Abstract
Predator-prey interactions are vital for organismal survival. They shape anti-predator mechanisms and often depend on sensory abilities. Tadpoles use chemical cues, such as injury cues (alarm cues), to assess predation risks and modify their life-history, morphology, and behaviours accordingly. However, the prevalence of chemically mediated anti-predator responses in species with distinct ecological niches (e.g. within phytotelmata) remains unknown, hindering our understanding of the ecological significance and evolution of alarm substances. Therefore, our study aimed to investigate chemically mediated anti-predator responses in tadpoles of two Neotropical poison dart frogs, Ranitomeya sirensis and Epipedobates anthonyi (and compare their responses to two Palearctic model organisms, Rana temporaria and Bufo bufo, which are known to utilise alarm substances). Through behavioural bioassays, we exposed predator-naïve tadpoles to extracts of each species (i.e. con- and heterospecific cues), including water as a control (i.e. five treatments per species). We assessed changes in their activity before and after stimulus introduction. Our results show that E. anthonyi did not respond to any of the stimuli, whereas R. sirensis displayed increased activity levels exclusively in response to conspecific cues, but not to heterospecific cues. With this, our findings suggest a specialized recognition system in R. sirensis, potentially directed at conspecific competitors but likely unrelated to anti-predator mechanisms. In contrast, E. anthonyi may be insensitive to injury cues or utilize alternative sensory modalities to respond to acute predation events. This study sheds light on the chemical alarm response system of Neotropical poison dart frog tadpoles, providing foundational understanding of how dendrobatids react to injury cues. It prompts questions about the ecological significance and evolutionary implications of chemical communication in species facing extreme resource limitation during development and underscores the importance of comparative research for understanding chemical communication in diverse aquatic ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation plays a crucial role in the survival of organisms, driving the evolution of sophisticated sensory abilities and plastic anti-predator mechanisms (Ferrari et al. 2010; Bradbury and Vehrencamp 2011; Davies et al. 2012). Olfaction plays a critical role in evading predation, especially in aquatic ecosystems, where other sensory modalities are limited or impaired (Ferrari et al. 2010; Bradbury and Vehrencamp 2011). Thus, aquatic prey organisms heavily rely on chemical cues to evaluate and efficiently respond to ambient predation risks (Kats and Dill 1998; Ferrari et al. 2010; Brönmark and Hansson 2012). Chemical cues from conspecifics and predators, in particular, provide valuable information about spatial and temporal predation risks (Kats and Dill 1998; van Buskirk and Arioli 2002; Ferrari et al. 2006; van Buskirk et al. 2014; Crane et al. 2023) as well as predator identity (Relyea 2003; Hawkins et al. 2007), enabling prey to regulate their short-term defences in a threat-, context- and species-dependent manner (Ferrari et al. 2010; Hossie et al. 2016; Mitchell et al. 2017). With this, accurate identification of chemical cues is imperative for survival and leads to the evolution of both innate (Petranka and Hayes 1998; Fendt 2006; Epp and Gabor 2008; Lau et al. 2021) and acquired cue identification (i.e. learning processes; Suboski 1990; Mirza and Chivers 2000; Dalesman et al. 2006; Mirza et al. 2006; Ferrari et al. 2010).
Amphibian larvae, due to their aquatic lifestyle and heightened vulnerability during early developmental stages (Duellman and Trueb 1994; McDiarmid and Altig 1999; Wells 2007), have evolved anti-predator mechanisms centred around the perception of predator- and prey-borne cues (Ferrari et al. 2010; Hettyey et al. 2015; Hossie et al. 2016; Mitchell et al. 2017). Tadpoles use predator kairomones (predator odours and diet cues; Mitchell et al. 2017) but also prey-borne cues emitted by stressed or injured conspecifics (disturbance cues or injury cues; Mitchell et al. 2017; see also Caballero-Díaz et al. 2023 for the use of social cues) to assess ambient predation risk. They use these cues to respond by altering morphology (van Buskirk and Mccollum 2000; Relyea 2001; Teplitsky et al. 2005; Hossie et al. 2010; Middlemis Maher et al. 2013) life-history (Chivers et al. 2001; Relyea and Auld 2004; Ireland et al. 2007; Gazzola et al. 2015), and behaviour (van Buskirk and Mccollum 2000; Relyea 2001; Ferrari et al. 2009a; Fraker et al. 2009; Hossie et al. 2010; Rödin-Mörch et al. 2011; Gazzola et al. 2015; Hettyey et al. 2015; Bairos-Novak et al. 2020). Behavioural response patterns towards prey-borne injury cues tend to emerge at the family level (Rödin-Mörch et al. 2011). For example, bufonid tadpoles predominantly display avoidance and vivacity upon perceiving conspecific injury cues, whereas ranid tadpoles exhibit a broader spectrum of behavioural responses, including reduced activity and avoidance (Rödin-Mörch et al. 2011). Although the study of chemical communication and anti-predator responses in tadpoles has gained significant attention (Schulte et al. 2023), there is still a notable gap in our understanding, particularly regarding species with specific ecological niches and environmental interactions.
Previous research has primarily focused on a few anuran families, predominantly studying species with high larval individual counts and gregarious tendencies (e.g. loose association and shoaling behaviour with kin or conspecifics; Rödin-Mörch et al. 2011; Hossie et al. 2016). This limited focus excludes families or species characterised by low larval individual counts, non-social behaviours (including latent aggression towards conspecifics), or habitats with extreme resource limitations (i.e. space and food availability) which are equally important to comprehensively understand chemical communication systems and alarm substance evolution in tadpoles (Summey and Mathis 1998; Chivers et al. 2012). Among these understudied groups, the Neotropical poison dart frogs (Dendrobatidae and Aromobatidae; Grant et al. 2006) offer intriguing prospects for investigating the ecological and evolutionary significance of alarm cues and chemically mediated anti-predator responses.
Neotropical poison dart frogs primarily inhabit the tropical rainforests of Central and South America (Lötters et al. 2007) and are renowned for their aposematic skin colouration, which deter predators and signal their toxicity (Maan and Cummings 2012; Saporito et al. 2012). In addition they exhibit complex parental care behaviours, including egg attendance, tadpole transport, and offspring provisioning (Summers and Tumulty 2014; Schulte et al. 2020; Schulte and Summers 2021). The tadpoles of many species rely on specific larval deposition habitats, such as phytotelmata, which are small water reservoirs in leaf axils, bromeliad pools, or water-filled cavities in trees (Brust 1993; Caldwell and De Araújo 1998; Summers 1999; Poelman and Dicke 2007; Wells 2007). This habitat and corresponding lifestyle (i.e. solitary or small groups) is in stark contrast to that of typical model organisms, most notably from the families Ranidae and Bufonidae, with distinctly larger clutch sizes, high degree of larval sociality and ecological interactions (Duellman and Trueb 1994; Wells 2007). While evidence suggests poison dart frog tadpoles are able to assess and respond to visual predator cues (i.e. predator presence; Stynoski and Noble 2012; Szabo et al. 2021) and potentially kairomones (Szabo et al. 2021), the prevalence and significance of chemically mediated anti-predator responses among Neotropical poison dart frog tadpoles remains unknown. Given the unique ecological characteristics and complex life history of Neotropical poison dart frogs, investigating the presence and effectiveness of chemically mediated anti-predator responses in their tadpoles can greatly contribute to our understanding of the evolution and adaptive significance of alarm substances in poison dart frog tadpoles and other anuran taxa (Summey and Mathis 1998; Hagman 2008; Chivers et al. 2012; Wisenden 2015).
In this comparative study, we primarily aimed to expand and investigate chemically mediated anti-predator behaviour in response to conspecific injury cues (i.e. prey-borne chemical cues that indicate acute predation events like predator attacks or capture of prey; Hettyey et al. 2015; Wisenden 2015) in poison dart frogs. Specifically, we focused on two Neotropical poison dart frogs of the family Dendrobatidae, Ranitomeya sirensis and Epipedobates anthonyi. While R. sirensis lays 2–4 eggs and transports their predatory (potentially cannibalistic) tadpoles individually into small phytotelmata (e.g. bromeliad leaf axils; Brown et al. 2011; Kahn et al. 2016), E. anthonyi lays 5–32 eggs and transports their tadpoles in groups into small streams, puddles or water-filled holes in boulders (Lötters et al. 2007). Unlike R. sirensis, tadpoles of E. anthonyi do not show aggressive behaviours towards other tadpoles (Walls 1994). Furthermore, we sought to compare the response (changes in activity) to conspecific injury cues of these two species with two commonly studied model organisms (within the context of chemical communication): Rana temporaria (Ranidae) and Bufo bufo (Bufonidae). Both species lay clutches of several thousand eggs in temporal and permanent ponds (Lardner 2000; Glandt 2018), and the resulting tadpoles exhibit aggregations characterised by non-aggressive behaviour towards each other (Wells 2007). In addition to our main goal, we sought to contribute to the understanding of the evolutionary and ecological significance of these substances in tadpoles by exploring the responses of all species involved to all possible heterospecific injury cues. Considering cross-species reactions and phylogenetic differences (especially concerning R. temporaria and B. bufo) helps refine hypotheses and develop future studies about the evolution of injury cues, their characteristics (i.e. phylogenetically conserved and/or convergent elements), and ecological significance (Summey and Mathis 1998; Hagman 2008; Chivers et al. 2012; Wisenden 2015).
We predict that (Hy1) Ranitomeya sirensis tadpoles will show no behavioural changes in response to both con- and heterospecific injury cues, reflecting absent evolutionary adaptations to discern and interpret these cues as heightened predation risk, given their solitary lifestyle, cannibalistic tendencies and limited options for action in their resource-limited environment (i.e. small water bodies in bromeliad leaf axis). We further predict that (Hy2) Epipedobates anthonyi, tadpoles will show decreased levels of activity in response to conspecific injury cues, reflecting evolutionary adaptations to enhance fitness by discerning and interpreting conspecific injury cues as heightened predation risks by sit-and-wait predators (Greeney 2001; McKeon and Summers 2013), facilitated by their gregarious lifestyle (i.e. loose aggregation with conspecifics and kin) in resource limited environments (e.g. small puddles or water-filled holes in boulders). Furthermore, we expect the strongest response (decrease in activity) towards conspecific injury cues and diminished anti-predator behaviour towards heterospecific injury cues, attributable to phylogenetic differences. Lastly, previous studies indicate that both Rana temporaria and Bufo bufo exhibit reduced activity levels as typical anti-predator responses to various predators (Van Buskirk 2001; Marquis et al. 2004; Maag et al. 2012; Nunes et al. 2013). Therefore, we predict that (Hy3a) R. temporaria and (Hy3b) B. bufo tadpoles will show reduced activity levels in response to conspecific injury cues and diminished anti-predator responses towards heterospecific injury cues, attributable to phylogenetic differences.
Methods
Animals
Ranitomeya sirensis (Aichinger, 1991) is a dendrobatid species endemic to the Serranía de Sira mountain range in east-central Peru (Lötters et al. 2007). Epipedobates anthonyi (Noble, 1921) occurs in southwestern Ecuador and northwestern Peru, extending west of the Andes at elevations ranging from 153–1387 m (Lötters et al. 2007). Tadpoles of R. sirensis and E. anthonyi used in this study were obtained from laboratory populations maintained at the Goethe University in Frankfurt am Main since 2018 (see supplementary material S1 for husbandry of adults). In laboratory populations, adult poison dart frogs deposited tadpoles in 50 mL falcon tubes or 280 mL beakers, which we then removed regularly and transferred to a dedicated tadpole housing environment. Newly hatched R. sirensis tadpoles were transferred into separate housing beakers individually (8.5 cm diameter, water level 4 cm), while E. anthonyi tadpoles were placed in tanks as sibling groups of 10 to 20 individuals (30 × 20 cm, water level 7 cm). Both tadpole housing environments were filled with demineralised water and included pieces of dried almond leaves (Prunus dulcis). Tadpoles of Rana temporaria (Linnaeus, 1758) and Bufo bufo (Linnaeus, 1758) were obtained by collecting and culturing eggs from a wild population where both species occur sympatrically. We collected eggs from 12 clutches annually (in each breeding season) from a permanent pond built by the Naturschutzbund Deutschland (NABU) in Steinau-Marborn, Hesse, Germany (coordinates: 50°19’34.28” N, 9°25’27.08” E). The eggs were transferred to cooling boxes with water from the collection site and transported within 1 h to the animal maintenance facilities at Goethe University of Frankfurt. In the laboratory, the eggs hatched, and the tadpoles were reared in large glass aquaria (40 × 50 × 100 cm) containing 200 L of well-aerated aged tap water (1–2 individual’s/L). The tanks were equipped with an air lift pump, sponge ceramic rings filter system and enriched with synthetic algae, clay pots, and stones to provide hiding places for the tadpoles. Months in advance, the filter system and water were inoculated with bacteria, phyto- and zooplankton from the respective collection sites.
Individuals of all species (breeding adults and tadpoles alike) were kept in the same room at constant temperatures (23–25 °C) and 12 L:12D photoperiod. Tadpoles of all species were fed with nettle powder every 2nd day and occasionally supplemented with rabbit chow. Water changes (25–50%) for tadpoles of R. temporaria and B. bufo were performed every third day, whereas tadpoles of R. sirensis and E. anthonyi did not require any water changes.
Behavioural assay
To investigate tadpole responses to conspecific and heterospecific injury cues, we conducted a series of behavioural bioassays between April 2021 and May 2023. The bioassays involved investigating the activity of predator-naïve tadpoles from Ranitomeya sirensis (N = 140), Epipedobates anthonyi (N = 141), Rana temporaria (N = 134) and Bufo bufo (N = 135), before and after the introduction of one of five different stimuli (i.e. five treatments). In each trial, tadpoles were individually placed in plastic cups (8 cm in diameter) filled with 495 mL of water and exposed to extracts of either R. sirensis (x-Rs), E. anthonyi (x-Ea), R. temporaria (x-Rt), B. bufo (x-Bb) or water (control). Thus, tadpoles of every species were exposed to (a) conspecific extracts, (b) each of one of three possible heterospecific extracts and (c) water as a control. Each trial comprised three phases: (1) pre-stimulus, (2) stimulus, and (3) post-stimulus (each lasting 5 min). After an acclimatisation period (1 h) the trials started with the pre-stimulus phase, followed by the administration of one of the five stimuli mentioned. To introduce the stimuli, we gently placed the syringes on the cups’ side without disturbing the animal. We then slowly dripped the content (5mL) into the cups, which marked the start of the stimulus phase. Prior observations (test with food dye) ensured 5 min time to be adequate for the stimulus to disperse in the second phase throughout the entire water body before initiating the post-stimulus phase; upon completion, a trial ended. Tadpole activity was recorded during the trials using a Panasonic camera and a mirror positioned at a suitable angle over the cups to capture the cups interior without obstructing the camera’s line of sight. Observer presence was found to have no impact on tadpole activity based on preliminary trials (see also McIntyre et al. 2004; Carlson et al. 2015). Therefore, we carefully stepped in front of the sides of the mirror to apply the stimuli via the syringes. To minimise a potential influence of “time-of-day effects” on tadpole behaviour (e.g. Ferrari et al. 2008b), the trials were conducted alternately at one of five different time slots between 10:00 and 17:00 each day. Moreover, we ensured that the same species-stimulus combination was not tested on two consecutive days during the same time slot. Each individual underwent a single testing session. The specific developmental stages of tadpoles in bioassays according to Gosner (1960), were: R. sirensis (28–36), E. anthonyi (28–36), R. temporaria (28–32), and B. bufo (28–32).
Extraction of injury cues
To obtain injury cues indicating acute predatory events (Hettyey et al. 2015; Wisenden 2015), we randomly selected tadpoles from husbandry environments and euthanised them with a blow to the head (Ferrari et al. 2007; Lucon-Xiccato 2019a,b). This physical method was chosen to preserve the activity of alarm substances, as previous studies have shown negative effects of chemical euthanasia methods (i.e. anaesthetics) on alarm substance activity in fish (Losey and Hugie 1994) and tadpoles (Achtymichuk et al. 2022; own unpublished data). Moreover, we prepared extracts immediately before each trial (within 30 min of euthanasia) to avoid the degradation of substances mediating anti-predatory responses (van Buskirk et al. 2014; Crane et al. 2023). Euthanised tadpoles were pooled and homogenised in 2 ml of water using a handheld rotor-stator homogeniser (TissueRuptor II, Quiagen). The crude extracts were then centrifuged (10,000 ×g for 10 min at 4 °C) to separate water-soluble from insoluble fractions. The aqueous supernatant was diluted with water (5mL per euthanised tadpole), split up and loaded into 5mL syringes, and used immediately in the bioassay. The choice of water type for extract preparation (demineralised or aged tap-water) depended on the species used in the bioassay due to differences in the husbandry of tadpoles. Demineralised water was used for bioassays with dendrobatid tadpoles, while aged tap-water was used for bioassays with ranid and bufonid tadpoles. Given the lack of available information regarding the dose-dependent effects of alarm substances in poison dart frogs, we opted for sacrificing one tadpole per individual participating in the bioassay (tadpoles euthanized: Ranitomeya sirensis (N = 140), Epipedobates anthonyi (N = 141), Rana temporaria (N = 134) and Bufo bufo (N = 135). This approach allowed us to obtain preliminary insights into the perception of alarm substances in poison dart frog tadpoles within an anti-predator context, while also ensuring comparability between species in this study. To record the applied biomass in bioassays but reduce handling of animals, we conducted regular weight measurements of participating tadpoles every 3rd day. For this, we used a microscale and a small beaker with a specific volume of water to introduce the tadpoles, and then calculated the differences to determine the wet biomass of tadpoles used for extract preparation. The final concentrations of extracts per trial were as follows: x-Rs (0.20–0.48 mg/mL), x-Ea (0.28–0.64 mg/mL), x-Rt (0.11–0.44 mg/mL), and x-Bb (0.09–0.38 mg/mL). With this, the applied end concentrations were above concentrations used in studies with evident responses from ranid and bufonid tadpoles (e.g. Hagman and Shine 2008; Fraker et al. 2009; Lucon-Xiccato 2019a,b; Crossland et al. 2019). The specific developmental stages of the tadpoles used for extract preparation, according to Gosner (1960), were: R. sirensis (28–38), E. anthonyi (28–38), R. temporaria (28–32), and B. bufo (28–32).
Video evaluation and statistical analysis
To compare the recorded tadpole behaviour before and after stimulus application, we quantified tadpole activity (total time being active) in the (1) pre- and (3) post-stimulus phases by scoring their total active time (in seconds) using the behaviour coding software “CowLog.“ (Version 3.0.2; Hänninen and Pastell 2009). Tadpoles were considered active when moving (i.e. swimming or exhibiting tail movement). Tadpoles showing slight tail movements that did not result in forward body movement were also considered active, as dendrobatid tadpoles often inhabit spatially limited environments (Brust 1993; Caldwell and De Araújo 1998; Summers 1999; Poelman and Dicke 2007). We then calculated a response index (response activity) for each individual within a treatment as the difference between the activity in the (3) post- and (1) pre-stimulus phase: [post - pre = response activity]. Therefore, the response activity represented the change in activity following exposure to the stimuli in phase (2), with positive numbers indicating increases and negative numbers indicating decreases in activity. Animals that had been inactive in both phases were excluded from the analysis. Due to data non-normality (assessed by the Kolmogorov-Smirnov test), tadpole responses of each species were compared using a Kruskal-Wallis one-way ANOVA (k samples). The dependent variable was “response activity” and the groups included in the analysis were “treatments” (i.e. five stimuli tested). Significant test results of species were subsequently assessed using Mann-Whitney U pairwise comparisons of each extract (x-Rs, x-Ea, x-Rt, x-Bb) against the control (water), including a Bonferroni-adjusted alpha value for four comparisons (P = 0.0125). Each species was analysed individually (i.e. no statistical analysis of treatments between species). All statistical analyses were conducted using SPSS 20 (SPSS Inc., Chicago, IL, USA).
Results
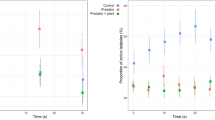
We quantified the activity and calculated the median response activity for tadpoles of Ranitomeya sirensis (N = 129), Epipedobates anthonyi (N = 131), Rana temporaria (N = 131) and Bufo bufo (N = 135; Table 1). Kruskal-Wallis one-way ANOVA rejected the null hypothesis for R. sirensis (H = 9.532, df = 4, P < 0.05), R. temporaria (H = 29.989, df = 4, P < 0.0001) and B. bufo (H = 9.703, df = 4, P < 0.05), indicating significant differences between tadpole responses towards introduced stimuli. Kruskal-Wallis test, however, did not reject the null hypothesis for tadpole responses of E. anthonyi (H = 3.527, df = 4, P = 0.474), indicating no significant differences towards any of the introduced stimuli. Therefore, Mann-Whitney U post hoc tests were conducted to compare response activities among treatments for R. sirensis, R. temporaria, and B. bufo (Table 2).
In R. sirensis a significant difference in tadpole activity was observed between the control and the group exposed to x-Rs (Z = -2.587, P = 0.010). Tadpoles exposed to extracts of conspecifics exhibited higher median response activity than the control, indicating increased activity (Table 1; Fig. 1). No significant difference in R. sirensis tadpole activity was observed between the control group and those exposed to heterospecific extracts (x-Ea, x-Rs, and x-Bb).
In R. temporaria, significant differences were found in tadpole activity between the control and the groups exposed to x-Rs (Z = -3.086, P = 0.002), x-Ea (Z = -2.848, P = 0.004) and x-Rt (Z = -4.731, P < 0.001) Tadpoles exposed to extracts of conspecifics, as well as heterospecific extracts of both poison dart frog species (R. sirensis, E. anthonyi) showed decreased median response activity compared to the control group, indicating reduced activity. No significant difference in R. temporaria tadpole activity was found between the control and the group exposed to x-Bb.
In B. bufo a significant difference in tadpole activity was observed between the control group and the group exposed to x-Rt (Z = -2.989, P = 0.004). Tadpoles exposed to heterospecific R. temporaria extracts exhibited higher median response activity than the control group, indicating increased activity. No significant difference in B. bufo tadpole activity was observed between the control and the groups exposed to conspecific extracts of x-Bb and heterospecific extracts of x-Rs and x-Ea.
Short term changes in activity (response activity) in tadpoles of Ranitomeya sirensis, Epipedobates anthonyi, Rana temporaria and Bufo bufo after experiencing extracts of: R. sirensis (x-Rs), E. anthonyi (x-Ea), R. temporaria (x-Rt), B. bufo (x-Bb) or water (i.e. control) during behavioural trials. Trials with extracts from conspecific tadpoles are arranged next to the control (C) on the left side, trials with heterospecific extracts on the right side of each panel. ***P < 0.001, ** < P 0.01, in post hoc Mann-Whitney U pairwise comparisons of treatments vs. control (alpha value adjusted for four comparisons per species; P = 0.0125)
Discussion
In this study we focused on the perception and responses to injury cues (alarm cues) in dendrobatid tadpoles, specifically emphasizing Ranitomeya sirensis and Epipedobates anthonyi. Additionally, we broaden our study to include tadpoles and injury cues from Rana temporaria and Bufo bufo, enabling us to explore the perception of heterospecific responses among all the aforementioned species, each with distinct ecological niches and degrees of sociality. We hypothesized diverse responses to con- and heterospecific injury cues among tadpoles, with variations attributable to differences in their degree of sociality, habitat constraints and phylogenetic relationships.
Our results diverge from the predictions made in hypotheses (Hy1), (Hy2), and (Hy3b). Contrary to expectations, R. sirensis tadpoles increased their activity when exposed to conspecific injury cues. Furthermore, E. anthonyi and B. bufo tadpoles did not show reduced activity levels as anticipated in response to con- and heterospecific cues (with the exception of increased activity of B. bufo tadpoles towards R. temporaria cues). Our findings do, however, support hypothesis (Hy3a), as R. temporaria tadpoles exhibited reduced activity in response to both con- and heterospecific cues, with varying levels of intensity.
Tadpole response - Ranitomeya sirensis
Contrary to our prediction (Hy1), R. sirensis tadpoles increased not decreased their activity towards conspecific injury cues. The increased activity towards conspecific injury cues may suggest a chemically mediated agitation or cannibalistic behavioural response (potentially related to feeding behaviour; Spieler and Linsenmair 1999; Hagman 2008), considering the species’ solitary lifestyle and latent aggressive behaviour towards conspecific competitors (personal observation). Previous studies have reported facultative, opportunistic cannibalism in several genera of poison dart frogs (Caldwell and De Araújo 1998; Gray et al. 2009; Rojas 2014; Dugas et al. 2016), including Ranitomeya (Summers 1999; Summers and Symula 2001; Poelman and Dicke 2007; Schulte et al. 2011; Brown et al. 2011; Schulte 2014). Meaning that due to the risk of reducing inclusive fitness by consuming close relatives, many tadpoles consume only conspecifics that are already deceased or weakened. The evolution of such behaviour in dendrobatids is likely driven by increased parental investments and limited resources for tadpoles in their environment (Caldwell and De Araújo 1998; Summers 1999; Summers and McKeon 2004; Carvajal-Castro et al. 2021). Since injury cues are involuntary released substances during predation events (Hettyey et al. 2015; Wisenden 2015) and likely associated with potential nutrient-rich carcasses, the perception of these stimuli likely triggers an altered perception of increased nutrient availability in facultative cannibalistic tadpoles. This, in turn, induces feeding behaviour, which is associated with increased levels of activity.
Surprisingly, however, R. sirensis tadpoles did not respond to heterospecific injury cues, raising the question of the adaptive advantage of this discriminatory behaviour towards injury cues. If aggressive behaviour enhances survivorship and reproductive output, aggressive cannibalism, as seems to be the case in R. sirensis, may be favoured by natural selection (Bobisud 1976; Jones 1982; Stenseth 1985). This is because traits accelerating tadpole development or increasing body size at metamorphosis can confer advantages such as earlier reproductive maturity or larger body size at maturity (Wilbur 1980; Werner 1986; Smith 1987), rendering in-discriminatory elimination of competitors (Crump 1992) and potential predators (Caldwell and De Araújo 1998) a beneficial fitness increasing trait (Bobisud 1976; Jones 1982; Stenseth 1985). As many poison dart frog species deploy their offspring individually and often actively avoid phytotelmata with conspecific competitors (Brust 1993; Caldwell and De Araújo 1998; Summers 1999; Poelman and Dicke 2007), the potential cost of reducing their inclusive fitness, should become minimal, thus facilitating the evolution of in-discriminatory aggression or cannibalism even more. Our results, however, suggest a specialised recognition system that targets conspecific competitors and might be the results of an isolated evolutionary history mediated by selective individual tadpole deposition in unoccupied phytotelmata (Brust 1993; Caldwell and De Araújo 1998; Summers 1999; Poelman and Dicke 2007; Schulte et al. 2011) that left tadpoles of R. sirensis unable to perceive or interpret heterospecific cues appropriately (but see Schulte and Lötters 2014). Although evidence suggests that conspecific cannibalism may provide greater benefits compared to consuming heterospecifics due to proper proportion of nutrients (Nagai et al. 1971; Meffe and Crump 1987; Crump 1990; Wildy et al. 1998), future studies should delve deeper into the factors influencing these responses (e.g. naturally occurring heterospecific interactions among dendrobatids).
Tadpole response - Epipedobates anthonyi
Contrary to our hypothesis (Hy2), E. anthonyi tadpoles did not exhibit any observable behavioural response to the tested injury cues (i.e. neither con- nor heterospecific). Our results suggest that chemical cues associated with acute predation events do not mediate anti-predator responses in this species. A potential reason would be associated with the information transmission characteristics. While chemical cues possess advantageous long-lasting properties that facilitate the perception and fine-tuning of anti-predator mechanisms, their information transmission may be insufficient (i.e. too slow and non-directional) compared to other modalities such as acoustic and visual cues (Bradbury and Vehrencamp 2011). This limitation could reduce chemical communication’s utility and adaptive benefits in anti-predator contexts in small water bodies, especially in situations with rapidly escalating predator-prey interactions. In contrast, the rapid transmission of information within a short timeframe becomes crucial in spatially limited environments, such as puddles or low-current bays in rivers. However, evidence suggests that tadpoles have poor vision (Manteuffel et al. 1977; Hoff et al. 1999; Eluvathingal et al. 2009), which can be further impaired in murky, turbulent, or densely vegetated water bodies where tadpoles are commonly found (McDiarmid and Altig 1999; Wells 2007). Indeed, E. anthonyi tadpoles appeared unaffected by approaching shrimp nets, shadows, or movements just above the water level until close proximity (personal observation).
In spatially limited aquatic environments, like puddles or low-current bays in tropical rainforests, alternative modalities such as short-range communication may play a more significant role. Unlike chemical cues, stimuli like touch and hydrodynamic cues can rapidly convey information and are highly effective over short distances, particularly when vision is impaired (Bradbury and Vehrencamp 2011). Similar to chemical cues, these cues possess advantageous properties and are vital for many aquatic predators and prey organisms (Bradbury and Vehrencamp 2011). Generating hydrodynamic cues, including currents, vortices, and surface disturbances, is inherent to locomotion in predominantly aquatic organisms (i.e. involuntary generated) and can provide valuable information to prey animals about the size, speed, and shape of the generating organism (Hanke et al. 2000; Coombs 2001). In fact, numerous aquatic organisms rely on hydrodynamic cues to track prey (Bleckmann 1994; Dehnhardt et al. 1998, 2001; Pohlmann et al. 2001, 2004; Schulte-Pelkum et al. 2007) or detect nearby predators and navigate their environments through hydrodynamic communication (Hassan 1986; Budelmann and Bleckmann 1988; Gray and Denton 1991; Budelmann 1995; Coombs and Montgomery 1999; Mogdans et al. 2002; Burt De Perera 2004; Janssen and Strickler 2006; Platvoet et al. 2007; Bradbury and Vehrencamp 2011).
Given that tadpoles have well-developed lateral line systems (Lannoo 1987; McDiarmid and Altig 1999), and considering dendrobatid interactions in which visual and tactile cues are of more importance (e.g. mother offspring communication; Stynoski and Noble 2012), the potential involvement of short-range modalities in anti-predator mechanisms of E. anthonyi tadpoles remains plausible. Moreover, blind cave fish actively generate turbulences while moving and rely on distortions in reflected eddies and vortices to detect and warn conspecifics about obstacles in their proximity (Hassan 1986; Burt De Perera 2004), leaving room for speculation about sophisticated use and interpretation of hydrodynamic cues in E. anthonyi tadpoles. Likewise, there is the possibility that responses to avoid predation may have remained undetected within the confines of our experimental design (e.g. limited space with no options for tadpoles to escape or hide and undetected behavioural changes immediately after the introduction of cues in the stimulus phase). Anti-predator behaviour is often more intricate and may also manifest in spatial avoidance and increased hiding behaviour (Teplitsky and Laurila 2007; Eterovick et al. 2010; Szabo et al. 2021).
Both assumptions regarding the utilization of short-range modalities and more intricate behavioural changes are supported by observations during husbandry activities. In these instances, E. anthonyi individuals displayed heightened sensitivity to water body movements and surface disturbances. Tadpoles exhibited rapid, agile swimming responses when encountering unusual water movements or disturbances in close proximity, often resulting in shelter-seeking behaviour. Therefore, our results lead us to postulate that tadpoles of E. anthonyi employ distinct short-range modalities and behavioural responses to avoid predation, which may extend beyond activity adjustments and include behaviours such as rapid shelter-seeking. Consequently, further investigations are necessary to explore alternative sensory modalities and anti-predator strategies employed by E. anthonyi tadpoles in their natural environment.
Tadpole responses - Rana temporaria & Bufo bufo
Contrary to our expectations outlined in hypotheses (Hy3a) and (Hy3b), our study reveals notable inter-species differences between R. temporaria and B. bufo. Specifically, our results provide support only for hypothesis (H3a). Tadpoles of R. temporaria exhibited a reduced activity response to conspecific injury cues, consistent with previous research (Laurila 2000; Laurila et al. 2004; Marquis et al. 2004; Mandrillon and Saglio 2009; Hettyey et al. 2015), while demonstrating a milder reaction to heterospecific cues. In contrast (Hy3b), B. bufo tadpoles did not exhibit the expected reduction in activity in the presence of either conspecific or heterospecific cues, deviating from prior research findings with conspecific injury cues (Marquis et al. 2004; but see Rödin-Mörch et al. 2011). Instead, B. bufo tadpoles displayed an increase in activity when exposed to cues from R. temporaria.
Literature suggests that the phylogenetic relationship and shared evolutionary history among species contribute to heterospecific responses due to the presence of similar metabolites and specialised mixture compositions (Mirza and Chivers 2001; Dalesman et al. 2007; Ferrari et al. 2007, 2008a, 2009a; Ferland-Raymond and Murray 2008; Mitchell et al. 2012, 2017). Therefore, the diminished response of R. temporaria to heterospecific cues may be attributed to the phylogenetic distance between the species involved and the presence of phylogenetically conserved or convergent elements of injury cues mediating anti-predator behavioural responses (Ferrari et al. 2010). However, the evolution of injury cues remains largely unclear but may be facilitated through kin-selection (Ferrari et al. 2010; Chivers et al. 2012; see also Wisenden 2015 for a comprehesive list of hypothesis).
The responses of bufonid tadpoles to heterospecific injury cues have been shown to be quite variable in previous studies, ranging from “no response” (Hagman and Shine 2009), decreased activity (Summey and Mathis 1998; Hagman and Shine 2009), attraction/foraging (Petranka 1989; Hagman and Shine 2009), to avoidance behaviour (Hews and Blaustein 1985). In our experimental setup using cups, behavioural responses such as attraction/foraging and avoidance would translate into increased activity levels. This is because, increased movement may be utilized by feeding tadpoles to churn up food particles from the pond floor (Spieler and Linsenmair 1999; Hagman 2008), or as an attempt to escape the area (i.e. the cup) associated with elevated predation risk (Rödin-Mörch et al. 2011). Consequently, it remains uncertain whether the response exhibited by B. bufo tadpoles to injury cues from R. temporaria represents attraction to a food source or an escape strategy.
Between-species differences in the context of anti-predator behaviour between R. temporaria and B. bufo have previously been reported by Laurila et al. (1997). Although they investigated behavioural responses to different types of cues (i.e. predator-borne cues), their findings align with those of our study (i.e. no activity reduction in B. bufo tadpoles but observed reductions in R. temporaria tadpoles), and suggest that such differences may result from variations in species-specific predation risks as well as costs associated with anti-predator behaviour (e.g. energetic and opportunity costs; Lima and Dill 1990; Laurila et al. 1997; Lima and Bednekoff 1999; Ferrari et al. 2009b). Studies have shown that anti-predator behaviour, such as decreased activity, can lead to reduced growth rates and smaller size at metamorphosis (Holomuzki 1986; Skelly and Werner 1990; Skelly 1992), ultimately impacting lifelong reproductive success (Smith 1987; Semlitsch et al. 1988; Berven 1990; Scott 1994). Given that R. temporaria and B. bufo tadpoles can coexist in dense populations (Laurila et al. 1997; Bardsley and Beebee 1998; Nyström and Åbjörnsson 2000; personal observation of our study system), the expression of behavioural anti-predator behaviour might lower their competitive abilities. Therefore, maintaining high activity levels in B. bufo (Chovanec 1992), may be favoured to prevent a loss of competitive ability (Laurila et al. 1997). Furthermore, B. bufo possesses inherent physiological defence mechanisms (i.e. noxious skin compounds; Flier et al. 1980) that render it unpalatable to certain predators (Griffiths and Denton 1992; Semlitsch and Gavasso 1992). In contrast, R. temporaria lacks such additional defence mechanisms and therefore might face a generally higher risk of consumption by predators compared to B. bufo (Álvarez and Nicieza 2009). This disparate predation risk could explain why B. bufo maintains its activity levels while R temporaria exhibits anti-predator behaviour (Laurila et al. 1997).
Study limitations
Given tadpoles in this study were all predator naïve, our results point towards innate response and thus do not account for potential undetected behavioural responses mediated by learning (i.e. past experiences with additional predator-borne cues; Suboski 1990; Mirza and Chivers 2000; Dalesman et al. 2006; Mirza et al. 2006; Ferrari et al. 2010). Moreover, although prey-borne injury cues can elicit behavioural responses in isolation (e.g. Marquis et al. 2004; Fraker et al. 2009; Rödin-Mörch et al. 2011; Hettyey et al. 2015), evidence suggests that additional presence of predator kairomones may be necessary for a complete manifestation of short term induced defences (Petranka and Hayes 1998; van Buskirk and Arioli 2002; Schoeppner and Relyea 2005, 2009; Richardson 2006; Hettyey et al. 2010, 2015; Hemnani et al. 2022). Future research should therefore also strongly consider including predator odours and diet cues (Mitchell et al. 2017), to be able to formulate more reliable conclusions about chemically induced short term anti-predator mechanisms in poison dart frog tadpoles (e.g. Szabo et al. 2021).
Lastly, our study is limited to conclusions about individual tadpole responses. In fish, predator avoidance behaviour triggered by injury cues can be influenced by group size (Surova et al. 2009) and disturbance cues can lead to tighter group cohesion and increased coordination (Bairos-Novak et al. 2019; Crane et al. 2020). Given that tadpoles also exhibit group formation based on familiarity or kinship (Waldman and Adler 1979; Halverson et al. 2006; Eluvathingal et al. 2009; Pizzatto et al. 2016), tadpoles of socially oriented species involved in our study (i.e. E. anthonyi, R. temporaria, and B. bufo) may demonstrate different group-level responses compared to individual responses. Future research should therefore incorporate more complex bioassays (e.g. testing groups of tadpoles) and deploy more complex quantitative measures to elucidate the full range of anti-predator responses in these species. Particularly exploring the degree of sociality of E. anthonyi and the extent of their gregarious behaviour would provide valuable insights into their microhabitat preferences and potential kin associations mechanisms (e.g. attraction to conspecifics vs. repulsion from heterospecifics; Eluvathingal et al. 2009).
Conclusion
Our study provides preliminary insights into the chemical alarm cue response system of Neotropical poison dart frog tadpoles in comparison to species inhabiting temperate climates. In contrast to species such as ranids and bufonids, which exhibit social behaviour and inhabit large permanent pools characterised by intricate ecological communities and significant predator pressure, dendrobatid tadpoles, residing solitarily or in small sibling groups within small or ephemeral pools, solely responded to conspecific cues or displayed no response to chemical cues altogether. Being the first investigation of perception and responses to con- and heterospecific injury cues (alarm cues) in dendrobatid tadpoles, this research prompts new questions and ideas regarding the ecological significance and evolution of chemical communication in species facing limited resources and reduced predator pressure during development. Further investigations are needed to explore the factors influencing these responses, investigate alternative sensory modalities, and elucidate the anti-predator strategies employed by different tadpole species in their natural environments. Moreover, our study underscores the necessity of comparative research for a comprehensive understanding of chemical communication in different aquatic ecosystems and developing new ideas for the evolution of chemical alarm systems and the substances involved in conveying information’s about environmental risks.
Data Availability
Upon acceptance the data underlying this article is available on request from the corresponding author.
References
Achtymichuk GH, Crane AL, Simko OM, Stevens HEF, Ferrari MCO (2022) The choice of euthanasia techniques can affect experimental results in aquatic behavioural studies. Anim Behav 185:1–8. https://doi.org/10.1016/j.anbehav.2021.11.013
Álvarez D, Nicieza AG (2009) Differential success of prey escaping predators: tadpole vulnerability or predator selection? Copeia 2009:453–457. https://doi.org/10.1643/CE-08-105
Bairos-Novak KR, Ferrari MCO, Chivers DP (2019) A novel alarm signal in aquatic prey: familiar minnows coordinate group defences against predators through chemical disturbance cues. J Anim Ecol 88:1281–1290. https://doi.org/10.1111/1365-2656.12986
Bairos-Novak KR, Crane AL, Achtymichuk GH et al (2020) Forget the audience: tadpoles release similar disturbance cues regardless of kinship or familiarity. Behav Ecol Sociobiol 74:147. https://doi.org/10.1007/s00265-020-02936-8
Bardsley L, Beebee TJC (1998) Interspecific competition between larvae is not an important structuring force in mixed communities of Rana and Bufo on an English sand-dune system. Ecography (Cop) 21:449–456. https://doi.org/10.1111/j.1600-0587.1998.tb00435.x
Berven KA (1990) Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71:1599–1608. https://doi.org/10.2307/1938295
Bleckmann H (1994) Reception of hydrodynamic stimuli in aquatic and semiaquatic animals. Gustav Fischer Verlag, Stuttgart
Bobisud LE (1976) Cannibalism as an evolutionary strategy. Bull Math Biol 38:359–368. https://doi.org/10.1007/BF02462211
Bradbury JW, Vehrencamp SL (2011) Principles of Animal Communication, 2nd edn. Oxford University Press Inc, New York
Brönmark C, Hansson L-A (2012) Chemical ecology in aquatic systems, 1st edn. Oxford University Press Inc, New York, NY, US
Brown JL, Twomey E, Amézquita A, de Souza BM, Caldwell JP, Lötters S, von May RS, Sampaio PRM, Vargas DM, Pena PP, Pepper M, Poelmann EH, Rodriguez MS, Summers K (2011) A taxonomic revision of the neotropical Poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083:1. https://doi.org/10.11646/zootaxa.3083.1.1
Brust DG (1993) Maternal brood care by Dendrobates pumilio: a frog that feeds its young. J Herpetol 27:96–98
Budelmann BU (1995) Cephalopod sense organs, nerves and the brain: adaptations for high performance and life style. Mar Freshw Behav Physiol 25:13–33. https://doi.org/10.1080/10236249409378905
Budelmann BU, Bleckmann H (1988) A lateral line analogue in cephalopods: water waves generate microphonic potentials in the epidermal head lines of Sepia and Lolliguncula. J Comp Physiol A 164:1–5. https://doi.org/10.1007/BF00612711
Caballero-Díaz C, Arribas R, Polo-Cavia N (2023) Assessment of predation risk through conspecific cues by anuran larvae. Anim Cogn 26:1431–1441. https://doi.org/10.1007/s10071-023-01793-y
Caldwell JP, De Araújo MC (1998) Cannibalistic interactions resulting from indiscriminate predatory behavior in tadpoles of Poison frogs (Anura: Dendrobatidae). Biotropica 30:92–103. https://doi.org/10.1111/j.1744-7429.1998.tb00372.x
Carlson BE, Newman JC, Langkilde T (2015) Food or fear: hunger modifies responses to injured conspecifics in tadpoles. Hydrobiologia 743:299–308. https://doi.org/10.1007/s10750-014-2048-5
Carvajal-Castro JD, Vargas-Salinas F, Casas-Cardona S, Casas-Cardona S, Rojas B, Santos JC (2021) Aposematism facilitates the diversification of parental care strategies in Poison frogs. Sci Rep 11:19047. https://doi.org/10.1038/s41598-021-97206-6
Chivers DP, Kiesecker JM, Marco A et al (2001) Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92:135–142. https://doi.org/10.1034/j.1600-0706.2001.920116.x
Chivers DP, Brown GE, Ferrari MCO (2012) The evolution of alarm substances and disturbance cues in aquatic animals. In: Brönmark C, Hansson L-A (eds) Chemical Ecology in aquatic systems, 1st edn. Oxford University Press Inc, New York, NY, US, pp 127–139
Chovanec A (1992) The influence of tadpole swimming behaviour on predation by dragonfly nymphs. Amphibia-Reptilia 13:341–349. https://doi.org/10.1163/156853892X00049
Coombs S (2001) Smart skins: information processing by lateral line flow sensors. Auton Robots 11:255–261. https://doi.org/10.1023/A:1012491007495
Coombs S, Montgomery JC (1999) The enigmatic lateral line system. In: Fay RR, Popper AN (eds) Comparative hearing: Fish and amphibians. Springer-Verlag, New York:, pp 319–362
Crane AL, Feyten LEA, Ramnarine IW, Brown GE (2020) High-risk environments promote chemical disturbance signalling among socially familiar Trinidadian guppies. Oecologia 193:89–95. https://doi.org/10.1007/s00442-020-04652-6
Crane AL, Achtymichuk GH, Rivera-Hernández IAE et al (2023) Uncertainty about old information results in differential predator memory in tadpoles. Proc R Soc B Biol Sci 290. https://doi.org/10.1098/rspb.2023.0746
Crossland MR, Salim AA, Capon RJ, Shine R (2019) The effects of conspecific alarm cues on larval cane toads (Rhinella marina). J Chem Ecol 45:838–848. https://doi.org/10.1007/s10886-019-01111-2
Crump ML (1990) Possible enhancement of growth in tadpoles through cannibalism. Copeia 2:560–564. https://doi.org/10.2307/1446361
Crump ML (1992) Cannibalism in amphibians. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, pp 256–276
Dalesman S, Rundle SD, Coleman RA, Cotton PA (2006) Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Anim Behav 71:789–797. https://doi.org/10.1016/j.anbehav.2005.05.028
Dalesman S, Rundle SD, Bilton DT, Cotton PA (2007) Phylogenetic relatedness and ecological interactions determine antipredator behavior. Ecology 88:2462–2467. https://doi.org/10.1890/07-0403.1
Davies NB, Krebs JR, West SA (2012) An introduction to behavioural ecology, 4th edn. John Wiley & Sons, Chichister
Burt De Perera T (2004) Spatial parameters encoded in the spatial map of the blind Mexican cave fish, Astyanax fasciatus. Anim Behav 68:291–295. https://doi.org/10.1016/j.anbehav.2003.11.009
Dehnhardt G, Mauck B, Bleckmann H (1998) Seal whiskers detect water movements. Nature 394:5–6. https://doi.org/10.1038/28303
Dehnhardt G, Mauck B, Hanke W, Bleckmann H (2001) Hydrodynamic trail-following in harbor seals (Phoca vitulina). Sci (80-) 293:102–104. https://doi.org/10.1126/science.1060514
Duellman WE, Trueb L (1994) Biology of amphibians. The Johns Hopkins University Press, Baltimore and London
Dugas MB, Stynoski J, Strickler SA (2016) Larval aggression is Independent of food limitation in nurseries of a Poison frog. Behav Ecol Sociobiol 70:1389–1395. https://doi.org/10.1007/s00265-016-2148-5
Eluvathingal LM, Shanbhag BA, Saidapur SK (2009) Association preference and mechanism of kin recognition in tadpoles of the toad Bufo melanostictus. J Biosci 34:435–444. https://doi.org/10.1007/s12038-009-0050-2
Epp KJ, Gabor CR (2008) Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114:607–615. https://doi.org/10.1111/j.1439-0310.2008.01494.x
Eterovick PC, Oliveira FFR, Tattersall GJ (2010) Threatened tadpoles of Bokermannohyla alvarengai (Anura: Hylidae) choose backgrounds that enhance crypsis potential. Biol J Linn Soc 101:437–446. https://doi.org/10.1111/j.1095-8312.2010.01501.x
Fendt M (2006) Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol 32:2617–2627. https://doi.org/10.1007/s10886-006-9186-9
Ferland-Raymond B, Murray DL (2008) Predator diet and prey adaptive responses: can tadpoles distinguish between predators feeding on congeneric vs. conspecific prey? Can J Zool 86:1329–1336. https://doi.org/10.1139/Z08-117
Ferrari MCO, Messier F, Chivers DP (2006) The nose knows: minnows determine predator proximity and density through detection of predator odours. Anim Behav 72:927–932. https://doi.org/10.1016/j.anbehav.2006.03.001
Ferrari MCO, Gonzalo A, Messier F, Chivers DP (2007) Generalization of learned predator recognition: an experimental test and framework for future studies. Proc R Soc B Biol Sci 274:1853–1859. https://doi.org/10.1098/rspb.2007.0297
Ferrari MC, Messier F, Chivers DP (2008a) Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc R Soc B Biol Sci 275:1811–1816. https://doi.org/10.1098/rspb.2008.0305
Ferrari MCO, Messier F, Chivers DP (2008b) Degradation of chemical alarm cues under natural conditions: risk assessment by larval woodfrogs. Chemoecology 17:263–266. https://doi.org/10.1007/s00049-007-0381-0
Ferrari MCO, Brown GE, Messier F, Chivers DP (2009a) Threat-sensitive generalization of predator recognition by larval amphibians. Behav Ecol Sociobiol 63:1369–1375. https://doi.org/10.1007/s00265-009-0779-5
Ferrari MCO, Sih A, Chivers DP (2009b) The paradox of risk allocation: a review and prospectus. Anim Behav 78:579–585. https://doi.org/10.1016/j.anbehav.2009.05.034
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724. https://doi.org/10.1139/Z10-029
Flier J, Edwards MW, Daly JW, Myers CW (1980) Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Sci (80-) 208:503–505. https://doi.org/10.1126/science.6245447
Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J, Denver RJ (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529. https://doi.org/10.1016/j.yhbeh.2009.01.007
Gazzola A, Brandalise F, Rubolini D, Rossi P, Galeotti P (2015) Fear is the mother of invention: anuran embryos exposed to predator cues alter life-history traits, post-hatching behaviour and neuronal activity patterns. J Exp Biol 218:3919–3930. https://doi.org/10.1242/jeb.126334
Glandt D (2018) Praxisleitfaden Amphibien- Und Reptilienschutz. Springer Berlin Heidelberg, Berlin, Heidelberg
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Museum Nat Hist 0090:1–262. https://doi.org/10.1206/0003-0090(2006)299[1:PSODFA]2.0.CO;2
Gray JAB, Denton EJ (1991) Fast pressure pulses and communication between fish. J Mar Biol Assoc United Kingdom 71:83–106. https://doi.org/10.1017/S0025315400037413
Gray HM, Summers K, Ibáñez DR (2009) Kin discrimination in cannibalistic tadpoles of the green Poison frog, Dendrobates auratus. Dendrobatidae) Phyllomedusa 8:41–50. https://doi.org/10.11606/issn.2316-9079.v8i1p41-50. Anura
Greeney HF (2001) The insects of plant-held waters: a review and bibliography. J Trop Ecol 17:241–260. https://doi.org/10.1017/S026646740100116X
Griffiths RA, Denton J (1992) Interspecific associations in tadpoles. Anim Behav 44:1153–1157. https://doi.org/10.1016/S0003-3472(05)80327-6
Hagman M (2008) Behavioral responses by tadpoles of six Australian species to chemical cues from other tadpoles. Herpetol Conserv Biol 3:239–246
Hagman M, Shine R (2008) Understanding the toad code: behavioural responses of cane toad (Chaunus marinus) larvae and metamorphs to chemical cues. Austral Ecol 33:37–44. https://doi.org/10.1111/j.1442-9993.2007.01788.x
Hagman M, Shine R (2009) Species-specific communication systems in an introduced toad compared with native frogs in Australia. Aquat Conserv Mar Freshw Ecosyst 19:724–728. https://doi.org/10.1002/aqc.1045
Halverson MA, Skelly DK, Caccone A (2006) Kin distribution of amphibian larvae in the wild. Mol Ecol 15:1139–1145. https://doi.org/10.1111/j.1365-294X.2006.02819.x
Hanke W, Brücker C, Bleckmann H (2000) The ageing of the low-frequency water disturbances caused by swimming goldfish and its possible relevance to prey detection. J Exp Biol 203:1193–1200. https://doi.org/10.1242/jeb.203.7.1193
Hänninen L, Pastell M (2009) CowLog: open-source software for coding behaviors from digital video. Behav Res Methods 41:472–476. https://doi.org/10.3758/BRM.41.2.472
Hassan ES (1986) On the discrimination of spatial intervals by the blind cave fish (Anoptichthys jordani). J Comp Physiol A 159:701–710. https://doi.org/10.1007/BF00612042
Hawkins LA, Magurran AE, Armstrong JD (2007) Innate abilities to distinguish between predator species and cue concentration in atlantic salmon. Anim Behav 73:1051–1057. https://doi.org/10.1016/j.anbehav.2006.08.011
Hemnani M, Guimarães ISC, Kaefer IL, Pires THdaS (2022) Alarm reaction depends on multiple chemical cues in tadpoles of the cane toad (Rhinella marina). Ethol Ecol Evol 35:363–375. https://doi.org/10.1080/03949370.2022.2082537
Hettyey A, Tóth Z, Thonhauser KE, Frommen JG, Penn DJ, van Buskirk J (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710. https://doi.org/10.1007/s00442-015-3382-7
Hettyey A, Zsarnóczai S, Vincze K, Hoi H, Laurila A (2010) Interactions between the information content of different chemical cues affect induced defences in tadpoles. Oikos 119:1814–1822. https://doi.org/10.1111/j.1600-0706.2010.18563.x
Hews DK, Blaustein AR (1985) An investigation of the alarm response in Bufo boreas and Rana cascadae tadpoles. Behav Neural Biol 43:47–57. https://doi.org/10.1016/S0163-1047(85)91482-7
Hoff KV, Blaustein AR, Mcdiarmid RW, Altig R (1999) Behavior - interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles: Biology of Anuran Larvae. University of Chicago Press, Chicago, IL, pp 215–239
Holomuzki JR (1986) Effect of microhabitat on fitness components of larval tiger salamanders, Ambystoma tigrinum nebulosum. Oecologia 71:142–148. https://doi.org/10.1007/BF00377334
Hossie TJ, Ferland-Raymond B, Burness G, Murray DL (2010) Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Écoscience 17:100–108. https://doi.org/10.2980/17-1-3312
Hossie T, Landolt K, Murray DL (2016) Determinants and co-expression of anti-predator responses in amphibian tadpoles: a meta-analysis. Oikos 126:173–184. https://doi.org/10.1111/oik.03305
Ireland DH, Wirsing AJ, Murray DL (2007) Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152:162–168. https://doi.org/10.1007/s00442-006-0637-3
Janssen J, Strickler JR (2006) Hydromechanical communication via the lateral line: copepodology for the ichthyologist. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes, 1st edn. Science Publishers, Enfield, NH, pp 207–222
Jones JS (1982) On cannibals and kin. Nature 299:202–203
Kahn TR, Lötters S, La Marca E, Brown JL, Twomney E, Amezquita A (eds) (2016) Aposematic Poison frogs (Dendrobatidae) of the andean countries: Bolivia, Colombia, Ecuador, Perú and Venezuela. Conservation International
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394. https://doi.org/10.1080/11956860.1998.11682468
Lannoo MJ (1987) Neuromast topography in anuran amphibians. J Morphol 191:115–129. https://doi.org/10.1002/jmor.1051910203
Lardner B (2000) Morphological and life history responses to predators in larvae of seven anurans. Oikos 88:169–180. https://doi.org/10.1034/j.1600-0706.2000.880119.x
Lau MJ, Wilson CC, Neff BD (2021) Innate and learned predator recognition across populations of atlantic salmon, Salmo salar. Ethology 127:563–571. https://doi.org/10.1111/eth.13163
Laurila A (2000) Behavioural responses to predator chemical cues and local variation in antipredator performance in Rana temporaria tadpoles. Oikos 88:159–168
Laurila A, Kujasalo J, Ranta E (1997) Different behaviour antipredator in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40:329–336
Laurila A, Järvi-laturi M, Pakkasmaa S, Merilä J (2004) Temporal variation in predation risk: stage-dependency, graded defences and fitness costs in tadpole antipredator responses. Oikos 107:90–99
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659. https://doi.org/10.1086/303202
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losey GS, Hugie DM (1994) Prior anesthesia impairs a chemically mediated fright response in a gobiid fish. J Chem Ecol 20:1877–1883. https://doi.org/10.1007/BF02066229
Lötters S, Jungfer K-H, Henkel FW, Schmidt W (2007) Pfeilgiftfrösche: Biologie, Haltung, Arten, 1st edn. Chimaira, Frankfurt am Main, Germany
Lucon-Xiccato T (2019a) Chemical alarm cues allow prey to adjust their defensive behaviour to cover abundance. Behav Processes 162:86–89. https://doi.org/10.1016/j.beproc.2019.02.004
Lucon-Xiccato T (2019b) Tadpoles modulate antipredator responses according to the abundance of vegetation experienced during the embryonic stage. J Zool 308:259–265. https://doi.org/10.1111/jzo.12671
Maag N, Gehrer L, Woodhams DC (2012) Sink or swim: a test of tadpole behavioral responses to predator cues and potential alarm pheromones from skin secretions. J Comp Physiol A Neuroethol Sensory Neural Behav Physiol 198:841–846. https://doi.org/10.1007/s00359-012-0750-1
Maan ME, Cummings ME (2012) Poison frog colors are honest signals of toxicity, particularly for bird redators. Am Nat 179:E1–E14. https://doi.org/10.1086/663197
Mandrillon AL, Saglio P (2009) Effects of single and combined embryonic exposures to herbicide and conspecific chemical alarm cues on hatching and larval traits in the common frog (Rana temporaria). Arch Environ Contam Toxicol 56:566–576. https://doi.org/10.1007/s00244-008-9196-4
Manteuffel G, Wess O, Himstedt W (1977) Messungen am dioptrischen Apparat Von Amphibienaugen und Berechnung Der Sehrschärfe Im Wasser Und Luft. Zool Jahrbücher 81:395–406
Marquis O, Saglio P, Neveu A (2004) Effects of predators and conspecific chemical cues on the swimming activity of Rana temporaria and Bufo bufo tadpoles. Arch für Hydrobiol 160:153–170. https://doi.org/10.1127/0003-9136/2004/0160-0153
McDiarmid RW, Altig R (eds) (1999) Tadpoles: the biology of anuran larvae, 2nd edn. University of Chicago Press
McIntyre PB, Baldwin S, Flecker AS (2004) Effects of behavioral and morphological plasticity on risk of predation in a neotropical tadpole. Oecologia 141:130–138. https://doi.org/10.1007/s00442-004-1652-x
McKeon CS, Summers K (2013) Predator driven reproductive behavior in a tropical frog. Evol Ecol 27:725–737. https://doi.org/10.1007/s10682-013-9641-3
Meffe GK, Crump ML (1987) Possible growth and reproductive benefits of cannibalism in the mosquitofish. Am Nat 129:203–212. https://doi.org/10.1086/284630
Middlemis Maher J, Werner EE, Denver RJ (2013) Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc R Soc B Biol Sci 280:20123075. https://doi.org/10.1098/rspb.2012.3075
Mirza RS, Chivers DP (2000) Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can J Zool 78:2198–2208. https://doi.org/10.1139/cjz-78-12-2198
Mirza RS, Chivers DP (2001) Are chemical alarm cues conserved within salmonid fishes? J Chem Ecol 27:1641–1655. https://doi.org/10.1023/A:1010414426082
Mirza RS, Ferrari MCO, Kiesecker JM, Chivers DP (2006) Responses of American toad tadpoles to predation cues: behavioural response thresholds, threat-sensitivity and acquired predation recognition. Behaviour 143:877–889. https://doi.org/10.1163/156853906778017926
Mitchell MD, Cowman PF, McCormick MI (2012) Chemical alarm cues are conserved within the coral reef fish family Pomacentridae. PLoS ONE 7:E47428. https://doi.org/10.1371/journal.pone.0047428
Mitchell MD, Bairos-Novak KR, Ferrari MCO (2017) Mechanisms underlying the control of responses to predator odours in aquatic prey. J Exp Biol 220:1937–1946. https://doi.org/10.1242/jeb.135137
Mogdans J, Barenbrock J, Bleckmann H (2002) Sighted topminnows, Aplocheilus lineatus, use the lateral line for surface wave discrimination. Copeia 2002:190–194. https://doi.org/10.1643/0045-8511(2002)002[0190:stalut]2.0.co;2
Nagai Y, Nagai S, ichi, Nishikawa T (1971) The nutritional efficiency of cannibalism and an artificial feed for the growth of tadpoles of Japanese toad (Bufo vulgaris Sp). Agric Biol Chem 35:697–703. https://doi.org/10.1271/bbb1961.35.697
Nunes AL, Richter-Boix A, Laurila A, Rebelo R (2013) Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171:115–127. https://doi.org/10.1007/s00442-012-2389-6
Nyström P, Åbjörnsson K (2000) Effects of fish chemical cues on the interactions between tadpoles and crayfish. Oikos 88:181–190. https://doi.org/10.1034/j.1600-0706.2000.880120.x
Petranka J, Hayes L (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 42:263–271. https://doi.org/10.1007/s002650050438
Petranka J, Sep N (1989) Response of toad tadpoles to conflicting chemical stimuli: predator avoidance versus optimal foraging. Herpetologica 45:283–292
Pizzatto L, Stockwell M, Clulow S, Clulow J, Mahony M (2016) How to form a group: effects of heterospecifics, kinship and familiarity in the grouping preference of green and golden bell frog tadpoles. Herpetol J 26:157–164
Platvoet D, Song Y, Li S, van der Velde G (2007) Description of the lateral line grgan of Dikerogammarus Villosus (Sowinsky, 1894), with discussion on its function (Peracarida, Amphipoda) Amphipod Pilot species Project (Ampis) — report 4. Crustaceana 80:1373–1392. https://doi.org/10.1163/156854007782605619
Poelman EH, Dicke M (2007) Offering offspring as food to cannibals: oviposition strategies of amazonian Poison frogs (Dendrobates ventrimaculatus). Evol Ecol 21:215–227. https://doi.org/10.1007/s10682-006-9000-8
Pohlmann K, Grasso FW, Breithaupt T (2001) Tracking wakes: the nocturnal predatory strategy of piscivorous catfish. Proc Natl Acad Sci 98:7371–7374. https://doi.org/10.1073/pnas.121026298
Pohlmann K, Atema J, Breithaupt T (2004) The importance of the lateral line in nocturnal predation of piscivorous catfish. J Exp Biol 207:2971–2978. https://doi.org/10.1242/jeb.01129
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540. https://doi.org/10.2307/2679877
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test empirical test. Ecology 84:1827–1839. https://doi.org/10.1890/0012-9658(2003)084[1827:HPRTCP]2.0.CO;2
Relyea RA, Auld JR (2004) Having the guts to compete: how intestinal plasticity explains costs of inducible defences. Ecol Lett 7:869–875. https://doi.org/10.1111/j.1461-0248.2004.00645.x
Richardson JL (2006) Novel features of an inducible defense system in larval tree frogs (Hyla chrysoscelis). Ecology 87:780–787. https://doi.org/10.1890/05-0536
Rödin-Mörch P, Forsman A, Hagman M (2011) Taxonomic patterns of tadpole behavioral responses to alarm cues. In: Murray JL (ed) Frogs: Biology, Ecology and uses. Nova Science Publishers, New York, pp 123–140
Rojas B (2014) Strange parental decisions: fathers of the dyeing Poison frog deposit their tadpoles in pools occupied by large cannibals. Behav Ecol Sociobiol 68:551–559. https://doi.org/10.1007/s00265-013-1670-y
Saporito RA, Donnelly MA, Spande TF, Garraffo HM (2012) A review of chemical ecology in Poison frogs. Chemoecology 22:159–168. https://doi.org/10.1007/s00049-011-0088-0
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512. https://doi.org/10.1111/j.1461-0248.2005.00744.x
Schoeppner NM, Relyea RA (2009) Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences. Funct Ecol 23:1114–1121. https://doi.org/10.1111/j.1365-2435.2009.01578.x
Schulte LM (2014) Feeding or avoiding? Facultative egg feeding in a Peruvian Poison frog (Ranitomeya variabilis). Ethol Ecol Evol 26:58–68. https://doi.org/10.1080/03949370.2013.850453
Schulte LM, Lipkowski K, Abondano Almeida D (2023) Chemical communication and semiochemical recognition in frogs – from eggs to adults. In: Schaal, B., Rekow, D., Keller, K. & Damon F (ed) In Chemical Signals in Vertebrates 15, 1st edn. Springer, Cham. https://doi.org/10.1007/978-3-031-35159-4_5
Schulte LM, Lötters S (2014) A danger foreseen is a danger avoided: how chemical cues of different tadpoles influence parental decisions of a neotropical Poison frog. Anim Cogn 17:267–275. https://doi.org/10.1007/s10071-013-0659-2
Schulte LM, Ringler E, Rojas B, Stynoski JL (2020) Developments in amphibian parental care research: history, present advances, and future perspectives. Herpetol Monogr 34:71. https://doi.org/10.1655/HERPMONOGRAPHS-D-19-00002.1
Schulte LM, Summers K (2021) Who cares for the eggs? Analysis of egg attendance behaviour in Ranitomeya imitator, a Poison frog with biparental care. Behaviour 159:603–614. https://doi.org/10.1163/1568539X-bja10142
Schulte LM, Yeager J, Schulte R, Veith M, Werner P, Beck LA, Lötters S (2011) The smell of success: choice of larval rearing sites by means of chemical cues in a Peruvian Poison frog. Anim Behav 81:1147–1154. https://doi.org/10.1016/j.anbehav.2011.02.019
Schulte-Pelkum N, Wieskotten S, Hanke W, Dehnhardt G, Mauck B (2007) Tracking of biogenic hydrodynamic trails in harbour seals (Phoca vitulina). J Exp Biol 210:781–787. https://doi.org/10.1242/jeb.02708
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396. https://doi.org/10.2307/1937462
Semlitsch RD, Scott DE, Pechmann JHK, Pechmann HK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192. https://doi.org/10.2307/1943173
Semlitsch RDD, Gavasso S (1992). Behavioural responses of bufo bufo and bufo calamita tadpoles to chemical cues of vertebrate and invertebrate predators. Ethol Ecol Evol 4(2):165–173.https://doi.org/10.1080/08927014.1992.9525337
Skelly DK (1992) Field evidence for a cost of behavioral antipredator response in a larval Amphibian. Ecology 73:704–708. https://doi.org/10.2307/1940779
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322. https://doi.org/10.2307/1938642
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350. https://doi.org/10.2307/1939265
Spieler M, Linsenmair KE (1999) Aggregation behaviour of Bufo maculatus tadpoles as an antipredator mechanism. Ethology 105:665–686. https://doi.org/10.1046/j.1439-0310.1999.00446.x
Stenseth NC (1985) On the evolution of cannibalism. J Theor Biol 115:161–177. https://doi.org/10.1016/S0022-5193(85)80093-X
Stynoski JL, Noble VR (2012) To beg or to freeze: multimodal sensory integration directs behavior in a tadpole. Behav Ecol Sociobiol 66:191–199. https://doi.org/10.1007/s00265-011-1266-3
Suboski MD (1990) Releaser-induced recognition learning. Psychol Rev 97:271–284. https://doi.org/10.1037/0033-295X.97.2.271
Summers K (1999) The effects of cannibalism on amazonian Poison frog egg and tadpole deposition and survivorship in Heliconia axil pools. Oecologia 119:557–564. https://doi.org/10.1007/s004420050819
Summers K, McKeon CS (2004) The evolutionary ecology of phytotelmata use in neotropical Poison frogs. In: Lehtinen RM (ed) Ecology and Evolution of Phytotelm-breeding anurans. University of Michigan, Museum of Zoology, pp 55–73
Summers K, Symula R (2001) Cannibalism and kin discrimination in tadpoles of the amazonian Poison frog, Dendrobates ventrimaculatus, in the field. Herpetol J 11:17–21
Summers K, Tumulty J (2014) Parental care, sexual selection, and mating systems in neotropical Poison frogs. In: Macedo RH, Machado G (eds) Sexual selection - perspectives and models from the neotropics. Elsevier, pp 289–320
Summey MR, Mathis A (1998) Alarm responses to chemical stimuli from damaged conspecifics by larval anurans: tests of three neotropical species. Herpetologica 54:402–408
Surova GS, Mukhina TV, Bezryadnov DV (2009) Group effect on individual locomotor activity of common toad (Bufo bufo L.) and brown frog (Rana temporaria L.) tadpoles. Russ J Ecol 40:280–285. https://doi.org/10.1134/S1067413609040092
Szabo B, Mangione R, Rath M, Pasukonis A, Reber SA, Oh J, Ringler M, Ringler E (2021) Naive Poison frog tadpoles use bi-modal cues to avoid insect predators but not heterospecific predatory tadpoles. J Exp Biol 224. https://doi.org/10.1242/jeb.243647
Teplitsky C, Laurila A (2007) Flexible defense strategies: competition modifies investment in behavioral vs. morphological defenses. Ecology 88:1641–1646. https://doi.org/10.1890/06-1703.1
Teplitsky C, Plenet S, Lena J-P, Mermet N, Malet E, Joly P (2005) Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. J Evol Biol 18:180–190. https://doi.org/10.1111/j.1420-9101.2004.00790.x
van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489. https://doi.org/10.1046/j.1420-9101.2001.00282.x
van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580. https://doi.org/10.2307/3071977
van Buskirk J, Mccollum SA (2000) Functional mechanisms of an inducible defence in tadpoles: morphology and behaviour influence mortality risk from predation. J Evol Biol 13:336–347. https://doi.org/10.1046/j.1420-9101.2000.00173.x
van Buskirk J, Krügel A, Kunz J, Miss F, Stamm A (2014) The rate of degradation of chemical cues indicating predation risk: an experiment and review. Ethology 120:942–949. https://doi.org/10.1111/eth.12266
Waldman B, Adler K (1979) Toad tadpoles associate preferentially with siblings. Nature 282:611–613. https://doi.org/10.1038/282611a0
Walls JG (1994) Jewels of the rain forest - Poison frogs of the family Dendrobatidae. Tfh Pubns Inc, New York, US
Wells KD (2007) The ecology and behavior of amphibians, 1st edn. University of Chicago Press
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341. https://doi.org/10.1086/284565
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93. https://doi.org/10.1146/annurev.es.11.110180.000435
Wildy EL, Chivers DP, Kiesecker JM, Blaustein AR (1998) Cannibalism enhances growth in larval long-toed salamanders, (Ambystoma macrodactylum). J Herpetol 32:286. https://doi.org/10.2307/1565312
Wisenden BD (2015) Chemical cues that indicate risk of predation. In: Sorensen PW, Wisenden BD (eds) Fish Pheromones and related cues. John Wiley & Sons, Inc, Hoboken, NJ, pp 131–148
Acknowledgements
We gratefully acknowledge Carmen Meyer, Evan Twomey, and the animal caretaker team at the Biologicum, Goethe University Frankfurt, for their support in the animal room. We also thank David Wenzel for assisting with the experiments. Furthermore, we thank Dirk Höpfner, Rolf Weber and Thomas Mathias from the “Naturschutzbund Deutschland (NABU)” for their years long contribution in managing the collection sites and providing guidance at the start of every season. Lastly, we thank Birgit Szabo, Maria Thaker and an anonymous reviewer for their helpful comments on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors gratefully acknowledge the Wilhelm Peters Fond granted by the “Deutsche Gesellschaft für Herpetologie und Terrarienkunde (DGHT)” to support this study.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
KL & LMS developed the study idea. KL collected tadpole egg masses. DAA established the poison frog breeding colonies & KL the tadpole husbandry. KL and DAA established the research protocol and conducted experiments. KL extracted and analysed the data. KL and LMS conceptualised the framework and outline of this manuscript. KL took the lead in writing the manuscript. All authors provided critical feedback, helped finalising the manuscript, and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All co-authors have approved the manuscript for publication.
Ethics approval
All animal handling and research in this study followed the guidelines of the country where they were performed (Germany, Hesse) and were approved by the “Regierungspräsidium Darmstadt” (references: V54-19c20/21I-FR Biologicum & V54-19c18-FR/1022) and the “Hessisches Landesamt für Naturschutz, Umwelt und Geologie” (reference: N2-103s0601/2020-3-06).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lipkowski, K., Almeida, D.A. & Schulte, L.M. Perception of con- and heterospecific injury cues in tadpoles of dendrobatid, ranid and bufonid frogs (Anura). Evol Ecol 38, 37–59 (2024). https://doi.org/10.1007/s10682-023-10275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-023-10275-z