Abstract
An improved understanding of the contribution of a preceding forage crop to a subsequent potato crop can improve nitrogen (N) utilization in potato production. This study used two rotation experiments to estimate the N contribution from labelled shoot and root of red clover (RC, Trifolium pratense), timothy (T, Phleum pratense) and a red clover/timothy mixture (M) to a subsequent potato crop using microplots in the field. Forage crops were grown with 14NH414NO3 and 15NH415NO3 (98 atom %). The residue exchange technique was used to compare residue treatments of (i) whole plant labelled; (ii) labelled shoot only; and (iii) labelled root only in Experiment 1, and residue treatments of (i) whole plant labelled; (ii) labelled shoot/unlabelled root; and (iii) labelled root/unlabelled shoot in Experiment 2. Averaged across forage treatments, recoverable root biomass represented 64 and 37% of total forage biomass, and the total 15N recovery from labelled roots was 52 and 62% of the total 15N recovery from shoots, in Experiments 1 and 2, respectively. Therefore, forage roots represented a substantial source of N for the subsequent crop. However, less than 5% of the 15N from crop residues was recovered in the potato vines plus tubers, and most of the 15N was recovered in the soil, regardless of the forage or residue treatments. Potato tuber and vine dry matter was greater for the RC than the T treatment for all residue treatments, a finding attributed to greater potato N accumulation for the RC treatment. It is therefore important to consider the contribution of forage roots when studying N cycling in potato systems. Potato N requirements were satisfied more by soil-derived N rather than from fall incorporated forage residues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato is a crop with a high nitrogen (N) demand (Zebarth and Rosen 2007) but with a low N use efficiency, especially on coarse-textured soils (Cambouris et al. 2016), resulting in an increased risk of adverse effects on environmental and human health due to groundwater nitrate contamination (Zebarth et al. 2015). A study that assessed potato yield response and nitrate leaching in response to N management reported N fertilizer recovery to average 33% under intensive leaching conditions and 56% under less intensive leaching conditions (Errebhi et al. 1998). Matching the fertilizer N inputs to the potato crop N demand is an important strategy to improve N use efficiency, however, uncertainty in the quantity of N supplied by the soil makes such a strategy challenging (Zebarth et al. 2012). This is particularly true in humid environments like eastern Canada where residual nitrate from the previous growing season is leached from the root zone over the fall and winter, and the supply of N from the soil is determined primary by net N mineralization from the soil organic matter and from the residues of the preceding crop (Zebarth et al. 2009). A better understanding of the fate of N in the preceding rotation crop would therefore assist in improving potato fertilizer N management.
Forage crops are known to be associated with several agronomic benefits such as improved soil and water quality (Dabney et al. 2001; Stark and Porter 2005). In Eastern Canada, fall-plowed legume forage crops rapidly increase soil nitrate availability in the soil after their incorporation, thereby increasing the potential for N loss from the system over the subsequent fall and winter, and reducing the potential N contribution to subsequent potato crop (Sanderson et al. 1999; Nyiraneza et al. 2021). In addition, high residual soil nitrate after potato harvest has been reported (Jiang et al. 2012; Zebarth et al. 2015; Clément et al. 2019), which also increases the quantity of nitrate susceptible to leaching. This high residual nitrate could be the result of asynchrony between the timing of mineralization of crop residue N and potato crop N demand over the growing season, or overestimation of N fertilizer requirements due to difficulties in estimating N credits for the preceding legume forage crop.
Combining the benefits of legume and non-legume forage species may have synergistic effects for soil quality and for subsequent potato crops. Timothy is a perennial grass with a high C:N ratio that grows well in cooler climates with short growing seasons, making it an attractive option to add into cover crop mixtures with legumes in eastern Canada (Holmstrom et al. 2001; Kunelius et al. 2006). Compared to a pure legume, mixing grasses with legumes could decrease the net N mineralization after fall plowing, thereby decreasing N losses over the subsequent winter, and increase cover crop biomass, thereby increasing C inputs (Sainju and Singh 1997; Nyiraneza et al. 2021). Studies on diversifying cropping systems to mitigate nitrate leaching while sustaining potato yields have been carried out in Eastern Canada (Lynch et al. 2008; Nyiraneza et al. 2015, 2021; Liang et al. 2019), but more studies are needed to elucidate the contribution of N from legumes, grasses and a mixture of grasses and legumes into subsequent potato crops. In particular, studies on N dynamics from forage crops have focused primarily on the effects of shoot biomass rather than root biomass (Clark et al. 2001).
There is a lack of sufficient information regarding the contribution of crop residues from both the above- and below-ground components to subsequent crops. Fine roots have been reported to contribute more than 50% of total net primary productivity in grasslands (Titlyanova et al. 1999; Steinaker and Wilson 2005). Below-ground N represented 39% of total plant N for faba bean (Vicia faba), 53% for chickpea (Cicer arietinum), 27% for mung bean (Vigna radiata) and 47% for pigeon pea (Cajanus cajan) (Khan et al. 2002a). Fine roots play an important role in nutrient uptake, have high N content (Pregitzer et al. 2002) and have a fast turnover (Gill et al. 2002; Ruess et al. 2003). Greubs and Robers (2020) reported that cereals with low shoot may be characterized with greater root growth and thus N sequestration would be underestimated when root uptake is not evaluated. Therefore, an accurate characterization of N dynamics from different forage crops needs to consider both above- and below-ground biomass to elucidate the full effect of forage crops on the subsequent cash crop in rotation systems (Khan et al. 2000a; b) to increase cash crop N use efficiency and reduce N losses in agricultural production (Karimi et al. 2020).
The use of stable isotopes in agricultural research has proven to be a useful approach for field-based experiments (Hauck and Bremner 1976; Follett 2001; Delgado et al. 2009) to quantify N cycling in the soil–plant system. Using 15N labelled fertilizer, it was demonstrated that the soil supplies equal or more N to the crops than fertilizer depending on the crop (Tran et al. 1997; Nyiraneza et al. 2010; Greub and Roberts (2020). Using 15N enriched fertilizer, Greub and Roberts (2020) reported that the highest N fertilizer by cover crops (cereal rye and radish) was 38%. Nitrogen-15-enriched fertilizer in the form of 15NH4+ or 15NO3− can be used to label crop residues to estimate the N contribution from the residue to the subsequent crop (Delgado et al. 2004; Mayer et al. 2003; Arcand et al. 2014a, b; Ding et al. 2019). The crop residue exchange technique, where shoots are exchanged between plants fertilized with 14 N or 15N, can be useful to separate the contribution of the above- and below-ground residues to the subsequent crop (Taveira et al. 2020). Previous studies reported that a large proportion of N from labelled crop residues was found in the soil rather than in the subsequent crop (Delgado et al. 2004; Smith and Chalk 2018; Taveira et al. 2020) implying that most N was derived from the soil especially when no N fertilizer is applied. There is, however, a large knowledge gap regarding the N contribution from forage roots to a subsequent potato crop.
The objectives of this study were to quantify the N dynamics from three forage types (red clover, timothy and a red clover/timothy mixture) and to use the crop residue exchange technique to evaluate the fate of N from forage shoots and roots in the subsequent potato crop and in the soil under the cool humid environmental conditions in eastern Canada. We hypothesize that: i) most of N from labelled residue will be recovered in the soil; ii) total 15N recovery from labelled root will represent at least 30% of that from shoot; and iii) 15N recovered by potatoes from labelled forage legumes (RC&M treatments) will be greater than that recovered from a grass (T treatment).
Materials and methods
Two experiments were conducted to assess the fate of forage N residues into a subsequent potato crop. In Experiment 1, forage red clover (RC, trifolium pratense), timothy (T, phleum pratense) and a red clover/timothy mixture (M) were seeded in the field in microplots in 2013, labelled in situ in 2014, crop residue exchange performed in the fall of 2014, and potatoes grown in 2015. To confirm our findings in a second growing season, we conducted Experiment 2. In Experiment 2, forages were grown and labelled in the greenhouse in 2017, the crop residue exchange done in microplots in the field in the fall of 2017, and potatoes grown in 2018. The ultimate goal of both experiments was to obtain labelled roots and shoots of different forage crops namely red clover (RC), timothy (T) and a red clover-timothy mixture (M) and to follow their N cycling in potato plants grown in containers in the field over two growing seasons. Therefore, differences in forage growing conditions in the greenhouse or in the field are not expected to affect the overall objective of the study.
Field site description for Experiments 1 and 2
The experiments were conducted between 2013 and 2018 at the Harrington Research Farm of Agriculture and Agri-Food Canada, 12 km northwest of the city of Charlottetown, PEI, Canada (46° 21′N, 63° 9′W). The soil is characterised as an Orthic Humo-Ferric Podzol in the Canadian soil classification system, which corresponds to Orthic Podzol in the FAO classification system (Soil classification working group 1998). Prince Edward Island has a humid temperate climate. The long-term (1981–2010) average mean air temperatures are − 7.7 and 18.7 °C for January and July, respectively (Environment and Climate Change Canada 2021). The long-term average annual total precipitation is 1158 mm, with 25% as snow. The frost-free period varies from 100 to 160 days (Carter et al. 1994).
Experiment 1: field experiment establishment and forage crop 15 N-labelling
In spring 2013, a small area of land (approximately 4 × 20 m) was plowed and tilled. A total of 36 hollow metal cylinders (height: 0.3 m, diameter: 0.46 m, volume: 0.05 m3) were installed. Each cylinder, along with the total plant and soil matter contained within, was considered as one microplot. For installation of each microplot, an insertion channel was created in the soil using a prototype cookie cutter saw blade cut specifically for this project for easier installation and to minimize soil disturbance. The blade was connected to a hydraulic drive attached to the prongs of a Super Boom skid steer loader (New Holland, New Holland, PA, USA) (Fig. S1). This was a unique research design to allow container installation with minimum soil disturbance. Insertions were made with approximately 0.5 m spacing between cylinders, a spacing assumed to be sufficient to avoid contamination between cylinders (Fig. S2). Each cylinder was inserted into the ground by hand and hammered down leaving approximately 0.1 m protruding above the soil surface to ensure they were buried at a uniform depth (burial depth: 0.2 m). Red clover (RC), timothy (T) or red clover/timothy mixture (M) were hand seeded in 12 microplots for each forage at a seeding rate of 34 kg ha−1 for RC, 18 kg ha−1 for T and 18/9 kg ha−1 red clover/timothy for M. Forage crops were left to grow in the field during the 2013 growing season with no NPK fertilizers applied.
On May 28, 2014, double-labelled N fertilizer (15NH415NO3, atom 98% 15N) was applied to 24 of the microplots (8 per forage treatment) at a rate of 20, 60 and 40 kg N ha−1 for the RC, T, and M treatments, respectively. Nitrogen fertilizer rates were different with pure legume (RC) receiving the lowest N fertilizer to account for biological N fixation by legumes. The remaining 12 microplots (4 per forage treatment) received 14NH414NO3 fertilizer at the same rates. Pre-weighed fertilizer was dissolved in 0.5 L of distilled water and sprinkled over each microplot using a watering can (Haws Elliott Ltd, West Midlands, England). An additional 0.5 L of distilled water was added to residual solution in the watering can and applied to ensure complete application of fertilizer treatments to each microplot. Non-labelled fertilizer was applied before labelled fertilizer to avoid cross-contamination. Metal watering cans were used to apply fertilizer due to the small area of microplots instead of spraying with a pressurized gas cylinder as was done in previous work (Follett 2001; Delgado et al. 2004).
Before fertilizer application, soil samples were taken to provide an estimate of NO3− distribution and background 15N in the soil. Five to six soil cores (2 cm in diameter) were taken at 0–15 cm and 15–30 cm depth around the perimeter of each microplot so as not to disturb soil in microplots and to prevent preferential flow of N fertilizer through the sampling holes. Individual samples around each microplot were mixed together per depth to obtain a composite sample.
Experiment 1: plant tissue sampling
In PEI, cool-season grasses in their second year are normally harvested two or three times with the first harvest in mid-June, the second in mid-August and the third in early to mid October depending on the growing season length (Kunelius 1990). During the 2014 growing season, forage crops in all microplots were harvested twice. The first harvest occurred on August 8, 2014 (72 days after 15N fertilizer application). Shoot cover crop tissue was cut to soil level from each microplot, put into labelled paper bags and weighed. Non-labelled forage crops were harvested before labelled forage crops to avoid cross-contamination. A representative subsample (~ 100 g) was taken from each microplot and dried at 55 °C for 48 h to determine cover crop dry matter content. The remaining biomass was cut into 5-cm-long segments, air-dried and then stored frozen (-20 °C) until crop residue exchange. Samples from the M treatment were not separated into red clover and timothy residues, and only the total biomass and N accumulation of the mixed forage was measured.
The second harvest of shoot biomass occurred on October 29, 2014 (154 days after 15N fertilizer application). The sampling method was identical to the first harvest, except that smaller subsamples (~ 60 g) of tissue were used to determine dry matter, because the collected biomass was low (< 400 g) for all microplots. The remaining biomass was air-dried and combined with biomass from the first harvest to create one composite sample per each microplot.
The 15N-labelled recoverable roots were collected immediately after the second harvest using a method similar to Bolinder et al. (2002). All soil and recoverable roots were excavated from microplots receiving 15N fertilizer to a depth of 0.2 m (depth of cylinder) and brought back to the lab in large plastic bags. The cylinders were left in place in the field. The soil was passed through a 4-mm sieve to separate the roots from the soil. The roots were then washed twice through a series of sieves (4 mm, 2 mm, 1 mm) with distilled water to remove additional soil. The collected root biomass per microplot was small (~ 1000 g), and the entire root sample was air-dried to determine dry weight, and stored until the date of the crop residue exchange. Recoverable roots were categorized as all the roots that could be collected through sieving and by picking them out with tweezers from the soil.
Air-dried shoot tissue and root subsamples were weighed to determine dry matter content and biomass accumulation. Shoot tissues were ground to pass a 1 mm screen with a Wiley Mill grinder (Arthur H. Thomas Co., Philadelphia, USA). Due to the small sample size, root samples were ground using liquid N inside a mortar and pestle until they formed a powder. Subsamples (5 to 10 mg to obtain around 100 g N) of each shoot and root sample from each microplot in each harvest were encapsulated in 5 × 8 mm tin capsules. Samples were sent for analysis at the Agriculture and Agri-Food Canada Stable Isotope Lab at the Lethbridge Research and Development Centre for total 14 N and 15N and total C using a gas chromatograph-mass spectrometer (Flash 2000 Elemental Analyzer, manufactured by Thermo Fisher Scientific, Voltaweg 22, 2627 BC Delft, The Netherlands).
After the final removal of plant biomass, soil samples were taken from all microplots with a soil probe (2 cm in diameter). Three samples were taken in each microplot to form one composite sample at a depth of 0–15 cm and 15–30 cm to measure background 15N in unlabelled plots and 15N fertilizer recovery in labelled plots. Soil samples were passed through a 2-mm sieve. A subsample of soil was ground by hand with a mortar and pestle to pass through a 1-mm sieve, weighed and encapsulated in tin capsules to be sent for analysis of total 14 N and 15N using a gas chromatograph-mass spectrometer as described above.
Experiment 2: forage crop 15 N-labelling in the greenhouse
Experiment 2 had a similar design to Experiment 1, except that forage growth and labelling was conducted in the greenhouse using a pro-mix growing media to facilitate the root retrieval. The temperature in the greenhouse was set at 22° C with 14 h of lighting and plants were manually watered as needed. Plastic containers (diameter: 38.1 cm, height: 92.2 cm) were used as the experimental unit (Fig. S2). Red clover, timothy and a red clover/timothy mixture were seeded on April 27, 2017, and 15N fertilizer (15NH415NO3) was split applied at 24 microplots through three weekly applications on June 1, June 8 and June 15, 2017. Unlabelled 14 N fertilizer (14NH414NO3) was applied to 12 microplots (4 per forage treatment). The N rate was the same as for the field experiment (20 kg N ha−1 for RC, 60 kg N ha−1 for T and 40 kg N ha−1 for M). Three harvests of the above-ground biomass were collected for the RC and M treatments on June 26 (26 days after the first 15N fertilizer application), July 13 (43 days after the first 15N fertilizer application) and August 9 (70 days after the first 15N fertilizer application), while only two harvests were collected for the T treatment on June 26 and August 9. Plant shoot and root tissues were collected, processed, analyzed and conserved as described for Experiment 1.
Crop residue exchange in Experiments 1 and 2
The crop residue exchange was performed in the fall of 2014 for Experiment 1 and in the fall of 2017 for Experiment 2. In both experiments, potatoes were grown in microplots in the subsequent year growing season (i.e., 2015 and 2018).
In Experiment 1, three residue treatments were implemented on November 21, 2014: (i) labelled shoot/unlabelled root; (ii) labelled shoot only; and (iii) labelled root only. The labelled shoot/unlabelled root treatment was implemented by adding labelled residues from each forage to microplots of the same forage which had not been labelled and which had the shoot tissue removed. The labelled shoot only treatment was implemented by adding residues from each forage to new microplots and the labelled root only treatment was implemented by adding residues from each forage to new microplots. Note that an unlabelled shoot/labelled root treatment was not implemented because both the soil and roots were labelled and as a result it would not be possible to distinguish the N provided to the subsequent potato crop by the labelled root tissue only.
In Experiment 2, three residue treatments were also implemented, but they differed from those used in Experiment 1: (i) whole plant labelled; (ii) labelled shoot/unlabelled root; (iii) labelled root/unlabelled shoot. Labelled residue components were added to new microplots in the field on October 31, 2017.
In both experiments, the top 10–15 cm of soil was excavated from each microplot with a hand shovel and carefully placed in a large plastic bag. The labelled plant biomass was placed evenly inside the microplot, and the soil was then placed on top of the biomass, to simulate how the soil is overturned during plowing. Therefore, the labelled residues were incorporated at a depth of approximately 10–15 cm. After incorporation, microplots were left undisturbed until the following spring in preparation for the potato seeding.
Potato phase
Prior to planting potatoes in 2015 and in 2018, the topsoil in each microplot was mixed by hand, making sure to change gloves between experimental units to minimize cross-contamination. Potatoes were planted on May 28, 2015 and June 21, 2018. Two whole seed potatoes (var. Russet Burbank) were planted in each microplot to ensure that at least one would emerge. Whole seeds were used to decrease the risk of disease (Nolte et al. 2003). A small hill was created within the microplot by moving the soil together towards the centre of the cylinder. As soon as the plants emerged, the smaller potato plant of the two in each microplot was carefully removed completely by hand.
Potatoes were managed as closely as possible to commercial practice except that no additional N fertilizer was applied. Granular fertilizer was applied by hand at a rate of 190 kg P2O5 ha−1 as triple superphosphate and 190 kg K2O ha−1 as potassium chloride to ensure they were non-limiting (Government of PEI 2017). The microplots in all treatments were managed according to normal growing practices in PEI, and fungicide and herbicide were applied accordingly. There was no supplemental irrigation throughout the growing season to reflect the rain-fed potato production in PEI.
The potato plants were harvested from each microplot (one plant per container) on September 3, 2015 and August 30, 2018 before potato vine senescence to determine root, vine and tuber dry matter biomass, N accumulation, and recovery of 15N from forage residues. The entire potato plant from each microplot was dug out by hand and placed in designated labelled bags. Tissues were separated into root plus stolon, tuber and vine plant components before being washed and weighed, except in 2018 when roots were not recovered. All the vine and root tissues were then cut by hand to 5-cm segments and dried at 55 °C for 48 h to determine biomass. Given that the large recoverable roots represented a small proportion of plant biomass and N accumulation in 2015 and in previous studies (Bélanger et al. 2001; Liang et al. 2019), readily recoverable roots were not collected in 2018. All collected tubers from each microplot were cut and weighed. A subsample of approximately 3–4 tubers from each microplot was dried and weighed in the same way as roots and vines for subsequent 15N analysis preparation. Total tuber yield (g m−2) was estimated based on the tuber mass for an individual microplot and assuming a plant density of 28,704 tubers per ha (i.e., to reflect a row spacing of 91 cm and within-row spacing of 38 cm).
The dried potato tissue samples were ground to pass a 1-mm screen using a Black and Decker Smart Grind™ Coffee and Spice Grinder (Miramar, USA). A coffee grinder was used due to the small amount of sample collected. Once tissue was ground, vine, tuber and root subsamples were encapsulated for 15N analysis as was done for forage crops and sent for analysis of total C and N concentration and 14 N and 15N enrichment as described above. These results were also used to calculate the partitioning of 15N in the potato crop.
Calculations
The N accumulation for individual plant components was calculated according to Eq. 1, then summed to calculate plant N accumulation:
Labelled fertilizer N recovery in plant and soil samples was calculated based on Nyiraneza et al. (2010). Labelled fertilizer N recovery was calculated according to Eq. 2 below:
where p (kg N ha−1) is the plant total N accumulation, f is the amount of N applied with fertilizer or labelled residues (kg N ha1), a is the abundance in the applied fertilizer or in the labelled residue (atom% 15N), b is the 15N natural abundance of unlabelled plants, and c is the atom% 15N of labelled plants. Labelled fertilizer N recovery in soil was calculated according to Eq. 3 below:
where s (kg N ha−1) is the quantity of N in soil (as calculated by Eq. [4]), f is the amount of N applied with fertilizer or labelled residue (kg ha−1), a is the abundance in the applied fertilizer or in labelled residue (atom% 15N), b is the 15N natural abundance of soil samples collected in unlabelled plots, and c is the atom% 15N of labelled soil sample.
Soil total N was calculated as Eq. 4 below:
where A is the area (10,000 m2 ha−1), BD is the soil bulk density (Mg soil m−3), D is the sampling depth (m), and TSN is the soil total N concentration (g N kg−1 soil).
In the potato phase, recovery of 15N was calculated in the same way for fertilizer 15N recovery (Eq. [2]), except f was the amount of 15N applied with labelled cover crop residues (kg N ha1) and a was the abundance in the applied residues.
Statistical analysis
For both forage and potato phases, statistical analyses were performed with R software package (version 3.2.4, R core Team, 2016). The nlme packages were used for Analysis of Variance (ANOVA) for a completely randomized design, and differences among treatments were compared with Tukey’s HSD at 0.05 probability level. Assumptions of normality and equal variances were checked before analysis with a Shapiro–Wilk and Levene’s Test for Homogeneity. In the forage phase of each experiment (2014; 2017), the statistical analysis was based on a completely randomized design with 3 treatments and 4 replicates where the treatments were three forages (RC, M or T). In the potato phase the statistical analysis was done separately for each residue treatment.
Results
Temperature and rainfall during the study period
Mean monthly air temperature during the growing season (May to October) was generally close to the 30-yr average (Fig. S3A). The exceptions were specific months (May 2015, June 2017, July 2014 and 2018, September 2015 and 2017 and October 2014 and 2017) when the temperature was warmer by an average of 1.7 °C than the 30-yr average.
Compared with the 30-yr average, monthly rainfall in May was lower than average in 2014, 2015 and 2018, but 71% higher than average in 2017 (Fig. S3B). In June, mean monthly rainfall was below average in 2014 and 2017, but 13 and 53% higher than average in 2015 and 2018, respectively. Mean monthly rainfall in July was below average in all years, at approximately 50% of the average in 2014, 2015 and 2017, and 87% of the average in 2018. In August, mean monthly rainfall was 3 to 26% greater than the 30-yr average in all years, whereas rainfall in September was 7 to 41% below average. Rainfall in October was near average in 2014 and 2015, 69% below average in 2017, and 53% above average in 2018.
Shoot and root dry matter biomass, N accumulation and C:N ratio
Total shoot dry matter (i.e., cumulative shoot dry matter across harvests) averaged across forage treatment was greater in 2014 (1.8 kg m−2) than in 2017 (1.2 kg m−2) (Table 1). Greater total shoot biomass in 2014 than 2017 likely reflects the fact that the former was the second year of forage growth (i.e., 2013–2014) whereas the latter was the first year of forage growth. Additionally, forage root growth in the greenhouse in 2017 may also have been limited in plastic containers in comparison to open cylinders used in the field in 2014. In both years, total shoot biomass did not differ significantly between RC and M, and was approximately 2 times greater than for T. Root dry matter biomass did not differ significantly among treatments in 2014, whereas in 2017, root biomass was greater for T than for RC.
Similar to shoot total dry matter accumulation, shoot total N accumulation in 2014 was greater for RC and M than for T (Table 1). In comparison, shoot N accumulation in 2017 followed the pattern RC > M > T. Root N accumulation in 2014 was greater for RC and M than for T, similar to root dry matter accumulation, whereas root N accumulation did not differ significantly among forage treatments in 2017.
Shoot C:N ratio for RC and M was below 20 for each harvest in both years (Table 1). Except for harvest 1 in 2017, shoot C:N ratio was significantly greater for T than for RC and M. Root C:N ratio was greater for T than for RC and M in 2014, and followed the pattern T > M > RC in 2017.
The S:R ratios (i.e., total shoot dry matter/root dry matter) were 1.94, 1.77 and 0.98 in 2014 and 5.17, 3.33 and 1.15 in 2017 for RC, M and T, respectively (Table 1). The greater S:R for all forage crops in 2017 was likely a result of less root dry matter accumulation due to one season of growth versus two seasons of growth (2013–2014) for 2014.
Nitrogen-15 derived from fertilizer recovered in forage tissues and soil
In 2014, when averaged across forage treatments, the overall 15N recovery in plant tissues and soil was 99.6% (Table 2). On average, 54% of 15N was recovered in the plant tissues and 45% recovered in the soil. Of the 15N recovered in the plant, an average of 41% was recovered in the shoot in harvest 1, 3% in the shoot in harvest 2, and 10% in the roots. Forage treatment had a significant effect on 15N recovery in the shoot biomass and in the soil, but not in the root biomass. At the first harvest, greater recovery of 15N was observed for T than for RC treatment, whereas at the second harvest, greater recovery of 15N was observed for M than for RC and T. As expected, increased 15N recovery in plant tissues was associated with lower 15N recovery in the soil, and followed the pattern RC > M > T.
In 2017, when averaged across forage treatments, 15N recovery in plant tissues was 113% with an average recovery of 81% in the first harvest, 18% in the second plus third harvests and 14% in the roots (Table 2). Recovery of 15N in soil was not determined in 2017 because forage crops were grown using a pro-mix growing media instead of soil. The > 100% recovery of 15N fertilizer was attributed primarily to unrealistically high recovery in the first harvest for the M treatment, likely due to the difficulty in obtaining a representative sample from this heterogeneous forage tissue for determination of 15N labelling. There was a significant effect of forage treatment on 15N recovery, with greater recovery for M than RC and T for harvest 1, and greater recovery for T than RC in the roots.
Potato vine and tuber dry matter and N accumulation
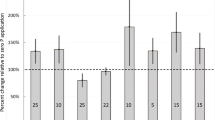
In 2015, potato tuber and total biomass averaged across forage treatments were of similar magnitude for each of the three residue treatments (Table 3). Averaged across all residue treatments and forages, tubers, vines and roots accounted for 74, 24 and 2% of total dry matter, respectively. There was a significant effect of forage on tuber and vine dry matter, with greater values for RC than T for all residue treatments. In comparison, tuber dry matter for M was not significantly different than RC for the Labelled shoot/unlabelled root treatment, not significantly different from T for the Labelled root only treatment, and intermediate between RC and T for the Labelled shoot only treatment. Vine dry matter was greater for RC and M than for T for the Labelled shoot/unlabelled root and Labelled shoot only treatments, and greater for RC than T for the Labelled root only. When averaged across forage treatments, values of N accumulation in tubers, vines and roots varied slightly across residue treatments. Averaged over forage and residue treatments, the tubers, vines and roots accounted for 48, 49 and 3% of the total plant N accumulation. The N accumulation was significantly greater for RC and M than T for vines and roots in the Labelled shoot/unlabelled root treatment, and tubers and vines in the Labelled shoot only treatment, whereas there was no effect of forage treatment on N accumulation in all other cases.
In 2018, the potato vine and tuber dry matter and N accumulation in tubers and vines were of comparable magnitude across the residue treatments (Table 4). Root dry matter and N accumulation was not determined in 2018. Averaged across the forages and residue treatments, 79 and 21% of total dry matter, and 64 and 46% of N accumulation, were in tubers and vines, respectively. Tuber dry matter for the Labelled shoot/unlabelled root and Labelled roots/unlabelled shoot treatments, and N accumulation for the Labelled roots/unlabelled shoot treatment, were greater for the RC and M than T. There was no significant effect of forage treatment in any other case.
Nitrogen-15 recovery from labelled residue components in potato tubers and vines
Regardless of the forage or residue treatments in either experiment, less than 5% of the 15N from crop residues was recovered in the potato vines plus tubers (Tables 5, 6). In 2015, 15N recovery from labelled residues in vines was significantly greater for forage treatments containing legumes (i.e., RC and M) than T (Table 5). The 15N recovery from labelled residues in tubers was greater for RC and M than T for the Labelled shoot only treatment, greater for RC than M or T for the Labelled root only treatment, and did not differ among forage treatments for the Labelled shoot/unlabelled root treatment. In 2018, 15N recovery from labelled residues in potato vines and tubers did not differ significantly among forage treatments (Table 6).
Nitrogen-15 recovery from labelled residue components in the soil after potato harvest
In 2015, 15N recovery at 0–15 cm depth in soil was greater for RC than M or T for the Labelled shoot/unlabelled root and Labelled root only treatments, but did not differ among treatments for the Labelled shoot only treatment (Table 5). At 15–30 cm depth in soil, 15N recovery was greater for RC than T for the Labelled shoot only treatment, but did not differ significantly among forage treatments for the other residue treatments. When averaged across forage and residue treatments, average 15N recovery at 0–30 cm depth in soil was of 64, 39 and 47% for RC, M and T, respectively.
In 2018, 15N recovery at 0–15 cm soil depth was greater for T than RC or M for the Labelled shoot/unlabelled root treatment, greater for T than M for the Whole plant labelled treatment, and did nor differ among forage treatments for the Labelled roots/unlabelled shoot treatment (Table 6). The 15N recovery at 15–30 cm soil depth did not differ among forage treatments for any residue treatment. When averaged across forage and residue treatments, average 15N recovery at 0–30 cm depth in soil was 71, 51 and 78% for RC, M and T, respectively (Table 6).
Total 15 N recovery from labelled residue components in the plant-soil system
In 2015, total 15N recovery in potato plants plus in the soil (0–30 cm) after potato harvest ranged from 36 to 73% for the Labelled shoot/unlabelled root treatment, 57 to 85% for the Labelled shoot only treatment, and 31 to 45% for the Labelled root only treatment (Table 5). This supports our hypothesis stating that total 15N recovery from labelled root will represent at least 30% of that from the shoot. In all cases, higher numerical values were observed with RC than M or T. In 2018, total 15N recovery ranged from 65 to 93% for the Whole plant labelled treatment, 63 to 95% for the Labelled shoot/unlabelled root treatment, and 36 to 57% for the Labelled root/unlabelled shoot treatment (Table 6). Averaged across forage and residue treatments in 2015 and 2018, about 95% of the total 15N recovery from labelled residues was in soil.
Discussion
Nitrogen accumulation by forage crops is underestimated when root contribution is not included
Greater forage dry matter biomass and total N accumulation in the current study compared to previous studies in the region was likely attributed to a greater seeding rate. In 2014, total shoot biomass was approximately 3 times greater than a yield of 0.637 kg m−2 reported by Bolinder et al. (2002) with a seeding rate ranging from 10 to 15 kg ha−1 in comparison to seeding rates of 33 kg ha−1 for red clover and of 18/9 kg ha−1 for red clover/timothy in this study. Similarly, N accumulation was approximately 2–3 times greater than the values recorded in this region (Gaudin et al. 2013; Jiang et al. 2015; Liang et al. 2019). Greater N accumulation in legume (RC, M) than grass (T) forages reflects the greater total biomass but also the greater N concentration due to the biological fixed nitrogen from the atmosphere, whereas T relied only on soil available N from in-season mineralization of SOM and thus the timothy crop was likely N limited.
Overall, root biomass was lower in 2017 (plants grown in the greenhouse for one growing season) than in 2014 (plants grown in the container in the field for 2 growing seasons), partially due to a shorter growth period (only one growing season in 2017 compared to 2 years in 2013–2014). The S:R values in the current study in 2014 (1.94, 1.77 and 0.98 for RC, M and T, respectively) are within the range reported by Bolinder et al. (2002) in a 2-yr field study with S:R values for timothy of 0.90 and 2.2 in the first and second years of growth and S:R values for red clover of 1.1 and 1.3 in the first and second years of growth. The greater S:R values in 2017 in the current study (5.2, 3.3 and 1.1 for RC, M and T, respectively), compared with 2014 may be attributed to rapid shoot growth in the greenhouse under optimal conditions of temperature and humidity. Averaged across forage types, recoverable root biomass represented 64% of total forage biomass in 2014 while it represented 37% in 2017 (Table 1). Root N accumulation accounted for 39% of total forage N accumulation in 2014 and 16% in 2017 when averaged across treatments. Our study suggests that the contribution of forage roots needs to be taken into account when studying N cycling from residues.
Nitrogen losses were limited after 15N labelled fertilizer application which resulted in high 15N fertilizer recovery in the soil-forage system.
There was an overall trend of increased 15N fertilizer recovery in the forage crops with decreasing legume content in 2015 (RC < M ≈ T), which was also found by Ranells and Wagger (1997). This was attributed partially to the labelled 15N fertilizer dilution through biological N fixation with legumes. In addition, less 15N in forage with legumes may be due to lower efficiency of uptake of the labelled fertilizer N from the soil by the RC than the T due to differences in root architecture. In 2014, forage shoot 15N fertilizer recoveries were within the range reported previously by Nyiraneza et al. (2010) on corn silage of 40 to 60% and by Tran et al. (1997) on corn of 43 to 54%. In 2017, forage shoot 15N fertilizer recovery values tended to be numerically higher than in 2014, and this is attributed to the fact that 15N fertilizer was applied at the early stage of the forage growth in the greenhouse and that the fertilizer was applied in three fractions, which may have increased N use efficiency. Additionally, N leaching might have been greater from the open cylinders in the field in Experiment 1 than in the plastic containers in the greenhouse in Experiment 2 which had only a few holes to allow drainage. The differences in growing conditions in Experiment 1 (open cylinders in the field) and Experiment 2 (containers in the greenhouses) could have affected the 15N fertilizer use efficiency and 15N lost below the root zone but should not impact the overall objective of the study which is to obtain labelled residues and to trace 15N fate from residues in the following potato crop and the soil. The 15N fertilizer recovered by forage roots was within the range reported by McNeil et al. (1997) of 7% to 26% on pasture legumes [(clover and serradella (ornitropus compressus L.)]. Soil 15N recovery in the T treatment was comparable to the average 27% recovery of applied 15N fertilizer reported by Delgado et al. (2004) for spring-planted barley, white wheat and hard red wheat. Values of 15N fertilizer recovery in the soil in this study are also comparable to the 31 to 58% reported by Nyiraneza et al. (2010) at a depth of 40 cm. Our results are also aligned with those reported by Gerub and Roberts (2020) with highest overall 15N fertilizer recovery by cover crops being around 38%, the remainder being recovered in the soil. Overall, the high total 15N recovery in the plant-soil system in the current study suggests that N losses were limited.
15 N recovery from labelled residues by the potato crop was less than 5%
Dry matter tuber values in this study are in the low range compared with those in a previous 2-yr study by Zebarth et al. (2004) at vine senescence where no N fertilizer was applied with total dry matter ranging from 980 to 1120 g m−2 and N accumulation ranging from 5.7 to 12.8 g m−2. This may be attributed to the better conditions of potato growth in the field in comparison to a container.
In this study, 15N recovery from labelled forage crops into the subsequent potato vine plus tuber was low, at less than 5%. We discuss below reasons which may explain this low recovery.
First, the low 15N recovery from labelled residues by potatoes may be the result of fall incorporation of the forage residues. Rapid release of N from labile components after incorporation may have resulted in N loss from the root zone, and therefore less-labile organic N components that were resistant to decomposition remained (Janzen et al. 1990). This is particularly true in humid environments like Eastern Canada where nitrate leaching is common in the fall to spring period when precipitation exceeds evapotranspiration (Jiang et al. 2015; Clément et al. 2019, 2020). This is consistent with relatively greater 15N recovery from residues reported in studies where the crop residues were incorporated in spring right before seeding the subsequent cash crop. For example, Harris et al. (1994) found that corn recovered 15 to 16% of 15N from labelled red clover shoot tissue incorporated right before corn planting while Collins et al. (2007) reported that 29% of 15N was recovered from labelled shoot mustard grown as a winter cover crop and incorporated in spring prior to potato planting. Similarly, wheat and canola recovered 8.6 to 12% from pea-residue N (Mayer et al. 2003), wheat recovered 16.9% from corn-residue-N, and canola recovered 22% of pea-residue-N (Arcand et al. 2014a, b) when spring incorporated. In comparison, much lower 15N recoveries from labelled residues, ranging from 6 to 14%, were reported when residues were incorporated in fall or in summer (Janzen et al. 1990; Delgado et al. 2004).
Second, the availability of the forage 15N for potato N uptake may have been reduced due to mineralization-immobilization processes in soil. This may reflect the rapid N cycling which may occur within soil, particularly when the rate of soil microbial activity is increased as the result of labile C sources (Berthrong et al. 2013) under N limited conditions given that no N fertilizer was applied during potato phase. An addition of N fertilizer was reported to stimulate the rate of N release from incorporated residues (Ta and Faris 1990) particularly for high C:N residues.
Additionally, the low 15N recovery from labelled forage crops may have been underestimated due to a limited recovery of potato root tissues in the current study. Even if Opena and Porter (1999) reported that around 85% of the potato root length was concentrated within 30 cm soil depth, some roots extend up to 100 cm depth (Iwama 2008) and fine root were reported to represent a large portion of the potato root system (Munoz-Arboleda et al. 2006). Finally, the difference in 15N recovery from labelled residues by potatoes in this study compared to previous studies on corn, canola and wheat may be attributed to differences in crop N uptake efficiency and thus our study represents a unique opportunity to assess the N contribution from forage shoot and root to potato N nutrition.
The addition of a labelled N source from residues may result in an increased N uptake by the subsequent crop from unlabelled native soil N sources in the absence of N fertilizer application. As a result, the 15N method likely underestimates the effective benefit of the legume on the subsequent potato crop. In comparison, indirect methods which estimate the apparent N contribution of a preceding crop (Zebarth et al. 2005), may over-estimate the N contribution from preceding legume crops. The indirect method estimates potentially available N pools (Ranells and Wagger 1997) and all N effects plus rotation effects (Harris and Hesterman 1990), while the 15N-labelled residues method estimates only the N contribution from residue alone. In addition, indirect methods assume that net N mineralization of the soil organic N is not affected by the presence of the forage legume. For example, fresh residues added to the soil constitute a good energy source for microbes and thus forage residues may have an indirect role in stimulating N mineralization from the soil organic matter.
A large proportion of total 15 N recovered from labelled residues was in the soil
A large proportion of the total 15N recovery from labelled residues occurred in the soil rather than the subsequent crop, regardless of the forage or residue treatment. This supports the hypothesis stating that most of N from labelled residues will be recovered in the soil. Averaged across forage types in 2015 and 2018, approximately 95% of total N recovery from labelled residues was in the soil. This finding is consistent with previous studies (Delgado et al. 2004; Collins et al. 2007; Smith and Chalk 2018). Approximately 66% of crop residue 15N from labelled mustard was found in the soil in the following year after potato harvest (Collins et al. 2007). Depending on the climate, residual soil 15N from residues may become available during subsequent years, even though previous studies have suggested that most residual N will be lost from cropping systems within the first 2 years (Harris et al. 1994; Kumar and Goh 2002). A study by Ta and Faris (1990) evaluated 15N recovery from labelled alfalfa applied in the fall to a barley crop seeded in following spring and reported average 15N recoveries of 15%, 6% and 5% in the first, second, and third year following residue incorporation, respectively. The results demonstrated that a greater proportion of N derived from crop residues is recovered in the soil in the subsequent year. Total 15N recovery from labelled residues ranged from 31.3 to 85.3% in 2015 and from 36.2 to 94.9% in 2018. The unaccounted labelled N from labelled residues may have been lost through leaching or denitrification.
In the absence of N fertilizer application, potato N requirement was satisfied primarily by N from the soil rather than from forage residues.
The 15N recovery in the potato crop (vine plus tuber) was statistically lower from T than RC or M in 2015, but values were comparable in 2018. Therefore, our hypothesis stating that 15N recovered by potatoes from labelled forage legumes will be higher than that recovered from T was partially supported. This is attributed to residue quality, where the timothy C:N ratio was greater in 2014 (2-yr forage growth, average C:N = 46) than in 2017 (one season forage growth, average C:N = 35). Potato 15N recovery values from RC and M were comparable, implying that red clover was predominant in the M treatment.
The relative contribution from the shoot versus root tissue can be compared by examining the 15N recovery in the potato tubers plus vines for the Labelled shoot only and Labelled root only treatments. When averaged over forage treatments, average 15N recovery in the potato crop was 2.65 and 3.88% for the Labelled shoot only treatment compared with 2.21 and 1.43% for the Labelled root only treatment in 2015 and 2018, respectively. The relative N contribution from shoot tissues to potatoes was therefore much greater than from root tissues in 2018 than in 2015. This may be due to greater immobilization of root tissue N in 2018 than in 2015 as a result of the greater C:N ratios of root tissues in 2017 than in 2014.
Much of N from crop residues is immobilized by soil microorganisms (Broadbent and Nakashima 1974). The newly immobilized N is less available to mineralization and most of mineralized N comes from native humus N (Kelley and Stevenson 1987). For example, in a 5-year incubation study using labelled barley residues on fine sandy loam soil, Broadbent and Nakashima (1974) reported average annual turnover rate of soil N to be 7.0% for soil alone, 7.4% with shoot added and 6.3% with root added. This study evaluated the N residue contribution to a subsequent potato over a single growing season, and showed that potato N requirements were satisfied more by soil-derived N than from N from previously incorporated forage residues crops. A summary of the highlights from this study is reported in Appendix 1.
Conclusion
This study assessed the N contribution from contrasting forage crops and their respective residue components (whole forage crops versus root or shoot biomass) to a subsequent potato crop which received no fertilizer N. Less than 5% of the N in the forage residues was recovered in the potato crop, and thus most of the N from the forage residue was recovered in the soil. Fall incorporation may have resulted in the rapid release of labile organic N components from the residues, and their subsequent loss over the fall and winter through nitrate leaching or denitrification, whereas a slower release of the less labile organic N components occurred during the potato growth period. When crop residues are incorporated in fall prior to seeding a cash crop in the following spring, results from this study indicate that crop N requirement is satisfied more from soil-derived N than N derived from previously incorporated crop residues. On average, 15N recovery from labelled roots was over 50% of that from labelled shoots. The results of our study therefore suggest that N accumulation by forage crops can be underestimated when roots are excluded. Future studies might explore N availability from forage residues on a subsequent crop over time, N cycling from residues in different soil N pools, and the fate of N from forage residues incorporated at different times (i.e., spring versus fall).
References
Arcand MM, Lemke R, Farell RE, Knight JD (2014a) Nitrogen supply from belowground residues of lentil and wheat to a subsequent wheat crop. Biol Fert Soils 50:507–515
Arcand MM, Knight JD, Farrell RE (2014b) Differentiating between the supply of N to wheat from above and belowground residues of preceding crops of pea and canola. Biol Fertil Soils 50:563–570
Bélanger G, Walsh JR, Richards JE, Milburn PH, Ziadi N (2001) Tuber growth and biomass partitioning of two potato cultivars grown under different N fertilization rates with and without irrigation. Am J Pot Res 78:109–117
Berthrong ST, Buckley DH, Drinkwater LE (2013) Agricultural management and labile carbon additions affect soil microbial community structure and interact with carbon and nitrogen cycling. Microb Ecol 66:158–170
Bolinder MA, Angers DA, Bélanger G, Michaud R, Laverdière MR (2002) Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can J Plant Sci 82:731–737
Broadbent FE, Nakashima T (1974) Mineralization of carbon and nitrogen in soil amended with carbon-13 and nitrogen-15 labelled plant material. Soil Sci Soc Amer Proc 38:313–315
Cambouris AN, St. Luce, M., Zebarth, B. J., Ziadi, N., Grant, C. A., Perron, I (2016) Potato response to nitrogen sources and rates in an irrigated sandy soil. Agron J 108(1):391–401
Carter MR, Angers DA, Kunelius HT (1994) Soil structural form and stability and organic matter under cool-season perennial grasses. Soil Sci Soc Am J 58:1194–1199
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J (2001) Measuring net primary production in forest: concepts and field methods. Ecol Appl 11:356–370
Clément CC, Cambouris AN, Ziadi N, Zebarth BJ, Karam A (2019) Nitrogen source and rate effects on residual soil nitrate and overwinter NO3-N losses for irrigated potatoes on sandy soils. Can J Soil Sci 100:44–57
Clément CC, Cambouris AN, Ziadi N, Zebarth BJ, Karam A (2020) Growing season nitrate leaching as affected by nitrogen management in irrigated potato production. Agron J 112:377–3787
Collins HP, Delgado JA, Alva AK, Follett RF (2007) Use of nitrogen-15 isotopic techniques to estimate nitrogen cycling from a mustard cover crop to potatoes. Agron J 99:27–35
Dabney SM, Delgado JA, Reeves DW (2001) Using winter cover crops to improve soil and water quality. Commun Soil Sci Plant Anal 32:1221–1250
Delgado JA, Dillon MA, Sparks RT, Follett RF (2004) Tracing the fate of 15N in a small-grain potato rotation to improve accountability of nitrogen budgets. J Soil Water Conserv 59:271–276
Delgado JA, Del Grosso SJ, Ogle SM (2009) 15N isotopic crop residue cycling studies and modeling suggest that IPCC methodologies to assess residue contributions to N2O-N emissions should be re-evaluated. Nutr Cycl Agroecosyst 86:383–390
Ding W, Li S, He P, Huang S (2019) Contribution and fate of maize residue-15N and urea-15N as affected by N fertilization regime. PlosOne 14:1–17
Environment and climate change Canada (2021) Canadian climate normals 1981–2010 at the Charlottetown airport weather station. Available at: https://climate.weather.gc.ca/climate_normals/index_e.html (Accessed on July 22, 2021)
Errebhi M, Rosen CJ, Gupta SC, Birong DE (1998) Potato yield response and nitrate leaching as influence by nitrogen management. Agron J 90:10–15
Follett RF (2001) Innovation 15N microplot research techniques to study nitrogen use efficiency under different ecosystems. Commun Soil Sci Plant Anal 32:951–979
Gaudin AACMA, Westra S, Loucks CES, Janovicek K, Martin RC, Deen W (2013) Improving resilience of northern field crop systems using inter-seeded red clover: a review. Agron 3:148–180
Gill RA, Burke IC, Lauenroth WK, Milchunas DG (2002) Longevity and turnover of roots in the short-grass steppe: influence of diameter and depth. Plant Ecol 159:241–251
Government of PEI (2017) Nutrient recommendation tables PEI Department of Agriculture and Fisheries. Available at: pei nutrient recommendations - Google Search.ne (accessed on 15 June, 2021)
Greub LH, Roberts TL (2020) 15N fertilizer recovery and partitioning by cover crops under greenhouse conditions. Agron J 112:5300–5311
Harris GH, Hesterman OB (1990) Quantifying the nitrogen contribution from alfalfa to soil and two succeeding crops using Nitrogen 15. Agron J 82:129–134
Harris GH, Hesterman OB, Eldor PA, Peters SE, Janke RR (1994) Fate of legume and fertilizer nitrogen-15 in a long-term cropping systems experiment. Agron J 86:910–915
Hauck RD, Bremner JM (1976) Use of tracers for soil and fertilizer nitrogen research. Adv Agron 28:219–261
Holmstrom DA, Kunelius HT, Ivany JA (2001) Forages underseeded in barley for residue management for potatoes. Can J Plant Sci 81:205–210
Iwama K (2008) Physiology of the potato: new insights into root systems and repercussions for crop improvements. Potato Res 51:333–353
Janzen HH, Bole JB, Biederbeck VO, Slinkard AE (1990) Fate of N applied as green manure or ammonium fertilizer to soil subsequently cropped with spring wheat at three sites in western Canada. Can J Soil Sci 70:313–323
Jiang Y, Zebarth BJ, Somers GH, Macleod JA, Savard MM (2012) Nitrate leaching from potato production in Eastern Canada. In: He Z, Larkin R, Honeycutt W (eds) Sustainable potato production: global case studies. Springer, Dordrecht, pp 233–250
Jiang Y, Jamieson T, Nyiraneza J, Somers GH, Thompson B, Murray B, Grimmett M, Geng X (2015) Effects of fall vs. spring plowing forages on nitrate leaching losses to groundwater. Ground Water Monit Remediat 35:43–54
Karimi R, Pogue SJ, Kröbel R, Beauchemin KA, Schwinghamer T, Janzen HH (2020) An updated nitrogen budget for Canadian agroecosystems. Agric Ecosyst Environ 304:107046
Kelley KR, Stevenson FJ (1987) Effects of carbon source on immobilization and chemical distribution of fertilizer nitrogen in soil. Soil Sci Soc Am J 51:946–951
Khan WDF, Peoples MB, Chalk PM, Herridge DF (2002a) Quantifying below-ground nitrogen of legumes. 2. A comparison of 15N and non isotopic methods. Plant Soil 239:277–289
Khan WDF, Peoples MB, Herridge DF (2002b) Quantifying below-ground nitrogen of legumes. 1. Optimising procedures for 15N shoot-labelling. Plant Soil 245:327–334
Kumar K, Goh KM (2002) Recovery of 15N-labelled fertilizer applied to winter wheat and perennial ryegrass crops and residual 15N recovery by succeeding wheat crops under different crop residue management practices. Nutr Cycl Agroecosyst 62:123–130
Kunelius HT (1990) Dry matter production, fibre composition and plant characteristics of cool-season grasses under two harvest systems. J Agr Sci 115:321–326
Kunelius HT, Dürr GH, McRae KB, Fillmore SAE (2006) Performance of timothy-based grass/legume mixtures in cold winter region. J Agron Crop Sci 192:159–167
Liang K, Jiang Y, Nyiraneza J, Fuller K, Murnaghan D, Meng FR (2019) Nitrogen dynamics and leaching potential under conventional and alternative potato rotations in Atlantic Canada. Field Crops Res 242:107603
Lynch DH, Zheng Z, Zebarth BJ, Martin RC (2008) Organic amendment effects on tuber yield, plant N uptake and soil mineral N under organic potato production. Renew Agric Food Syst 23:250–259
Mayer J, Buegger F, Jensein ES, Schoter M, Heß J (2003) Residual nitrogen contribution from grain legumes to succeeding wheat and rape and related microbial process. Plant Soil 255:541–554
McNeil AM, Zhu C, Fillery IRP (1997) Use of in situ 15N-labelling to estimate the total below ground nitrogen of pasture legumes in intact soil-plant systems. Aust J Agric Res 48:295–304
Munoz-Arboleda F, Mylavarapu RS, Hutchinson CM, Portier KM (2006) Root distribution under seepage-irrigated potatoes in northeast Florida. Am J Potato Res 83:463–472
Nolte P, Bertram M, Bateman M, Mclntosh CS (2003) Comparative effects of cut and treated seed tubers vs untreated whole seed tubers on seed decay, Rhizoctonia stem canker, growth, and yield of Russet Burbank potatoes. Am J Potato Res 80:1–8
Nyiraneza J, Chantigny MH, N’Dayegamiye A, Laverdière MR (2010) Long-term manure application and forages reduce nitrogen fertilizer requirements of silage corn–cereal cropping systems. Agron J 102:1244–1251
Nyiraneza J, Peters RD, Rodd VA, Grimmett MG, Jiang Y (2015) Improving productivity of managed potato cropping systems in Eastern Canada: crop rotation and nitrogen source effects. Agron J 107:1447–1457
Nyiraneza J, Chen D, Fraser TD, Comeau L-P (2021) Improving soil quality and potato productivity with manure and high-residue cover crops in Eastern Canada. Plants 10:1436
Opena GB, Porter GA (1999) Soil management and supplemental irrigation effects on potato: II. Root Growth Agron J 91:426–431
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monogr 72:293–309
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Ranells NN, Wagger MG (1997) 15N recovery and release by rye and crimson clover cover crops. Soil Sci Soc Am J 61:943–948
Ruess RW, Hendrick RL, Burton AJ, Pregitzer KS, Sveinbjornsson B, Allen MF, Maurer G (2003) Coupling fine root dynamics with ecosystem carbon cycling in black spruce forests of interior Alaska. Ecol Monogr 73:643–662
Sainju UM, Singh BP (1997) Winter cover crops for sustainable agricultural systems- Influence on soil properties, water quality, and crop yields. Hortic Sci 32:21–28
Sanderson JB, MacLeod JA, Kimpinski J (1999) Glyphosate application and timing of tillage of red clover affects potato response to N, soil N profile, and root and soil nematodes. Can J Soil Sci 79:65–72
Smith CJ, Chalk PM (2018) The residual value of fertiliser N in crop sequences: an appraisal of 60 years of research using 15N tracer. Field Crops Res 217:66–74
Soil Classification Working Group (1998). The Canadian System of Soil Classification, 3rd ed. Agriculture and Agri-Food Canada Publication 1646, pp 187 Available at: https://sis.agr.gc.ca/cansis/publications/manuals/1998-cssc-ed3/cssc3_manual.pdf (accessed on 15 June, 2021)
Stark JC, Porter GA (2005) Potato nutrient management in sustainable cropping systems. Am J Potato Res 82:329–338
Steinaker DF, Wilson SD (2005) Belowground litter contributions to nitrogen cycling at a northern grassland-forest boundary. Ecol 86:2825–2833
Ta TC, Faris MA (1990) Availability of N from 15N-labelled alfalfa residues to three succeeding barley crops under field conditions. Soil Biol Biochem 22:835–838
Taveira CJ, Farrell RE, Wagner-Riddle C, Machado PVF, Deen B, Congreves KA (2020) Tracing crop residue N into subsequent crops: insight from long-term crop rotations that vary in diversity. Field Crops Res 255:107904
Titlyanova AA, Romanova IP, Kosykh NP, Mironycheva Tokareva NP (1999) Pattern and process in above-ground and below-ground components of grassland ecosystems. J Veg Sci 10:307–320
Tran TS, Giroux M, Cescas MP (1997) Effect of N rate and application methods of 15N-labelled fertilizer use by corn. Can J Soil Sci 77:9–19
Zebarth BJ, Rosen CJ (2007) Research perspective on nitrogen BMP development for potato. Am J Potato Res 84:3–18
Zebarth BJ, Leclerc Y, Moreau G (2004) Rate and timing of nitrogen fertilization of Russet Burbank potato: Nitrogen use efficiency. Can J Plant Sci 84:845–854
Zebarth BJ, Leclerc Y, Moreau G, Sanderson JB, Arsenault WJ, Botha E, Wang-Pruski G (2005) Estimation of soil nitrogen supply in potato fields using a plant bioassay approach. Can J Soil Sci 85:377–386
Zebarth BJ, Drury CF, Tremblay N, Cambouris AN (2009) Opportunities for improved fertilizer nitrogen management in production of arable crops in eastern Canada: a review. Can J Soil Sci 89:113–132
Zebarth BJ, Bélanger G, Cambouris AN, Ziadi N (2012) Nitrogen fertilization strategies in relation to potato tuber yield, quality, and crop N recovery. In: He Z, Larkin RP, Honeycutt W (eds) Sustainable potato production: global case studies. Springer, Dordrecht, pp 165–186
Zebarth BJ, Danielescu S, Nyiraneza J, Ryan MC, Jiang Y, Grimmett M, Burton DL (2015) Controls on nitrate loading and implications for BMPs under intensive potato production systems in Prince Edward Island, Canada. Ground Water Monit Remediat 35:30–42
Acknowledgements
The assistance in field and/or laboratory work provided by Danielle Murnaghan, Irene Power, Barbara Enman and Dorothy Gregory is highly appreciated. The study was funded by Agriculture and Agri-Food Canada.
Funding
Open Access provided by Agriculture & Agri-Food Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whittaker, J., Nyiraneza, J., Zebarth, B.J. et al. Potato and soil 15N recoveries from different labelled forage root and shoot. Nutr Cycl Agroecosyst 125, 187–204 (2023). https://doi.org/10.1007/s10705-022-10245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-022-10245-x