Abstract
We aimed to investigate phosphorus (P) mobilization by different plant species from organic and inorganic sources in relation to different P mobilization mechanisms. Knowledge about P mobilization is important for producing crops on P sources other than phosphate rock-derived fertilizers. We conducted a greenhouse experiment with four plant species (maize, soy, lupin, mustard) and three P sources (FePO4, phytate, struvite). We determined pH and phosphomonoesterase activity in the rhizosphere using pH imaging and soil zymography. At harvest, root exudates were analyzed for phosphomonoesterase activity, pH, organic acids, and dissolved organic carbon (DOC). Plants were analyzed for biomass, root length, and P content. Struvite was more plant-available than phytate and FePO4 as indicated by higher plant P contents. Soy had the highest biomass and P content, irrespective of P source. Soy exuded up to 12.5 times more organic acids and up to 4.2 times more DOC than the other plant species. Lupin had a 122.9 times higher phosphomonoesterase activity than the other plant species with phytate. The pH in the exudate solution of mustard was on average 0.8 pH units higher than of the other plant species. P uptake by mustard and soy seemed to have also benefited from large root lengths. Taken together, our study indicates that soy has a particularly high potential to mobilize P from struvite and phytate, while mustard has a high potential to mobilize P from FePO4. Therefore, soy and mustard seem to be good options for agricultural production that relies less on phosphate rock-derived fertilizers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is essential for crop production since it is an integral constituent in the structural and cellular metabolism of plants as component of, e.g., adenosine triphosphate (ATP), nucleic acids, and phospholipids of biomembranes, and is thus involved in transferring information (DNA, RNA) and energy (ATP, ADP) (Ashley et al. 2011; George et al. 2016; Marschner 2012). To increase crop yields and avoid P limitation, huge amounts of P fertilizers, i.e., about 46 million metric tons of P2O5 per year, mainly derived from phosphate rock, are applied globally in agricultural production (Cordell and White 2014; IFA 2022). However, the application of phosphate rock-derived fertilizer is increasingly problematic since phosphate rock represents a finite and geographically unevenly distributed resource (Ashley et al. 2011; George et al. 2016). Thus, there is a need to reduce the reliance on phosphate rock-derived fertilizers, for instance through cultivation of crops that effectively mobilize less easily available soil P forms and recycled P sources (George et al. 2016; Sulieman and Mühling 2021).
Plants have developed various mechanisms to mobilize P from different sources, which might be used to reduce the reliance on phosphate rock-derived fertilizers in agriculture. These mechanisms can be summarized as ‘root foraging’ via morphological adaptations and ‘P mining’ via physiological adaptations to P deficiency (Lyu et al. 2016; Richardson et al. 2011; Wen et al. 2021). Root foraging allows plants to acquire nutrients from a greater soil volume and minimizes the distance between roots and plant-available orthophosphate anions in soil by extending the root system and increasing the root surface (Ma et al. 2018; Richardson et al. 2011; Wen et al. 2019). Especially Brassicaceae exhibit a ramified root system with thin, long, and intensively branched roots, whereas Lupinus species form thick and comparatively short taproots (Lyu et al. 2016; Wen et al. 2019). In contrast to root foraging, P mining refers to the mobilization of phosphate anions from sparingly soluble P sources by exuding various substances, such as phosphatases and low molecular weight organic acid anions (LMWOA), into the soil (Richardson et al. 2011; Wen et al. 2021).
Up to 80% of soil P is present as organic P in the topsoil, of which the majority (60–90%) exists as orthophosphate monoesters, with myo-inositol hexakisphosphate (phytate) being the most abundant form (Dalal 1977; Liu et al. 2022; Turner et al. 2002). Mobilizing P from phytate involves two steps: organic P needs to be released from precipitates and adsorption sites before it can be mineralized through hydrolysis of the ester bonds via extracellular phosphomonoesterase enzymes, namely phytases (Liu et al. 2022; Menezes-Blackburn et al. 2018; Richardson et al. 2009). However, a part of the organic soil P pool and particularly phytate is stabilized against mineralization by adsorption to mineral surfaces and is therefore only slowly hydrolyzed by enzymes (Jarosch et al. 2015; Menezes-Blackburn et al. 2018; Nannipieri et al. 2011). Moreover, plant species differ considerably in their capacity to exude phosphomonoesterases including phytases. Legumes, and particularly Lupinus species, are known for a high phosphomonoesterase activity in the rhizosphere, thus likely mobilizing organic P more effectively than Gramineae and Brassicaceae (Sulieman and Mühling 2021; Wen et al. 2019). However, the amount of released phytases differ among different legumes, both in absolute terms and relative to other phosphatases (Dong et al. 2020; Gilbert et al. 1999; Tang et al. 2021).
Orthophosphate anions have a high affinity for metal cations, e.g., of iron (Fe2+ and Fe3+), with which they precipitate, forming sparingly soluble P minerals such as iron phosphate (FePO4) (Chang and Jackson 1958; Richardson et al. 2009). FePO4 is also formed during wastewater treatment and its use in agriculture as a fertilizer can thus increase P recycling (Li et al. 2020b; Wilfert et al. 2015). Mobilizing P from FePO4 requires changes in the precipitation-dissolution equilibrium, which can be achieved by increases in the soil pH (Hinsinger 2001; Lindsay 1979; Richardson et al. 2009). Plants can modify the rhizosphere pH by releasing protons or hydroxyl ions and/or by exuding LMWOA. The latter can also mobilize inorganic P by modifying the surface characteristics of soil colloids, by successfully competing with phosphate for sorption sites, or by chelating cations bound to P (Hinsinger et al. 2003; Richardson et al. 2009; Wang and Lambers 2020). However, only some plant species exude LMWOA at high rates or change the rhizosphere pH substantially. Legumes, and particularly Lupinus species, are known for a high LMWOA exudation (Wang and Lambers 2020; Wen et al. 2019), while different Brassicaceae have been shown to substantially alkalize their rhizosphere, thus increasing P mobilization from FePO4 (Marschner et al. 2007). However, former research has also shown that LMWOA exudation is not necessarily strongly aligned with a plant species’ capacity to mobilize P from FePO4 (Pearse et al. 2007).
Struvite (ammonium magnesium phosphate; NH4MgPO4) is frequently formed as a byproduct of wastewater and sludge treatment (Kataki et al. 2016; Talboys et al. 2016). Compared to other recycling products, struvite has a high P content, contains also N, and tends to have a low concentration of heavy metals and other contaminants (Faucon et al. 2015; Schneider et al. 2019). Yet, the capacities of different plant species to mobilize P from struvite are still not fully understood. Lupin and buckwheat have been shown to mobilize P from struvite through the exudation of LMWOA (Robles-Aguilar et al. 2019; Talboys et al. 2016). Moreover, lupin has been shown to be more efficient in P uptake from struvite than maize due to rhizosphere acidification (Robles-Aguilar et al. 2020). However, the capacities of several other common crop species to mobilize P from struvite have not yet been investigated.
Our study aimed to investigate the mechanisms of plant P mobilization from different P sources in relation to plant P uptake by different plant species in order to identify plant species that effectively use P sources other than phosphate rock-derived fertilizers. For this purpose, we conducted a greenhouse experiment with three different P sources (phytate, FePO4, and struvite) that require different P mobilization mechanisms and four different plant species (maize, soy, lupin, and mustard) that likely have contrasting mechanisms of P mobilization. We hypothesized that (i) plant species with a high LMWOA exudation effectively mobilize P from struvite and FePO4, (ii) rhizosphere alkalinization mobilizes P from FePO4, and (iii) plant species with a high phosphomonoesterase activity in the rhizosphere effectively mobilize P from phytate. To test these hypotheses, we analyzed the spatial distribution of rhizosphere pH and phosphomonoesterase activities during plant growth using the in situ techniques pH imaging and soil zymography. Additionally, we collected root exudates at harvest, in which pH, phosphomonoesterase activity, LMWOA, and dissolved organic carbon (DOC) were measured, before plants were analyzed for biomass production, P content, and root length.
Materials and methods
Experimental setup
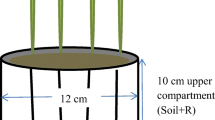
We conducted a greenhouse experiment with four plant species grown in rhizoboxes in a mineral substrate with three different P sources. The mineral substrate consisted of 20% (vol.) perlite and 80% (vol.) quartz sand, from which 50% (wt.) had a grain size of 0.1–0.4 mm and 50% (wt.) had a grain size of 0.7–1.2 mm. A mix of micronutrients (RADIGEN® Micronutrient mixed fertilizer, Terraflor GmbH, Iserlohn, Germany) was added to the mineral substrate (320 mg rhizobox−1), containing 5.0% MgO, 2.0% Fe, 1.5% Cu, 1.0% Mn, 0.8% Mo, 0.6% B, and 0.5% Zn. One of the following P sources was added to the mineral substrate (150 mg P rhizobox−1): iron phosphate (iron(III) phosphate dihydrate: FePO4 × 2H2O; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), phytate (phytic acid sodium salt hydrate: C6H18O24P6 × xNa+ × yH2O; Sigma-Aldrich), and struvite (ammonium magnesium phosphate hydrate: NH4MgPO4 × xH2O; Sigma-Aldrich). Further nutrients were supplied with a P-free nutrient solution as described below. The mineral substrate was filled into the rhizoboxes to a final bulk density of 1.1 g cm−3, which equals 1.7 kg of mineral substrate (dry weight) per rhizobox. Rhizoboxes were made of PVC and had an inner size of 39.2 × 19.2 × 2.2 cm (h × w × d).
In each rhizobox, one out of four plant species was sown: maize (Zea mays L. cv. Golden Bantam, Bingenheimer Saatgut AG, Echzell, Germany), soy (Glycine max (L.) Merr. cv. Lica, Marktgesellschaft der Naturland Bauern AG, Hohenkammer, Germany), blue lupin (Lupinus angustifolius L. cv. Rumba, Templiner Kräutergarten, Templin, Germany), and white mustard (Sinapis alba L., Bingenheimer Saatgut AG). All seeds except for mustard were soaked in water for 24 h before seeds of a consistent size were sown at a rate of one seed per rhizobox. All treatments were replicated four times summing up to a total number of 48 rhizoboxes (four plant species × three P sources × four replicates). However, one rhizobox of lupin supplied with struvite failed shortly before harvest. The plants were sown in August 2019 and harvested after 10 weeks in October 2019.
An inoculum was applied to the mineral substrate at the beginning of the experiment in order to introduce a soil microbial community. For this purpose, fresh soil with a loamy sandy texture was sampled from an agricultural soil cultivated with the same plant species used here. In the field, soy and lupin seeds had been inoculated with commercial Bradyrhizobium sp. inoculants, which are assumed to be also part of the microbial community introduced here (for details, see Schwerdtner and Spohn 2021). The soil was sieved (< 2 mm), mixed with tap water (1:2), and shaken on an overhead shaker for 1 h before being filtered through cellulose filters (Rotilabo®, type 113P, Carl Roth GmbH & Co. KG, Karlsruhe, Germany). The filtrate was mixed with tap water to a final soil:water ratio of 1:4 and stored at 20 °C over night before being applied to all rhizoboxes. The final soil inoculum had the following chemical properties (l−1 inoculum): 15.3 mg organic C, 4.4 mg N, 0.9 mg P, and pH 7.9. Each rhizobox received 180 ml of soil inoculum and 50 ml of tap water to adjust the mineral substrate to 75% water holding capacity (WHC).
The rhizoboxes were placed in an open greenhouse at the University of Bayreuth under ambient conditions and without artificial light. The rhizoboxes were placed in a randomized block design on a wooden rack that kept them inclined by 50° throughout the experiment, and they were rearranged randomly after 5 weeks. The inclination of the rhizoboxes made the roots grow along the bottom wall of the rhizoboxes, which made it possible to conduct imaging analyses (see below) and to remove the entire plant at harvest with very limited damage to the root system. Rhizoboxes were watered every two days with tap water to 75% WHC as measured by weight. In addition, a P-free nutrient solution was applied regularly. For this purpose, an adapted Ruakura solution (Smith et al. 1983) was used, where KH2PO4 was substituted by KNO3 and K2HPO4 by K2SO4. The final nutrient solution applied to the rhizoboxes contained (l−1): 220 mg Mg(NO3)2 × 6 H2O, 746 mg Ca(NO3)2 × 4 H2O, 377 mg NH4NO3, 189 mg KNO3, 367 mg K2SO4, 27 mg Na2SO4, and 15 mg NaCl. In total, 9.2 mg N kg−1 substrate were applied in the form of inoculum and nutrient solution.
Six weeks after plant emergence, pH imaging and soil zymography were performed to determine the spatial distribution of pH and phosphomonoesterase activity (see below). At harvest, 70 days after plant emergence, root exudates were collected, and plants were analyzed for biomass production, root length, and P concentrations (see below).
pH imaging
The distribution of pH in the rhizosphere was analyzed in situ by pH imaging, following Marschner and Römheld (1983) with modifications. The pH indicator bromocresol purple (Sigma-Aldrich) was dissolved in deionized water (0.6%). NaOH was added dropwise for better dissolution as described by Nkebiwe et al. (2016). The day before analysis, a boiled agar solution (1.3% agarose; Sigma-Aldrich) was mixed with the pH indicator solution (final pH indicator concentration of 0.006%), adjusted to mineral substrate pH with NaOH, and cast in glass systems usually used for gel electrophoresis with an inner size of 24.5 × 18.5 × 0.1 cm. Gels were plastic-wrapped to prevent drying and stored overnight at 20 °C to allow acclimatization. Rhizoboxes were transferred to the 20 °C climate chamber 1 h before analyses to allow acclimatization of the mineral substrate. The pH indicator gels were cut into three pieces, each with a size of 8 × 18 cm. Each gel was attached to the soil surface of one rhizobox to a soil depth of 18 cm (from the top, box-centered) and covered with a plastic sheet. After 12 min of incubation in the dark at 20 °C, gels were photographed with a digital camera (EOS 1100D, Canon). No quantitative image analysis was performed since the mineral substrate gave no uniform background values due to the mixing with perlite. Instead, representative pH images of each plant species supplied with one of the three P sources are presented in Fig. S1 (Supplement). Photos of the root systems are not included in the study as it was practically impossible to photograph the roots accordingly since mineral substrate and roots had very similar colors.
Soil zymography
Directly after pH imaging, the distribution of phosphomonoesterase activity was measured in situ by soil zymography following Spohn and Kuzyakov (2013) with modifications. No agarose gels were used as in Holz et al. (2019) as we used a mineral substrate and thus the gel, which is thought to protect the membrane from staining with organic material, was not required. The substrate 4-methylumbelliferyl phosphate (Sigma-Aldrich) was dissolved in deionized water to a concentration of 2 mM. Membrane filters of nylon (0.45 µm pore size; Nantong FilterBio Membrane Co. Ltd., Jiangsu, China) with a size of 8 × 31 cm were coated with this solution. The membranes were allowed to dry flat for 1 min at room temperature (20 °C) on aluminum foil, before being attached to the soil surface of one rhizobox to a soil depth of 31 cm (from the top, box-centered). After 30 min of incubation at 20 °C in the dark, the membrane was removed from the soil surface, cut into two equal pieces, and each piece was photographed with a digital camera (D60, Nikon) on an epi-UV-desk (Desaga, Heidelberg, Germany) at 366 nm wavelength. The cutting was done to ensure equal distribution of UV light all over the zymogram.
For calibration, membranes were soaked in 4-methylumbelliferone (MUF; Sigma-Aldrich) of different concentrations (0, 25, 75, 125, 200 µM). The membranes were also allowed to dry for 1 min and then photographed as described for the zymograms. Phosphomonoesterase activity was calculated based on a linear correlation between the different MUF concentrations and the corresponding gray values of the images (Spohn and Kuzyakov 2013).
The zymograms were analyzed using the open-source software ImageJ (version 1.52a; Rasband 2018). For this purpose, the photographs were converted into 8-bit, i.e., grayscale images, and a digital grid with cells of 10 × 10 pixels was laid on the images, similar as in Hofmann et al. (2016). The mean gray value of each grid cell was determined, and the twenty highest gray values of each rhizobox (considering both pieces of the zymogram) were arithmetically averaged to obtain one average value per rhizobox, i.e., per plant. The corresponding phosphomonoesterase activities were calculated based on the calibration line and the incubation time. Representative zymograms of each plant species supplied with phytate are presented in Fig. S2 (Supplement).
Root exudate collection
Root exudates were collected in sterile deionized water using the soil-hydroponic-hybrid sampling approach (Oburger and Jones 2018). For this purpose, the bottom walls of the rhizoboxes were opened at harvest and plants were removed as carefully as possible to prevent root damage. Roots were gently shaken and washed with deionized water to remove adhering substrate particles and potential metabolites (Oburger and Jones 2018). The entire root system of the intact plant was then transferred to a sterile beaker that was filled with a known volume of sterile deionized water (between 50 and 125 ml) so that roots were completely submerged. We used sterile deionized water instead of a CaCl2 solution since this reduces the background matrix for the analyses while not altering exudation patterns (Egle et al. 2003; Oburger and Jones 2018). Plants in beakers were stored at 20 °C in a climate chamber with artificial lighting (650 µmol m−2 s−1). After 4 h, plants were removed, and beakers were swayed to homogenize the exudates in the solution. The exudate solutions were filtered through 0.2 μm syringe filters and four aliquots were frozen for subsequent analyses of pH, phosphomonoesterase activity, LMWOA, and DOC. All plants were sampled in a way ensuring that exudate collection took place during peak metabolic activity, i.e., collection started 3.5 ± 1 h after sunrise, as recommended in Oburger and Jones (2018). Since we used a relatively short exudate collection period and maintained very similar temperatures during plant growth and exudate collection, we assume that plant metabolism and therefore exudation patterns do not differ between growth and sampling conditions (Oburger and Jones 2018).
Biomass analyses
After the plants were removed from the exudate collection beakers, aboveground biomass (AGB) was separated from belowground biomass (BGB). AGB was immediately dried at 60 °C for 24 h, then weighed and milled. The BGB was washed again with deionized water and stored over night at 2 °C. For root length determination, two plant individuals per species and P source (24 individuals in total) were chosen (due to time constraints during harvest). The root length was determined using a flatbed scanner (Epson Perfection V700, Seiko Epson Corporation, Nagano, Japan) and the software WinRhizo™ 2008 (Regent Instruments Inc., Québec, Canada). The BGB was submerged in a water bath, neatly arranged to avoid root overlapping, and scanned at 400 dpi resolution. After root length analysis, BGB of all plants was dried at 60 °C, weighed, and milled.
The biomass samples (AGB and BGB of each plant) were analyzed for total P concentrations after pressure digestion in concentrated nitric acid using inductively coupled plasma-optical emission spectroscopy (Vista-Pro radial, Varian Inc., Palo Alto, USA).
Exudate analyses
pH The pH in the exudate solution was measured with a pH electrode (WTW SenTix 51, Xylem Analytics GmbH & Co. KG, Weilheim, Germany).
Phosphomonoesterase activity Phosphomonoesterase activity in the exudate solution was measured using the fluorogenic substrate 4-methylumbelliferyl phosphate following Marx et al. (2001), German et al. (2011), and Herold et al. (2014). In brief, the exudate samples (50 µl) were pipetted into black polystyrene 96-well microplates (BRANDplates®, Brand GmbH & Co. KG, Wertheim, Germany) having four replicates. Sterile deionized water (50 µl) and substrate solution (100 µl) were added. Microplates were covered and pre-incubated in the dark at 15 °C for 30 min and measured fluorometrically after 0, 60, and 180 min with 360 nm excitation and 460 nm emission filters (Herold et al. 2014) by a microplate reader (Infinite® 200 PRO, Tecan Trading AG, Männedorf, Switzerland). Between measurements, microplates were incubated in the dark at 15 °C. Enzyme activities were calculated according to German et al. (2011) modified by Widdig et al. (2019). Fluorescence values were corrected for quenching, sample fluorescence, and substrate fluorescence.
LMWOA LMWOA were analyzed using high-performance liquid chromatography-mass spectrometry (HPLC–MS). For this purpose, the exudate samples were loaded on a HPLC RP-C18 column (Luna Omega 1.6 µm PS C18, 100 Å, 100 × 2.1 mm, Phenomenex Inc., Torrance, USA; operated as part of an Ultimate 3000 HPLC, Thermo Fisher Scientific GmbH, Bremen, Germany), which was connected to a Q Exactive mass spectrometer (Thermo Fisher Scientific GmbH) equipped with a hybrid quadrupole orbitrap mass analyzer (maximum mass range 50–6000 Da, resolution 140.000 @ m/z = 200). A 10 min isocratic elution with pure water (HPLC-grade, spiked with 0.2% formic acid) at a flow rate of 0.3 ml min−1 was applied. Mass spectra were acquired after electrospray ionization (ESI negative) in full scan mode (50 < m/z < 750) recording the total ion current. For evaluation (i.e., identification and integration/quantitation) of the LMWOA, their characteristic mass traces were used (Table S1; Supplement).
DOC Dissolved organic carbon (DOC) was analyzed using a Total Carbon Analyzer (TOC-TN Analyzer, multi N/C 2100, Analytik Jena GmbH, Jena, Germany).
Calculations
The total biomass (TBM) was calculated as the sum of the dry weights (DW) of AGB and BGB for each plant. The plant P concentration (in mg P g−1 DW) was calculated based on the P concentrations of AGB and BGB and the DW of AGB and BGB for each plant. The plant P content (in mg P plant−1) was calculated as the sum of AGB and BGB P content (calculated by multiplying the P concentrations of AGB and BGB with the DW of AGB and BGB, respectively).
The pH in the exudate solution was converted into the H+ concentration, and subsequently the H+ concentration was multiplied by the volume of sterile deionized water and reconverted into pH in order to correct for the different volumes. The phosphomonoesterase activity and DOC concentration in the exudate solution were multiplied by the volume of sterile deionized water, in which roots were submerged in order to correct for the different volumes and gain results per plant.
The exudation of each LMWOA (in μmol plant−1) was calculated by multiplying the LMWOA concentrations in the exudate solution (in mg l−1) with the volume of sterile deionized water and dividing by the molar mass of the respective LMWOA. The concentration of each LMWOA (in μmol plant−1) was multiplied with the number of carboxyl groups (1, 2, or 3, respectively; Table S1; Supplement), and all numbers were totaled up to calculate the total number of carboxyl groups in the exudate solution. The carboxyl groups (in μmol plant−1) were divided by the BGB DW in order to gain results per g root DW. For two plant individuals per treatment, the carboxyl groups (in μmol plant−1) were also divided by the root length in order to gain results per cm root length.
Statistical analyses
Data were tested for significant differences both among plant species (tested separately for each P source) and among P sources (tested separately for each plant species). Prior to all statistical analyses, normality was checked with Shapiro–Wilk normality tests, and homogeneity of variances was tested with Levene’s tests. Where normality and homogeneity assumptions were met, analyses of variance followed by Tukey’s post-hoc tests were conducted to identify significant differences. Where normality and homogeneity assumptions were not met, Kruskal–Wallis tests followed by post-hoc tests using the criterium Fisher’s least significant difference and Holm correction for p adjustment were conducted to identify significant differences. All statistical analyses were performed in R (version 3.5.2; R Core Team 2018) using the packages agricolae (version 1.3–2; Mendiburu 2020), car (version 3.0–7; Fox and Weisberg 2019), dplyr (version 0.8.5; Wickham et al. 2020), and ggplot2 (version 3.3.0; Wickham 2016). Data on root length were not tested for significant differences since the sample size was too low (n = 2).
Results
Biomass production
AGB was 870 ± 353 mg plant−1 and BGB was 244 ± 104 mg plant−1, averaged across all plant species and P sources (Table 1). When P was provided in the form of phytate, TBM of soy was highest and was significantly higher, by a factor of 2.5, than TBM of lupin (p = 0.005; Table 1). When P was provided in the form of struvite, TBM of soy was also highest, and TBM of soy and mustard were significantly higher than of lupin and maize (p ≤ 0.007; Table 1). When P was provided in the form of FePO4, no significant difference in TBM among the plant species was found (Table 1).
Root length of soy, and to a lesser extent of mustard, tended to be larger than of maize and lupin, especially when supplied with phytate or struvite (Fig. S3; Supplement). Irrespective of the P source, mustard and soy had a thin and intensively branched root system that already filled the whole rhizobox 6 weeks after plant emergence. Maize had thicker roots that penetrated almost exclusively the upper third of the substrate in the rhizoboxes, whereas lupin formed thick tap roots that reached the bottom of the rhizoboxes but did not fill the whole volume of the rhizoboxes 6 weeks after plant emergence.
Plant phosphorus
Plant P concentrations (in mg g−1) differed significantly among all plant species and decreased in the order mustard > lupin > soy > maize when P was provided in the form of FePO4 (p < 0.003; Table 1). More precisely, plant P concentration of mustard was significantly higher, by a factor of 2.8, compared to maize (p < 0.001; Table 1). When P was provided in the form of phytate, plant P concentrations of mustard and soy were significantly higher than of maize (p < 0.001) and lupin (p ≤ 0.009; Table 1). When P was provided in the form of struvite, plant P concentrations of soy (p = 0.028), lupin (p = 0.052), and mustard (p = 0.052) were higher, by a factor of 2.4 to 2.6, than those of maize (Table 1). Lupin was the only plant species for which the P concentration was significantly higher when supplied with FePO4 than when supplied with phytate (p < 0.001; Table 1).
Plant P content (in mg plant−1) of soy was significantly higher, by a factor of 3.8, 3.3, and 1.6, respectively, than of lupin (p < 0.001), maize (p < 0.001), and mustard (p = 0.009) when P was provided in the form of phytate. Moreover, plant P content of mustard was significantly higher, by a factor of 2.5 and 2.1, respectively, than of lupin (p = 0.005) and maize (p = 0.013) when P was provided in the form of phytate (Fig. 1). When P was provided in the form of struvite, plant P contents significantly differed among all plant species and decreased in the order soy > mustard > lupin > maize (p < 0.007). More precisely, plant P content of soy was significantly higher, by a factor of 4.5, than of maize (p < 0.001; Fig. 1). When P was provided in the form of FePO4, no significant difference in plant P content was found among the plant species. Plant P contents of soy, lupin, and mustard were significantly higher when supplied with struvite than when supplied with phytate (p < 0.004) and FePO4 (p ≤ 0.020). Soy was the only plant species for which the plant P content was slightly higher with phytate than FePO4 (p = 0.097; Fig. 1). The P contents of all plant species were higher in AGB than in BGB (Table S2; Supplement).
Plant P content of the total biomass which is the sum of P in AGB and BGB. Columns show means and error bars indicate standard deviations. Different lowercase letters indicate significant differences (p < 0.05) among the plant species, tested separately for each P source. Different capital letters indicate significant differences among the P sources, tested separately for each plant species. Absence of letters indicates that there was no significant difference
Exudate pH and rhizosphere pH
The pH in the exudate solution of mustard was 0.8 pH units higher than in the exudate solution of the other plant species, when averaged across P sources (Fig. 2). When P was provided in the form of phytate or struvite, the pH in the exudate solution of mustard was significantly higher, by 1.0 and 0.7 pH units, respectively, than the pH of all other plant species (p ≤ 0.001; Fig. 2). When P was provided in the form of FePO4, the pH in the exudate solution of mustard was significantly higher, by 1.0 and 0.8 pH units, respectively, than of lupin (p = 0.004) and maize (p = 0.014), and it tended to be higher than of soy (p = 0.117; Fig. 2). The pH in the exudate solution of mustard was slightly higher when supplied with phytate than when supplied with FePO4 (p = 0.061) or struvite (p = 0.060; Fig. 2).
In addition, the pH images, taken 6 weeks after plant emergence, revealed a comparatively strong rhizosphere acidification by maize and an intermediate rhizosphere acidification by soy, irrespective of the P source (Fig. S1; Supplement).
Phosphomonoesterase activity
When P was provided in the form of phytate, phosphomonoesterase activity of lupin was significantly higher, by a factor of on average 122.9, compared to the other plant species (p < 0.022; Fig. 3). When P was provided in the form of FePO4, phosphomonoesterase activity of lupin was significantly higher, by a factor of 33.8, compared to soy and mustard (p ≤ 0.011), and slightly higher than of maize (p = 0.084; Fig. 3). When P was provided in the form of struvite, phosphomonoesterase activity of lupin was also significantly higher, by a factor of 8.5, compared to the other plant species (p < 0.001; Fig. 3).
Phosphomonoesterase activity in the exudate solution, including a zoom onto low values (right). Columns show means and error bars indicate standard deviations. Different lowercase letters indicate significant differences (p < 0.05) among the plant species, tested separately for each P source. Different capital letters indicate significant differences among the P sources, tested separately for each plant species. Absence of letters indicates that there was no significant difference
Soil zymography, conducted 6 weeks after plant emergence, revealed that lupin and soy had similarly high maximum phosphomonoesterase activities in the rhizosphere (Figs. S2 and S4; Supplement). When P was provided in the form of phytate, the phosphomonoesterase activities of lupin and soy were higher, by a factor of on average 1.8, compared to mustard (p < 0.001 and p = 0.005, respectively) and maize (p = 0.002 and p = 0.126, respectively; Fig. S4; Supplement).
Exudation of LMWOA
When P was provided in the form of struvite, the exudation of carboxyl groups by soy was significantly higher, by a factor of 12.5 and 7.3, respectively, compared to mustard (p < 0.001) and maize (p = 0.002), and slightly higher than of lupin (p = 0.061; Fig. 4). When P was provided in the form of FePO4, the exudation of carboxyl groups by soy was significantly higher, by a factor of 10.1 and 3.3, respectively, compared to mustard (p = 0.003) and lupin (p = 0.019; Fig. 4). When P was provided in the form of phytate, the exudation of carboxyl groups by lupin was significantly higher, by a factor of 17.5 and 4.2, respectively, compared to mustard (p < 0.001) and maize (p = 0.011), while LMWOA exudation did not differ significantly among lupin and soy (p = 0.253; Fig. 4). Moreover, lupin exuded significantly more carboxyl groups when supplied with phytate than when supplied with FePO4 (p = 0.008) or struvite (p = 0.024; Fig. 4). Similar findings were obtained when LMWOA data were normalized to BGB DW (Fig. S5a; Supplement). In contrast, maize, soy, and lupin exuded similar amounts of carboxyl groups per cm root length, which tended to be higher than of mustard, when P was provided in the form of struvite or FePO4. When P was provided in the form of phytate, lupin tended to exude substantially more carboxyl groups per cm root length than the other plant species (Fig. S5b; Supplement).
Total number of LMWOA carboxyl groups in the exudate solution. Columns show means and error bars indicate standard deviations. Different lowercase letters indicate significant differences (p < 0.05) among the plant species, tested separately for each P source. Different capital letters indicate significant differences among the P sources, tested separately for each plant species. Absence of letters indicates that there was no significant difference
Irrespective of P source, maize exuded mainly aconitate, followed by citrate and malate. Soy exuded mainly malonate, followed by citrate and malate. Lupin exuded mainly citrate, followed by malate. Mustard exuded mainly malate, followed by citrate. Succinate, fumarate, and gluconate played a minor role in all plant species (Table 2).
DOC exudation
When P was provided in the form of phytate, DOC exudation of soy was significantly higher, by a factor of 3.1, 2.4, and 2.0, respectively, than of mustard, maize, and lupin (p < 0.001; Table 2). When P was provided in the form of struvite, DOC exudation of soy was significantly higher, by a factor of 3.2, than DOC exudation of maize (p = 0.009), and slightly higher than DOC exudation of lupin and mustard (p = 0.052; Table 2). When P was provided in the form of FePO4, DOC exudation of soy was slightly higher than of mustard (p = 0.056; Table 2).
Discussion
In the present study, we found indications that soy had a particularly high potential to mobilize P from struvite and phytate, while mustard had a high potential to mobilize P from FePO4. The underlying mechanisms of plant P mobilization from the different P sources are discussed in the following.
Soy effectively mobilized P from struvite likely through LMWOA exudation
Our finding that soy had the highest P content of all investigated plant species when supplied with struvite (Fig. 1) is in accordance with a recent study showing that soy mobilized P from struvite nearly as effectively as from the highly soluble triple superphosphate and more effectively than wheat (Rech et al. 2019). We further found that soy exuded more LMWOA, mostly malonate, citrate, and malate, than the other plant species when supplied with struvite (Fig. 4; Table 2). Thus, soy might have mobilized P from struvite via a high LMWOA exudation, since in water only 1–2% of struvite P is soluble while in citric acid solution about 50–100% of struvite P is soluble (Ahmed et al. 2018; Cabeza et al. 2011; Möller et al. 2018; Rech et al. 2019). Moreover, dicarboxylic LMWOA (malate and oxalate) have been shown to mobilize slightly more P from struvite than citrate (Talboys et al. 2016). Further, the low LMWOA exudation of lupin found here (Fig. 4) might explain the comparatively low P content of lupin when P was provided in the form of struvite (Fig. 1).
We further found that the root length of soy tended to be larger than of the other plant species (Fig. S3; Supplement). This might have been advantageous for P uptake by soy once P was mobilized, since a strong positive linear correlation between root length and P uptake has been reported earlier (Pang et al. 2015). This is further supported by our finding that mustard had both the second highest plant P content (Fig. 1) and the second largest root length when supplied with struvite (Fig. S3; Supplement), while LMWOA exudation of mustard was low (Fig. 4). The advantage of a large root length for P mobilization from finely ground struvite has also been suggested for other Brassicaceae (Brennan and Bolland 2001; Lyu et al. 2016; Wen et al. 2021). By contrast, maize and lupin had comparatively low root lengths (Fig. S3; Supplement), which might be the reason for the lower P content of maize and lupin (Fig. 1). Our finding that mustard had the second highest plant P content when supplied with struvite (Fig. 1) is in accordance with studies on other Brassicaceae such as canola, in which P mobilization from struvite was high (Ahmed et al. 2018; Brennan and Bolland 2001; Katanda et al. 2016).
Mustard effectively mobilized P from FePO4 likely through rhizosphere alkalinization
Our finding that mustard had the highest P concentration of all investigated plant species when supplied with FePO4 (Table 1) indicates that mustard has a high potential to mobilize P from FePO4. This is in accordance with a previous study reporting that the Brassica oilseed rape mobilized more P from FePO4 than wheat and different legumes (Pearse et al. 2007). We further found that the pH in the exudate solution of mustard was higher than of the other plant species (Fig. 3). Thus, mustard might have mobilized P from FePO4 via rhizosphere alkalinization since the solubility of FePO4 increases with increasing pH (Hinsinger 2001; Lindsay 1979). Such pH increases in the rhizosphere have also been found for other Brassicaceae, which increased the rhizosphere pH by up to one pH unit compared to bulk soil (Marschner et al. 2007). Moreover, a significant positive correlation between the pH of rhizosphere extracts and leaf P concentrations has been reported for the Brassica oilseed rape when supplied with various P sources including FePO4 (Pearse et al. 2007). We further found that the root length of mustard tended to be larger than of maize and lupin when supplied with FePO4 (Fig. S3; Supplement), which might have been advantageous for P uptake by mustard, as discussed above. A significant positive correlation between shoot P uptake and total root length of different Brassicaceae and Poaceae supplied with FePO4 has been reported earlier (Marschner et al. 2007; Wang et al. 2007a). The authors suggested that the large root length allowed the Brassicaceae to access a greater soil volume than wheat, resulting in root foraging for P in addition to P mining. However, it was also reported that P uptake and root length of the Brassicaceae correlated mainly in early growth stages (Marschner et al. 2007; Wang et al. 2007a).
The P concentration of lupin supplied with FePO4 was significantly higher than of lupin supplied with phytate (Table 1). A higher capacity to mobilize P from FePO4 than from phytate has also been reported for white lupin and chickpea (Shu et al. 2007; Wang et al. 2007b). However, since pH in the exudate solution of lupin was significantly lower than of mustard (Fig. 3) and LMWOA exudation of lupin was significantly lower than of soy and lower with FePO4 than phytate (Fig. 4), the mechanisms of P mobilization from FePO4 by lupin remained largely unclear. This indicates that LMWOA amount and composition as well as pH in the exudate solution alone do not explain lupins’ ability to mobilize P from FePO4, as has also been found in another experiment comparing different plant species and their utilization of different P sources (Pearse et al. 2007). However, lupin has been shown to effectively mobilize P from FePO4 in another experiment, likely through a high LMWOA exudation (Schwerdtner et al. 2022).
Legumes differed in their response to phytate
When P was provided in the form of phytate, plant P content of lupin was significantly lower than of soy (Fig. 1), while phosphomonoesterase activity was considerably higher in the rhizosphere of lupin than of soy (Fig. 3). One explanation for the contrasting findings among lupin and soy might be that soy exuded more phytases capable of catalyzing phytate hydrolysis, whereas lupin exuded mainly other phosphomonoesterases, not capable of hydrolyzing phytate. This is in accordance with former studies showing that the phytase activity of lupin (and other plant species) contributed less than 5% to total phosphatase activity (Gilbert et al. 1999; Hayes et al. 1999; Richardson et al. 2000), whereas soy has been shown to have a high phytase activity relative to other phosphatases (Ramesh et al. 2011). We further found that the maximum phosphomonoesterase activity per root surface, determined by soil zymography, was similar in the rhizosphere of lupin and soy (Figs. S2 and S4; Supplement), indicating that soy also had a few root regions with very high phosphomonoesterase activities. We cannot exclude that the phytase activity by soy was not (fully) detected by our analyses since (some) phytases might specifically catalyze the hydrolysis of phytate, but not of other phosphomonoesters (such as 4-methylumbelliferyl phosphate used in our analyses), thus potentially underestimating the total phosphomonoesterase activity of soy (German et al. 2011; Oh et al. 2004; Turner et al. 2002).
We further found that soy exuded significantly more DOC than the other plant species (Table 2). These organic substances (including LMWOA) might act as substrate for microorganisms, which produce additional phytases that effectively hydrolyze phytate in the rhizosphere of soy, as demonstrated earlier (Lambers et al. 2008; Wang and Lambers 2020; Wu et al. 2018). This is supported by previous studies showing that the addition of commercial fungal phytases (Hayes et al. 2000; Sun et al. 2021) as well as the inoculation with phytate-mineralizing bacteria (Ramesh et al. 2011; 2014; Richardson et al. 2000) increased P availability from phytate for soy, wheat, and several pasture species. Similarly, mycorrhizal symbionts have been found to effectively mobilize P from phytate (Wang et al. 2017; Zhang et al. 2016). Thus, in the case of soy (but not of non-mycorrhizal lupin), the so-called tripartite symbiosis, i.e., a double symbiosis with rhizobia and mycorrhizal fungi, might have contributed to phytate mineralization, as reported earlier (Bai et al. 2017; Jia et al. 2004).
Taken together, the P content of soy was highest among the investigated plant species in our study indicating effective P mobilization by soy. Soy mobilized P from phytate likely via high enzyme activity and high DOC exudation, and from struvite likely via high LMWOA exudation.
Implications
Overall, our study indicates that plant responses to different P sources were plant species-specific rather than P source-specific. The plant species-specific differences in P mobilization could be utilized to design multi-species plant communities that sustainably improve plant P nutrition in agriculture. For instance, the reliance on phosphate rock-derived fertilizers in agriculture could be reduced in intercropping systems with plant species that have complementary P mobilization capacities (Homulle et al. 2022; Honvault et al. 2021; Sulieman and Mühling 2021). The plant P content of maize was generally lower than of soy, mustard, and lupin (Fig. 1) and maize P concentrations (Table 1) indicate that maize P acquisition was relatively low (Reuter and Robinson 1997). Thus, maize plants could potentially benefit from intercropping with one of the other plant species if the different plant species have a joint rhizosphere in which complementary and facilitative interactions can occur. Such positive intercropping effects on maize have been proposed earlier in various studies on intercropping (Homulle et al. 2022; Li et al. 2020a; Schwerdtner and Spohn 2021; Tang et al. 2021). Moreover, plant species that effectively mobilize P from different soil P pools (such as soy and mustard) could potentially be used in crop rotations and/or as cover crops with likely positive effects on the P uptake of subsequent crops (Hallama et al. 2019).
Conclusion
In the present study, we found plant species-specific responses to different P sources. In particular, we found that soy had a high potential to mobilize P from struvite and phytate, while mustard had a high potential to mobilize P from FePO4. Our findings suggest that soy effectively mobilized P from struvite via a high LMWOA exudation, which might be further promoted by its long roots. This partly confirms our first hypothesis on P mobilization via LMWOA from struvite, while it needs to be rejected for FePO4. Our findings further suggest that mustard effectively mobilized P from FePO4 via rhizosphere alkalinization, confirming our second hypothesis. We further found that soy but not lupin was capable of effectively mobilizing P from phytate, while phosphomonoesterase activity was considerably higher in the rhizosphere of lupin than of soy, indicating that the phosphomonoesterases that were determined here likely do not hydrolyze phytate. Our third hypothesis that a high phosphomonoesterase activity in the rhizosphere effectively mobilizes P from phytate needs, therefore, to be rejected.
Taken together, particularly soy and mustard were capable of mobilizing P from inorganic and organic sources through species-specific mechanisms. Thus, these plant species with their specific P mobilization mechanisms offer a chance to reduce the reliance of agricultural production on phosphate rock-derived fertilizers.
Data availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed N, Shim S, Won S, Ra C (2018) Struvite recovered from various types of wastewaters: characteristics, soil leaching behaviour, and plant growth. Land Degrad Dev 29(9):2864–2879. https://doi.org/10.1002/ldr.3010
Ashley K, Cordell D, Mavinic D (2011) A brief history of phosphorus: from the philosopher’s stone to nutrient recovery and reuse. Chemosphere 84(6):737–746. https://doi.org/10.1016/j.chemosphere.2011.03.001
Bai B, Suri VK, Kumar A, Choudhary AK (2017) Tripartite symbiosis of Pisum–Glomus–Rhizobium leads to enhanced productivity, nitrogen and phosphorus economy, quality, and biofortification in garden pea in a Himalayan acid Alfisol. J Plant Nutr 40(4):600–613. https://doi.org/10.1080/01904167.2016.1263320
Brennan RF, Bolland MDA (2001) Comparing fertilizer phosphorus requirements of canola, lupin, and wheat. J Plant Nutr 24(12):1885–1900. https://doi.org/10.1081/PLN-100107601
Cabeza R, Steingrobe B, Römer W, Claassen N (2011) Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr Cycl Agroecosyst 91(2):173–184. https://doi.org/10.1007/s10705-011-9454-0
Chang SC, Jackson ML (1958) Soil phosphorus fractions in some representative soils. J Soil Sci 9(1):109–119. https://doi.org/10.1111/j.1365-2389.1958.tb01903.x
Cordell D, White S (2014) Life’s bottleneck: sustaining the world’s phosphorus for a food secure future. Annu Rev Environ Resour 39(1):161–188. https://doi.org/10.1146/annurev-environ-010213-113300
Dalal RC (1977) Soil organic phosphorus. Adv Agron 29:83–117. https://doi.org/10.1016/S0065-2113(08)60216-3
Dong Q, Echigo K, Raboy V, Saneoka H (2020) Seedling growth, physiological characteristics, nitrogen fixation, and root and nodule phytase and phosphatase activity of a low-phytate soybean line. Plant Physiol Biochem 149:225–232. https://doi.org/10.1016/j.plaphy.2020.02.015
Egle K, Römer W, Keller H (2003) Exudation of low molecular weight organic acids by Lupinus albus L., Lupinus angustifolius L. and Lupinus luteus L. as affected by phosphorus supply. Agronomie 23(5–6):511–518. https://doi.org/10.1051/agro:2003025
Faucon M-P, Houben D, Reynoird J-P, Mercadal-Dulaurent A-M, Armand R, Lambers H (2015) Advances and perspectives to improve the phosphorus availability in cropping systems for agroecological phosphorus management. Adv Agron 134:51–79. https://doi.org/10.1016/bs.agron.2015.06.003
Fox J, Weisberg S (2019) An R companion to applied regression. SAGE Publications Inc., Los Angeles
George TS, Hinsinger P, Turner BL (2016) Phosphorus in soils and plants: facing phosphorus scarcity. Plant Soil 401(1–2):1–6. https://doi.org/10.1007/s11104-016-2846-9
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Gilbert GA, Knight JD, Vance CP, Allan DL (1999) Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ 22(7):801–810. https://doi.org/10.1046/j.1365-3040.1999.00441.x
Hallama M, Pekrun C, Lambers H, Kandeler E (2019) Hidden miners: the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 434:7–45. https://doi.org/10.1007/s11104-018-3810-7
Hayes JE, Richardson AE, Simpson RJ (1999) Phytase and acid phosphatase activities in extracts from roots of temperate pasture grass and legume seedlings. Aust J Plant Physiol 26(8):801–809. https://doi.org/10.1071/PP99065
Hayes JE, Simpson RJ, Richardson AE (2000) The growth and phosphorus utilisation of plants in sterile media when supplied with inositol hexaphosphate, glucose 1-phosphate or inorganic phosphate. Plant Soil 220(1/2):165–174. https://doi.org/10.1023/A:1004782324030
Herold N, Schöning I, Berner D, Haslwimmer H, Kandeler E, Michalzik B, Schrumpf M (2014) Vertical gradients of potential enzyme activities in soil profiles of European beech, Norway spruce and Scots pine dominated forest sites. Pedobiologia 57(3):181–189. https://doi.org/10.1016/j.pedobi.2014.03.003
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237(2):173–195. https://doi.org/10.1023/A:1013351617532
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248(1/2):43–59. https://doi.org/10.1023/A:1022371130939
Hofmann K, Heuck C, Spohn M (2016) Phosphorus resorption by young beech trees and soil phosphatase activity as dependent on phosphorus availability. Oecologia 181(2):369–379. https://doi.org/10.1007/s00442-016-3581-x
Holz M, Zarebanadkouki M, Carminati A, Hovind J, Kaestner A, Spohn M (2019) Increased water retention in the rhizosphere allows for high phosphatase activity in drying soil. Plant Soil 443(1–2):259–271. https://doi.org/10.1007/s11104-019-04234-3
Homulle Z, George TS, Karley AJ (2022) Root traits with team benefits: understanding belowground interactions in intercropping systems. Plant Soil 471:1–26. https://doi.org/10.1007/s11104-021-05165-8
Honvault N, Houben D, Nobile C, Firmin S, Lambers H, Faucon M-P (2021) Tradeoffs among phosphorus-acquisition root traits of crop species for agroecological intensification. Plant Soil 461(1–2):137–150. https://doi.org/10.1007/s11104-020-04584-3
IFA (2022) Consumption database of the International Fertilizer Association (IFASTAT). https://www.ifastat.org/databases/plant-nutrition. Accessed 05 September 2022
Jarosch KA, Doolette AL, Smernik RJ, Tamburini F, Frossard E, Bünemann EK (2015) Characterisation of soil organic phosphorus in NaOH-EDTA extracts: a comparison of 31P NMR spectroscopy and enzyme addition assays. Soil Biol Biochem 91:298–309. https://doi.org/10.1016/j.soilbio.2015.09.010
Jia Y, Gray VM, Straker CJ (2004) The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Ann Bot 94(2):251–258. https://doi.org/10.1093/aob/mch135
Kataki S, West H, Clarke M, Baruah DC (2016) Phosphorus recovery as struvite: recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour Conserv Recycl 107:142–156. https://doi.org/10.1016/j.resconrec.2015.12.009
Katanda Y, Zvomuya F, Flaten D, Cicek N (2016) Hog-manure-recovered struvite: effects on canola and wheat biomass yield and phosphorus use efficiencies. Soil Sci Soc Am J 80(1):135–146. https://doi.org/10.2136/sssaj2015.07.0280
Lambers H, Chapin FS, Pons TL (2008) Plant physiological ecology. Springer, New York
Li C, Hoffland E, Kuyper TW, Yu Y, Zhang C, Li H, Zhang F, van der Werf W (2020a) Syndromes of production in intercropping impact yield gains. Nat Plants 6(6):653–660. https://doi.org/10.1038/s41477-020-0680-9
Li R-H, Cui J-L, Hu J-H, Wang W-J, Li B, Li X-D, Li X-Y (2020b) Transformation of Fe-P complexes in bioreactors and P recovery from sludge: investigation by XANES spectroscopy. Environ Sci Technol 54(7):4641–4650. https://doi.org/10.1021/acs.est.9b07138
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Liu X, Han R, Cao Y, Turner BL, Ma LQ (2022) Enhancing phytate availability in soils and phytate-P acquisition by plants: a review. Environ Sci Technol 56(13):9196–9219. https://doi.org/10.1021/acs.est.2c00099
Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7:1939. https://doi.org/10.3389/fpls.2016.01939
Ma Z, Guo D, Xu X, Lu M, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018) Evolutionary history resolves global organization of root functional traits. Nature 555(7694):94–97. https://doi.org/10.1038/nature25783
Marschner P (ed) (2012) Marschner’s mineral nutrition of higher plants. Academic Press, London
Marschner H, Römheld V (1983) In vivo measurement of root-induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Z Pflanzenphysiol 111(3):241–251. https://doi.org/10.1016/S0044-328X(83)80083-X
Marschner P, Solaiman Z, Rengel Z (2007) Brassica genotypes differ in growth, phosphorus uptake and rhizosphere properties under P-limiting conditions. Soil Biol Biochem 39(1):87–98. https://doi.org/10.1016/j.soilbio.2006.06.014
Marx M-C, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33(12–13):1633–1640. https://doi.org/10.1016/S0038-0717(01)00079-7
Mendiburu F de (2020) agricolae: Statistical procedures for agricultural research, https://cran.r-project.org/package=agricolae
Menezes-Blackburn D, Giles C, Darch T, George TS, Blackwell M, Stutter M, Shand C, Lumsdon D, Cooper P, Wendler R, Brown L, Almeida DS, Wearing C, Zhang H, Haygarth PM (2018) Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant Soil 427(1):5–16. https://doi.org/10.1007/s11104-017-3362-2
Möller K, Oberson A, Bünemann EK, Cooper J, Friedel JK, Glæsner N, Hörtenhuber S, Løes A-K, Mäder P, Meyer G, Müller T, Symanczik S, Weissengruber L, Wollmann I, Magid J (2018) Improved phosphorus recycling in organic farming: navigating between constraints. In: Sparks DL (ed) Advances in agronomy. Elsevier, pp 159–237
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action: biological processes in soil phosphorus cycling. Springer, Berlin, pp 215–243
Nkebiwe PM, Weinmann M, Müller T (2016) Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: from lab to field. Chem Biol Technol Agric 3(1):15. https://doi.org/10.1186/s40538-016-0065-5
Oburger E, Jones DL (2018) Sampling root exudates: Mission impossible? Rhizosphere 6:116–133
Oh B-C, Choi W-C, Park S, Kim Y-o, Oh T-K (2004) Biochemical properties and substrate specificities of alkaline and histidine acid phytases. Appl Microbiol Biotechnol 63(4):362–372. https://doi.org/10.1007/s00253-003-1345-0
Pang J, Yang J, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2015) Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol Plant 154:511–525. https://doi.org/10.1111/ppl.12297
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173(1):181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
R Core Team (2018) R: A language and environment for statistical computing. https://www.r-project.org/
Ramesh A, Sharma SK, Joshi OP, Khan IR (2011) Phytase, phosphatase activity and P-nutrition of soybean as influenced by inoculation of Bacillus. Indian J Microbiol 51(1):94–99. https://doi.org/10.1007/s12088-011-0104-7
Ramesh A, Sharma SK, Yadav N, Joshi OP (2014) Phosphorus mobilization from native soil P-pool upon inoculation with phytate-mineralizing and phosphate-solubilizing Bacillus aryabhattai isolates for improved P-acquisition and growth of soybean and wheat crops in microcosm conditions. Agric Res 3(2):118–127. https://doi.org/10.1007/s40003-014-0105-y
Rasband W (2018) ImageJ. https://imagej.net/
Rech I, Withers PJA, Jones DL, Pavinato PS (2019) Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability 11(1):134. https://doi.org/10.3390/su11010134
Reuter DJ, Robinson JB (1997) Plant analysis: an interpretation manual. CSIRO Publishing, Collingwood
Richardson AE, Hadobas PA, Hayes JE (2000) Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ 23(4):397–405. https://doi.org/10.1046/j.1365-3040.2000.00557.x
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci 60(2):124–143. https://doi.org/10.1071/CP07125
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349(1–2):121–156. https://doi.org/10.1007/s11104-011-0950-4
Robles-Aguilar AA, Pang J, Postma JA, Schrey SD, Lambers H, Jablonowski ND (2019) The effect of pH on morphological and physiological root traits of Lupinus angustifolius treated with struvite as a recycled phosphorus source. Plant Soil 434(1–2):65–78. https://doi.org/10.1007/s11104-018-3787-2
Robles-Aguilar AA, Schrey SD, Postma JA, Temperton VM, Jablonowski ND (2020) Phosphorus uptake from struvite is modulated by the nitrogen form applied. J Plant Nutr Soil Sci 183(1):80–90. https://doi.org/10.1002/jpln.201900109
Schneider KD, Thiessen Martens JR, Zvomuya F, Reid DK, Fraser TD, Lynch DH, O’Halloran IP, Wilson HF (2019) Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J Environ Qual 48(5):1247–1264. https://doi.org/10.2134/jeq2019.02.0070
Schwerdtner U, Spohn M (2021) Interspecific root interactions increase maize yields in intercropping with different companion crops. J Plant Nutr Soil Sci 184(5):596–606. https://doi.org/10.1002/jpln.202000527
Schwerdtner U, Lacher U, Spohn M (2022) Lupin causes maize to increase organic acid exudation and phosphorus concentration in intercropping. J Sustain Agric Environ 1(3):191–202. https://doi.org/10.1002/sae2.12026
Shu L, Shen J, Rengel Z, Tang C, Zhang F, Cawthray GR (2007) Formation of cluster roots and citrate exudation by Lupinus albus in response to localized application of different phosphorus sources. Plant Sci 172(5):1017–1024. https://doi.org/10.1016/j.plantsci.2007.02.006
Smith GS, Johnston CM, Cornforth IS (1983) Comparison of nutrient solutions for growth of plants in sand culture. New Phytol 94(4):537–548. https://doi.org/10.1111/j.1469-8137.1983.tb04863.x
Spohn M, Kuzyakov Y (2013) Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation: coupling soil zymography with 14C imaging. Soil Biol Biochem 67:106–113. https://doi.org/10.1016/j.soilbio.2013.08.015
Sulieman S, Mühling KH (2021) Utilization of soil organic phosphorus as a strategic approach for sustainable agriculture. J Plant Nutr Soil Sci. https://doi.org/10.1002/jpln.202100057
Sun M, He Z, Jaisi DP (2021) Role of metal complexation on the solubility and enzymatic hydrolysis of phytate. PLoS ONE 16(8):e0255787. https://doi.org/10.1371/journal.pone.0255787
Talboys PJ, Heppell J, Roose T, Healey JR, Jones DL, Withers PJA (2016) Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 401:109–123. https://doi.org/10.1007/s11104-015-2747-3
Tang X, Zhang C, Yu Y, Shen J, van der Werf W, Zhang F (2021) Intercropping legumes and cereals increases phosphorus use efficiency; a meta-analysis. Plant Soil 460(1–2):89–104. https://doi.org/10.1007/s11104-020-04768-x
Turner BL, Papházy MJ, Haygarth PM, McKelvie ID (2002) Inositol phosphates in the environment. Phil Trans r Soc Lond B 357(1420):449–469. https://doi.org/10.1098/rstb.2001.0837
Wang Y, Lambers H (2020) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447(1–2):135–156. https://doi.org/10.1007/s11104-019-03972-8
Wang D, Marschner P, Solaiman Z, Rengel Z (2007a) Belowground interactions between intercropped wheat and Brassicas in acidic and alkaline soils. Soil Biol Biochem 39(4):961–971. https://doi.org/10.1016/j.soilbio.2006.11.008
Wang D, Marschner P, Solaiman Z, Rengel Z (2007b) Growth, P uptake and rhizosphere properties of intercropped wheat and chickpea in soil amended with iron phosphate or phytate. Soil Biol Biochem 39(1):249–256. https://doi.org/10.1016/j.soilbio.2006.07.013
Wang X-X, Hoffland E, Feng G, Kuyper TW (2017) Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front Plant Sci 8:684. https://doi.org/10.3389/fpls.2017.00684
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H, Pang J, Ryan MH, Lambers H, Shen J (2019) Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223(2):882–895. https://doi.org/10.1111/nph.15833
Wen Z, White PJ, Shen J, Lambers H (2021) Linking root exudation to belowground economic traits for resource acquisition. New Phytol. https://doi.org/10.1111/nph.17854
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, Cham
Wickham H, François R, Henry L, Müller K (2020) dplyr: A grammar of data manipulation, https://cran.r-project.org/package=dplyr
Widdig M, Schleuss P-M, Weig AR, Guhr A, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2019) Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front Environ Sci 7:185. https://doi.org/10.3389/fenvs.2019.00185
Wilfert P, Kumar PS, Korving L, Witkamp G-J, van Loosdrecht MCM (2015) The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: a review. Environ Sci Technol 49(16):9400–9414. https://doi.org/10.1021/acs.est.5b00150
Wu L, Kobayashi Y, Wasaki J, Koyama H (2018) Organic acid excretion from roots: a plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci Plant Nutr 64(6):697–704. https://doi.org/10.1080/00380768.2018.1537093
Zhang L, Xu M, Liu Y, Zhang F, Hodge A, Feng G (2016) Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol 210(3):1022–1032. https://doi.org/10.1111/nph.13838
Acknowledgements
Ulrike Schwerdtner thanks the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU) for granting her a PhD scholarship. Marie Spohn thanks the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) for funding her position through the Emmy Noether-program (Grant SP1389/6-1). We thank Bettina Engelbrecht and Elisabeth Obermaier for co-mentoring the PhD project and giving helpful comments. The authors gratefully acknowledge Renate Krauß, Uwe Hell, Fabian Nützel, Heike Deinlein, Markus Winkler, Tom Zimmermann, and Theophilus Awoyele for technical assistance. We thank the chemical analytics (CAN) of the Bayreuth Center of Ecological and Environmental Research (BayCEER) for performing parts of the chemical analyses.
Funding
Open Access funding enabled and organized by Projekt DEAL. US was supported by the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU) with a PhD scholarship. MS was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through the Emmy Noether-program (Grant SP1389/6-1).
Author information
Authors and Affiliations
Contributions
US and MS designed the study. US performed the greenhouse experiment. US and UL performed plant and exudate analyses. US wrote a first draft of the manuscript, US and MS worked on the manuscript. All authors read, contributed to, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwerdtner, U., Lacher, U. & Spohn, M. Soy and mustard effectively mobilize phosphorus from inorganic and organic sources. Nutr Cycl Agroecosyst 124, 211–226 (2022). https://doi.org/10.1007/s10705-022-10234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-022-10234-0