Abstract
A plankton eco-evolutionary model with an alga that has the metabolic pathways to allow it to function as an autotroph or heterotroph is considered. Ecological constraints dictate that the traits that describe the feeding preferences and abilities of the alga naturally have bounded distributions. The trait distributions are then non-normal, and evolve with the population as it changes its trophic behaviour from an autotroph to a heterotroph. A key result of the simulations is that the populations remain in ecological stasis for many generations while the trait mean slowly adapts—only at the conclusion of this transition does herbivory emerge. After initially adapting to improve its competitive performance as an autotroph, the adapting population eventually emerges as a heterotroph having maximised its share of the resources at the expense of its prey, previously its competitor.


Similar content being viewed by others
References
Abrams P (1991) The effects of interacting species on predator-prey coevolution. Theor Popul Biol 39:241–262
Abrams P (1999) The adaptive dynamics of consumer choice. Am Nat 153:83–97
Abrams P, Matsuda H (2004) Consequences of behavioral dynamics for the population dynamics of predator-prey systems with switching. Popul Ecol 46:13–25
Abrams P, Matsuda H, Harada Y (1993) Evolutionarily unstable fitness maxima and stable fitness minima of continuous traits. Evol Ecol 7:465–487
Bell G (2012) Experimental evolution of heterotrophy in a green alga. Evolution 67(2):468–476
Bengtson S (2002) Origins and early evolution of predation. Paleontol Soc Pap 8:289–318
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Cortez M (2016) How the magnitude of prey genetic variation alters predator-prey eco-evolutionary dynamics. Am Nat 188:329–341
Cortez M, Ellner S (2010) Understanding rapid evolution in predator-prey interactions using the theory of fast–slow dynamical systems. Am Nat 176(5):E109–E127
Cortez M, Patel S (2017) The effects of predator-evolution and genetic variation on predator-prey population-level dynamics. Bull Math Biol 79:1510–1538
Coutinho R, Klauschiies T, Gaedke U (2016) Bimodal trait distributions with large variances question the reliability of trait-based aggregate models. Theor Ecol 9:389–408
Cropp R, Norbury J (2015a) Mixotrophy: the missing link in consumer-resource-based ecologies. Theor Ecol 8:245–260
Cropp R, Norbury J (2015b) Population interactions in ecology: a rule-based approach to modeling ecosystems in a mass-conserving framework. SIAM Rev 57(3):437–465
Cropp R, Norbury J (2019) An eco-evolutionary system with naturally bounded traits. Theor Ecol https://doi.org/10.1007/s12080-019-0407-6
De Meester L, Brans K, Govaert L, Souffreau C, Mukherjee S, Vanvelk H, Korzeniowski K, Kilsdonk L, Decaestecker E, Stoks R, Urban M (2019) Analyzing eco-evolutionary dynamics–the challenging complexity of the real world. Funct Ecol 33:43–59. https://doi.org/10.1111/1365-2435.13261
Flynn K, Stoecker D, Mitra A, Raven J, Glibert P, Hansen P, Graneli E, Burkholder J (2013) Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J Plankton Res 35:3–11
Fussman G, Ellner S, Hairston NJ (2002) Evolution as a critical component of plankton dynamics. Proc R Soc Lond Ser B Biol Sci 270:1015–1022
Fussman G, Loreau M, Abrams P (2007) Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol 21(3):465–477
Gaedke U, Klauschies T (2017) Analysing the shape of observed trait distributions enables a data-based moment closure of aggregate models. L&O Methods 15:979–994
Govaert L, Fronhofer E, Lion S, Eizaguirre C, Bonte D, Egas M, Hendry A, De Brito Martins A, Melian C, Raeymaekers J, Ratikainen I, Saether B, Schweitzer J, Matthews B (2018) Eco-evolutionary feedbacks-theoretical models and perspectives. Funct Ecol https://doi.org/10.1111/1365-2435.13241
Klauschies T, Coutinho R, Gaedke U (2018) A beta distribution-based moment closure enhances the reliability of trait-based aggregate models for natural populations and communities. Ecol Model 381:46–77
Lande R (1976) Natural selection and random genetic drift in phenotype evolution. Evolution 30:314–334
Mitra A, Flynn K, Burkholder J, Berge T, Calbet A, Raven J, Graneli E, Glibert P, Hansen P, Stoecker D, Thingstad F, Tillmann U, Vage S, Wilken S, Zubkov M (2014) The role of mixotrophic protists in the biological carbon pump. Biogeosciences 11:995–1005
Osmond M, Otto S, Klausmeier C (2017) When predators help prey adapt and persist in a changing environment. Am Nat 190:83–98
Pastor J (2017) Ecosystem ecology and evolutionary biology, a new frontier for experiments and models. Ecosystems 20:245–252
Pelletier F, Garant D, Hendry A (2009) Eco-evolutionary dynamics. Philos Trans Roy Soc B 364:1483–1489
Post D, Palkovacs E (2009) Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Philos Trans Roy Soc B 364:1629–1640
Schoener T (2011) The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331:426–429
Selosse M, Charpin M, Not F (2017) Mixotrophy everywhere on land and in water: the grand ecart hypothesis. Ecol Lett 20:246–263
Vasseur D, Amarasekare P, Rudolf V, Levine J (2011) Eco-evolutionary dynamics enable coexistence via neighbor-dependent selection. Am Nat 178(5):E96–E109
Vitousek P, Matson P (2012) Nutrient cycling and biogeochemistry. In: Levin S (ed) The Princeton guide to ecology, vol 1. Princeton University Press, Princeton, pp 330–339
Acknowledgements
The authors thank two very thorough and constructive anonymous reviewers for their contributions to publishing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Equilibrium points
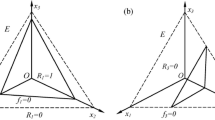
The full eco-evolutionary model (1) has eco-evolutionary coexistence equilibrium points \(\{x_1^*,x_2^*,a_{21}^*,\rho ^*\}\), that are potentially stable and are the focus of our investigation. One coexistence equilibrium point \({\textit{EP}}_1\) is:
For the parameter values used in the simulations (Table 1) this point lies inside the feasible ecospace \(E\equiv \{0<x_1<1,0<x_2<1;0<x_1+x_2<1\}\), and is relevant to the early eco-evolutionary dynamics of the system. Note that when \(\rho ^*=1\) the value of \(a_{21}^*\) is not fixed as it does not appear in the model Eqs. (1) or (13). Substituting \(\rho ^*=1\) into the Jacobian matrix [Eq. (9)] of the model [Eq. (1)] and solving for the eigenvalues gives:
As the value of \(a_{21}^*>-a_{12}\) is not fixed for \({\textit{EP}}_1\), in the four dimensional eco-evo phase space this equilibrium point in E becomes a half-line of equilibrium points. Further, \(\lambda _4\) can change sign and its stability along the \(\rho\) manifold can change, and is negative when \(a_{21}^*\) is sufficiently positive, that is when \(a_{21}^*>-r_2N^*/x_1^*\).
The other coexistence equilibrium point \({\textit{EP}}_0\) is:
The eigenvalues of this point are:
The eigenvalues \(\lambda _{1,2}\) on the \(\{x_1,x_2\}\) manifold remain the same, and are negative for all values used in the numerical simulation. The eigenvalue along the \(a_{21}\) manifold \(\lambda _3\) is always negative for positive values of \(x_1\), and the eigenvalue along the \(\rho\) manifold \(\lambda _4\) is also negative as \(r_2N^*<a_{12}x_1^*\). This point is then stable. Note that the fourth eigenvalues \(\lambda _4\) of \({\textit{EP}}_1\) and \({\textit{EP}}_0\) have opposite signs.
Appendix 2: Parameter values
Although this work is conceptually based on the results of Bell (2012), the ecological model is heuristic, with population interactions represented in their simplest form, so we have not attempted to reproduce the timescale of evolution noted by Bell. The parameter values have been chosen to produce interesting, representative dynamics in a reasonable computational time. We do not report a parameter sensitivity analysis here, but note that the evolutionary time scales are sensitive to both the evolutionary and ecological parameters. In particular, we note that the \(x_2\) density-independent mortality parameter \(m_2\) has a significant effect on the evolutionary timescale, supporting the view that adaptation is driven by the rate at which less-fit individuals are removed from the population.
Rights and permissions
About this article
Cite this article
Cropp, R., Norbury, J. The emergence of heterotrophy in an eco-evolutionary model: modelling trophic transitions in a resource-based framework with naturally-bounded trait distributions. Evol Ecol 33, 313–328 (2019). https://doi.org/10.1007/s10682-019-09981-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09981-4