Abstract
Resource sharing within clonal plant networks can occur via the translocation of water, nutrients, and photoassimilates through rhizomes and stolons. Similar mechanisms may mediate the sharing of information (e.g., about herbivory or other environmental stressors) among ramets via molecular or biochemical signals. The storage of such information in belowground structures could facilitate the transmission of appropriate phenotypic responses across growing seasons in perennial species. However, few previous studies have explored the potential transfer of ecologically relevant information within such networks. This study addresses the effects of foliar herbivory and belowground overwintering on the growth and flowering, physical defenses, and herbivore resistance in the clonally spreading species Solanum carolinense L. (Solanaceae). We used rhizomes from inbred and outbred plants that were repeatedly exposed to feeding damage by Manduca sexta L. (Sphingidae) caterpillars and rhizomes from undamaged control plants. These rhizomes were either planted immediately or exposed to overwintering conditions and allowed to produce new ramets (rhizomatous offshoots). We then assessed offshoot emergence, flowering, physical defense traits, and herbivore performance. Relative to controls, offshoots of herbivore-damaged plants exhibited greater spine and trichome densities, and reduced performance of M. sexta larvae. However, they also emerged and flowered significantly later, and produced fewer flowers than offshoots of undamaged plants. Inbreeding also negatively affected offshoot emergence, flowering, trichome production, and herbivore resistance. These effects of parental herbivory were more pronounced in outbred offshoots, indicating that inbreeding may compromise the trans-seasonal induction of plant defenses. Finally, exposure to overwintering conditions increased trichome production and reduced caterpillar performance on offshoots. Together, these results show that induced defenses can be transmitted through rhizomes and affect offshoot growth, flowering, defensive traits, and herbivore resistance. They also document fitness-related costs associated with defense induction in offshoots and suggest that the transfer of defenses across seasons can be compromised by inbreeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbaceous perennial plant species often spread via runners (e.g., stolons and rhizomes) that grow laterally from the parent plant and can produce new shoots (Cook 1985). When such shoots are produced within a single growing season, it is well established that the parent plant can share resources such as water, carbohydrates, and mineral nutrients with its lateral shoots (de Kroon et al. 1996; Alpert and Stuefer 1997; Alpert et al. 2002; Liu et al. 2016). In addition, there is some evidence that inducible defense traits can be transmitted to interconnected lateral shoots (Stuefer et al. 2004; Gómez and Stuefer 2006; Gómez et al. 2007, 2010; Chen et al. 2011; Jelínková et al. 2012). Rhizomes also frequently function as the overwintering organs of herbaceous perennials, producing new shoots in the next growing season (Cook 1985; de Kroon and van Groenendael 1997) that typically are no longer connected to the parent plant and are therefore physiologically independent (Hutchings and Bradbury 1986; Kelly 1995). In principle, information stored in rhizomes could allow the trans-seasonal transmission of defensive states from parental plants to these new shoots; however, such effects have not previously been documented.
Herbivore damage is a ubiquitous threat to plant growth, development, and reproduction (Louda 1989; Marquis 1992; Crawley 1997; Strauss and Zangrel 2002), and interconnected clonal plants may be especially vulnerable to herbivorous insects due to their close proximity to genetically identical individuals that harbor similar pests (Lei 2010). In response to the threat of herbivory, plants have evolved a great diversity of physical and chemical defense mechanisms. These defenses can affect herbivores directly, by deterring feeding, impeding movement, and limiting access to the surface of plants (Levin 1973; Hanley et al. 2007; Howe and Schaller 2008; War et al. 2012; Kariyat et al. 2017), or indirectly, by attracting predators of the herbivores (Paré and Tumlinson 1999; Kessler and Baldwin 2001; Heil 2008, 2015). Furthermore, some defenses are constitutively expressed, while others are induced only in response to herbivore feeding (Karban and Myers 1989; Karban and Baldwin 1997). Many studies have shown that chewing insect herbivores induce indirect defenses such as volatile organic compounds that attract natural enemies of the herbivores (Paré and Tumlinson 1997; De Moraes et al. 1998), as well as direct defenses, such as chemical feeding deterrents, and mechanical defenses, such as spines and trichomes (Holeski 2007; Kariyat et al. 2013; Barton 2015).
Induced plant defenses against herbivores can be expressed systemically throughout the plant, and there is evidence that belowground organs can contribute to aboveground plant defenses against foliar herbivores (Bezemer and van Dam 2005; Erb 2012; Nalam et al. 2013). Furthermore, plants may respond to herbivory by reallocating resources from leaf tissues to belowground storage tissues (Kaplan et al. 2008; Orians et al. 2011). These belowground tissues are critical for the winter survival and next season regrowth of rhizomatous plants in temperate climates and frequently experience prolonged cold exposure during overwintering (Bertrand and Castonguay 2003), a process similar to seed vernalization. In many species, this prior exposure to a cold period has important implications for flowering (Kim et al. 2009) and dormancy (Chouard 1960; Brunner et al. 2014), plant resistance to abiotic stress (Palva et al. 2001; Rinne et al. 2010), and pathogen resistance (Plazek and Zur 2003; Kuwabara and Imai 2009; Gaudet et al. 2011; Moyer et al. 2016). However, the effects of overwintering on plant defenses against herbivores remain unexplored.
There is strong evidence that inbreeding often compromises plant defenses against herbivores (Carr and Eubanks 2014; Johnson et al. 2015). Specifically, inbred individuals frequently exhibit reduced levels of physical and chemical defenses (Delphia et al. 2009a; Kariyat et al. 2012a, 2013; Campbell et al. 2013), experience higher levels of damage from insect herbivores (Hayes et al. 2004; Stephenson et al. 2004; Delphia et al. 2009b; Bello-Bedoy and Núñez-Farfán 2010; Muola et al. 2011; Campbell et al. 2013), recruit fewer herbivore natural enemies upon attack (Kariyat et al. 2012a), and have weaker induction of defenses compared to outbred plants (Kariyat et al. 2012b; Leimu et al. 2012; Campbell et al. 2014). Insect herbivores feeding on inbred plants have also been shown to outperform those feeding on outbred plants (Leimu et al. 2008; Delphia et al. 2009b; Portman et al. 2014, 2015). In addition to these deleterious effects on plant defenses, inbreeding has been repeatedly shown to have severe consequences for plant growth, development, and reproductive success (Charlesworth and Charlesworth 1987; Husband and Schemske 1996).
Extensive research has addressed the effects of herbivory on plant fitness and defense-related traits; however, it is not known whether herbivore-induced defensive states can persist across growing seasons in belowground structures and be transmitted to new shoots that are otherwise physiologically independent of the parent plant. The current study addresses this question in the clonal species Solanum carolinense. In addition, we explore how such trans-seasonal effects are affected by the overwintering process itself and by plant inbreeding, which as noted above has previously been shown to have deleterious effects on plant defense induction, including in S. carolinense. To address these questions, we assessed offshoot emergence, flower production, physical defense traits, and herbivore performance on the rhizomatous offshoots of inbred and outbred S. carolinense plants that have and have not experienced herbivory.
Materials and methods
Study system
Solanum carolinense L. (Solanaceae) is an herbaceous perennial weed common throughout the eastern United States and southeastern Canada (Britton and Brown 1970). It is a pioneer species of early successional habitats, waste places, crop fields, and pastures. Once established, S. carolinense spreads via horizontal rhizomes that can extend a meter or more from the parent plant (Ilnicki et al. 1962). These rhizomes overwinter belowground, separate from the parent plant, and produce new shoots in the spring. The parent plant dies over the winter and does not send up new stems during the following spring. Consequently, the rhizomes are independent of the parent plant during the next growing season. Flowering begins during the summer and continues until the first hard frost. The flowers are buzz-pollinated by bumblebees and carpenter bees, which vibrate the anthers to remove pollen (Hardin et al. 1972). Solanum carolinense is considered an economically and agriculturally important weed because it acts as an alternate host for insect herbivores and diseases of closely related crops in the genus Solanum (e.g., tomato, eggplant, and potato) (Ilnicki et al. 1962; Bassett and Munro 1986). Solanum carolinense is attacked by a variety of specialist herbivores (e.g. Epitrix fuscula [Chrysomelidae], Leptinotarsa junta [Chrysomelidae], and Manduca sexta [Sphingidae]) (Imura 2003; Wise 2007; Delphia et al. 2009a) and exhibits a variety of physical and chemical defense traits that likely play a role in defenses against herbivores. The leaves and stem are covered with spines, leaves are also covered with stellate trichomes, and all plant parts including leaves, flowers, and fruits contain constitutive, as well as inducible, toxic secondary compounds (e.g., glycoalkaloids) (Bassett and Munro 1986; Cipollini and Levey 1997; Cipollini et al. 2002).
Solanum carolinense exhibits a solanaceous-type, RNAse-mediated gametophytic self-incompatibility system controlled by the multiallelic S-locus (Richman et al. 1995). However, there is plasticity in this self-incompatibility system. The ability of S. carolinense to produce selfed seeds increases with the age of unpollinated flowers and when fruit production is low (Stephenson et al. 2003; Travers et al. 2004). Additionally, plants possessing certain S-alleles exhibit higher levels of self-compatibility (Mena-Ali and Stephenson 2007). Because of this plasticity in self-incompatability, S. carolinense can self-fertilize under field conditions and has been shown to experience inbreeding depression in both the field and greenhouse (Mena-Ali et al. 2008; Kariyat et al. 2011).
Insects
Manduca sexta is a specialist lepidopteran herbivore of solanaceous plants and is a common herbivore of S. carolinense throughout its range (Imura 2003; Wise 2007; Delphia et al. 2009a). Manduca sexta eggs (Carolina Biological, Burlington, North Carolina, USA) were hatched in translucent 32 oz. plastic containers and larvae were reared on an artificial wheat germ-based diet (Frontier Agricultural Sciences, Newark, Delaware, USA) for multiple generations. Each summer, wild M. sexta larvae were collected periodically from tomato fields at the Russell E. Larson Agricultural Research Farm in Rock Springs, Pennsylvania. Wild larvae were reared in the laboratory to adults. Adult M. sexta from the lab colony were then mated with wild adults to increase the genetic diversity of the lab colony. The lab colony underwent several generations per year, but because of asynchronies in developmental times, all four stages of the life cycle (eggs, larvae, pupae, and adults) were available at most times in the colony.
Plant material
Plants used in this experiment were collected from a large S. carolinense population occupying an approximate 180 ha area near State College, Pennsylvania. In order to reduce the possibility of collecting cuttings from the same genet (i.e., genotype), cuttings were taken from the rhizomes of 16 plants located at least 10 m apart. These cuttings were planted in 4-L pots in a greenhouse and allowed to resprout, grow, and flower. After flowering, shoots were removed and the pots were put in a 4 °C cold room for 6–8 weeks. Ramets were generated from the 16 plants by taking rhizome cuttings, replanting them in 4-L pots, and allowing them to resprout and grow in a greenhouse. Hand pollinations were performed on flowers from each ramet to produce self and cross seeds. Various subsets of the resulting selfed and crossed plants were used in a series of studies by our group. After completion of these studies, the plants were cut back and the rhizomes were stored in a cold room at 4 °C (for details see Mena-Ali 2006; Mena-Ali and Stephenson 2007).
Experimental design
Three (of the original 16) maternal families were selected for this study. None of the three families selected had an S-allele in common, indicating that they were not clonal replicates and that they are unlikely to be close relatives (see Mena-Ali and Stephenson 2007 for details). Within each family three self-pollinated and three cross-pollinated genets were selected for a total of 18 individual genets. Two ramets were produced from each genet by taking 1-inch rhizome cuttings and resprouting them in flats of potting soil (Pro-Mix, Premier Horticulture, Quakertown, Pennsylvania, USA) in an insect-free growth chamber (16 h light/8 h dark, 25 °C/22 °C, 65% RH). After 3 weeks, the resprouts were individually transplanted in 4-L pots and allowed to grow for 4 weeks.
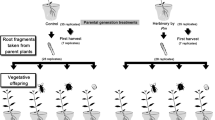
In order to examine whether herbivore-induced defenses persist in unconnected offshoots, and to determine if inbreeding alters this persistence, ramets were randomly assigned to either a control treatment (no damage) or herbivore-damage treatment (damaged by M. sexta larvae) (for experimental design, see Fig. 1). All plants in the herbivore-damage treatment were subjected to 18 bouts (2 bouts per week for 9 weeks) of caterpillar damage. Plants assigned to the control group did not receive any type of damage. Prior to each bout of damage, early 4th instar larvae were starved for 4 h. During each damage application, two randomly selected larvae were placed on lower leaves of each S. carolinense plant assigned to the herbivore-damage treatment group and were allowed to feed ad libitum for 4 h. No caterpillar was used on more than one plant.
Schematic of the experimental design to test the effects of Manduca sexta herbivory and plant inbreeding on the growth, reproduction, and defenses in rhizomatous offshoots of Solanum carolinense. X = outbred plants; S = inbred plants; DAM = parent plant subjected to repeated damage by M. sexta larvae; UD = parent plant was not damaged by M. sexta larvae (UnDamaged controls)
Plants were harvested 12 weeks after the start of herbivore-damage applications. At this point, all plants had produced a substantial number of mature fruit and belowground rhizomes. Aboveground shoot and belowground rhizome systems were separated and weighed. Six rhizome cuttings from each plant were taken and planted in flats of potting soil in a growth chamber (16 h light/8 h dark, 25 °C/22 °C, 65% RH). The remaining rhizomes were individually stored in bags in a cold room at 4 °C. After a 30-day overwintering treatment, six more rhizome cuttings from each plant were taken and planted in flats of potting soil in a growth chamber (16 h light/8 h dark, 25 °C/22 °C, 65% RH).
Growth, reproductive, and defense-related traits
The proportion of rhizome cuttings that produced offshoots and the average time to emergence were recorded for 30 days. Offshoots were then transplanted into 1-L pots with potting soil (Pro-Mix, Premier Horticulture, Quakertown, Pennsylvania, USA) and given 3 g of Osmocote Plus fertilizer (15–9–12 NPK, plus micronutrients, Scotts Co., Marysville, Ohio, USA). For plants that did not have an overwintering treatment, we counted and recorded the number of flowers produced per plant for 18 days starting at the onset of flowering. We did not record flower production for plants that received the 30-day overwintering treatment due to a Phytophthora sp. outbreak in our greenhouse that caused many individuals to die from root rot prior to flowering.
Leaf trichome density was assessed on offshoots 8 weeks after initial planting. A DinoLite digital microscope (Dunwell Tech, Inc., Torrance, California, USA) was used to take images of the adaxial surface of fully developed leaves from three offshoots of each ramet. Leaves of similar positions and size were selected on all plants. Trichomes were manually counted using the Preview software (Apple Inc., Cupertino, California, USA). Depending on availability of offshoots, one to three offshoots from the 30-day overwintering treatment were assessed for leaf trichome density. Internode spine density was assessed on offshoots 12 weeks after initial planting. Due to the disease outbreak, spine density was only assessed on offshoots that did not receive an overwintering treatment. Spines located on the third internode below the stem apex were counted and total internode length was measured.
No-choice assays were performed to assess the effects of parental plant herbivory, inbreeding, and overwintering on herbivore resistance in rhizomatous offshoots. To do so, three leaves of similar size were removed from each plant and placed in separate Petri dishes (100 × 15 mm) with moist filter paper. One newly molted 4th instar M. sexta larvae was then weighed and placed in each Petri dish. All larvae were starved for 4 h prior to the start of the assay. After 24 h, larvae were removed from the leaves, starved for 4 h (to clear the gut of the last meal), and reweighed. Larval relative growth rate (RGR) was calculated using the equation:
where \(\Delta B\) is the change in larval body mass and \(Bi\) is the initial body mass of the larva. Leaves were scanned and digitized at the beginning and end of the assay. Initial and final leaf areas were measured using the ImageJ v 1.46 software (Schneider et al. 2012) and total leaf area consumed was calculated.
Statistical analyses
All statistical analyses were performed using R statistical software (R Development Core Team 2013). A log-likelihood ratio test of independence (‘GTest’) was performed to examine the effects of parental herbivore-damage treatment (herbivore-damaged vs. undamaged), breeding type (outbred vs. inbred), and plant maternal family on the proportion of rhizomes that produced offshoots. Linear mixed-effects model ANOVAs (‘lmer’) were used to examine the effects of herbivore-damage treatment, breeding type, and plant maternal family on flower production, trichome density, spine density, larval mass gain, and larval relative growth rate (Bates et al. 2015). Total leaf area consumed by M. sexta larvae was analyzed by a linear mixed-effects model ANCOVA (‘lmer’) with larval initial mass as a covariate. All models included the main effects of parental herbivore-damage treatment (fixed), breeding type (fixed), the breeding type by damage interaction term (fixed), and plant maternal family (random). To assess the effect of overwintering on plant traits, full linear mixed-effects model ANOVAs and ANCOVAs were run with overwintering treatment as a main fixed effect in addition to the main effects of parental herbivore-damage treatment (fixed), breeding type (fixed), all interaction terms (fixed), and plant maternal family (random). Post hoc comparisons were performed using least square means multiple comparisons (‘lsmeans’) to examine differences among means for all fixed interactions terms with p values adjusted for multiple comparisons (Lenth 2016). Given that outbred and inbred S. carolinense plants are known to respond differently to herbivore damage (e.g., Kariyat et al. 2012a, b; 2013; Campbell et al. 2014), separate log-likelihood ratio tests of independence (G-test), ANOVA, and ANCOVA models were also run for outbred and inbred plants to further assess the effects of parental plant herbivory within each breeding type. To determine if the random effect of plant maternal family was significant in the models, performances for models with and without the random family effect were compared using likelihood ratio tests for all linear mixed-effects model ANOVAs and ANCOVAs.
Data transformations were performed when needed to meet the assumptions of each statistical test. Flower production was only followed for offshoots that were not given an overwintering treatment. Data for average number of flowers were log + 1 transformed and all plants were included in the analysis. Data for average time to first flower came from a small sample size and a data transformation did not correct for the violation of the assumption of normality for ANOVA, so a non-parametric Wilcoxon signed-rank test (‘wilcox.test’) was performed to test the effects of parental herbivore-damage treatment (herbivore-damaged vs. undamaged) and breeding type (outbred vs. inbred) on average time to first flower, and a non-parametric Kruskal–Wallis test (‘kruskal.test’) was performed to test the effect of plant maternal family on average time to first flower. All figures were created with the ‘ggplot2' package in R (Wickham 2016).
Results
Rhizome biomass of parental plants
Rhizome biomass was not affected by M. sexta herbivory on parental plants (F1,30 = 0.05, P = 0.818). However, inbreeding significantly affected rhizome biomass (F1,30 = 4.69, P = 0.038), with outbred plants producing rhizomes with a significantly greater biomass than inbred plants (outbred = 60.3 ± 4.9 g, inbred = 45.7 ± 5.1 g, mean ± SE). There was no effect of the breeding type by damage interaction on rhizome biomass (F1,30 = 0.18, P = 0.676). There was slight variation in rhizome biomass among maternal families (χ2(1) = 2.72, P = 0.099). A summary of these results and all results reported below are provided in Table 1.
Overall effects on offshoots
Our initial analyses of effects on offshoot traits employed a full statistical model, with parameters including overwintering treatment (fixed), herbivore-damage treatment (fixed), breeding type (fixed), their interactions, and plant maternal family (random) (see "Statistical analyses" section and Table 1). In these analyses, overwintering did not affect the likelihood of offshoot emergence (Table 2), but offshoots from overwintered rhizomes emerged significantly earlier than those from rhizomes that did not receive an overwintering treatment (overwintering treatment = 13.9 ± 0.5 days, no overwintering treatment = 20.1 ± 0.5 days, LSMeans ± SE; F1,363 = 248.16, P < 0.001; Fig. 2). Overwintering also led to significantly greater constitutive leaf trichome density (F1,167 = 37.55, P < 0.001; Fig. 3). Manduca sexta larvae feeding on offshoots of overwintered rhizomes gained significantly less mass (overwintering treatment = 151.0 ± 8.6 mg, no overwintering treatment = 219.0 ± 5.3 mg, LSMeans ± SE; Table 3) and had a lower relative growth rate (RGR) (Supplement Table 1; Fig. 4) than larvae feeding on offshoots of rhizomes without an overwintering treatment.
Mean offshoot emergence time (days) for breeding type by herbivore-damage treatments of S. carolinense offshoots that did and did not receive an overwintering treatment. Different letters indicate significant differences among overwintering by breeding type by herbivore-damage treatments determined by post hoc analysis using least square means multiple comparisons (P < 0.05). Error bars correspond to standard errors
Mean number of adaxial trichomes per 22 mm2 for breeding type by herbivore-damage treatments of S. carolinense offshoots that did and did not receive an overwintering treatment. Different letters indicate significant differences among overwintering by breeding type by herbivore-damage treatments determined by post hoc analysis using least square means multiple comparisons (P < 0.05). Error bars correspond to standard errors
Mean larval relative growth rate (g g−1 day−1) for inbred and outbred S. carolinense offshoots that did and did not receive an overwintering treatment. Different letters indicate significant differences among overwintering by breeding type treatments determined by post hoc analysis using least square means multiple comparisons (P < 0.05). Error bars correspond to standard errors
The full statistical model also revealed significant effects of parental herbivore-damage and breeding type on offshoot emergence, trichome density, and herbivore performance (Table 1). Herbivore damage on parental plants delayed offshoot emergence (F1,363 = 7.79, P = 0.017; Fig. 2), but led to higher constitutive trichome density in offshoots (F1,167 = 6.50, P = 0.012; Fig. 3) and reduced weight gain by M. sexta larvae feeding on offshoots (Table 3; Supplement Fig. 1). Relative to outbreeding, inbreeding of parental plants both reduced the likelihood of offshoot production (Table 2) and delayed offshoot emergence (F1,363 = 23.50, P < 0.001; Fig. 2).
Effects on offshoot traits with and without overwintering
To assess differences in the effects of parental plant herbivory and inbreeding within and across growing seasons, we separately analyzed our results for offshoots with and without overwintering via statistical models with parameters including herbivore-damage treatment (fixed), breeding type (fixed), the breeding type by damage interaction (fixed), and plant maternal family (random) (see "Statistical analyses" section and Table 1). The following sections describe the results of these models for specific offshoot traits.
Offshoot emergence
Without overwintering In the absence of an overwintering treatment, rhizomes of herbivore-damaged parental plants were more likely to produce offshoots (90%) than rhizomes from undamaged parental plants (81%) (Table 2); however, herbivore damage on parental plants also delayed offshoot emergence (herbivore-damaged = 20.7 ± 0.8 days, undamaged = 19.5 ± 0.8 days, LSMeans ± SE; F1,178 = 4.26, P = 0.041; Fig. 2). Inbreeding had no effect on the likelihood of offshoot emergence (Table 2), but offshoots from outbred parental plants emerged significantly earlier than offshoots from inbred plants (outbred = 19.0 ± 0.8 days, inbred = 21.2 ± 0.8 days, LSMeans ± SE; F1,178 = 11.50, P < 0.001; Fig. 2). There was no breeding type by damage interaction effect on offshoot emergence time (F1,178 = 0.002, P = 0.965). Finally, maternal plant family did not affect the likelihood of offshoot emergence (Table 2), but did have a significant effect on offshoot emergence time (χ2(1) = 5.16, P = 0.023).
When assessing the effects of parental herbivore-damage separately within each breeding type, herbivore damage reduced the likelihood of offshoot emergence from 94 to 83% (G2 = 3.51, df = 1, P = 0.061) for outbred parental plants; however, no similar effect was observed for inbred plants (G2 = 0.99, df = 1, P = 0.32). Manduca sexta herbivory on parental plants did not affect emergence time for outbred (F1,93 = 1.80, P = 0.183) or inbred (F1,85 = 2.13, P = 0.148) offshoots.
With overwintering With an overwintering treatment, herbivore damage on parental plants did not affect the likelihood of offshoot emergence (Table 2) or emergence time (F1,185 = 1.66, P = 0.199). Inbreeding reduced the likelihood of offshoot emergence (relative to outbreeding) from 95 to 80% (Table 2) and also delayed offshoot emergence (outbred = 13.0 ± 0.3 days, inbred = 14.7 ± 0.4 days, LSMeans ± SE; F1,185 = 12.05, P < 0.001; Fig. 2). Additionally, there was a significant breeding type by damage interaction effect on the likelihood (Table 2) and timing (F1,185 = 6.70, P = 0.01; Fig. 2) of offshoot emergence. Plant maternal family had a significant effect on the likelihood of offshoot emergence (Table 2), but not on offshoot emergence time (χ2(1) < 0.001, P = 1).
Parental herbivore-damage did not affect the likelihood of offshoot production from outbred (G2 = 0.21, df = 1, P = 0.646) or inbred (G2 = 0.23, df = 1, P = 0.633) plants. However, among outbred plants, parental herbivore-damage significantly delayed offshoot emergence (F1,101 = 9.62, P = 0.003; Fig. 2), while no similar effect was observed for inbred plants (F1,82 = 0.82, P = 0.416; Fig. 2).
Flowering
Without overwintering Herbivore damage on parental plants significantly reduced offshoot flower numbers (Table 4) and delayed flowering relative to offshoots of undamaged parental plants (W = 402, P = 0.043; Fig. 5a). Similarly, inbreeding reduced offshoot flower numbers (Table 4) and delayed flowering time (W = 347, P = 0.018; Fig. 5b). There was also a significant breeding type by damage interaction effect on flower production (Table 4). Offshoots from different maternal plant families did not differ significantly in flower numbers (χ2(1) = 0.08, P = 0.773), but did differ in flowering time (H = 6.97, df = 2, P = 0.031).
Mean time to first flower (days) for a herbivore-damage treatments and b breeding types of S. carolinense offshoots that did not receive an overwintering treatment. Different letters indicate significant differences between a herbivore-damage treatments and b breeding types determined by a non-parametric Wilcoxon signed-rank test. Error bars correspond to standard errors
Parental herbivore-damage significantly reduced flower numbers in offshoots from outbred plants (F1,93 = 6.51, P = 0.012); however, no similar effect was observed for inbred plants (F1,85 < 0.001, P = 0.976). Parental herbivore-damage did not affect flowering time in outbred (W = 187.5, P = 0.322) or inbred (W = 32.5, P = 0.108) offshoots.
With overwintering Due to the pathogen outbreak described in the methods section, we were unable to assess flower production for offshoots grown from rhizomes that received an overwintering treatment.
Defense traits: leaf trichome and internode spine densities
Without overwintering With no overwintering treatment, herbivore damage on parental plants led to significantly greater densities of constitutive trichomes (F1,98 = 6.58, P = 0.012; Fig. 3) and internode spines (F1,112 = 8.03, P = 0.005; Fig. 6) in offshoots, relative to offshoots from undamaged parental plants. Inbreeding of parental plants reduced the constitutive trichome density off offshoots relative to outbreeding (F1,98 = 3.85, P = 0.053; Fig. 3), but did not affect internode spine density (F1,112 = 0.02, P = 0.876; Fig. 6), perhaps because offshoots of outbred plants had significantly longer internodes (F1,112 = 0.01, P = 0.009). There was no significant breeding type by damage interaction effect on leaf trichome density (F1,98 = 1.17, P = 0.282) or internode spine density (F1,112 = 0.01, P = 0.679). There was significant variation among maternal plant families in internode spine density (χ2(1) = 19.84, P < 0.001), but not in leaf trichome density (χ2(1) < 0.001, P = 1).
Mean internode spine density (spines/cm) for breeding type by herbivore-damage treatments of S. carolinense offshoots that did not receive an overwintering treatment. Different letters indicate significant differences among breeding type by herbivore-damage treatments determined by post hoc analysis using least square means multiple comparisons (P < 0.05). Error bars correspond to standard errors
Among outbred parental plants, however, offshoots from herbivore-damaged plants had significantly greater densities of trichomes (F1,48 = 6.80, P = 0.012; Fig. 3) and internode spines (F1,75 = 9.20, P = 0.003; Fig. 6) than offshoots of undamaged outbred plants. In contrast, herbivore damage on inbred parental plants did not affect the density of trichomes (F1,47 = 0.85, P = 0.361) or internode spines (F1,37 = 1.98, P = 0.168).
With overwintering With an overwintering treatment, offshoot trichome densities were not affected by either parental herbivore-damage (F1,68 = 1.52, P = 0.222; Fig. 3) or inbreeding (F1,68 = 0.01, P = 0.942; Fig. 3). There was also no breeding type by damage interaction effect (F1,68 = 0.76, P = 0.386; Fig. 3) or maternal family effect on leaf trichome densities (χ2(1) = 0.43, P = 0.513). Among outbred parental plants, herbivore damage led to greater trichome density in offshoots (F1,42 = 3.37, P = 0.073); however, no similar effects were observed for inbred plants (F1,25 = 0.41, P = 0.528). Due to the pathogen outbreak described in the methods section, we were unable to assess internode spine densities for offshoots grown from rhizomes that received an overwintering treatment.
Herbivore performance traits
Without overwintering In the absence of overwintering, there was no effect of parental herbivore-damage, breeding type, or their interaction on the amount of leaf area consumed by 4th instar M.sexta larvae feeding on offshoots (Table 5). Additionally, maternal plant family did not affect total leaf area consumed by M. sexta larvae (χ2(1) = 1.62, P = 0.204). Larvae gained less mass when feeding on offshoots from herbivore-damaged parental plants compared to offshoots from undamaged parents (herbivore-damaged = 211.9 ± 15.6 mg, undamaged = 230.0 ± 15.6 mg, LSMeans ± SE; Supplement Fig. 1; Table 3); however, parental herbivore-damage did not affect larval RGR (Supplemental Table 1). In contrast, inbreeding had no effect on larval mass gain (Table 3), but did enhance larval RGR relative to larvae feeding on offshoots of outbred parental plants (Supplement Table 1; Fig. 4). There was no breeding type by damage interaction effect on either larval mass gain (Table 3) or RGR (Supplement Table 1), and maternal plant family also did not affect larval mass gain (χ2(1) < 0.001, P = 1) or RGR (χ2(1) = 2.43, P = 0.119).
For offshoots from parental outbred plants, M. sexta herbivory on parental plants did not affect larval mass gain or RGR (Mass: F1,104 = 0.13, P = 0.722; RGR: F1,104 = 0.01, P = 0.944). For offshoots of inbred parental plants, there was also no effect of parental herbivore-damage on RGR (F1,92 = 0.05 P = 0.819); however, larvae gained less mass on offshoots from herbivore-damaged inbred plants compared to offshoots from undamaged inbred plants (F1,92 = 3.47, P = 0.066).
With overwintering As with the no overwintering treatment, there was no effect of parental herbivore-damage, breeding type, or their interaction on the amount of leaf area consumed by 4th instar M. sexta larvae feeding on offshoots from rhizomes receiving an overwintering treatment (Table 5), and maternal family also did not affect total leaf area consumed by larvae (χ2(1) = 0, P = 1). Similar to offshoots that did not receive an overwintering treatment, M. sexta herbivory on parental plants reduced larval mass gain (Supplement Fig. 1; Table 3), but there was no effect of parental herbivore-damage on larval RGR (Supplemental Table 1). We did not observe an overall effect of breeding type or a breeding type by damage interaction on larval mass gain (Table 3) or RGR (Supplemental Table 1; Fig. 4). However, maternal plant family did significantly affect larval RGR on offshoots (χ2(1) = 6.32, P = 0.012).
Manduca sexta herbivory on parental plants significantly reduced larval leaf consumption on offshoots from outbred plants (F1,64 = 11.21, P = 0.001; Supplement Fig. 2 ), while no similar effects were observed for offshoots from inbred plants (F1,26 < 0.001, P = 0.994). The negative effects of parental herbivore-damage on larval mass gain were particularly pronounced for offshoots of outbred parental plants (F1,62 = 11.02, P = 0.002; Supplement Fig. 1), and herbivore-damage also affected larval RGR on offshoots of outbred plants (herbivore-damaged outbred = 0.78 ± 0.08 g g−1 day−1, undamaged outbred = 0.89 ± 0.08 g g−1 day−1, LSMeans ± SE, F1,61 = 3.27, P = 0.076). Meanwhile, for offshoots of inbred plants there were no significant effects of parental herbivore-damage on either larval growth (F1,26 = 0.14, P = 0.715; Supplemental Fig. 1) or RGR (F1,26 = 0.48, P = 0.495).
Discussion
Few previous studies have explored the effects of foliar herbivory on rhizomes of vegetatively spreading plants (but see Gómez et al. 2007, 2010; Dong et al. 2017; González et al. 2017), and none have demonstrated the ability of clonal plants to transmit herbivore-induced information from parental plants to unconnected offshoots. The current results demonstrate that information concerning plant herbivory can persist in unconnected offshoots grown from rhizomes of S. carolinense, which have never experienced herbivory, and that this information positively influences offshoot defenses against herbivores and negatively impacts herbivore (M. sexta) performance traits (Table 1). Furthermore, we found that these effects persisted even after 30 days of overwintering, strongly suggesting that the induction of such defenses is not only a plastic response of the parent plant, but one that can extend to clonal offshoots across growing seasons. Our results thus complement a handful of other recent studies showing that inducible defense can extend across generations and appear in seed-grown progeny that have yet to experience herbivory (see review by Holeski et al. 2012).
Despite possible fitness-associated costs in the absence of herbivores, the expression of induced defenses in undamaged rhizomatous offshoots of herbivore-damaged plants may be adaptive if offshoots face similar herbivore pressure as parental plants. Because offshoots of clonally spreading plants emerge in the same vicinity as the parent plant, this is likely to be the case for offshoots produced during a single growing season. Similarly, if local herbivore pressure is positively correlated across consecutive years, the trans-seasonal induction of defenses would tend to produce offshoots with defense phenotypes appropriate to local conditions. The current results reveal that herbivory on parental plants leads to increased expression of physical defenses in offshoots—and reduced herbivore performance—both with and without exposure to a (30-day) period of overwintering conditions. Somewhat surprisingly, we also found an independent positive effect of overwintering itself on offshoot defense traits and herbivore resistance. Offshoots exposed to our overwintering treatment sprouted new shoots faster, had higher trichome densities, and supported reduced growth of M. sexta larvae relative to those of plants that did not receive an overwintering treatment (Table 1). The mechanism underlying these effects remain unclear, and while numerous studies have shown that a cold period (i.e. vernalization) is required to promote flowering in herbaceous perennials (Chouard 1960; Kim et al. 2009), few, if any, previous studies have examined the consequences of overwintering on the defenses of clonal plants that spread via rhizomes.
If the transmission of induced defense phenotypes from parental plants to offshoots is indeed adaptive, we might expect it to be disrupted by inbreeding, which has been shown to have adverse effects on a wide range of defense traits in a number of plant species, including S.carolinense (Kariyat et al. 2013; Carr and Eubanks 2014; Portman et al. 2014, 2015). Indeed, inbreeding depression, which has been observed since the time of Darwin (1876), has been documented for a wide range of traits in scores of plant species (e.g., Husband and Schemske 1996). It is thought to arise because inbreeding exposes deleterious recessive alleles—which otherwise rarely occur in homozygous condition—to selection, while simultaneously decreasing the contributions of overdominance to fitness (Charlesworth and Charlesworth 1987). Recent, but limited, evidence suggests that epigenetic modifications may also play a role in inbreeding depression by altering DNA methylation patterns and altering patterns of gene expression (Vergeer et al. 2012). In the current study, we indeed found that the effects of herbivory on offshoot defense traits and herbivore performance were more pronounced when offshoots were grown from outbred rather than inbred parental plants (Table 1). More generally, we found adverse effects of inbreeding on the biomass of rhizomes produced by our parental plants, the emergence of the rhizomatous offshoots, time to flowering, and total flower production (Table 1). These findings support and complement those of Kariyat et al. (2011), which demonstrated under field conditions that outbred S. carolinense plants produced more rhizomatous offshoots and had greater flower and fruit production than inbred plants across 2 years.
We also found that herbivory on parental plants had implications for the growth and reproduction of offshoots, which may in turn be related to the expression of induced defense phenotypes. We found that the rhizomatous offshoots of undamaged plants emerged significantly earlier, flowered earlier, and produced more total flowers per plant than those of herbivore-damaged plants (Table 1). The negative relationship between vigor-related traits and defense traits in offshoots is consistent with potential trade-offs between investment in growth and defense. While induced defense responses benefit plants during herbivory, their production can be costly in terms of energy and nutrients that could be utilized for other plant metabolic processes (see reviews by Karban and Baldwin 1997; Strauss et al. 2002; Cipollini et al. 2003) and can also entail ecological costs, especially in the absence of herbivores (Baldwin et al. 1990; Mutikainen and Walls 1995; Redman et al. 2001; Glawe et al. 2003; Gómez et al. 2007; Sletvold et al. 2010).
The current study focused on physical defense traits, specifically the density of trichomes and internode spines. However, previous work on S. carolinense has shown that feeding by M. sexta larvae also elicits increased density of spines and trichomes on new growth in the “parental” generation (Kariyat et al. 2013), as well as the production of chemical feeding deterrents in leaves (Campbell et al. 2013, 2014), and the emission of volatile organic compounds that attract predaceous insects and parasitoids (Kariyat et al. 2012a). Consequently, the observed effects on spine and trichome densities in rhizomatous offshoots may be part of a broader suite of induced defensive traits expressed following herbivory on parental plants and then passed on to offshoots.
The mechanisms by which such defense phenotypes may be transmitted across seasons through rhizamatous offshoots (or via seeds) are not well understood, but it is likely that epigenetics plays a role via DNA methylation patterns that control gene expression patterns (Latzel and Klimešová 2010; Verhoeven and Preite 2014; Latzel et al. 2016; Thiebaut et al. 2019). Numerous studies across a variety of plant–herbivore systems have shown that herbivory by chewing insects results in the induction of a battery of direct and indirect defenses in plants, which are typically mediated by the phytohormone jasmonic acid (JA) (Howe 2004; Boughton et al. 2005; Howe and Jander 2008). A previous study has shown that JA-mediated induction of herbivore defenses in apomictic dandelion (Taraxacum officinale) resulted in differential methylation changes throughout the plant genome that were transmitted to asexually produced offspring (Verhoeven et al. 2010) and herbivores preferred to feed on leaves from offspring of untreated control plants over leaves from offspring of JA-treated plants (Verhoeven and van Gurp 2012). A recent study of alligator weed (Alternanthera philoxeroides) also revealed that epigenetic modifications of DNA can persist in belowground rhizomes for several asexual generations (Shi et al. 2019), but this study did not examine the function of the genes that were modified. Future studies of trans-seasonal induced defensive traits in clonal offshoots should focus on elucidating how herbivory affects the epigenetic signatures in the rhizomes, how these signatures affect the expression of induced defenses, and their impact on fitness across growing seasons. Finally, it should be noted that rhizomatous plants may provide a powerful tool for studying the epigenetics of environmental stress because multiple clones of the same genotype can be propagated and subjected to different treatments.
References
Alpert P, Stuefer JF (1997) Division of labour in clonal plants. In: Kroon H, Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys, Leiden, pp 137–154
Alpert P, Holzpfel C, Benson JM (2002) Hormonal modification of resource sharing in the clonal plant Fragaria chiloensis. Funct Ecol 16(2):191–197
Baldwin IT, Sims CL, Kean KE (1990) The reproductive consequence associated with inducible alkaloidal responses in wild tobacco. Ecology 71(1):252–262
Barton KE (2015) Tougher and thornier: general patterns in the induction of physical defence traits. Funct Ecol 30:181–187
Bassett IJ, Munro DB (1986) The biology of Canadian weeds. 78. Solanum carolinense L. and Solanum rostratum Dunal. Can J Plant Sci 66:977–991
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Bello-Bedoy R, Núñez-Farfán J (2010) Cost of inbreeding in resistance to herbivores in Datura stramonium. Ann Bot 105:747–753
Bertrand A, Castonguay Y (2003) Plant adaptation to overwintering stresses and implications of climate change. Can J Bot 81:1145–1152
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624
Boughton AJ, Hoover K, Felton GW (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol 31:2211–2216
Britton NL, Brown A (1970) An illustrated flora of the northeastern United States and Canada, vol 1. Dover Publications, New York
Brunner AM, Evans LM, Chuan-Yu H, Sheng X (2014) Vernalization and the chilling requirement to exit bud dormancy: shared or separate regulation? Front Plant Sci 5:732. https://doi.org/10.3389/fpls.2014.00732
Campbell SA, Thaler JS, Kessler A (2013) Plant chemistry underlies herbivore-mediated inbreeding depression in nature. Ecol Lett 16:252–260
Campbell SA, Halitschke R, Thaler JS, Kessler A (2014) Plant mating systems affect adaptive plasticity in response to herbivory. Plant J 78(3):481–490
Carr DE, Eubanks ME (2014) Interactions between insect herbivores and plant mating systems. Annu Rev Entomol 59:185–203
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Ann Rev Ecol Syst 18:237–268
Chen J-S, Lei N-F, Liu Q (2011) Defense signaling among interconnected ramets of a rhizomatous clonal plant, induced by jasmonic-acid application. Acta Oecol 37:355–360
Chouard P (1960) Vernalization and its relations to dormancy. Ann Rev Plant Physiol 11:191–238
Cipollini ML, Levey DJ (1997) Why are some fruits toxic? Glycoalkaloids in Solanum and fruit choice by vertebrates. Ecology 78:782–798
Cipollini ML, Paulk E, Cipollini DF (2002) Effect of nitrogen and water treatment on leaf chemistry in horsenettle (Solanum carolinense), and relationship to herbivory by flea beetles (Epitrix spp.) and tobacco hornworm (Manduca sexta). J Chem Ecol 28:2377–2398
Cipollini DF, Purrington CB, Bergelson J (2003) Costs of induced responses in plants. Basic Appl Ecol 4:79–85
Cook RE (1985) Growth and development in clonal plant populations. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 259–296
Crawley MJ (1997) Plant–herbivore dynamics. In: Crawley MJ (ed) Plant ecology. Blackwell Science, Oxford, pp 401–474
Darwin C (1876) The effects of cross and self fertilisation in the vegetable kingdom. John Murray, London
de Kroon H, Groenendael J (eds) (1997) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden
de Kroon H, Fransen B, van Rheenen JWA, van Dijk A, Kreulen R (1996) High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labeling. Oecologia 106:73–84
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Delphia CM, Rohr JR, Stephenson AG, De Moraes CM, Mescher MC (2009a) Effects of genetic variation and inbreeding on volatile production in a field population of horsenettle. Int J Plant Sci 170(1):12–20
Delphia CM, De Moraes CM, Stephenson AG, Meshcer MC (2009b) Inbreeding in horsenettle influences herbivore resistance. Ecol Ent 34:513–519
Dong B, Fu T, Luo F, Yu F (2017) Herbivory-induced maternal effects on growth and defense traits in the clonal species Alternanther philoxeroides. Sci Total Environ 605–606:114–123
Erb M (2012) The role of roots in plant defense. In: Mérillon JMM, Ramada KGG (eds) Plant defense: biological control. Springer, Dordrecht, pp 291–309
Gaudet DA, Wang Y, Frick M, Puchalski B, Penniket C, Ouellet T, Robert L, Singh J, Laroche A (2011) Low temperature induced defense gene expression in winter wheat in relation to resistance to snow moulds and other wheat diseases. Plant Sci 180:99–110
Glawe GA, Zavala JA, Kessler A, van Dam NM, Baldwin IT (2003) Ecological costs and benefits correlated with trypisin protease inhibitor production in Nicotiana ateenuata. Ecology 84(1):79–90
Gómez S, Stuefer JF (2006) Members only: induced systemic resistance to herbivory in a clonal plant network. Oecologia 147:461–468
Gómez S, Latzel V, Verhulst YM, Steufer JF (2007) Cost and benefits of induced resistance in a clonal plant network. Oecologia 153:921–930
Gómez S, van Dijk W, Stuefer JF (2010) Timing of induced resistance in a clonal plant network. Plant Biol 12:512–517
González APR, Dumalasová V, Rosenthal J, Skuhrovec J, Latzel V (2017) The role of transgenerational effects in adapation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol Ecol 31:345–361
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol 8:157–178
Hardin JW, Doerkense G, Herndon D, Hobson M, Thomas F (1972) Pollination ecology and floral biology of four weedy genera in southern Oklahoma. Southwest Nat 16:403–412
Hayes CN, Winsor JA, Stephenson AG (2004) Inbreeding influences herbivory in Cucurbita pepo ssp. texana (Cucurbitaceae). Oecologia 140(4):601–608
Heil M (2008) Indirect defences via tritrophic interactions. New Phytol 178:41–61
Heil M (2015) Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu Rev Entomol 60:213–232
Holeski LM (2007) Within and between generation phenotypic plasticity in trichome density of Mimulus guttatus. J Evol Biol 20:2092–2100
Holeski LM, Jander G, Agrawal AA (2012) Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol Evol 27(11):618–626
Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23:223–237
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Howe GA, Schaller A (2008) Direct defenses in plants and their induction by wounding and insect herbivores. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, Berlin, pp 7–29
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50(1):54–70
Hutchings MJ, Bradbury IK (1986) Ecological perspectives on clonal perennial herbs. Bioscience 36(3):178–182
Ilnicki RD, Tisdell TF, Fertig SN, Furrer AH (1962) Life history studies as related to weed control in the Northeast. 3. Horsenettle. Bulletin 368, University of Rhode Island Agricultural Experiment Station, Kingston, RI
Imura O (2003) Herbivoruos arthropod community of an alien weed Solanum carolinense L. Appl Entomol Zool 38:293–300
Jelínková H, Tremblay F, Desrochers A (2012) Herbivore-simulated induction of defenses in clonal networks of trembling aspen (Populus tremuloides). Tree Physiol 32:1348–1356
Johnson MTJ, Campbell SA, Barrett SCH (2015) Evolutionary interactions between plant reproduction and defense against herbivores. Annu Rev Ecol Evol Syst 46:191–213
Kaplan I, Halitschke R, Kessler A, Rehill BJ, Sardanelli S, Denno RF (2008) Physiological integration of roots and shoots in plant defense strategies link above- and belowground herbivory. Ecol Lett 11:841–851
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Karban R, Myers JH (1989) Induced plant responses to herbivory. Annu Rev Ecol Syts 20:331–348
Kariyat RR, Scanlon SR, Mescher MC, De Moraes CM, Stephenson AG (2011) Inbreeding depression in Solanum carolinense (Solanaceae) under field conditions and implications for mating system evolution. PLoS ONE 6(12):e28459
Kariyat RR, Mauck KE, De Moraes CM, Stephenson AG, Mescher MC (2012a) Inbreeding alters volatile signaling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol Lett 15(4):301–309
Kariyat RR, Mena-Ali J, Forry B, Mescher MC, De Moraes CM, Stephenson AG (2012b) Inbreeding, herbivory, and the transcriptome of Solanum carolinense. Entomol Exp Appl 144:134–144
Kariyat RR, Balogh CM, Moraski RP, De Moraes CM, Mescher MC, Stephenson AG (2013) Constitutive and herbivore-induced structural defences are compromised by inbreeding in Solanum carolinense (Solanaceae). Am J Bot 100(6):1014–1021
Kariyat RR, Hardison SB, De Moraes CM, Mescher MC (2017) Plant spines deter herbivory by restricting caterpillar movement. Biol Lett. https://doi.org/10.1098/rsbl.2017.0176
Kelly CK (1995) Thoughts on clonal integration: facing the evolutionary context. Evol Ecol 9:575–585
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Kim DH, Doyle MR, Sung S, Amasino RM (2009) Vernalization: winter and the timing of flowering plants. Annu Rev Cell Dev Biol 25:277–299
Kuwabara C, Imai R (2009) Molecular basis of disease resistance acquired through cold acclimation in overwintering plants. J Plant Biol 52:19–26
Latzel V, Klimešová J (2010) Transgenerational plasticity in clonal plants. Evol Ecol 24:1537–1543
Latzel V, González APR, Rosenthal J (2016) Epigenetic memory as a basis for intelligent behavior in clonal plants. Front Plant Sci 7:1354. https://doi.org/10.3389/fpls.2016.01354
Lei SA (2010) Benefits and costs of vegetative and sexual reproduction in perennial plants: a review of literature. ANAS 42(1):9–14
Leimu R, Kloss L, Fischer M (2008) Effects of experimental inbreeding on herbivore resistance and plant fitness: the role of history of inbreeding, herbivory and abiotic factors. Ecol Lett 11:1101–1110
Leimu R, Kloss L, Fischer M (2012) Inbreeding alters activities of the stress-related enzymes chitinases and β-1,3-glucanases. PLoS ONE 7(8):e42326
Lenth RV (2016) Least-square means: the R package lsmeans. J Stat Softw 69(1):1–33
Levin DA (1973) The role of trichomes in plant defense. Q Rev Biol 48:3–15
Liu F, Liu J, Dong M (2016) Ecological consequences of integration in plants. Front Plant Sci 7:770. https://doi.org/10.3389/fpls.2016.00770
Louda SM (1989) Predation in the dynamics of seed regeneration. In: Leck MA, Parker VT, Simpson RL (eds) Ecology of soil seed banks. Academic Press, San Diego, pp 24–51
Marquis RJ (1992) Selective impacts of herbivores. In: Fritz RS, Simms EL (eds) Ecology and evolution of plant resistance. University of Chicago Press, Chicago, pp 301–325
Mena-Ali JI (2006) Dynamics of the self-incompatibility alleles in populations of Solanum carolinenese. Dissertation, Pennsylvania State University
Mena-Ali JI, Stephenson AG (2007) Segregation analyses of partial self-incompatibility in self and cross progeny of Solanum carolinense reveals a leaky S-allele. Genetics 177:501–510
Mena-Ali JI, Keser LH, Stephenson AG (2008) Inbreeding depression in Solanum carolinense (Solanaceae), a species with a plastic self-incompatibility response. BMC Evol Biol 8:10. https://doi.org/10.1186/1471-2148-8-10
Moyer MM, Londo J, Gadoury DM, Cadle-Davidson L (2016) Cold stress-induced disease resistance (SIDR): indirect effects of low temperatures on host-pathogen interactions and disease progress in the grapevine powdery mildew pathosystem. Eur J Plant Pathol 144:695–705
Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R (2011) The role of inbreeding and outbreeding in herbivore resistance and tolerance in Vincetoxicum hirundinaria. Ann Bot 108:547–555
Mutikainen P, Walls M (1995) Growth, reproduction and defense in nettles—responses to herbivory modified by competition and fertilization. Oecologia 104:487–495
Nalam V, Shah J, Nachappa P (2013) Emerging role of roots in plant responses to aboveground insect herbivory. Insect Sci 20(3):286–296
Orians CO, Thorn A, Gómez S (2011) Herbivore-induced sequestration in plants: why bother? Oecologica 167:1–9
Palva ET, Welling A, Tähtiharju S, Tamminen T, Puhakainen T, Mäkelä P, Laitinen R, Li C et al (2001) Cold acclimniation and development of freezing and drought tolerance in plants. Acta Hortic 560(560):277–284
Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167
Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–331
Plazek A, Zur I (2003) Cold-induced plant resistance to necrotrophic pathogens and antioxidant enzyme activities and cell membrane permeability. Plant Sci 164:1019–1028
Portman SL, Kariyat RR, Johnston MA, Stephenson AG, Marden JH (2014) Inbreeding compromises host plant defenses gene expression and improves herbivore survival. Plant Signal Behav 10(5):e998548
Portman SL, Kariyat RR, Johnston MA, Stephenson AG, Marden JH (2015) Cascading effects of host plant inbreeding on the larval growth, muscle molecular composition, and flight capacity of an adult herbivorous insect. Funct Ecol 29(3):328–337
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org. Accessed Aug 2017
Redman AM, Cipollini DF, Schultz JC (2001) Fitness costs of jasmonic acid-induced defenses in tomato, Lycopersicuon esculentum. Oecologia 154:82–91
Richman AD, Kao TH, Schaeffer SW, Uyenoyama MK (1995) S-allele sequence diversity in natural populations of Solanum carolinense (Horsenettle). Heredity 75:405–415
Rinne LHP, Welling A, van der Schoot C (2010). Perennial life style of populus: dormancy cycling and overwintering. In: Jansson S, Bhalerao R, Groover A (eds) Genetics and genomics of Populus. Plant genetics and genomics: crops and models, vol 8. Springer, New York, pp 171–200
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Shi W, Chen X, Gao L, Xu C-Y, Ou X, Bossdorf O, Yang J, Geng Y (2019) Transient stability of epigenetic population differentiation in a clonal invader. Front Plant Sci 9:1851. https://doi.org/10.3389/fpls.2018.01851
Sletvold N, Huttunen P, Handley R, Karkkainen K, Agren J (2010) Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata. Evol Ecol 24:1307–1319
Stephenson AG, Travers SE, Mena-Ali JI, Winsor JA (2003) Pollen performance before and during the autotrophic-heterotrophic transition of pollen tube growth. Philos Trans R Soc Lond B Biol Sci 385:1009–1018
Stephenson AG, Leyshon B, Travers S, Hayes CN, Winsor JA (2004) Interrelationship among inbreeding, herbivory, and disease on reproduction in a wild gourd. Ecology 85(11):3023–3034
Strauss SY, Zangrel AR (2002) Plant-insect interactions in terrestrial ecosystems. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Science, Oxford, pp 77–106
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Stuefer JF, Gómez S, Van Molken T (2004) Clonal integration beyond resource sharing: implications for defence signaling and disease transmission in clonal plant networks. Evol Ecol 18:647–667
Thiebaut F, Hemerly AS, Ferreira PCG (2019) A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front Plant Sci 10:246. https://doi.org/10.3389/fpls.2019.00246
Travers SE, Mena-Ali JI, Stephenson AG (2004) Plasticity in the self-incompatibility of Solanum carolinense. Plant Species Biol 19:127–135
Vergeer P, Wagemaker N, Ouborg NJ (2012) Evidence for an epigenetic role in inbreeding depression. Biol Lett 8:798–801
Verhoeven KJF, Preite V (2014) Epigenetic variation in asexually reproducing organisms. Evolution 68:644–655
Verhoeven KJF, van Gurp TP (2012) Transgenerational effects of stress exposure on offspring phenotypes in apomictic dandelion. PLoS ONE 7(6):e38605
Verhoeven KJF, Jansen JJ, van Dijk PJ, Biere A (2010) Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol 185:1108–1118
War AR, Paulraj MG, Ahman T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7(10):1306–1320
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wise MJ (2007) The herbivores of Solanum carolinense (horsenettle) in Northern Virginia: natural history and damage assessment. Southeastern Nat 6:505–522
Acknowledgements
The authors thank Scott Diloreto and Steven Brown for assistance in the greenhouse. This project was funded in part by National Science Foundation Grant DEB-1050998 to AGS, MCM, and CMD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nihranz, C.T., Kolstrom, R.L., Kariyat, R.R. et al. Herbivory and inbreeding affect growth, reproduction, and resistance in the rhizomatous offshoots of Solanum carolinense (Solanaceae). Evol Ecol 33, 499–520 (2019). https://doi.org/10.1007/s10682-019-09997-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09997-w