc. (Fabaceae)] landraces cultivated in the Ivory Coast. Tropicultural 30:216–221
Vavilov NI (1926) Centers of origin of cultivated plants (Originally in Russian). Bull Appl Bot Genet Plant Breed Leningr VIR 16(2):248
Wen W, Franco J, Chavez-Tovar VH et al (2012) Genetic characterization of a core set of a tropical maize race Tuxpeño for further use in maize improvement. PLoS ONE 7:e32626. doi:10.1371/journal.pone.0032626
Will M (2008) Promoting value chain of neglected and underutilized species for pro-poor growth and biodiversity conservation. Global Facilitation unit for underutilized Species, Rome
Williams JT, Haq N (2002) Global research on underutilized crops Global research on underutilized crops-An assessment of current activities and proposal for enhanced cooperation. ICUC, UK
Zhang P, Li J, Li X et al (2011) Population structure and genetic diversity in a rice core collection (Oryza sativa L.) investigated with SSR markers. PLoS ONE 6:e27565. doi:10.1371/journal.pone.0027565
Acknowledgments
This work was (financially) supported by Crops For the Future (CFF) and the University of Nottingham Malaysia Campus (UNMC) through the CFF-UNMC Doctoral Training Partnership scholarship scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aliyu, S., Massawe, F. & Mayes, S. Genetic diversity and population structure of Bambara groundnut (Vigna subterranea (L.) Verdc.): synopsis of the past two decades of analysis and implications for crop improvement programmes. Genet Resour Crop Evol 63, 925–943 (2016). https://doi.org/10.1007/s10722-016-0406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-016-0406-z