Abstract
Bambara groundnut (Vigna subterranea L. Verdc) is the second most important indigenous food legume in Africa. The aim of this study was to evaluate plant growth, N2 fixation, N contribution, C accumulation, and plant water relations of Bambara groundnut grown in 26 farmers’ fields in Mpumalanga Province of South Africa. The data revealed marked (p ≤ 0.05) differences in plant dry matter (DM) yield, N concentration and content, δ15N, the proportion of N derived from symbiotic fixation (%Ndfa), and actual amounts of N-fixed between and among the 26 farms surveyed. Bambara groundnut plants obtained 33–98 % (mean = 72 %) of their N nutrition from symbiotic fixation and contributed 4–200 kg N-fixed ha−1 (mean = 102 kg N-fixed ha−1). Plant density correlated positively with %N (r = 0.31***), δ15N (r = 0.126***), and amount of N-fixed (r = 0.15*), indicating that the high %Ndfa values obtained for Bambara groundnut in this study and the low symbiotic N yield associated with some farms were due to low plant density rather than poor symbiotic functioning. Bambara groundnut obtained more N from soil (e.g., 173 kg N ha−1) than from symbiosis (e.g., 135 kg N-fixed ha−1) in some fields, implying that the N2-fixing efficacy of the microsymbionts nodulating Bambara groundnut was low at some locations in South Africa. The data from this study showed δ13C values ranging from −28.01 to −26.20 ‰, which indicates differences in plant water use efficiency on the different fields studied. Furthermore, the positive correlations between δ13C and N-fixed (r = 0.15*) and between δ13C and N content (r = 0.14*) suggest a functional relationship between water use efficiency and N2 fixation, just as the positively significant correlations between δ15N and DM yield (r = 0.24***), N-fixed and DM weight (r = 0.76**), and N content and DM yield (r = 0.99*), as well as N-fixed and C content (r = 0.76**) also indicate a functional relationship between N2 fixation and photosynthesis. In the same way, the positive correlation between δ13C and DM weight (r = 0.14*), or δ13C and C content (r = 0.15*), also implies a functional link between water use efficiency and plant growth. Thus, an increase in water use efficiency in Bambara groundnut, whenever it occurs, seems to functionally enhance plant growth, symbiotic N2 fixation, and photosynthetic activity, just as N2 fixation in nodules also seems to stimulate leaf photosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen and P deficiency limits crop plant growth and grain yield in global agriculture (Vance 2001; Eickhout et al. 2006). Although synthetic fertilizers can improve soil N fertility, and thus increase crop yields, their continuous use can negatively impact on soil health and cause environmental pollution (Mosier and Kroezen 2000; Eickhout et al. 2006). Additionally, chemical fertilizers are expensive and inaccessible to smallholder farmers with limited credit (Bockman 1997). Taken together, these constraints limit the use of chemical inputs by resource-poor farmers in African agriculture. Incorporating N2-fixing legumes into cropping systems is therefore a much cheaper and sustainable way of tapping atmospheric N2 for increased crop yields. Recent studies have shown that grain legumes such as cowpea (Vigna unguiculata L. Walp.), groundnut (Arachis hypogaea L.), and soybean (Glycine max L. Merr.) can contribute significant amounts of symbiotic N to traditional cropping systems in Africa (Belane and Dakora 2009; Naab et al. 2009; Pule-Meulenberg and Dakora 2009; Nyemba and Dakora 2010; Pule-Meulenberg et al. 2010). The 15N natural abundance, in particular, has been found to be one of the few techniques able to provide on-farm estimates of N2 fixation by legumes (Pule-Meulenberg and Dakora 2009; Nyemba and Dakora 2010).
Bambara groundnut is a food legume that is under-researched and underutilized in Africa. As a result, there are no improved varieties or recommended agronomic practices for this legume in Africa (Ntundu et al. 2006). Although Bambara groundnut is generally regarded as being drought tolerant, there is little information on its water use efficiency under field conditions. Currently, carbon isotope discrimination is recognized as a potential tool for studying water use efficiency in C3 plant species such as Bambara groundnut. Interestingly, Basu et al. (2007) have shown that, in the case of Bambara groundnut, carbon isotope discrimination is under multigene control and could therefore serve as a true indicator of plant water relations in this species.
The δ13C of C3 plants such as Bambara groundnut has been reported to reflect the water use efficiency of those species (Farquhar et al. 1989). During photosynthesis, plants discriminate against the heavier 13CO2 in favor of the lighter 12CO2; therefore, 13C becomes more abundant (or less negative) in plant tissues relative to 12C in the atmosphere. However, during periods of soil water deficit, a partial closure of stomata can occur, leading to an intercellular CO2 concentration that is just sufficient to saturate photosynthesis. Under those circumstances, less 13C discrimination occurs, and less water is also lost. This forms the basis for the use of δ13C as a tool for measuring water use efficiency in C3 species such as Bambara groundnut.
As a food crop, Bambara groundnut is cultivated throughout sub-Saharan Africa, stretching from the sahel regions of West Africa, across Central Africa to East Africa, and then down to Southern Africa. The ability of Bambara groundnut to grow in these different agro-ecologies (Jørgensen et al. 2010), with soils differing in pH, mineral nutrients, soil moisture, rhizosphere temperature, and N2-fixing microsymbionts, is rather intriguing. In the African continent, it is ranked the second most important indigenous food legume after cowpea (Azam-Ali et al. 2001). Nutritionally, the grain of Bambara groundnut is a complete meal with 14–24 % protein, 60 % carbohydrate, and 6–12 % oil (Brough and Azam-Ali 1992; Mahala and Mohammed 2010), components ideal for human health. Furthermore, the spread of its cultivation into new areas such as Malaysia, Indonesia, Thailand, and the Papuan region of Southeast Asia (Ilyas and Sopian 2013; Redjeki et al. 2013; Somta et al. 2013) suggests that Bambara groundnut is a major food security crop in the world. Yet, little is known about its nodulation, N2 fixation, microsymbiont biodiversity, plant water relations, and nutritional adaptation to different environments. These alone ought to justify renewed research interest in this species, in addition to the superior dietary value of its grain as food. The aim of this study was to evaluate plant growth, N2 fixation, water use efficiency, pod yield, and net N returns to soil of Bambara groundnut plants sampled from farmers’ fields in Mpumalanga Province of South Africa, using 15N and 13C natural abundance. The report presented here is part of a wider study on Bambara groundnut symbiosis, including its microsymbiont biodiversity in Africa.
Material and methods
Site description, plant sample collection, and processing
The selection of fields and plants was done, as described by Nyemba and Dakora (2005). Ten plants were randomly sampled from each farm by walking in a zigzag manner across the length of the field starting from one corner. The plants were dug up using a shovel and separated into nodulated roots and shoots plus pods. In all, 26 farmers’ fields were surveyed in six villages (namely, Machipe, Majakaneng, Dikgwale, Malekutu, Phameni, and Skhwahlane) within the Mpumalanga Province of South Africa in 2009. The number of farmers’ fields sampled varied from village to village depending on the willingness of farmers to allow plants to be collected from their farms. A paired legume–reference plant sampling procedure was used, as described by Unkovich et al. (1994). The shoots of non-legume species growing near the Bambara groundnut plants in each farm were harvested as reference plants for the estimation of soil N uptake by the legume. All plant materials (i.e., shoots plus pods of Bambara groundnut and shoots of reference plants) were oven dried at 60 °C for 48 h, weighed, and milled to fine powder (0.85 mm sieve) for 15N and 13C isotope analysis.
Soil sampling and analysis
Bulk soil (i.e., soil not associated with plant roots) was sampled from a 0 to 20 cm depth on wide empty spaces in between plants in each farm using an augur. The soil samples were taken to the laboratory, sieved (2 mm), and stored in the fridge (4 °C) prior to analysis for chemical properties.
Measurement of N2 fixation
15N/14N isotopic analysis
Finely ground plant material was weighed into Al tin capsules (1–2 mg/legume sample and 2–3 mg/reference plant) and analyzed for %N and 15N/14N ratio using a Carlo Erba NA1500 elemental analyzer (Fisons Instruments SpA, Strada, Rivoltana, Italy) coupled to a Finnigan MAT252 mass spectrometer via Conflo II open-split device.
The 15N natural abundance is expressed as δ (delta) notation and is the per mille deviation of the 15N natural abundance of the sample from atmospheric (atm) N2 (0.36637 atom % 15N). The isotopic composition (δ15N) was measured as described by Unkovich et al. (2008):
where 15N/14Nsample is the abundance ratio of 15N and 14N in the Bambara groundnut sample, and 15N/14Natm is the abundance ratio of 15N and 14N in the atmosphere.
The combined 15N natural abundance of shoots plus pods was calculated as an average of the 15N natural abundance values of shoots and pods weighted by their respective total N contents as (Robinson et al. 2000):
Nitrogen content
The N content of each plant organ was determined as the product of %N and organ weight as (Pausch et al. 1996):
Percent N derived from fixation (%Ndfa)
The percent N derived from symbiotic fixation of atmospheric N2 was estimated using the equation (Shearer and Kohl 1986; Unkovich et al. 2008):
where the δ15Nref is the 15N natural abundance of reference plants, δ15Nleg is the 15N natural abundance of legume, and the B value is the 15N natural abundance of test legume wholly dependent on N2 fixation for its N nutrition. The B value replaces the value of atmospheric N2 as it incorporates the isotopic fractionation associated with N2 fixation (Unkovich et al. 2008). In this study, the B value used (−1.4 ‰) was obtained from Nyemba and Dakora (2010).
The amount of N-fixed was calculated as (Maskey et al. 2001):
N-fixed per hectare was estimated as the product of fixed N in shoots + pods per plant and plant density per hectare. The plant density in each field was measured by counting the number of Bambara groundnut plants in a 4-m2 quadrant area and using it to estimate plant population per hectare.
13C/12C isotopic analysis
To analyze for 13C/12C ratio, shoot samples of Bambara groundnut (2–3 mg) were weighed into tin capsules and run on a mass spectrometer, as described for 15N/14N isotopic ratio. The ratio of 13C/12C in each sample was used to calculate the 13C natural abundance (δ13C) as (Stout and Rafter 1978):
where 13C/12Csample is the isotopic ratio of the sample, and 13C/12Cstandard is the isotopic ratio of Belemnite Pee Dee limestone formation (Craig 1957), a universally accepted standard. Shoot C content per plant was calculated as the product of %C and shoot DM weight.
Statistical analysis
Statistical analyses were carried out with the STATISTICA (StatSoft Inc., Tulsa, OK, USA) package. Data were subjected to a normal distribution test, followed by one-way analysis of variance (ANOVA). Where there were significant differences, treatment means were separated using the Duncan multiple range test.
Results
Soil chemical properties
In general, Skhwahlane and Dikgwale sites appeared to have intermediate to high levels of C, N, Ca, Mg, B, and Fe relative to the other sites (Table 1).
δ15N values of reference plants
Several plant species, including grasses, herbaceous weeds, and cereals, were collected from farmers’ fields in the six villages (namely, Machipe, Majakaneng, Dikgwale, Malekutu, Phameni, and Skhwahlane) in Mpumalanga Province. Where more than one reference plant was sampled per farm, the average value of the combined δ15N values was used to estimate %Ndfa of Bambara groundnut on that farm. A recent study has shown that the combined mean δ15N value of herbaceous weeds can be used to estimate a legume’s dependence on symbiotic N2 fixation for its N nutrition (Nyemba and Dakora 2010).
Plant growth
A comparison of Bambara groundnut plant growth between and among the 26 fields surveyed in Mpumalanga revealed marked differences in DM yield (Table 3). Across the board, DM yield was highest at Dikgwale (field 9, 162 g plant−1), followed by Skhwahlane (field 25, 141.9 g plant−1), Malekutu (field 18, 109.1 g plant−1), Skhwahlane (field 23, 89.2 g plant−1), and Skhwahlane (field 24, 86.5 g plant−1). The lowest DM (6.0 g plant−1) was obtained at Malekutu (field 12).
Dry matter yield of shoots plus pods differed significantly (p ≤ 0.05) between fields around each village surveyed. For example, at the Machipe site, DM production was highest in field 4 (64 g plant−1) and lowest in field 5 (17 g plant−1), while at Majakaneng, DM yield was 81 g plant−1 in field 6 compared to 20 g plant−1 in field 8 (Table 3). Similarly, Bambara groundnut showed better growth at Dikgwale in field 9 (163 g dry mass plant−1) compared to field 8 (68.6 g plant−1).
Field 10 at Dikgwale similarly showed greater plant growth (163.3 g DM plant−1) than field 9 (68.6 g DM plant−1). Of the 9 farms sampled at Malekutu, farm 12 produced 5.9 g DM plant−1 in contrast to farm 18, which yielded 109.1 g DM plant−1 (Table 3). Significant differences were similarly found in the DM yield of shoots plus pods at Phameni. For example, shoot plus pod biomass production ranged from 7.8 g DM plant−1 (field 22) to 53.4 g plant−1 (field 19) at the Phameni site, while it ranged from 73 g DM plant−1 (field 26) to 142 g DM plant−1 (farm 25) at Skhwahlane (Table 3).
N concentration and N content
N concentration and content of Bambara groundnut plants sampled across Mpumalanga Province also differed significantly between fields. The N concentration of shoots plus pods ranged from 1.7 to 3.9 % across the 26 farmers’ fields studied (Table 3). As a result of those differences in N concentration, N content of shoots plus pods also differed between and among fields. For example, the N content of Bambara groundnut shoots plus pods was much greater at Skhwahlane field 25 (4.4 g plant−1), followed by Dikgwale field 9 (3.9 g plant−1), Malekutu field 18 (3.6 g plant−1), and Skhwahlane field 24 (3.2 g plant−1). In contrast, the lowest DM yield was produced at Malekutu (see fields 12, 14, and 13).
δ15N values and %Ndfa
The δ15N of Bambara groundnut plants sampled in Mpumalanga Province showed marked differences across the 26 farmers’ fields surveyed and varied from −1.77 to +1.92 ‰ for shoots and −1.29 to +2.61 ‰ for shoots plus pods (Table 3). The highest δ15N value was obtained at Malekutu (field 10), followed by Skhwahlane (fields 26 and 25) and Dikgwale (field 8). In contrast, the lowest δ15N values for shoots plus pods were recorded at Malekutu (field 12) and Phameni (field 20). The %Ndfa of Bambara groundnut shoots plus pods also differed markedly between farmers’ fields and ranged from 33 % (field 10) to 98 % at Malekutu (field 12). Except for Malekutu (field 10) and Skhwahlane (field 25), where the legume derived below 50 % N (i.e., 33 and 43 %, respectively) from symbiotic fixation, Bambara groundnut was able to obtain between 52 and 98 % of its N nutrition on all the other fields studied (Table 4).
Amounts of N-fixed
The amounts of N-fixed by Bambara groundnut plants on farmers’ fields differed significantly (p ≤ 0.05) between and among the 26 farms surveyed. As shown in Table 4, symbiotic N contribution by Bambara groundnut across the 26 fields ranged from 4 to 200 kg ha−1, with both minimum and maximum values being recorded at Malekutu fields 12 and 18, respectively. The sites where Bambara groundnut plants made a greater N contribution (116–144 kg N-fixed ha−1) to the N economy of the cropping system included Malekutu (field 16), Skhwahlane (fields 23, 24, and 25), and Machipe (field 4). By contrast, the areas with very low N contribution were Malekutu (field 14) and Phameni (field 21).
Net N returns to soil
The net N returns to soil from Bambara groundnut shoots ranged from 7 to 177 kg N ha−1 (Table 4). Despite the low plant density, the estimated N returns to soil were quite substantial at Skhwahlane (fields 23 and 24), Malekutu (field 16), Machipe (field 4), and Malekutu (field 18) (i.e., 177, 170, 137, 125, and 124 kg N ha−1, respectively; see Table 4), indicating the potential of Bambara groundnut to contribute to the N economy of cropping systems.
Soil mineral N uptake
Soil N uptake differed markedly between and among Bambara groundnut plants sampled from the 26 farmers’ fields (Table 4) and ranged from 0.01 kg N ha−1 at Malekutu (field 12) to 173 kg N ha−1 at Skhwahlane (field 25). Other locations where soil N uptake was high included Skhwahlane (116 kg N ha−1 at field 24), Malekutu (89 kg N ha−1 at field 4), and Skhwahlane (63 kg N ha−1 at field 26). Soil N uptake by Bambara groundnut plants was however below 10 kg N ha−1 in nine other farmers’ fields (Table 4).
Plant density and its relationship with biomass production and N2 fixation
There were large differences in Bambara groundnut plant density across the 26 farmers’ fields surveyed (Table 3). Relative to other sites, plant density was highest at Malekutu (e.g., fields 13 and 10 with 16 and 12 plants m−2, respectively). In fact, 15 out of the 26 fields surveyed had very low plant densities, ranging between 3 and 6 plants m−2 (Table 3).
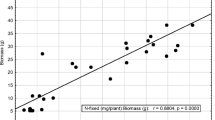
As shown in Fig. 1, there was evidence to show that low plant density affected DM yield and hence N2 fixation. Thus, plant biomass increased where legume plant density was high (Fig. 1a). The amount of N-fixed was however also much greater where Bambara groundnut density was higher in the cropping system (Fig. 1b).
Carbon accumulation
C concentration in Bambara groundnut shoots plus pods differed markedly across the 26 farmers’ fields studied and ranged from 40 to 49 % (Table 5). Plants at Skhwahlane (field 23) exhibited the highest C concentration (48.9 %), followed by Phameni (46 % in each of fields 20 and 22), Skhwahlane (46 % in field 26), and Majakaneng (46 % in field 6). The lowest C concentration was recorded at Malekutu (40 % at field 10), followed by Machipe (43 % in each of fields 2 and 4), Phameni (43 % in field 19), Dikgwale (43 % in field 9), Majakaneng (43 % in field 7), and Malekutu (43 % in field 18).
As a result, the C content of Bambara groundnut shoots plus pods also differed significantly across all fields and ranged from 2.6 to 70.9 g plant−1 (Table 5). The highest C content was found at Dikgwale (70.9 g plant−1 in field 9), followed by Skhwahlane (62.5 g plant−1 in field 25) and Malekutu (46.8 g plant−1 in field 18). In contrast, the lowest C content was recorded in plants harvested from Malekutu (2.6 g plant−1 in field 12), Phameni (3.6 g plant−1 in field 21), and Malekutu (4.3 g plant−1 in each of fields 13 and 14).
C/N ratio and photosynthetic fixed N use efficiency
The C/N ratio is a measure of the C and N relationship in plants and is therefore an estimate of photosynthetic N use efficiency. As with other parameters, there were significant differences in C/N ratios across all 26 fields where Bambara groundnut was sampled. Overall, the C/N ratio ranged from 10.7 g g−1 in field 13 to 26.6 g g−1 in field 12 at Malekutu (Table 5).
The photosynthetic N-fixed use efficiency (measured as shoot C per unit N-fixed in shoots) also varied with the amounts of symbiotic N and C accumulated by field-grown Bambara groundnut plants. As shown in Table 5, the Bambara groundnut material sampled from Malekutu (field 10) showed the highest photosynthetic fixed N use efficiency (154 g C g−1 N-fixed), followed by Malekutu (83 g C g−1 N-fixed in field 12), Majakaneng (58 g C g−1 N-fixed in field 6), and Phameni (54 g C g−1 N-fixed in field 22). The Bambara groundnut shoots with the lowest photosynthetic N-fixed use efficiency were sampled from Skhwahlane (field 24), Dikgwale (fields 8 and 9), and Skhwahlane (field 23), and they respectively recorded 5.0, 5.9, 6.5, and 6.1 g C g−1 N-fixed (Table 5).
δ13C values
A one-way ANOVA of δ13C from Bambara groundnut shoots revealed significantly marked variation between and among farmers’ fields at the different study sites. The δ13C values of Bambara groundnut shoots were much greater (i.e., less negative) at Skhwahlane (fields 23 and 24, −26.20 and −26.68 ‰, respectively) when compared to the other sites/fields (Table 5). However, the fields that showed much lower, or more negative, δ13C values in Bambara groundnut shoots include Phameni (−28.24 ‰ in field 21), Malekutu (−28.06 ‰ in field 13), and Dikgwale (−28.01 ‰ in field 9). Thus, the δ13C values of Bambara groundnut shoots ranged from −28.24 to −26.20 ‰ across the 26 farmers’ fields studied (Table 5).
Correlation analysis
Bambara groundnut plant density in farmers’ fields was found to correlate positively with N-fixed (r = 0.15*), with %N (r = 0.31***) and with δ15N (r = 0.126***). The δ13C of Bambara groundnut shoots plus pods also correlated positively with dry matter (r = 0.14*), with C content (r = 0.15*), with N content (r = 0.14*), and with N-fixed (r = 0.15*). Furthermore, N-fixed correlated positively with DM yield (r = 0.76**) and with C content (r = 0.76**), just as shoot N content correlated positively with DM yield (r = 0.86*), with C content (r = 0.0.95**), and with δ13C (r = 0.0.14*).
Discussion
Symbiotic N nutrition by Bambara groundnut in farmers’ fields
The 15N natural abundance technique was used to evaluate N2 fixation and N contribution by Bambara groundnut plants grown in farmers’ fields in Mpumalanga Province of South Africa. In estimating soil N uptake by legume, many different non-legume plant species were sampled from study sites and analyzed for their 15N/14N ratios (Table 2). The number of weeds, grasses, and crop species sampled ranged from 2 to 10/field, with mean δ15N values ranging from +2.22 to +7.83 ‰ (Table 2). The difference between combined mean δ15N value of reference plants and that of legume was generally above +2.00 ‰ (Unkovich et al. 1994), indicating the potential for high precision in the use of the δ15N natural abundance technique for measuring N2 fixation in Bambara groundnut (Nyemba and Dakora 2005).
Due to marked differences in legume plant density across the 26 farmers’ fields surveyed, the growth of Bambara groundnut plants (measured as DM yield) also differed between and among experimental fields of each study site (Table 3). In most instances, farms with lower legume density produced greater shoot and pod biomass on an individual plant basis (Table 3). But the number of pods per plant appeared to be genetically controlled, as some farms with fewer Bambara groundnut stands produced low number of pods per plant than those with high density.
As to be expected, N concentration in pods (containing seeds) was higher than shoots (Table 3) due to N translocation from green leaves to the developing ovules. There was however no difference in N concentration of shoots and pods between fields (Table 3). But N content and %N derived from fixation differed markedly between and among farms due to variations in δ15N values of shoots and pods (Table 4). The shoot δ15N ranged from −1.77 to +1.92 ‰ and that of pods from −1.29 to +2.51 ‰ (Table 4). Calculating the average of δ15N in shoots and pods weighted by their total N contents (Robinson et al. 2000) produced mean δ15N data ranging from −1.29 to +2.61 ‰, and it is these mean δ15N values of shoots plus pods that were used to estimate %Ndfa. Because of the low δ15N values obtained in this study, the Bambara groundnut plants derived between 33 and 98 % of their N nutrition from symbiotic fixation, levels comparable to those obtained for this species in Zambia (Nyemba and Dakora 2010). This clearly indicates that, in South Africa, Bambara groundnut relies heavily on N2 fixation for meeting its N nutrition.
Perhaps as a result of factors such as low plant density (see Fig. 1) and the efficacy of the microsymbionts nodulating Bambara groundnut (Dakora and Keya 1997; Pule-Meulenberg et al. 2010), N contribution by the species varied hugely between and among fields and ranged from 4 to 200 kg ha−1 (mean = 102 kg N-fixed ha−1; Table 4). This variation in symbiotic performance was perhaps not unexpected as the bacterial symbionts isolated from root nodules of Bambara groundnut plants displayed a similarly wide range in symbiotic efficacy (data not shown). The locations where Bambara groundnut made significant N contribution to the cropping system included Skhwahlane fields 23, 24, and 25, which recorded 116, 143, and 135 kg N-fixed ha−1, respectively (Table 4). Incidentally, those fields with greater N contribution either had relatively high pH (e.g., pH 7.6 in fields 23 and 24), greater soil concentrations of Ca, P, and Fe needed for symbiosis (e.g., fields 23 and 24), or high levels of Mn (e.g., field 25). The endogenous soil factors mentioned here could account for the increased N contribution made by Bambara groundnut plants at those sites (Table 4). However, the differences in symbiotic performance by Bambara groundnut could also be due to its bacterial partner as legumes growing in nutrient-poor, low pH soils in Africa have been found to form root nodules with both α- and β-proteobacteria (Hassen et al. 2012; Kanu and Dakora 2012; Angus et al. 2013; Howieson et al. 2013). It is important to note that even though Bambara groundnut plants derived as high as 98 % of their N nutrition from symbiotic fixation at Malekutu (field 12), the actual amount of N-fixed was very low, only 4 kg ha−1 (Table 4). This small amount of N contributed by plants with 98 % Ndfa value was likely due to low plant density (Table 3) rather than poor symbiotic functioning (Table 4) as positive correlations were found between plant density and N-fixed (r = 0.15*), plant density and %N (r = 0.31***), as well as plant density and δ15N (r = 0.126***). While the N contribution made by Bambara groundnut on some fields could be considered high (e.g., Skhwahlane fields 23, 24, and 25), the actual amounts of N-fixed were nevertheless underestimated by the N content in roots and nodules, which were not harvested.
Similar studies done by Nyemba and Dakora (2010) in farmers’ fields found that N contribution by Bambara groundnut also varied with location and was 21, 39, and 68 kg N ha−1 in Southern, Central, and Northern Zambia, respectively. Compared to the data from this study, N contribution by Bambara groundnut is much greater in South Africa relative to Zambia. It was also interesting to note that, at some sites in South Africa, Bambara groundnut obtained more N from soil than symbiosis (e.g., 173 kg N from soil vs. 135 kg N ha−1 from symbiosis on farm 25 at Skhwahlane and 116 kg N from soil vs. 143 kg N ha−1 from symbiosis on farm 24 at Skhwahlane; see Table 4). Although these high levels of N uptake might have, to some extent, inhibited nodule functioning in Bambara groundnut (Streeter 1988; Ayisi et al. 2000), earlier studies have shown that some landraces of the species are tolerant of mineral N in the rhizosphere (Dakora et al. 1992; Dakora 1998). Whatever the case, even under farmer conditions, the net N returns by Bambara groundnut to the cropping system was quite significant, ranging from 7 to 177 kg N ha−1 (Table 4). The mean net N returns of 92 kg N ha−1 from Bambara groundnut grown in farmers’ fields indicate the potential of this species for use as a biofertilizer in cropping systems of resource-poor African farmers.
Carbon assimilation and photosynthetic fixed N use efficiency in Bambara groundnut
Carbon accumulation in plant species (whether measured as concentration or content) is directly linked to photosynthetic activity (Belane and Dakora 2011). In this study, C concentration in Bambara groundnut ranged from 40 to 49 % (Table 5), levels comparable to those reported for symbiotic legumes (Sprent et al. 1996). As to be expected, Bambara groundnut plants that exhibited greater C content (Table 5), always accumulated higher DM (Tables 3), a finding consistent with the report that photosynthetic C accumulation accounts for a substantial amount of plant total biomass (Taiz and Zeiger 2002).
The level of photosynthetic C accumulation in plants is regulated by N nutrition. The C/N ratio is therefore a good measure of the N status in plants and indicates the C-to-N relationship in tissues. Because bacteroids in root nodules of symbiotic legumes reduce atmospheric N2 to NH3, N2-fixing species tend to have C/N values less than 24 g g−1 and non-legumes more than 24 g g−1 (Hobbie et al. 1998). Except for two fields where C/N values were above 24 g g−1, all the other fields exhibited C/N ratios below 24 g g−1 (Table 5), which are consistent with those of symbiotic legumes (Hobbie et al. 1998). In all instances, the C/N value increased (Table 5) with decreasing δ15N values (Table 4), suggesting that photosynthetic C supply was increased by higher levels of N2 fixation in Bambara groundnut.
Furthermore, Bambara groundnut plants sampled from Malekutu (field 10) showed the highest photosynthetic fixed N use efficiency (154 g C g−1 N-fixed), followed by Malekutu field 12 (83 g C g−1 N-fixed), Majakaneng field 6 (58 g C g−1 N-fixed), and Phameni field 22 (54 g C g−1 N-fixed). By contrast, Bambara groundnut shoots from Skhwahlane field 24, Dikgwale fields 8 and 9, and Skhwahlane field 23 displayed the lowest photosynthetic fixed N use efficiency (5.0, 5.9, 6.5, and 6.1 g C g−1 N-fixed, respectively; see Table 5). These findings seem to support the view that photosynthetic activity in Bambara groundnut was stimulated by symbiotic N nutrition, as sites producing low C/N-fixed ratios (Table 5) yielded high symbiotic N (Table 4; also see Belane and Dakora 2011).
δ13C and its relationship with N and C nutrition in nodulated Bambara groundnut
The δ13C of C3 plants is a known measure of water use efficiency in those species (Farquhar et al. 1989; Basu et al. 2003). In this study, the variation in δ13C of Bambara groundnut plants ranged from −28.24 to −26.20 ‰ for the 26 fields (Table 5), thus indicating differences in water use efficiency between and among farmers’ fields. Interestingly, Bambara groundnut from Skhwahlane (fields 23, 24, and 25), which showed relatively greater δ13C values (−26.20, −26.68, and −27.57 ‰, respectively, see Table 5) made the highest N contribution to the cropping system (Table 4), while Phameni (field 21), which recorded the lowest δ13C (Table 5), made the least N contribution (Table 4). This can be interpreted to mean that there is a functional relationship between plant water use efficiency and symbiotic N2 fixation, and that improved water use in nodulated legumes supports nodule functioning. This argument is reinforced by the finding that δ13C correlated positively with N content (r = 0.14*), just as δ13C also correlated with amount of N-fixed (r = 0.15*). Furthermore, there was a positive correlation between δ13C and DM yield (r = 0.14*), δ13C and C content (r = 0.15*), δ15N and DM yield (r = 0.24***; N content and DM yield (r = 0.99**)), amount of N-fixed and DM yield (r = 0.76**), as well as N-fixed and C content (r = 0.76**). Taken together, these results suggest the existence of a functional relationship between symbiotic N nutrition and plant water use efficiency, between N2 fixation and photosynthesis, and between water use efficiency and plant growth in Bambara groundnut.
C3 plant species increase their δ13C (or water use efficiency) with drought events (Vandoorne et al. 2012), in the same way that they increase their δ13C values when experiencing soil water deficit (Naumburg et al. 2003; Hartman and Danin 2010; Wang et al. 2010). It is therefore likely that the high δ13C values or greater water use efficiency of Bambara groundnut plants harvested from fields around Skhwahlane and other locations were due to reduced soil moisture from low or uneven rainfall at those study sites. Unfortunately, no rainfall data could be obtained from farmers’ fields to help explain these variations in shoot δ13C.
In conclusion, we have used the 15N and 13C natural abundance to assess symbiotic functioning and plant water relations in Bambara groundnut grown in farmers’ fields. The data revealed about 33 to 98 % dependency on symbiotic N2 fixation for the species’ N nutrition, an N contribution of 4 to 200 kg N-fixed ha−1 to the cropping system, and net N returns of 7 to 177 kg fixed N ha−1. The sometimes small symbiotic N yield per hectare obtained in this study for Bambara groundnut was due to low plant density rather than poor symbiotic functioning in farmers’ fields. With proper management of plant density, this legume has the potential to become a significant food security crop and a biofertilizer in cropping systems of resource-poor farmers in Africa. Furthermore, it is important to screen and identify Bambara groundnut landraces with superior symbiosis and improved water use efficiency for increased food security in a climate change scenario.
References
Angus AA, Lee A, Lun MR, Shehayeb M, Hessabi R, Fujishige NA, Yerrapragada S, Kano S, Song N, Yang P, De la Santos PE, De Faria SM, Dakora FD, Weinstock G, Hirsch A (2013) Nodulation and effective nitrogen fixation of Macroptilium atropurpureum (siratro) by Burkholderia tuberum, a nodulating and plant growth promoting beta-proteobacterium, are influenced by environmental factors. Plant Soil 369:543–562. doi:10.1007s11104.013.1590.7
Ayisi KK, Nkgapele RJ, Dakora FD (2000) Nodule formation and function in six varieties of cowpea (Vigna unguiculata L. Walp.) grown in a nitrogen–rich field soil in South Africa. Symbiosis 28:17–31
Azam-Ali SN, Sesay A, Karikari SK, Massawe FJ, Aguitar-Manjarrez J, Bannayan M, Hampson KJ (2001) Assessing the potential of an underutilized crop, a case study using Bambara groundnut. Exp Agric 37:433–472
Basu SM, Roberts JA, Davey MR, Azam-Ali SN, Mithen RF (2003) Towards genetic linkage mapping in Bambara groundnut. In: Proc. International Bambara Groundnut Symposium, Gaborone Botswana College of Agriculture, pp 211–222
Basu SM, Mayes S, Davey M (2007) Inheritance of ‘domestication’ traits in Bambara groundnut (Vigna subterranea L. Verdc.). Euphytica 157:59–68
Belane AK, Dakora FD (2009) Measurement of N2 fixation in 30 cowpea (Vigna unguiculata L. Walp.) genotypes under field conditions in Ghana using 15N natural abundance technique. Symbiosis 48:47–57
Belane AK, Dakora FD (2011) Photosynthesis, symbiotic N and C accumulation in leaves of 30 nodulated cowpea genotypes grown in the field at Wa in the Guinea savanna of Ghana. Field Crop Res 24:279–287
Bockman OC (1997) Fertilizers and biological nitrogen fixation as sources of plant nutrients: perspectives for future agriculture. Plant Soil 194:81–98
Brough SH, Azam-Ali SN (1992) The proximate composition of Bambara groundnut (Vigna subterranea L. Verdc.) in response to soil moisture. J Sci Food Agric 60:197–203
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim 12:133–149
Dakora FD (1998) Nodule function in symbiotic Bambara groundnut (Vigna subterranea L. Verdc.) and Kersting’s Bean (Macrotyloma geocarpum L.) is tolerant of nitrate in the root medium. Ann Bot 82:687–690
Dakora FD, Keya SO (1997) Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa. Soil Biol Biochem 29:809–817
Dakora FD, Atkins CA, Pate JS (1992) Effect of NO3 on N2 fixation and nitrogenous solutes of xylem in two nodulated West African geocarpic legumes, Kersting’s bean (Macrotyloma geocarpum L.) and Bambara groundnut (Vigna subterranea L. Verdc). Plant Soil 140:255–262
Eickhout B, Bouwman AF, Van Zeijts H (2006) The role of nitrogen in world food production and environmental sustainability. Agric Ecosyst Environ 116:4–14
Farquhar GD, Hubick KT, Condon AG, Richards RA (1989) Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotope in ecological research. Springer, New York, pp 21–40
Hartman G, Danin A (2010) Isotopic values of plants in relation to water availability in the Eastern Mediterranean region. Oecologia 162:837–852
Hassen AI, Bopape FL, Habig J, Lamprecht SC (2012) Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-Proteobacteria and β-Proteobacteria. Biol Fertil Soils 48:295–303
Hobbie EA, Macko SA, Shugart HH (1998) Patterns in N dynamics and N isotopes during primary succession in Glacier Bay, Alaska. Chem Geol 152:3–11
Howieson JG, De Meyer SE, Vivas-Marfisi A, Ratnayake S, Ardley JK, Yates RJ (2013) Novel Burkholderia bacteria isolated from Lebeckia ambigua—a perennial suffrutescent legume of the fynbos. Soil Biol Biochem 60:55–64
Ilyas S, Sopian O (2013) Effect of seed maturity and invigoration on seed viability and vigor, plant growth, and yield of Bambara groundnut (Vigna subterranea (L.) Verdcourt). In: Massawe F, Mayes S, Alderson P (eds) Proc. 2nd Int. Symp. on Underutilized Plants: Crops for the Future—Beyond Food Security. Acta Hort. (ISHS) 979:695–702
Jørgensen ST, Liu F, Oue draogo M, Ntundu WH, Sarrazin J, Christiansen JL (2010) Drought responses of two Bambara groundnut (Vigna subterranea L. Verdc.) landraces collected from a dry and a humid area of Africa. J Agron Crop Sci 196:412–422
Kanu SA, Dakora FD (2012) Symbiotic nitrogen contribution and biodiversity of bacteria nodulating Psoralea species in the Cape Fynbos of South Africa. Soil Biol Biochem 54:68–76
Mahala AG, Mohammed AAA (2010) Nutritive evaluation of Bambara groundnut (Vigna subterranea L. Verdc.) pods, seeds and hull as animal feeds. J Appl Sci Res 6:383–386
Maskey SL, Bhattarai S, Peoples MB, Herridge DF (2001) On-farm measurements of nitrogen fixation by winter and summer legumes in the Hill and Terai regions of Nepal. Field Crop Res 70:209–221
Mosier A, Kroezen C (2000) Potential impact on the global atmospheric N2O budget of the increased nitrogen input required to meet future global food demands. Chemosphere 2:465–473
Naab JB, Chimpango SMB, Dakora FD (2009) N2 fixation in cowpea plants grown in farmers’ fields in the Upper West Region of Ghana, measured using 15N natural abundance. Symbiosis 48:37–46
Naumburg E, Housman DC, Charlet TN, Loik ME, Smith SD (2003) Photosynthetic responses of Mojave Desert shrubs to free air CO2 enrichment are greatest during wet years. Glob Chang Biol 9:276–285
Ntundu WH, Shillah SA, Marandu WYF, Christiansen JL (2006) Morphological diversity of Bambara groundnut (Vigna subterranea L. Verdc.) landraces in Tanzania. Genet Resour Crop Evol 53:367–378
Nyemba RC, Dakora FD (2005) Identifying suitable arable weeds as reference plants for measuring N2 fixation. Symbiosis 40:79–86
Nyemba RC, Dakora FD (2010) Evaluating N2 fixation by food grain legumes in farmers’ fields in the three agro-ecological zones of Zambia, using 15N natural abundance. Biol Fertil Soils 46:461–470
Pausch RC, Charles L, Mulchi CL, Lee EH, Meisinger JJ (1996) Use of 13C and 15N isotopes to investigate O3 effects on C and N metabolism in soybeans. Part II. Nitrogen uptake, fixation, and partitioning. Agric Ecosyst Environ 60:61–69
Pule-Meulenberg F, Dakora FD (2009) Assessing the symbiotic dependency of grain and tree legumes on N2 fixation for their N nutrition in five agroecological zones of Botswana. Symbiosis 48:68–77
Pule-Meulenberg F, Belane A, Krasova-Wade T, Dakora FD (2010) Symbiotic functioning and bradyrhizobial biodiversity of cowpea (Vigna unguiculata L. Walp.) in Africa. BMC Microbiol 10:89
Redjeki ES, Mayes S, Azam-Ali S (2013) Evaluating the stability and adaptability of Bambara groundnut (Vigna subterranea (L.) Verd.) landraces in different agro-ecologies. In: Massawe F, Mayes S, Alderson P (Ed) Proc. 2nd Int. Symp. on Underutilized Plants: Crops for the Future—Beyond Food Security. Acta Hort. (ISHS) 979:389–400
Robinson D, Handley LL, Scrimgeour CM, Gordon DC, Forster BP, Ellis RP (2000) Using stable isotope natural abundance (δ15N and δ13C) to integrate the stress response of wild barley (Hordeum spontaneum C. Kock.) genotypes. J Exp Bot 51:50–51
Shearer G, Kohl DH (1986) N2-fixation in field settings: estimations based on 15N natural abundance. Aust J Plant Physiol 13:699–756
Somta P, Chankaew S, Srinives P, Rungnoi O (2013) Cross-species amplification of microsatellite markers in Bambara groundnut (Vigna subterranea) and their application in diversity study. In: Massawe F, Mayes S, Alderson P (Ed) Proc. 2nd Int. Symp. on Underutilized Plants: Crops for the Future—Beyond Food Security. Acta Hort. (ISHS) 979:431–435
Sprent JI, Geoghegan IE, Whitty PW (1996) Natural abundance of 15N and 13C in nodulated legumes and other plants in the cerrado and neighbouring regions in Brazil. Oecologia 105:440–446
Stout JD, Rafter TA (1978) The 13C/12C isotopic ratios of some New Zealand tussock grassland soils. In: Stable isotopes in the earth sciences. New Zealand Department of Scientific and Industrial Research, DSIR Bulletin 220, INS Contribution no. 806. pp 75–83
Streeter J (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. CRC Crit Rev Plant Sci 7:1–23
Taiz L, Zeiger E (2002) Photosynthesis: carbon reactions. In Plant physiology, 3rd edn. Sinauer, Sunderland, Chapter 8, pp. 145–170
Unkovich MJ, Pate JS, Armstrong EL, Sanford P (1994) Selection of reference plants for 15N natural abundance assessment of N2 fixation by crop and pasture legumes in South-west Australia. Aust J Agric Res 45:133–147
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R, Giller K., Alves B, Chalk P (2008) Measuring plant-associated nitrogen fixation in agricultural systems. ACIAR. Monograph No 136. Canberra, Australia.
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Vandoorne B, Mathieu AS, Van den Ende W, Vergauwen R, Pe’rilleux C, Javaux M, Lutts S (2012) Water stress drastically reduces root growth and inulin yield in Cichorium intybus (var. sativum) independently of photosynthesis. J Exp Bot 63:4359–4373
Wang X, Yan X, Liao H (2010) Genetic improvement for phosphorus efficiency in soybean: a radical approach. Review: part of a highlight section on plant–soil interactions at low pH. Ann Bot 106:215–222
Acknowledgments
We are grateful to the South African Research Chair in Agrochemurgy and Plant Symbioses, the National Research Foundation, and the Tshwane University of Technology for financial support to FDD’s research and for bursaries to KCM and AKB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mohale, K.C., Belane, A.K. & Dakora, F.D. Symbiotic N nutrition, C assimilation, and plant water use efficiency in Bambara groundnut (Vigna subterranea L. Verdc) grown in farmers’ fields in South Africa, measured using 15N and 13C natural abundance. Biol Fertil Soils 50, 307–319 (2014). https://doi.org/10.1007/s00374-013-0841-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-013-0841-3