Abstract
Biological soil crusts (biocrusts) are widespread in dryland ecosystems worldwide and were only recently discovered in agroecosystems. As agroecosystem biocrusts complete biological nitrogen (N) fixation, this research investigated if biocrust N could be released to biocrust subsoil and assimilated by a perennial tree crop. Biocrust was pulse labeled with 15NH4Cl (15N) in the greenhouse for eight weeks prior to application in a citrus (Citrus spp.) orchard in Southwest Florida, USA. Patches of labeled biocrust were applied to twelve citrus trees in September, with twelve trees in the same orchard not receiving biocrust (controls). The 15N in the biocrust, subsoil, crop fibrous roots, and crop leaves was quantified at seven dates leading up to fruit harvest 158 days after application. Sampling dates overlapped with citrus root (September–October) and shoot (March) flush periods. Isotopically labeled biocrust N was present in subsoil and crop roots one day after biocrust application and in leaves after three days. Results revealed that N was released from biocrust at a rate of 0.63% 15N released per day leading up to fruit harvest when 91.7% of 15N had been released. The amount of biocrust 15N in the fibrous roots increased during a root flush period (September–October) until 28 days after biocrust application and then decreased leading up to a leaf flush period and fruit harvest. Approximately 23.8% of total biocrust 15N was in the crop tissue at fruit harvest. Overall, these results indicate agroecosystem biocrusts have the potential to support tree crop growth during N demanding growth stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is one of the most limiting nutrients for plant growth and crop production worldwide. In agriculture systems, this limitation is alleviated through the application of synthetic or organic N fertilizer to maximize plant growth and productivity. The total amount of N applied to the agroecosystem to optimize production depends on the crop type. For example, in Florida, USA citrus (Citrus spp.) agroecosystems, the N requirement for the growth of the perennial tree crop is met by applying 100–300 kg N ha−1 annually (Morgan et al. 2019; Quaggio et al. 2019). It is common for this N to be applied through several applications to meet tree N requirements during fall root flushes, spring leaf flushes, and fruit flushes (Alva et al. 2006). In addition, daily irrigation is used to maintain soil water at levels ideal for crop growth (Hamido et al. 2017; Hamido and Morgan 2021). These management practices are especially prominent in Florida sandy soil agroecosystems which are prone to water and N losses through leaching (Kadyampakeni et al. 2018; Morgan et al. 2006a, b). Growers are increasingly using adaptive nutrient management approaches, such as cover crops, compost, and fertigation, to meet N and water requirements and build soil health (Allar and Maltais-Landry 2022; Roth et al. 2023; Strauss et al. 2020a, b).
Biological soil crusts (biocrusts) are soil surface dwelling communities of cyanobacteria, algae, fungi, and bryophytes that are found globally and can coexist with vascular plants (Zhang et al. 2016). Biocrusts are currently estimated to cover 12% of Earth’s terrestrial surface and account for approximately 15% and 40–85% of global terrestrial carbon (C) and N fixation, respectively (Rodriguez-Caballero et al. 2018). Though extensively studied in arid and semi-arid ecosystems worldwide, biocrusts have only recently been discovered and studied in agroecosystems (Peng and Bruns 2019a, b; Strauss et al. 2020a, b; Nevins et al. 2022a). Initial investigations of these biocrusts indicate the potential to influence plant growth and soil health as biocrusts in cropping systems in Pennsylvania and Florida, USA have filamentous Cyanobacteria capable of biological N fixation (Nevins et al. 2021; Peng and Bruns 2019a; Sorochkina et al. 2022). In addition, Florida agroecosystem biocrusts can increase soil bacterial and fungal community diversity in the root zone below the biocrust (Nevins et al. 2021, 2022b). There is also potential for agroecosystem biocrusts to increase plant-available N and C concentrations in the subsurface soil below the biocrust (Nevins et al. 2020). Despite the potential for biocrust to impact the subsurface microbiome and nutrient cycling in crop root zones, it is unknown if biocrusts supply N to crops.
Biocrusts in arid and semi-arid ecosystems can have a positive impact on plant growth (Havrilla et al. 2019; Zhang et al. 2016). As a result of fungal loop transfer and nutrient mass flow transfer, biocrust presence has led to increased plant N tissue content relative to plants grown in bare soil (Defalco et al. 2001; Langhans et al. 2009; Zhang & Nie 2011), and increased concentrations of phosphorus (P) and other mineral nutrients have also been observed (Concostrina-Zubiri et al. 2013; Guo et al. 2008; Harper and Belnap 2001). In addition, improved plant performance in the form of increased seedling dry weight was found for smooth mesquite (P. Laevigata) under the influence of Cyanobacteria crust in a tropical desert ecosystem (Godínez-Alvarez et al. 2011), and biocrust presence has been associated with increased leaf N concentrations (% N) and biomass in grasses from a cool desert ecosystem (Havrilla et al. 2020). However, neutral and negative impacts of biocrusts on plants have been observed in grassland and arid ecosystems (Godínez-Alvarez et al. 2011; Havrilla and Barger 2018), and plant growth in arid ecosystems has been inhibited due to reduced moisture content with biocrust presence compared to non-crusted soils (Kidron 2014).
While studies have found that biocrusts can facilitate plant growth under specific contexts, fewer studies have investigated the translocation of N between biocrusts and plants. In a dryland ecosystem, 15N added to biocrust was transferred from biocrust to nearby shrub roots, with subsequent N retention in the plant (Dettweiler‐Robinson et al. 2019). Similarly, herb species coexisting with biocrust in a temperate desert ecosystem resulted in the herbs assimilating biocrust N in a 15N labelling field experiment (0.5–7.3% of N recovered) (Zhuang et al. 2020). Also, vascular plants growing in biocrust covered plots in a temperate desert accumulated more applied 15N than plants growing in bare soil in this ecosystem (Zhuang et al. 2015). Collectively, these studies indicate there is potential for biocrusts to facilitate plant growth and N uptake, though the studies did not illustrate that biocrusts can add external N to the system.
Subtropical ecosystems have different climate conditions than semi-arid and arid ecosystems, where biocrusts have routinely been studied. Greater precipitation and daily temperatures in subtropical ecosystems could impact N transformations in the biocrust and subsurface soil and plant N uptake of biocrust N. In addition, biocrust interactions with shrubs and grasses have been examined in several ecosystems (Dettweiler‐Robinson et al., 2019; Havrilla et al. 2020), but the interactions between biocrust and woody perennial plants have been less studied. To our knowledge, there are no studies that have investigated the role of biocrusts in agroecosystems where N supplied by biocrusts could replace synthetic N fertilizer applications. This is especially important in agroecosystems with perennial crops that require high year-round nutrient inputs to reach maximum plant productivity (Esteves et al. 2022; Zheng et al. 2023). While biocrusts can increase plant-available N in the subsurface soil compared to bare soil areas in agroecosystems (Nevins et al. 2020), no studies have investigated crop N uptake and distribution of biocrusts N in subtropical systems. Therefore, this study was conducted with biocrusts in a citrus agroecosystem to document and quantify direct plant uptake of biocrust N and distribution of biocrust N through plant tissues, including roots, leaves, and fruit at harvest.
Materials and methods
Biocrust harvest and 15 N labelling
Biocrusts were harvested intact from a commercial citrus orchard (biocrust collection site) in Felda, FL, USA (26.593, -81.440) in Southwest Florida on July 21, 2020 (Fig. 1A).
A Biological soil crust that was harvested at a commercial citrus orchard (biocrust collection site) in Felda, Florida, USA, prior to a precipitation event. B Biocrust after being harvested intact from a commercial citrus grove, sprayed with deionized water, and transported to the greenhouse in preparation for 15N isotopic labeling. C, D Biocrust transplanted from the greenhouse into the citrus orchard at the University of Florida Southwest Florida Research and Education Center (Immokalee, Florida, USA) on September 21, 2020
Biocrusts were collected using sterilized plastic garden trays (38.1 × 22.9 × 6.4 cm) (Midi Garden Tray, Greenhouse Megastore, Danville, IL, USA) (Fig. 1B). Prior to collection, one side of each tray was removed using a handsaw. This allowed the trays to be pushed under biocrust and approximately 5 cm of biocrust subsoil in the field, enabling intact biocrust collection. Total surface area of biocrust in each tray was 38.1 cm × 17.8 cm (678.2 cm2) (Fig. 1B), and there was approximately 4 cm of biocrust subsurface soil. A total of 24 trays of biocrust were collected and immediately transported to a greenhouse at the University of Florida (UF)—Institute of Food and Agricultural Sciences (IFAS)—Southwest Florida Research and Education Center (SWFREC) (Immokalee, FL, USA). Biocrusts were sprayed with deionized water every three days while in the greenhouse using a 7.5-L pump sprayer (HDX, The Home Depot, Atlanta, GA, USA).
Biocrusts were labeled with 15NH4Cl once weekly beginning on July 31, 2020, and ending on September 18, 2020 (eight applications). During each application, 80 mL of 10 mM 98 atom % 15NH4Cl was sprayed on each biocrust. At the end of the labeling period the biocrust was 9.17 ± 1.49 atom % 15N. Prior to this research, a preliminary study revealed that approximately 90% of 15NH4Cl applied to intact biocrusts under greenhouse conditions was converted to an organic form of N in the biocrust (Nevins 2021).
Field trial site description and management
The citrus field trial site was located at the UF/IFAS SWFREC in Immokalee, FL, USA (26.463, − 81.444) on an Immokalee fine sand soil (sandy, siliceous, hyperthermic Arenic Alaquods). Average annual precipitation and temperature for the site are 124.0 cm and 23.2 °C, respectively (Köppen climate classification is Aw). The field trial was six rows wide with six trees in each row (Figure S1). Half of the trees (first three rows: 18 trees) were four-year old ‘Valencia’ orange trees (Citrus sinensis (L.) Osbeck) grafted on US 802 (C. sinensis × Poncirus trifoliata) rootstock. The other half of the trees (last three rows: 18 trees) were four-year old ‘Valencia’ orange trees (C. sinensis (L.) Osbeck) grafted on X639 (C. sinensis × P. trifoliata) rootstock. Trees were spaced at 2.4 m by 3.6 m in the field trial. Each row of trees was a field plot replicate, with the first two trees in the row receiving 15N labeled biocrust and the last two trees in the row serving as the control without biocrust. Trees were not randomly assigned treatments because it was necessary to have border trees between biocrust-treated and untreated control trees.
All trees received non-isotopically labeled fertilizations twice during the study period to support crop growth. Each tree received 0.45 kg of 8–4–8 (N–P–K) conventional fertilizer on October 30, 2020, and 0.23 kg of 12–3–9 (N–P–K) controlled release fertilizer on November 20, 2020 (Table 1). Trees were watered daily using 360-degree micro-sprinklers (Maxijet Inc., Dundee, FL, USA), with one emitter per tree at approximately 30 cm from the tree trunk. Irrigation was not used after precipitation events greater than 17 mm per day. Precipitation totals for the research site (Table 1) were retrieved from the Florida Automated Weather Network (https://fawn.ifas.ufl.edu/).
Biocrusts (labeled with 15N) were applied to the first two trees of each row (n = 6 rows × 2 trees per row = 12 trees) on September 21, 2020 (Fig. 1C). One tray of biocrust (approximately 665 mg 15N) was applied to each side of these trees (4 biocrust trays row−1 × 6 rows = 24 biocrust trays total). Biocrust were applied approximately 20 cm from the drip line and 30 cm from the tree base. Prior to biocrust application, the top 6 cm of surface soil was removed from the 38.1 × 17.8 cm surface area where the biocrust was being applied. To apply, the biocrust sample was gently slid out of the tray until the biocrust was flush with the soil surface (Fig. 1C).
Sample collection and processing
Biocrust samples were collected from the field trial using a sterilized scoopula utensil (ThermoFisher, Wilmington, DE, USA) on September 21, 2020, September 22, 2020, September 24, 2020, September 28, 2020, October 19, 2020, November 16, 2020, January 11, 2020, and February 26, 2020 (Table 1). At each sampling date, a 0.5 cm × 0.5 cm × 1 cm subsample of biocrust was removed from each biocrust patch (24 patches total). Biocrust from each isotopically labeled row (four subsamples per row) were combined to form one composite sample (six rows total). Biocrust samples were stored on ice in the field and immediately returned to the laboratory for processing after sampling. In the laboratory, biocrust was homogenized and a subsample was oven dried at 70 °C for 72 h.
Soil samples were collected using a sterilized 2 cm diameter soil corer to a depth of 15 cm. Soil cores were collected at the edge of each biocrust patch for subsoil and fibrous root collection. One soil core was collected per biocrust patch in each row (four cores per row) and these cores were combined to form one composite soil sample per row (n = 4). Similarly, one soil core was collected on each side of the last two trees in each row (control trees) in the same location (20 cm below the drip line). These four soil cores near control trees were combined to form one composite sample per row (n = 4). Composite soil cores were stored on ice in the field and immediately transported to the laboratory after collection.
In the laboratory, all roots were manually removed from each composite soil sample using sterilized scoopula utensils and forceps (ThermoFisher, Wilmington, DE, USA). Fibrous roots are commonly concentrated near the soil surface in sandy soil citrus orchards (Morgan et al. 2006a, b; Quiñones et al. 2007), and these roots are more metabolically active than larger, older roots (Lea-Cox et al. 2001; Mattos et al. 2003). To focus on fibrous roots, the roots greater than 2 mm diameter were discarded and small roots (< 2 mm diameter) were washed with deionized water and dried at 60 °C until a constant weight (at least 3 d). A subsample of each composite soil core was oven dried at 70 °C for 72 h after removal of roots, detrital material, and rocks.
Medium roots (4–20 mm) were sampled by collecting soil cores (2 cm diameter corer) 15 cm away from the tree base to a depth of 15 cm (March 1, 2021). One core was collected from each side of each tree and cores from the first two trees of the row (trees with biocrust application) were combined to form one composite sample (n = 4 cores). Cores from the last two trees of the row (control trees) were combined to form one composite sample (n = 4 cores). After collection, composite samples were taken to the laboratory where medium sized roots (2–20 mm) were manually removed from the soil using sterilized scoopula utensils and forceps (ThermoFisher, Wilmington, DE, USA).
Large roots (> 20 mm) of each tree were exposed using a shovel to manually remove surface soil 0.5 m surrounding the base of each tree for sampling (Rossi et al. 2006) (March 2, 2021). Soil was brushed from the large roots and exposed roots were sprayed with deionized water. Four cores were taken from large, exposed roots in each quadrant of the tree root system (four cores per quadrant x four quadrants = 16 cores per tree) (Trephor tool, Costruzioni Meccaniche Carabin C., Valle di Cadore, Belluno, Italy). Cores from the first two trees in each row (trees with biocrust application) were combined to form a composite sample (32 cores total). Cores from the last two trees in each row were combined to form a composite sample (32 cores total). Medium and large roots were oven dried at 60 °C for at least seven days.
Tree canopies were divided into four quadrants and four leaves were collected from each quadrant (16 leaves per tree) on September 21, 2020, September 22, 2020, September 24, 2020, September 28, 2020, October 19, 2020, November 16, 2020, January 11, 2021, and February 26, 2021. Leaves were combined to form one composite sample for the two trees per row that received biocrust applications (n = 32 total leaves per composite sample). Similarly, leaves were combined to form a composite sample for the two control trees in each row. The leaves were washed with deionized water and oven dried at 60 °C for 72 h, or until constant weight.
Two twigs (< 7 mm diameter) were collected from each quadrant of each tree, similar to leaves (8 twigs per tree, 16 twigs per row) (March 1, 2021). Leaves were removed and the twigs were cut into 5 cm length pieces. The twig pieces were oven dried for at least seven days at 60 °C. One medium branch (7–30 mm diameter) and one large branch (> 30 mm diameter) were collected from each quadrant of each tree. Twigs and leaves were removed from the branches and branches were oven dried for at least ten days at 60 °C until constant weight. Trunk subsamples were collected using a Trephor tool (Costruzioni Meccaniche Carabin C., Valle di Cadore, Belluno, Italy) (cores 15 mm in length and 2 mm in diameter) (Rossi et al. 2006) (March 2, 2021). Sixteen cores were collected from each tree and cores from the first two trees of each row and last two trees of each row were combined to form composite samples (32 cores per composite sample). Trunk cores were oven dried at 60 °C for at least 10 days.
Fruit samples were collected by hand harvesting all mature fruit from each tree. A subsample of eight fruit from each tree were cut into quarters using a sterilized knife (February 26, 2021). Quarters of fresh fruit were oven dried at 60 °C for 10 days.
Biocrust chlorophyll a biomass determination
Chlorophyll a (Chl a) in biocrust was assessed on the day of biocrust collection from the biocrust collection site (July 21, 2020) and after 15N labelling prior to application in the field trial site (September 23, 2020). Chl a was measured as a proxy for phototroph biomass and biocrust health (Ayuso et al. 2017; Chiquoine et al. 2016). After the 15N labeling period in the greenhouse, 1 × 1 cm diameter cores were collected from each biocrust tray in the greenhouse (24 cores). Every four cores collected were combined to form one composite core (6 composite cores total). After collection, cores were stored at 4 °C in the dark until analysis. Chl a was measured according to Castle et al. (2011) using a double ethanol extraction technique (Caesar et al. 2018; Schwabedissen et al. 2017) with negative controls. A subsample (~ 1.0 g) of each fresh biocrust composite sample was ground for 3 min with a mortar and pestle in 95% ethanol neutralized with MgCO3 (0.3 g MgCO3 L−1 ethanol). The resultant paste was transferred to a 15 mL polypropylene centrifuge tube (Corning, Glendale, AR, USA) and 6 ml of 95% ethanol was added. Centrifuge tubes were then placed in an 80 °C water bath until boiling (~ 5 min). After boiling, tubes were placed in an ice bath for 10 min, followed by 20 min of horizontal shaking and subsequent centrifugation at 3000 × G for 10 min at 15 °C. The supernatant was transferred to a separate 15 mL vial. Another 6 mL of neutralized ethanol was added to the paste in the original vial and the extraction procedure (boiling, cooling, shaking, centrifuging) was repeated. The supernatants were combined and extractants were measured in triplicate on a spectrophotometer (SpectraMax 190 Absorbance Microplate Reader, Molecular Devices, Sunnyvale, CA, USA) at 649, 665, and 750 wavelengths with an acidification correction of 0.1 M HCl (Parker et al. 2016). Chl a is reported as ug Chl a g dry biocrust−1 cm−2.
Biomass and nitrogen analyses
Dry weight biomass estimations for tree organs were predicted using allometric equations based on tree canopy volume (Table S1) (Morgan et al. 2006a, b). Tree canopy volumes (TCVs) were calculated using the model (Morgan et al. 2006a, b; Whitney et al. 1991) (Eq. 1):
where TCV = tree canopy volume (m3), PaD = parallel diameter of tree canopy (m), PeD = perpendicular diameter of tree canopy (m), CanHT = canopy height (m), SkirtHT = skirt height (m).
After oven drying, biocrust and soil samples were ground using a ball mill for analysis of total N (TN) and atom % 15N. Plant tissues were ground using a Wiley mill (model 1; Arthur Thomas Manufacturing Co., Philadelphia, PA, USA) (leaf, fruit, twig, and branch samples) or a Cyclotec mill (1093 Sample Mill; Tecator Manufacturing, Hoganas Sweden) followed by a mortar and pestle (trunk samples). Total N content for biocrust, soil, and plant tissue was determined using an ECS 4010 CHN analyzer (Costech Analytical Technologies, Inc., Valencia, CA, USA) (dry combustion method) (Tables S2, S3). Atom % 15N was determined using a Finnigan MAT Delta PlusXL isotopic ratio mass spectrometer (Finnigan Corporation, San Jose, CA, USA) (Inglett et al. 2007). The total N content of each tree organ was calculated as (Eq. 2):
The total amount of 15N recovered by the soil and plant components and the percentage of N in the soil and plant components derived from the labeled biocrust were calculated using isotopic dilution equations (Hauck and Bremner 1976; Mattos et al. 2003). Predicted tree tissue dry weights (determined using Eq. 1) were used to calculate the amount of 15N in each plant tissue as a percentage of the original amount of 15N applied (Eq. 3):
The amount of 15N from the biocrust that was assimilated by each tree organ or the subsurface soil was calculated as [Eq. 4]:
Where [Eq. 5],
Statistical analysis
Statistical modeling was conducted in R (R Development Core Team 2021) with the nlme v. 3.1.149 (Pinheiro et al. 2021) and emmeans v. 1.5.3 (Lenth 2021) packages. Welch’s t-test was used to test for significant differences between biocrust Chl a biomass before and after 15N labeling. This test was also used to test for significant differences in fresh fruit yield between trees that received biocrust and trees that did not (control). A generalized least squares linear model with an unstructured covariance matrix was used to identify significant differences in 15N content in biocrust, subsoil, and plant tissue (fibrous roots and leaves) over time. Sampling time was modeled as a fixed effect and field replicate was a covariate.
Results
Chlorophyll a biomass
Chlorophyll a biomass in biocrust was assessed before and after 15N labeling. There was no significant difference in biocrust Chl a biomass before 15N application (July 31, 2020) and after 15N application (September 21, 2020), indicating biocrust phototroph biomass was not impacted by greenhouse incubation and pulse 15N labeling (p < 0.05) (Fig. 2).
Biocrust Chl a biomass (ug Chl a g dry biocrust−1 cm−2) before 15Nitrogen labeling in the greenhouse (July 31, 2020) and after labeling (September 21, 2020). No significant differences between bars (p > 0.05) (n = 6 box−1). Whiskers represent maximum and minimum values. Median represented by line inside box and mean represented by diamond inside box. The top and bottom of boxes represent the 75th and 25th percentiles, respectively
Biocrust N release to subsoil
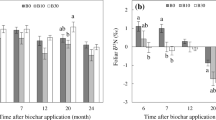
The amount of 15N in the biocrust was significantly impacted by sampling date (p < 0.001). Approximately 92% of biocrust N (biocrust 15N) was released from the time of biocrust application (665.0 ± 121.2 mg 15N) (September 21, 2020) through fruit harvest (55.2 ± 28.0 mg 15N) (February 26, 2021) (Fig. 3A). The largest difference in biocrust N between two sampling dates occurred from three days after biocrust application to seven days after application (189.8 mg released).
A Average atom% 15N of biocrust (solid line; primary y-axis) and biocrust 15N (mg) (dashed line; secondary y-axis) from time of application in the field (September 21, 2020; 0 Days) through fruit harvest (February 26, 2021; 158 Days) (n = 6, mean ± SE). B Amount of N (mg) recovered from biocrust in subsoil (primary y-axis), leaves (primary y-axis), and fibrous roots (secondary y-axis) (n = 6, mean ± SE)
The amount of 15N in the subsoil below the biocrust was significantly impacted by sampling date (p < 0.001). Of the initial biocrust N, approximately 2.8% was present in the subsoil below biocrust one day after application (Fig. 3B). The amount of biocrust N in the subsoil peaked three days after biocrust application when 3.5% of biocrust N available for release was in the subsoil. There was significantly more 15N in the subsoil three days after biocrust application than 112 and 156 days after biocrust application (p < 0.05).
Uptake of biocrust N by fibrous roots and leaves
Approximately 2.3% of biocrust N was assimilated by the fibrous roots one day after application (Fig. 3B). The amount of biocrust N in the fibrous roots increased until 28 days after biocrust application when 14% of biocrust N from the time of application was in the fibrous roots. This was significantly more 15N in the fibrous roots than at fresh fruit harvest at 158 days after application (p < 0.05). Biocrust N in the fibrous roots decreased linearly from 28 days after biocrust application through fruit harvest (158 days after biocrust application) (m = -51, R2 = 0.989).
There was a significant effect of sampling date on the amount of 15N in the leaves (p < 0.001). Biocrust N was first observed in the tree leaves six days after it was assimilated by the fibrous roots and seven days after biocrust application (Fig. 3B). The amount of biocrust N in the leaves peaked 28 days after application (1.2% of biocrust N) and remained at these levels through fruit harvest.
Translocation of 15N within the plant
At fruit harvest (158 days after biocrust application) approximately 23.8% of biocrust N available at transplant (0 days after application) was in tree tissue (Table 2). The most biocrust N was in the leaves (49 ± 10.8 mg) (7.4% of biocrust N), followed by fibrous roots (26.9 ± 8.6 mg) (4.0%) and large branches (26.4 ± 2.7 mg) (4.0%). There was significantly more biocrust N in the aboveground biomass (111.2 mg) (16.7% of biocrust N available at application) compared to belowground biomass (46.7 mg) (7.0% of biocrust N available at application). At fruit harvest, approximately 0.7% of biocrust N was in the tree fruit. There was no difference in fresh fruit yield between trees that received biocrust and trees that did not (p > 0.05) (Fig. 4).
Fruit yield (kg fresh fruit biomass tree−1) of the trees with biocrust application and trees without biocrust application (control). No significant difference between boxes (p > 0.05) (n = 6 box−1). Whiskers represent maximum and minimum values. Median represented by line inside box and mean represented by diamond inside box. Top and bottom of boxes represent the 75th and 25th percentiles, respectively
Discussion
Nitrogen release from biocrust
Biological soil crusts have higher concentrations of N compared to nearby bare soil without crusts (Barger et al. 2016; Wu et al. 2009), and there is potential for this N to be transported to biocrust subsoil (Evans and Lange 2001; Nevins et al. 2020). This source and potential transfer of N has implications in ecosystems with plant root zones that overlap with biocrust subsoil. For example, it is common for crops in sandy soil agroecosystems to have shallow root systems (Kadyampakeni et al. 2017), especially in citrus orchards where upwards of 75% of citrus root mass can be found in the top 15 cm of the soil profile (Quiñones et al. 2007; Zhang et al. 1996). These perennial tree crops have high N demand with up to 300 kg N ha−1 commonly applied to achieve maximum plant yield (Morgan et al. 2006a, b; Quaggio et al. 2019), so a biocrust mediated increase of plant-available N in the crop root zone could reduce the amount of synthetic fertilizer needed to reach maximum plant productivity. Therefore, this research investigated the potential for biocrust N to be transported to the biocrust subsoil and subsequently into a citrus crop from fruit set (September) through harvest (March).
Nitrogen was slowly released from the biocrust over time from the crop fruit set period through harvest (Fig. 3A), as indicated by 15N movement from biocrust to subsurface soil and tree tissue over time. Biocrusts released N to the subsoil in the crop root zone at a rate of 3.86 mg N d−1 over 158 days from September through March. This gradual release of N indicated there was likely not an immediate leaching of N after biocrust application, and instead, the biocrust N was slowly mineralized into plant-available forms (ON, NH4+, and NO3−), released to subsoil, and taken up by the plant. Other studies have found that a one-time injection of 15N into biocrusts in the field is immediately (within hours) leached into the subsurface soil for plant uptake, however, much of the N at the injection sites could have directly passed through the root zone (Dettweiler‐Robinson et al. 2019; Green et al. 2008; Zhuang et al. 2015). Our study used a greenhouse incubation and pulse 15N labeling technique to apply 15NH4Cl to biocrust prior to biocrust field application and confirmed that biocrust Chl a biomass was not significantly impacted by the greenhouse incubation or pulse labeling. This signaled that the biocrust was alive (Raggio et al. 2022) and that the added N was present mostly in the biomass of the biocrust. Unlike field application of 15N in other studies, we measured only a small release of 15N from the biocrust into the subsoil after 24 h. At the end of the experiment (158 days after biocrust application), 8% of 15N remained in the biocrust for future release. Therefore, our study is the first to illustrate that biocrust N can be slowly released over time (158 days), potentially acting as a slow-release N fertilizer in an agroecosystem.
Nitrogen uptake by tree crop
Biocrust N flowed through the system starting from the biocrust to the subsurface soil and fibrous roots (after 24 h) and into the tree leaves (after 72 h) (Fig. 3). Biocrust N accumulation peaked in the roots and leaves 28 days after application and began to subside in these tissues leading up to harvest when biocrust N was present in tree fruit. More than five times the amount of 15N was taken up by the fibrous roots 72 h after biocrust application compared to the amount of 15N in the subsoil at the same time. This indicated that biocrust N does not build up in the subsoil and instead is either assimilated by the plant or lost from the subsoil through leaching, denitrification, or nitrous oxide production. This sampling period (72 h after application/September) overlaps with a period of citrus root flush when trees are actively assimilating N and transporting it to the root system for root growth and development (Duncan and Noling 1987). Thus, biocrusts have the potential to support citrus root growth during the fall root flush period. However, because this is a N demanding growth period for citrus, this also indicates that additional N from other sources is important for the trees during this period to supplement biocrust N.
Other studies have found that biocrusts support root growth of nearby plants, either through nutrient mass flow (Green et al. 2008) or fungal loop transfer between biocrust and plants (Aanderud et al. 2018; Dettweiler‐Robinson et al. 2019; Hawkes 2003). Ectomycorrhizal fungi (EMF) and AMF can move N from plant to plant (Addy et al. 2005; Simard and Durall 2004) and crust to plant, respectively (Aanderud et al. 2018; Hawkes 2003), in arid and scrubland ecosystems. Saprotrophic fungi can also transfer N from areas of net mineralization (Frey et al. 2000), including those near biocrusts. Citrus can form mutualistic relationships with AMF (Song et al. 2015; Wu et al. 2013), and a recent work found Glomeromycota (AMF) and fungal saprotrophs in biocrust subsoil in citrus orchards (Nevins et al. 2021, 2022b). Despite this, it is likely that the initial N transfer (within 72 h) from biocrusts to roots in this study was through nutrient mass flow as fungal connections and associations with biocrusts can take weeks or months to fully development (Morriën et al. 2017). Nitrogen, however, continued to be taken up by roots through the duration of this study (158 days), and it is possible that fungal associations facilitated N transfer later in the study during fruit maturation. For example, N translocation from biocrust to plants occurred in drylands despite disrupting soil fungi (Dettweiler-Robinson et al. 2019).
Biocrust N was present in citrus leaves three days after biocrust application and biocrust N content initially peaked in the leaves 28 days after application (Fig. 3B). Biocrust N was in all plant tissues at fruit harvest (Table 2), and the rise of biocrust N in leaves and other aboveground tissue coincided with the beginning of a vegetative flush period in the spring (Syvertsen et al. 1981). Although biocrust N can be translocated to nearby shrubs in desert ecosystems (Green et al. 2008; Zhuang et al. 2015), this is the first study to indicate that N can be transferred from biocrust to crop aboveground biomass and fruit in an agroecosystem. Approximately 23.8% of total biocrust 15N applied was in the crop tissue at fruit harvest (March), with 0.65% of that applied 15N in the fruit tissue. This 15N in the fruit was 2.7% of the total 15N taken up by the tree.
Biocrust support tree crop nitrogen requirements
Stable 15N isotope tracing has previously been used as a tool to quantify N uptake and partitioning in citrus trees. When N was not limited, citrus trees can recover 16–61% of 15N supplied through synthetic fertilizer in the spring (Dasberg 1987; Feigenbaum et al. 1987; Menino et al. 2007; Roccuzzo et al. 2017). Comparably, in our study, approximately 23% of biocrust 15N was recovered in tree tissue at harvest. In this current experiment, the labelled N was not broadcast as granular synthetic fertilizer, as done in other studies. It is possible that by concentrating the labeled N to a limited soil volume below the biocrust patches, we may have saturated the ability of the roots to assimilate biocrust N (Fernández-Escobar et al. 2014). As already mentioned, leaching of labelled N could also have contributed to the reduced N recovery in this study compared to others. Southwest Florida agroecosystems have highly permeable sandy soils with shallow citrus root zones and experience high intensity rain events (Shukla et al. 2010).
Assuming an agroecosystem has 300 trees ha−1 on average, each tree would receive approximately 1.2 kg N annually. In this study, biocrusts had the potential to supply approximately 0.008 kg N tree−1, or 0.7% of tree total annual N requirements at 250 kg N ha−1 yr−1. It is common for biocrusts in sandy soil agroecosystems to cover approximately 30–70% of the soil surface surrounding plants (Nevins et al. 2021), and up to 70–80% of the soil surface in drylands (Chen et al. 2020; Ding and Eldridge 2020). In this study, biocrust covered approximately 1.2% of the bare soil surface between trees (under canopies). Therefore, this research should serve as a conservative estimate of the amount of N that can be transferred from biocrust to nearby trees. Furthermore, according to this study, biocrusts could supply up to 17% of crop N requirements of a young tree assuming the biocrust occupies 30% of the soil surface in an agroecosystem. This study should serve as a starting point for future research to determine the amount of biocrust N that can be assimilated by different crops under varying environmental conditions, soil types, and biocrust types. Future studies should focus on the potential role of fungal communities and mutualistic fungal relationships in the translocation of N from biocrust to crops, potentially using DNA stable isotope probing techniques (Wang et al. 2020). The methods in this work did not allow more time to facilitate potential connections between the biocrust, soil fungal community, and crop roots. Should fungi facilitate the transfer of N from biocrust to crops in agroecosystems, management practices could be adopted by growers to support this translocation. Alternative nutrient management strategies that increase N mineralization and quantities in agroecosystem soil could foster growth and presence of these mutualistic fungal communities.
Conclusions
This research indicates that biocrust in agroecosystems have the potential to support crop growth by supplying N that is available for plant assimilation during root and leaf flush periods. Biocrusts released N to the subsoil in the crop root zone over 158 days from September through March. Biocrust N was present in fibrous roots and leaves as soon as one and three days after adding labeled biocrusts, respectively. Over 20% of total biocrust 15N applied was in the crop tissue at fruit harvest (March), including in the fruit tissue. Overall, these results indicate biocrusts have the potential to supplement other N applications to assist in meeting crop N demand. Future research should investigate how different nutrient management strategies, including different rates of synthetic and organic fertilizers, interact with biocrusts in agroecosystems. Biological N fixation rates of biocrusts in arid ecosystems are impacted by environmental conditions, such as precipitation and temperature (Ochoa-Hueso et al. 2016; Yeager et al. 2012). Recent work indicated that biocrusts in citrus and grape agroecosystems can fix atmospheric N, despite crop fertilization, however fertilization and irrigation rates and phosphorus management impacted biocrust N fixation rates (Sorochkina et al. 2022). Future work should further characterize the impact of management on biocrust functional potential to better understand the potential to manage biocrusts to support crop growth and soil health. This could include how different fertilizer management practices impact plant assimilation of biocrust N. Collectively, this work indicates that managing agroecosystems to support biocrust functioning could overlap between peak biological N fixation activity of agroecosystem biocrusts with crop N demanding growth periods, increasing potential for biocrust support of crop growth.
References
Aanderud ZT, Smart TB, Wu N, Taylor AS, Zhang Y, Belnap J (2018) Fungal loop transfer of nitrogen depends on biocrust constituents and nitrogen form. Biogeosciences 15:3831–3840. https://doi.org/10.5194/bg-15-3831-2018
Addy HD, Piercey MM, Currah RS (2005) Microfungal endophytes in roots. Can J Bot 83(1):1–13. https://doi.org/10.1139/B04-171
Allar J, Maltais-Landry G (2022) Limited benefits of summer cover crops on nitrogen cycling in organic vegetable production. Nutr Cycl Agroecosyst 122:119–138
Alva AK, Paramasivam S, Obreza TA, Schumann AW (2006) Nitrogen best management practice for citrus trees: I. Fruit yield, quality, and leaf nutritional status. Sci Hortic (Amsterdam) 107:233–244. https://doi.org/10.1016/j.scienta.2005.05.017
Ayuso SV, Silva AG, Nelson C, Barger NN, Garcia-Pichel F (2017) Microbial nursery production of high-quality biological soil crust biomass for restoration of degraded dryland soils. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02179-16
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J (2016) Patterns and Controls on Nitrogen Cycling of Biological Soil Crusts. In: Weber B, Budel B, Belnap J (eds) Biological soil crusts: an organizing principle in drylands. Springer, Cham, pp 257–285. https://doi.org/10.1007/978-3-319-30214-0_14
Caesar J, Tamm A, Ruckteschler N, Lena Leifke A, Weber B (2018) Revisiting chlorophyll extraction methods in biological soil crusts: methodology for determination of chlorophyll a and chlorophyll a Cb as compared to previous methods. Biogeosciences 15:1415–1424. https://doi.org/10.5194/bg-15-1415-2018
Castellano-Hinojosa A, Nevins C, Strauss S (2021) Influence of cover crops on nitrogen cycling and the soil microbial community. In: Gonzalez-Lopez J, Gonzalez-Martinez A (eds), Nitrogen cycle: ecology, biotechnological applications and environmental impacts, First, pp 264–284. CRC Press
Castle SC, Morrison CD, Barger NN (2011) Extraction of chlorophyll a from biological soil crusts: a comparison of solvents for spectrophotometric determination. Soil Biol Biochem 43:853–856. https://doi.org/10.1016/j.soilbio.2010.11.025
Chen N, Yu K, Jia R, Teng J, Zhao C (2020) Biocrust as one of multiple stable states in global drylands. Sci Adv 6:eaay3763. https://doi.org/10.1126/sciadv.aay3763
Chiquoine LP, Abella SR, Bowker MA (2016) Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem. Ecol Appl 26:1260–1272. https://doi.org/10.1002/15-0973
Concostrina-Zubiri L, Huber-Sannwald E, Martínez I, Flores Flores JL, Escudero A (2013) Biological soil crusts greatly contribute to small-scale soil heterogeneity along a grazing gradient. Soil Biol Biochem 64:28–36. https://doi.org/10.1016/j.soilbio.2013.03.029
Dasberg S (1987) Nitrogen fertilization in citrus orchards. Plant Soil 100:1–9. https://doi.org/10.1007/BF02370928
Defalco LA, Detling JK, Tracy CR, Warren SD (2001) Physiological variation among native and exotic winter annual plants associated with microbiotic crusts in the Mojave Desert. Plant Soil 234:1–14. https://doi.org/10.1023/A:1010323001006
Dettweiler-Robinson E, Sinsabaugh RL, Rudgers JA (2019) Fungal connections between plants and biocrusts facilitate plants but have little effect on biocrusts. J Ecol 1365–2745:13310. https://doi.org/10.1111/1365-2745.13310
Ding J, Eldridge DJ (2020) Biotic and abiotic effects on biocrust cover vary with microsite along an extensive aridity gradient. Plant Soil 450:429–441. https://doi.org/10.1007/s11104-020-04517-0
Duncan L, Noling J (1987) The relationship between development of the citrus root system and infestation by Tylenchulus semipenetrans. Rev Nematol 10:61–66
Esteves E, Kadyampakeni DM, Zambon F, Ferrarezi R, Maltais-Landry G (2022) Magnesium fertilization has a greater impact on soil and leaf nutrient concentrations than nitrogen or calcium fertilization in Florida orange production. Nutr Cycl Agroecosyst 122:73–87
Evans RD, Lange OL (2001) Biological soil crusts and ecosystem nitrogen and carbon dynamics. Springer, Berlin, Heidelberg, pp 263–279. https://doi.org/10.1007/978-3-642-56475-8_20
Feigenbaum S, Bielorai H, Erner Y, Dasberg S (1987) The fate of 15N labeled nitrogen applied to mature citrus trees. Plant Soil 97:179–187. https://doi.org/10.1007/BF02374940
Fernández-Escobar R, Antonaya-Baena MF, Sánchez-Zamora MA, Molina-Soria C (2014) The amount of nitrogen applied and nutritional status of olive plants affect nitrogen uptake efficiency. Sci Hortic (Amsterdam) 167:1–4. https://doi.org/10.1016/j.scienta.2013.12.026
Frey SD, Elliott ET, Paustian K, Peterson GA (2000) Fungal translocation as a mechanism for soil nitrogen inputs to surface residue decomposition in a no-tillage agroecosystem. Soil Biol Biochem 32:689–698. https://doi.org/10.1016/S0038-0717(99)00205-9
Godínez-Alvarez H, Morín C, Rivera-Aguilar V (2011) Germination, survival and growth of three vascular plants on biological soil crusts from a Mexican tropical desert. Plant Biol 14:23. https://doi.org/10.1111/j.1438-8677.2011.00495.x
Green LE, Porras-Alfaro A, Sinsabaugh RL (2008) Translocation of nitrogen and carbon integrates biotic crust and grass production in desert grassland. J Ecol 96:1076–1085. https://doi.org/10.1111/j.1365-2745.2008.01388.x
Guo Y, Zhao H, Zuo X, Drake S, Zhao X (2008) Biological soil crust development and its topsoil properties in the process of dune stabilization, Inner Mongolia, China. Environ Geol 54:653–662. https://doi.org/10.1007/s00254-007-1130-y
Hamido SA, Morgan KT (2021) The effect of irrigation rate on the water relations of young citrus trees in high-density planting. Sustainability 13:1759. https://doi.org/10.3390/su13041759
Hamido S, Morgan K, Kadyampakeni D (2017) The effect of huanglongbing on young citrus tree water use. Horttechnology 27:659–665
Harper KT, Belnap J (2001) The influence of biological soil crusts on mineral uptake by associated vascular plants. J Arid Environ 47:347–357. https://doi.org/10.1006/jare.2000.0713
Hauck RD, Bremner JM (1976) Use of tracers for soil and fertilizer nitrogen research. Adv Agron 28:219–266. https://doi.org/10.1016/S0065-2113(08)60556-8
Havrilla CA, Barger NN (2018) Biocrusts and their disturbance mediate the recruitment of native and exotic grasses from a hot desert ecosystem. Ecosphere 9:e02361. https://doi.org/10.1002/ecs2.2361
Havrilla CA, Chaudhary VB, Ferrenberg S, Antoninka AJ, Belnap J, Bowker MA, Eldridge DJ, Faist AM, Huber-Sannwald E, Leslie AD, Rodriguez-Caballero E, Zhang Y, Barger NN (2019) Towards a predictive framework for biocrust mediation of plant performance: a meta-analysis. J Ecol 1365–2745:13269. https://doi.org/10.1111/1365-2745.13269
Havrilla C, Leslie AD, Di Biase JL, Barger NN (2020) Biocrusts are associated with increased plant biomass and nutrition at seedling stage independently of root-associated fungal colonization. Plant Soil 446:331–342. https://doi.org/10.1007/s11104-019-04306-4
Hawkes CV (2003) Nitrogen cycling mediated by biological soil crusts and arbuscular mycorrhizal fungi. Ecology 84:1553–1562. https://doi.org/10.1890/0012-9658(2003)084[1553:NCMBBS]2.0.CO;2
Inglett PW, Reddy KR, Newman S, Lorenzen B (2007) Increased soil stable nitrogen isotopic ratio following phosphorus enrichment: Historical patterns and tests of two hypotheses in a phosphorus-limited wetland. Oecologia 153:99–109. https://doi.org/10.1007/s00442-007-0711-5
Kadyampakeni DM, Nkedi-Kizza P, Leiva JA, Muwamba A, Fletcher E, Morgan KT (2018) Ammonium and nitrate transport during saturated and unsaturated water flow through sandy soils. J Plant Nutr Soil Sci 181:198–210. https://doi.org/10.1002/jpln.201700405
Kadyampakeni DM, Morgan KT, Zekri M, Ferrarezi R, Schumann AW, Obreza TA (2017) Citrus irrigation management. UF/IFAS Electron. Data Inf Source SL446
Kidron GJ (2014) Do mosses serve as sink for rain in the Negev Desert? A theoretical and experimental approach. CATENA 121:31–39. https://doi.org/10.1016/j.catena.2014.05.001
Langhans TM, Storm C, Schwabe A (2009) Biological soil crusts and their microenvironment: impact on emergence, survival and establishment of seedlings. Flora Morphol Distrib Funct Ecol Plants 204:157–168. https://doi.org/10.1016/j.flora.2008.01.001
Lea-Cox JD, Syvertsen JP, Graetz DA (2001) Springtime 15nitrogen uptake, partitioning, and leaching losses from young bearing Citrus trees of differing nitrogen status. J Am Soc Hortic Sci 126:242–251
Lenth R (2021) emmeans: estimated marginal means, aka least-squares means. (R package version 1.3.5.1.). https://cran.r-project.org/package=emmeans
Mattos D, Graetz DA, Alva AK (2003) Biomass distribution and nitrogen-15 partitioning in citrus trees on a sandy entisol. Soil Sci Soc Am J 67:555–563. https://doi.org/10.2136/sssaj2003.5550
Menino MR, Carranca C, de Varennes A (2007) Distribution and remobilization of nitrogen in young non-bearing orange trees grown under Mediterranean conditions. J Plant Nutr 30:1083–1096. https://doi.org/10.1080/01904160701394543
Morgan K, Scholberg J, Obreza T, Wheaton T (2006a) Size, biomass, and nitrogen relationships with sweet orange tree growth. J Am Soc Hortic Sci 131(1):149
Morgan KT, Obreza TA, Scholberg JMS, Parsons LR, Wheaton TA (2006b) Citrus water uptake dynamics on a sandy Florida Entisol. Soil Sci Soc Am J 70:90. https://doi.org/10.2136/sssaj2005.0016
Morgan KT, Kadyampakeni DM, Zekri M, Shumann A, Vashisth T, Obreza T (2019) 2019–2020 Florida citrus production guide: nutrition management for citrus trees. UF/IFAS Electron. Data Inf. Source CMG13. https://edis.ifas.ufl.edu/cg091
Morriën E, Hannula SE, Snoek LB, Helmsing NR, Zweers H, De Hollander M, Soto RL, Bouffaud ML, Buée M, Dimmers W, Duyts H, Geisen S, Girlanda M, Griffiths RI, Jørgensen HB, Jensen J, Plassart P, Redecker D, Schmelz RM, Van Der Putten WH et al (2017) Soil networks become more connected and take up more carbon as nature restoration progresses. Nat Commun 8:1–10. https://doi.org/10.1038/ncomms14349
Nevins CJ, Strauss SL, Inglett PW (2020) Biological soil crusts enhance moisture and nutrients in the upper rooting zone of sandy soil agroecosystems. J Plant Nutr Soil Sci 183:615–626. https://doi.org/10.1002/jpln.202000218
Nevins CJ, Inglett PW, Strauss SL (2021) Biological soil crusts structure the subsurface microbiome in a sandy agroecosystem. Plant Soil 462:311–329. https://doi.org/10.1007/s11104-021-04868-2
Nevins CJ, Inglett PW, Reardon CL, Strauss SL (2022a) Seasonality drives microbiome composition and nitrogen cycling in soil below biocrusts. Soil Biol Biochem 166:108551. https://doi.org/10.1016/J.SOILBIO.2022.108551
Nevins CJ, Strauss SL, Inglett PW (2022b) Contrasting effects of agroecosystem biocrusts on seedling growth and nitrogen accumulation in a greenhouse environment. Agrosyst Geosci Environ 5:295
Nevins CJ (2021) Biological soil crusts impact nutrient cycling and soil microbiome in agroecosystems. Doctoral disseration, University of Florida.
Ochoa-Hueso R, Delgado-Baquerizo M, Gallardo A, Bowker MA, Maestre FT (2016) Climatic conditions, soil fertility and atmospheric nitrogen deposition largely determine the structure and functioning of microbial communities in biocrust-dominated Mediterranean drylands. Plant Soil 399:271–282. https://doi.org/10.1007/s11104-015-2695-y
Parker S, Bowden W, Flinn M (2016) The effect of acid strength and postacidification reaction time on the determination of chlorophyll a in ethanol extracts of aquatic periphyton. Limnol Oceanogr Methods 14:839–852
Peng X, Bruns MA (2019a) Cyanobacterial soil surface consortia mediate N cycle processes in agroecosystems. Front Environ Sci 6:156. https://doi.org/10.3389/fenvs.2018.00156
Peng X, Bruns MA (2019b) Development of a nitrogen-fixing cyanobacterial consortium for surface stabilization of agricultural soils. J Appl Phycol 31:1047–1056. https://doi.org/10.1007/s10811-018-1597-9
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2021) nlme: linear and nonlinear mixed effects models (3.1-152). https://cran.r-project.org/web/packages/nlme/nlme.pdf
Quaggio JA, Souza TR, Zambrosi FCB, Mattos D, Boaretto RM, Silva G (2019) Citrus fruit yield response to nitrogen and potassium fertilization depends on nutrient-water management system. Sci Hortic (Amsterdam) 249:329–333. https://doi.org/10.1016/j.scienta.2019.02.001
Quiñones A, Martínez-Alcántara B, Legaz F (2007) Influence of irrigation system and fertilization management on seasonal distribution of N in the soil profile and on N-uptake by citrus trees. Agric Ecosyst Environ 122:399–409. https://doi.org/10.1016/j.agee.2007.02.004
R Development Core Team (2021) R: a language and environment for statistical computing R foundation for statistical computing
Raggio J, Pescador D, Gozalo B, Ochoa V, Valencia E, Sancho LG, Maestre F (2022) Continuous monitoring of chlorophyll a fluorescence and microclimatic conditions reveals warming-induced physiological damage in biocrust-forming lichens. Plant Soil 482:261–276
Roccuzzo G, Scandellari F, Allegra M, Torrisi B, Stagno F, Mimmo T, Zanotelli D, Gioacchini P, Millard P, Tagliavini M (2017) Seasonal dynamics of root uptake and spring remobilisation of nitrogen in field grown orange trees. Sci Hortic (Amsterdam) 226:223–230. https://doi.org/10.1016/j.scienta.2017.08.010
Rodriguez-Caballero E, Belnap J, Büdel B, Crutzen PJ, Andreae MO, Pöschl U, Weber B (2018) Dryland photoautotrophic soil surface communities endangered by global change. Nat Geosci 11:185–189. https://doi.org/10.1038/s41561-018-0072-1
Rossi S, Anfodillo T, Menardi R (2006) Trephor: A new tool for sampling microcores from tree stems. IAWA J 27:89–97
Roth RT, Lacey CG, Camberato JJ et al. (2023) Quantifying the fate of nitrogen from cereal rye root and shoot biomass using 15N. Nutr Cycl Agroecosyst 125:219–234. https://doi.org/10.1007/s10705-022-10213-5
Schwabedissen SG, Lohse KA, Reed SC, Aho KA, Magnuson TS (2017) Nitrogenase activity by biological soil crusts in cold sagebrush steppe ecosystems. Biogeochemistry 134:57–76. https://doi.org/10.1007/s10533-017-0342-9
Shukla S, Boman BJ, Ebel RC, Roberts PD, Hanlon EA (2010) Reducing unavoidable nutrient losses from Florida’s horticultural crops. Horttechnology 20:52–66. https://doi.org/10.21273/horttech.20.1.52
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Can J Bot 82:1140–1165. https://doi.org/10.1139/B04-116
Song F, Pan Z, Bai F, An J, Liu J, Guo W, Bisseling T, Deng X, Xiao S (2015) The scion/rootstock genotypes and habitats affect arbuscular mycorrhizal fungal community in citrus. Front Microbiol 6:1372. https://doi.org/10.3389/fmicb.2015.01372
Sorochkina K, Strauss SL, Inglett PW (2022) Contrasting seasonal patterns and factors regulating biocrust N2-fixation in two Florida agroecosystems. Front Microbiol. https://doi.org/10.3389/FMICB.2022.892266
Strauss S, Castellano-Hinojosa A, Kadyampakeni D, Kanissery R, Wade T (2020a) Improving soil health with cover crops: an update. EDIS 2020: https://journals.flvc.org/edis/article/view/123731
Strauss S, Sorochkina K, Nevins C, Reardon C, Inglett P (2020b) Exploring the roles of biological soil crusts in agroecosystems. In: ASA, CSSA SSSA International Annual Meetings
Syvertsen J, Smith M, Allen J (1981) Growth rate and water relations of citrus leaf flushes. Ann Bot 47:97–105
Wang X, Bei Q, Yang W, Zhang H, Hao J, Qian L, Feng Y, Xie Z (2020) Unveiling of active diazotrophs in a flooded rice soil by combination of NanoSIMS and 15N2-DNA-stable isotope probing. Biol Fertil Soils 56:1189–1199. https://doi.org/10.1007/s00374-020-01497-2
Whitney JD, Elezaby A, Castle WS, Wheaton TA, Littell RC (1991) Citrus tree spacing effects on soil water use, root density and fruit yield. Trans Am Soc Agric Eng 34:129–134
Wu N, Zhang YM, Downing A (2009) Comparative study of nitrogenase activity in different types of biological soil crusts in the Gurbantunggut Desert, Northwestern China. J Arid Environ 73:828–833. https://doi.org/10.1016/j.jaridenv.2009.04.002
Wu QS, Srivastava AK, Zou YN (2013) AMF-induced tolerance to drought stress in citrus: a review. Scientia horticulturae, vol 164. Elsevier, pp 77–87. https://doi.org/10.1016/j.scienta.2013.09.010
Yeager CM, Kuske CR, Carney TD, Johnson SL, Ticknor LO, Belnap J (2012) Response of biological soil crust diazotrophs to season, altered summer precipitation, and year-round increased temperature in an arid grassland of the Colorado Plateau, USA. Front Microbiol 3:358. https://doi.org/10.3389/fmicb.2012.00358
Zekri M, Schumann AW, Vashisth T, Kadyampakeni DM, Morgan KT, Bowman BJ, Obreza TA, Boman BJ, Obreza TA (2017) Fertigation for citrus trees. UF/IFAS Electron Data Inf Source 2017. https://doi.org/10.32473/edis-hs1306-2017
Zhang Y-M, Nie H-L (2011) Effects of biological soil crusts on seedling growth and element uptake in five desert plants in Junggar Basin, western China. Chin J Plant Ecol 35:380–388. https://doi.org/10.3724/sp.j.1258.2011.00380
Zhang M, Alva AK, Li YC, Calvert DV (1996) Root distribution of grapefruit trees under dry granular broadcast vs. fertigation method. Plant Soil 183:79–84. https://doi.org/10.1007/BF02185567
Zhang Y, Aradottir AL, Serpe M, Boeken B (2016) Interactions of biological soil crusts with vascular plants. In: Weber B, Budel B, Belnap J (eds) Biological soil crusts: an organizing principle in drylands. Springer, London, pp 385–406. https://doi.org/10.1007/978-3-319-30214-0_19
Zheng Z, Zhang Q, Zhang A, Wang T, Zhao Z, Sompouviset T, Cai S, Zheng W, Zhai B (2023) Distinguishing the contribution of nitrogen fertilizer and native soil nitrogen to young apple trees. Nutr Cycl Agroecosyst 126:101–114
Zhuang WW, Downing A, Zhang Y (2015) The influence of biological soil crusts on 15N translocation in soil and vascular plant in a temperate desert of northwestern China. J Plant Ecol 8:420–428. https://doi.org/10.1093/jpe/rtu033
Zhuang W, Li J, Yu F, Dong Z, Guo H (2020) Seasonal nitrogen uptake strategies in a temperate desert ecosystem depends on N form and plant species. Plant Biol 22:386–393. https://doi.org/10.1111/plb.13083
Acknowledgements
The authors thank Dr. Antonio Castellano-Hinojosa, Dr. Kira Sorochkina, and Brittney Monus of the UF/IFAS Southwest Florida Research and Education Center Soil Microbiology Laboratory for assistance with sample collection, processing, and analysis. We thank Dr. Kathryn Curtis of the UF Soil and Water Sciences Department for helping with stable isotope measurements. We also thank Dr. Catherine Reardon of the USDA-ARS for assistance with planning the research.
Funding
This work was supported by the United States Department of Agriculture- National Institute of Food and Agriculture- Agriculture and Food Research Initiative grant (2018–67019-27707) awarded to Sarah L. Strauss and Patrick W. Inglett, as well as Hatch Grant (7000945) (Sarah L. Strauss).
Author information
Authors and Affiliations
Contributions
P.W.I. and S.L.S. conceived of the main study and secured funding. C.J.N. adapted field design, carried out the work and conducted data analysis. C.J.N. and P.W.I. wrote the manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nevins, C.J., Strauss, S.L. & Inglett, P.W. Uptake of biocrust nitrogen by tree crops in a sandy soil agroecosystem. Nutr Cycl Agroecosyst 128, 115–130 (2024). https://doi.org/10.1007/s10705-023-10334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-023-10334-5