Abstract
Under intense breeding, modern wheats, such as durum (Triticum turgidum L. ssp. durum), are believed to have lost nutritional quality and protein content while increasing productivity. Emmer (Triticum turgidum ssp. dicoccum Thell) and wild emmer (Triticum turgidum ssp. dicoccoides) are alternative resources for breeding programs by offering favorable alleles to be introgressed into modern materials and thus broadening their genetic diversity. Studies conducted so far have shown that durum wheat has better performance in agronomical qualities and protein quality than T. dicoccum and T. dicoccoides. However, its grain protein content (GPC) and Fe/Zn concentrations are lower. Several QTL for yield, GPC, and nutrient content in T. dicoccoides have been described, demonstrating its potential for transfer of important genes such as Gpc-B1 into modern cultivars. The Gpc-B1 gene increased the grain protein and Fe and Zn contents, but the agronomic performance of some of the modern recipients was reduced. Understanding the correlations and relationships between agronomic, chemical, and nutritional qualities would simplify selection through breeding for a single trait. Combining this knowledge with conventional breeding, MAS, and new breeding techniques would facilitate the QTL studies in these ancestral wheats and the development of new durum cultivars while retaining the agronomic qualities. In this review, we compare some grain parameters of T. durum, T. dicoccum, and T. dicoccoides wheats, including Fe and Zn content and their genetic aspects, and the existing information is analyzed and integrated for the future prospects of durum wheat improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is a staple food for nearly one-third of the world's population, making it relevant for global food security (Sing et al. 2022). In fact, most of the products found in the market derive from bread wheat (Triticum aestivum L.–a hexaploid species; 2n = 6x = 42 chromosomes) and durum wheat (Triticum turgidum L. ssp. durum–a tetraploid species; 2n = 4x = 28 chromosomes). Even though the area cultivated with durum wheat constitutes only about 5% of the total wheat growing area, durum is important for human nutrition, and it is mainly used for pasta, couscous, and other semolina-based products widely consumed in many areas of the world (Sharma et al. 2019).
Durum grain contains vitamins and micronutrients, which may contribute to a healthy diet. However, their levels are insufficient; considering that a large number of people around the world rely on durum as their main staple, which is strongly related to inadequate food intake and the low nutrient content of staple foods such as wheat (Swamy et al. 2021). Currently, there is a demand for healthy and nutritious crops that are not intensively bred and produced (Longin et al. 2016). In this context, ancient tetraploid wheats (2n = 4x = 28, AABB genome) such as wild emmer (Triticum turgidum ssp. dicoccoides) and its domesticated form, emmer (Triticum turgidum ssp. dicoccum Thell.) are gaining popularity, marketed as an alternative to improve the grain quality and the nutritional value. These stocks can be used to introgress favorable alleles into modern cultivars, to broaden their genetic diversity. Much of this appears to be happening in durum breeding programs (Cakmak et al. 2004; Kuznetsova et al. 2019; Biradar et al. 2022).
Despite the popularity and usefulness of these ancient tetraploid wheats, there is limited and dispersed information on their qualities. This review aims to compile and compare the existing information about agronomic, chemical, and nutritional grain parameters between modern (T. durum) and ancient tetraploid wheats (T. dicoccum and T. dicoccoides). This may benefit durum wheat breeding programs, growers, the food industry, and consumers.
T. durum, T. dicoccum, and T. dicoccoides domestication and generalities about their qualities
Durum wheat, T. dicoccum, and T. dicoccoides belong to the Poaceae family. They evolved naturally through hybridization of a wild diploid wheat T. urartu (2n = 2x = 14, AA genome), with goat grass Aegilops speltoides (2n = 2x = 14, BB genome). Neither of the two was ever domesticated (Dvorák et al. 1993), The first hybridization event resulted in a hulled type of wheat known as T. dicoccoides, some 300,000–500,000 years before present (yr BP). T. dicoccoides naturally grows in the Fertile Crescent and it was rediscovered in 1906 by Aaron Aaronsohn in eastern Galilee. Domestication of T. dicoccoides appears to have occurred at multiple sites independently in the Levant region (Peng et al. 2011). Through the process of domestication and selection made by hunter-gatherers, the cultivated form known as emmer (T. dicoccum or dicoccon), another hulled wheat, appeared about 10,000 years BP (Peng et al. 2011), most likely in the southeast Turkey (Özkan et al. 2005). Around 8,500 years ago, a more easily thresheable form appeared, probably as a consequence of selection and spontaneous mutations (Özkan et al. 2005). Geographic distribution of T. dicoccum and T. dicoccoides has changed through the domestication process; currently, T. dicoccum is mainly present in Ethiopia, Iran, Turkey, Central Europe, Italy, Spain, and India (Biradar et al. 2022) while T. dicoccoides is narrowly distributed in Turkey, Iraq, and Iran (Özkan et al. 2011). Due to the long history of cultivation in a wide range of environments, broad genetic diversity is evident in these ancient wheats (Biradar et al. 2022). This offers avenues for enhancing quality and nutritional value of durum wheat.
The quality traits of durum wheat meet the requirements of farmers, food industries, and consumers. It is used mainly to produce pasta products (Araya-Flores et al. 2020). Durum is expected to produce high vitreous kernels content ≥ 90%, test weight ≥ 80 kg hL−1, hardness, moisture content ≤ 14%, protein content ≥ 13%, and bright yellow color, and high semolina extraction yield (Colasuonno et al. 2019). These parameters do not directly address the nutritional value, such as the content of minerals, like zinc (Zn) and iron (Fe). Which can mitigate micronutrient deficiencies, known as "hidden hunger"; a consequence of the low intake and absorption of these micronutrients (Lowe 2021).
The quality of T. dicoccum and T. dicoccoides is related to their high protein content (Stehno 2007) with high degree of digestibility and an appropriate amino acid profile (Kuznetsova et al. 2019) and serving as a source of antioxidants and minerals (Cakmak et al. 2004) with high fiber content and resistant starch (Mohan and Malleshi 2006), and low glycemic index. These are valuable for diabetic nutrition (Buvaneshwari et al. 2003). Making wild tetraploid species attractive resources for durum wheat breeding programs, which aim at the improvement of quality and nutritional value of the grain (Anuarbek et al. 2020; Biradar et al. 2022).
Agronomic quality traits
Yield
Increased grain yield has been one of the most important selection goals in crop breeding, including wheat. However, because yield is a multifactorial trait regulated by multiple genes, environmental factors, and their interactions, phenotypic selection has never been simple (Arriagada et al. 2020). With a large number of genotypes and a wide range of environments in which durum, T. dicoccum, and T. dicoccoides are grown, grain yield ranges reported worldwide are wide. The largest producer of durum wheat is the European Union (EU), followed by Canada, Turkey, the United States, Algeria, Mexico, Kazakhstan, Syria, and India (EUROSTAT 2022; AAFC 2022; USDA 2022). Reported average durum grain yields range between 1.6 and 6.5 t ha−1 of grains (Longin et al. 2016; Atar and Kara 2017; Özberk 2018; Rachoń et al. 2020; Chaieb et al. 2020; USDA 2022; AAFC 2022; EC 2022; ODEPA 2022). This includes rainfed and irrigated areas; for example, during the 2021/22 season the average grain yield in the Northern Plains of the USA, under rainfed conditions, was 1.6 t ha−1, while in the same year, in Chile, on irrigated area, the average grain yield was 6.5 t ha−1 (ODEPA 2022).The range for grain yield of T. dicoccum was 1.8–4.3 t ha−1 (Troccoli and Codianni 2005; De Vita et al. 2006; Pagnotta et al. 2009; Marino et al. 2016; Longin et al. 2016; Atar and Kara 2017; Rachoń et al. 2020; Biel et al. 2021; Ehsanzadeh et al. 2021; Biradar et al. 2022). Unfortunately, no recent data for T. dicoccoides is available.
In general, grain yields reported for durum were higher than for T. dicoccum (Table 1). Rachoń et al. (2020) lists a difference of 46% between the average yield of durum (6.0 t ha−1) and T. dicoccum (3.2 t ha−1) in a study performed in different environments from 2015 to 2017, in Poland. Longin et al. (2016) observed a 41% lower yield in T. dicoccum (3.6 t ha−1) than durum (6.1 t ha−1) in a study at four locations in Germany. Lower T. dicoccum yields compared to durum could be attributed to a less intensive breeding process, resulting in "the primitiveness" of the T. dicoccum, as described by Ehsanzadeh et al. (2021). This results in smaller grain (Pagnotta et al. 2009) with lower remobilization of assimilates into the grain, a high number of non-reproductive tillers (Ehsanzadeh et al. 2021), taller plants prone to lodging (Longin et al. 2016), and, on the other hand, high protein/nutrient content (Pagnotta et al. 2009) relative to the cultivated materials.
Despite its lower grain yields, studies have shown that the performance of T. dicoccum is more stable than durum across different environments, even under (abiotic) stresses such as drought (Ehsanzadeh et al. 2021). This suggests better adaptation to environmental stressors (Longin et al. 2016; Rachoń et al. 2020; Ehsanzadeh et al. 2021) or higher plasticity. Durum wheat, and modern wheats in general, are more susceptible to abiotic and biotic stressors perhaps as a consequence of extensive breeding selection over time for good adaptation to specific environments. For example, weather conditions such as temperature and rainfall during anthesis, and when the crop is close to harvest, are critical factors for the crop development (Villegas et al. 2016; Cabas-Lühmann and Manthey 2020). Villegas et al. (2016) found that final durum grain yields were reduced when the average daily temperature during anthesis was above 6.9 °C and below 10.8 °C during grain filling. The optimum temperatures for high grain yields in durum should be between 18 and 24 °C during grain filling; higher temperatures at that stage, even for short periods, can result in losses of 20% or more. Temperatures ≥ 24 °C, and rainfall during durum harvest time had a detrimental effect on test weight because of the swollen and contraction of the endosperm and pericarp (Cabas-Lühmann and Manthey 2020).
Test weight (TW)
Test weight is one of the oldest parameters used to classify grain. It is given as the grain weight per unit volume (density). A higher TW indicates large and plump kernels, which means higher starch content and less bran, therefore higher semolina extraction, indicating the milling potential (Dexter and Marchylo 2001). Test weight is a quantitative trait; it depends on the expression of several genes, and it is influenced by the genotype and by the genotype × environment interactions (Sissons et al. 2020). Durum TW fluctuates between 77.8 and 83.2 kg hL−1 (De Vita et al. 2007, 2010; Bilgin et al. 2009; Fu et al. 2018; Özberk 2018; Rachoń et al. 2020; US wheat commission 2022; Canadian Cereals 2022) while T. dicoccum ranges from 70.4–75.6 kg hL−1 (De Vita et al. 2006, 2007; Pagnotta et al. 2009; Rachoń et al. 2020; Kalathunga et al. 2021). In Turkey, Özberk (2018) reported a mean value of 77.8 kg hL−1 for various durum wheat cultivars. Rachoń et al. (2020) in Poland determined that durum wheat has the highest TW with 82.8 kg hL−1 versus 75.2 kg hL−1 of T. dicoccum, whereas in a study made by Pagnotta et al. (2009) the mean TW of T. dicoccum was 72.9 kg hL−1 in Italy. At a commercial level, the durum TW were 81.4 and 79.7 kg hL−1 during the 2021/22 season in Canada and the United States, respectively. The mean and SD for TW per species are summarized in Table 1. In general, the TWs reported for durum were higher than for T. dicoccum; however, the SD showed a very similar variation after analyzing the data. There is no data for T. dicoccoides TW in this review.
Thousand kernel weight (TKW)
TKW is a measure of grain size. Dexter et al. (2001) observed that values of TKW below 40 g were associated with reduced milling quality and flour extraction rates. The average TKW for durum ranges from 38.8 to 49.3 g (De Vita et al. 2007; Bilgin et al. 2009; Taneva et al. 2015; Fatiukha et al. 2020a; Rachoń et al. 2020; Wang and Fu 2020; Akman and Karaduman 2021; US wheat commission 2022; Canadian cereals 2022). For T. dicoccum, the values of TKW range from 33.6 to 49.2 (De Vita et al. 2006, 2007; Marino et al. 2009; Mondini et al. 2014; Taneva et al. 2015; Desheva et al. 2016; Rachoń et al. 2020; Akman and Karaduman 2021; Kalathunga et al. 2021); for T. dicoccoides, Nevo et al. (1986) reported a value of 29.9 g. The mean and SD for TKW per species are summarized in Table 1. Some of the TKW values are higher for T. dicoccum than for durum; however, the SD showed a higher variability for T. dicoccum. The wide variability of this trait has been described by Desheva et al. (2016) in T. dicoccum, ranging from 21.8 to 50 g for a set of 38 genotypes. In parallel, Marino et al. (2009) reported values of 41.8 g for no nitrogen fertilization and 56.6 g for the N fertilized treatment. Rachoń et al. (2020) reported 33.9 g for T. dicoccum, which was significantly lower compared to the value of 40.7 g of durum wheat. Similar results were obtained by Akman and Karaduman (2021), who compared different Triticum species, determining lower values of ~ 30 g for T. dicoccum versus ~ 40 g for durum which means that T. dicoccum has lower milling yield since the ratio between endosperm and bran is smaller relative to durum.
Vitreous kernel content (VK)
Vitreous kernels have a translucent and shiny appearance since there are no air spaces between the starch and the protein matrix, which allows light to pass directly through the seed endosperm. Vitreousness is associated with grain hardness and high protein levels (Oury et al. 2015). The pasta industry prefers highly vitreous grain because the fracture of the endosperm during milling results in semolina with greater granulation, extraction, and intense yellow color. The range of kernel vitreousness in durum can oscillate between 60 and 99% (Subira et al. 2014; Fu et al. 2018; Taneva et al. 2015; Özberk 2018; Cabas-Lühmann and Manthey 2020; Vecherska et al. 2021; US Wheat Commission 2022; Canadian cereals 2022). However, at least 80% of vitreous kernels are expected for milling purposes (Petrova et al. 2009). Nevertheless, some sub-classes of durum can have less than 60% vitreous kernels, mainly because of wet conditions when the grain is ready to be harvested with about 12% moisture content (Cabas-Lühmann and Manthey 2020). Subira et al. (2014) reported that old and contemporary cultivars had above 80% of vitreousness, whereas old cultivars in Spain ranged from 90 to 92%. In T. dicoccum, a range of 56–94% was reported (Desheva et al. 2016; Taneva et al. 2015; Rachoń et al. 2020; Vecherska et al. 2021). The mean and SD for VK per species are summarized in Table 1. Vitreous kernel content for both species is quite similar in general, with T. dicoccum having a higher level of variability. Interestingly, different studies have shown a greater VK content for T. dicoccum than for durum, as did Taneva et al. (2015), who identified that T. dicoccum had a higher vitreousness than durum, which among 15 tested accessions ranged from 79 to 99% in T. dicoccum to 88% in durum. Rachoń et al. (2020) reported a 6% higher vitreousness in T. dicoccum than in durum, which showed a VK value of 86%. In another study, a collection of T. dicoccum characterized by Desheva et al. (2016) showed grain vitreousness in the range of 66–99%. As explained Sieber et al. (2015), VK depends on the protein content, therefore, as observed by Taneva et al. (2015), the T. dicoccum accessions with higher VK content also had the highest protein content. Research is needed to determine how the kernel water absorption affects the VK content of T. dicoccum and T. dicoccoides.
Genetic architecture of grain yield and other agronomical traits
Ancient wheats have long been recognized as a source of favorable alleles for wheat improvement, and so the wild germplasm of tetraploid wheats remain a valuable resource and useful discoveries can be made. Some studies have shown that wild emmer wheat can be exploited to improve some agronomic traits of present-day cultivars, by the introgression of desirable alleles (Ahmadi et al. 2007; Lu et al. 2022). Recombinant inbred lines (RIL) or single chromosome-arm substitution lines (CASLs) have been used to locate genetic loci in wild germplasm that carry favorable QTL alleles (Klymiuk et al. 2019). For example, Peleg et al. (2009a) mapped several QTL associated with plant productivity and drought-adaptive traits in a RIL population derived from a cross between durum wheat (‘Langdon’) and T. dicoccoides (‘G18-16’). Subsequently, some of these QTL were introgressed from ‘G18-16’ into elite Israeli durum (cv. ‘Uzan’) and bread wheat (‘Bar Nir’ and ‘Zahir’) via marker assisted selection. They were located on chromosomes 1B and 2B for durum and 7A in bread wheat. The introgressed QTL improved grain yield, biomass, photosynthetic capacity, and root development across different environments, particularly under drought conditions (Merchuk-Ovnat et al. 2016a, b, 2017). Collected information about selected QTL for grain yield (GY), grain weight (GW), and other agronomical quality traits is summarized in Table 2.
Heading date is a fundamental agronomic trait that affects the maturation time and grain yield. In this sense, Zhou et al. (2016), using CASLs of T. dicoccoides chromosomes (‘TDIC140’) in the genetic background of common wheat (‘Chinese Spring’), found QTL associated with late (on chromosomes 4A and 2B) and early (on chromosomes 3A and 7B) heading dates in the ‘TDIC140’ genome. Interestingly, the QTL detected on chromosome 2B related to the later heading explained the largest portions of phenotypic variation (57.2–76.7%) in different environments in Australia. In contrast, Lu et al. (2022) found a QTL flanked by C268 and C309 markers with an interval of 8.9 cM on T. dicoccoides chromosome 7B, explaining 9.1 and 12.4% of the phenotypic variation for the early heading date, which is useful for wheat breeding for rainfed conditions (Shavrukov et al. 2017).
Grain weight (GW) per plant is an essential component of wheat yield; therefore, several QTL have been identified in wild emmer wheat. For example, Golan et al. (2015) mapped a major QTL associated with GW increase on chromosome 2A positioned at 57.8 cM and linked to the Xhbg494 marker on T. dicoccoides ‘FA-15-3’. This QTL is associated with the Grain Number Increase 1 (GNI-A1) gene, a regulator of floret fertility, and it was introgressed into durum wheat var. ‘Langdon’, where it increased GW without significantly affecting the grain (Golan et al. 2019). Additionally, Avni et al. (2018) performed a QTL analysis for GW using a RIL population derived from a cross between T. dicoccoides (‘Zavitan’) and durum wheat (‘Svevo’). A total of 22 QTL were identified for TKW, of which eight were conferred by ‘Zavitan’. A meta-QTL analysis identified a locus on chromosome 6A associated with the Growth-Regulating Factor 4 (GRF4-A) gene; its introgression from ‘Zavitan’ into the ‘Svevo’ increased GW by up to 8%. In this same genomic region, Peleg et al. (2009b) located a QTL for TKW in T. dicoccoides acc. ‘G18-16’. These results suggest that the alleles from ‘Zavitan’ and ‘G18-16’ on chromosome 6A have the potential to increase wheat yields in breeding programs (Avni et al. 2018).
Chemical quality traits
Grain protein content
The grain protein content (GPC) and gluten quality are important quality traits for pasta manufacturing due to their effect on the firmness of cooked pasta and tolerance of overcooking. The average GPC in durum wheat ranges from 11.8 to 18.0% (Pagnotta et al. 2009; Blanco et al. 2012; Sayaslan et al. 2012; Hassan et al. 2016; Uppal and Bhise 2018; Geisslitz et al. 2018; Fatiukha et al. 2020a; Rachoń et al. 2020), in T. dicoccum from 11.1 to 21.6% (De Vita et al. 2006; Stehno 2007; Pagnotta et al. 2009; Giuliani et al. 2009; Oak et al. 2011; Giacintucci et al. 2014; Konvalina et al. 2012; Lacko et al. 2015; Kuznetsova et al. 2019; Geisslitz et al. 2018; Rachoń et al. 2020; Biel et al. 2021; Biradar et al. 2022) while for T. dicoccoides it ranges from 14.8 to 23.6% (Ciaffi et al. 1992; Hassan et al. 2007; Peleg et al. 2009a; Tonk et al. 2010; Liu et al. 2019; Fatiukha et al. 2020a). For high-quality pasta the GPC is expected at ≥ 13%; GPC at or below 11% results in poor-quality pasta regardless of the drying conditions in processing (Delcour and Hoseney 2010). The protein content is a quantitative trait with low heritability, largely influenced by the environment, and it is often negatively correlated with the wheat grain yield (Blanco et al. 2012).
The means and SD for GPC per species were summarized in Table 1. In general, durum data cluster at the lowest level, followed by T. dicoccum, and T. dicoccoides with the highest. However, durum had the most stable data compared to the ancestral wheats. The SD for durum was ± 1.9% vs ± 3.0% for T. dicoccum and ± 3.1% for T. dicoccoides. When T. dicoccum and T. dicoccoides are compared to modern wheats, these ancestral crops list among those with the highest grain protein content (Joppa et al. 1991; Stehno 2007; Pagnotta et al. 2009; Tonk et al. 2010; Konvalina et al. 2012; Kuznetsova et al. 2019; Rachoń et al. 2020; Biradar et al. 2022). Kuznetsova et al. (2019) found that the GPC of T. dicoccum was 3.9% higher than the common wheat, while Rachoń et al. (2020) found that the GPC of T. dicoccum was 4.7% higher than the durum wheat (19.2 vs. 14.5%, respectively). Conversely, Akman and Karaduman (2021) found that T. dicoccum and durum had almost equal grain protein contents (~ 15%). Hassan et al. (2007) determined a protein content of 15% in T. dicoccoides vs. 9.5% in durum wheat. Tonk et al. (2010) reported a GPC in T. dicoccoides of 21.4%, which was 6% higher than the values in bread wheat (15.7%) in Turkey. Newer cultivars have lower GPC than the old ones, mainly because the breeding techniques have prioritized the increase in yields, which is negatively correlated to GPC. According to Subira et al. (2014), this reduced GPC from 16% to 14.2–14.7% in newer wheat cultivars during the twentieth century.
Protein quality
Protein quality is related to gluten strength and cooked pasta quality. In wheat, there are two major protein fractions of prolamins: glutenins and gliadins. Glutenins and gliadins represent about 80% of the total protein in the endosperm of wheat. The ratio of glutenins: gliadins which upon hydration and mixing form gluten, is responsible for the gluten strength (Edwards et al. 2003). The glutenins confer the elasticity of the dough, while extensibility is mainly conferred by the gliadins (Shewry et al. 1995). In wheat breeding, the evaluation of gluten strength is based mainly on the sodium dodecyl sulfate (SDS)-sedimentation volume (Axford et al. 1978). It requires less material and is considerably faster than the gluten index, alveograph, and mixograph tests (Zhang et al. 2008). The SDS-sedimentation volume estimates the glutenin quality and, indirectly, the gluten strength (Peña et al. 2003). In tests by Oak et al. (2011) in India, the micro-sedimentation values for all 10 T. diccoccum accessions were lower than those of durum, with the mean of 28.5 mm vs. 32 mm, respectively. Stehno (2007), reported 29 mL for T. dicoccum vs. 61 mL for T. aestivum. On the other hand, Akman and Karaduman (2021) identified T. dicoccum ‘kavilca’ as having a higher macro-SDS than the durum ‘Meram 2002’ and ‘Kunduru 1149’, with values of about 35 mL and 15 mL, respectively. On the other hand, Ciaffi et al. (1992) reported that about 10% of the 315 accessions of T. dicoccoides had considerably higher SDS-sedimentation values compared to the best-performing durum wheat tested.
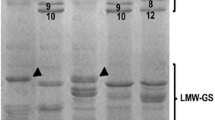
Glutenins are divided into two subunits: low molecular weight subunits (LMW-GS) and high molecular weight subunits (HMW-GS) (Dick and Quick 1983; Shewry et al. 1986). A significant association between the SDS-sedimentation values and HMW-GS and LMW-GS was reported by Dick and Quick (1983). Recent studies have shown that the LMW-GS may have a higher effect on the final protein quality and gluten strength than the HMW-GS (Zhen et al. 2014; Araya-Flores et al. 2020). However, because of their high heterogeneity and the abundance of allelic variants, characterization of LMW-GS is still incomplete and requires further investigation (Roncallo et al. 2021). Interestingly, durum wheats with strong gluten showed higher expression levels of the LMW glutenin-related genes between 21 and 35 days post anthesis (DPA) and had up to 43.5% more alanine than the weak gluten lines at 42 DPA, suggesting that alanine may have a positive impact gluten strength at a particular developmental stage (Araya-Flores et al. 2020). The most used criteria to classify LMW-GS are based on their mobility in the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and that is a consequence of differences in the molecular weight. There are three types of LMW-GS: type B (42–51 kD), type C (20–40 kD), and type D (50–70 kD). The B-type is a sulfur-rich prolamin known also as the “m” and “s” subunits; the C-type and D-type are forms of α-/γ-gliadins and ω-gliadins, respectively; they have cysteine residues with sulfur bonds (Rutsgi et al. 2019). Studies on durum wheat have shown that the γ-gliadins 45 (genetically associated with LMW-2GS) were positively correlated to al dente pasta and good cooking characteristics, while γ -gliadins 42 (genetically associated with LMW-1GS) had poor cooking properties (D’Ovidio and Masci 2004; Edwards et al. 2007). However, the B-type LMW-GS has been pointed out as ultimately responsible for the gluten strength in durum wheat (Ruiz et al. 2018; Chacón et al. 2020).
T. dicoccum gluten quality and technological performance is generally inferior to modern wheats, with low elasticity, high extensibility, and softer dough (De Vita et al. 2006; Kuznetsova et al. 2019). However, some studies have shown that some accessions of this species have promising characteristics with similar gluten performance when compared to durum (Oak et al. 2002; 2011). Oak et al. (2011) reported that among all accessions of T. dicoccum tested only ‘DDK 1025’ showed the presence of γ -gliadins 45 linked to Glu-B3 LMW-GS, resulting in dough properties similar to durum wheat. On the other hand, the electrophoretic profiles of Giacintucci et al. (2014) on non-vitreous of T. dicoccum did not identify LMW-GS between 16–23 kDa. The difference among the studies points to a need for additional studies in this area, which could lead to the improvement of the crop and its future uses. Further experiments could focus on the development of new T. dicoccum cultivars with LMW-GS (LMW-2) like durum wheat, for better dough properties.
Genetic architecture of grain protein content and quality
As noted above, modern wheat cultivars contain relatively low to moderate GPC. In this sense, wild and domesticated emmer species are a valuable genetic resource, rich in allelic variants, to improve grain quality through introgression of favorable alleles from the wild accessions (Marcotuli et al. 2020; Colasuonno et al. 2021; Biradar et al. 2022). Ruiz et al. (2018) and Chacón et al. (2020) identified new alleles at the Glu-B3 locus with a positive effect on gluten strength. The B type LMW-GS is controlled by loci Glu-B2, Glu-A3, and Glu-B3 on the short arms of homoeologous group-1 chromosomes (Bellil et al. 2014). Looking only the B-type LMW-GS at the Glu-3 loci, T. dicoccum shows five different binding patterns in Indian genotypes (Oak et al. 2002) while in a similar study, Degaonkar et al. (2005 observed eight different binding patterns. Liu and Shepherd (1996) identified large variation in the LMW-GS of two T. dicoccum accessions, with four to six B subunits with variations in band mobility and staining intensity.
T. dicoccoides has not only been identified as an appropriate source for the transfer of genes to improve the GPC, but some genotypes within this species appear as a promising source for the improvement of gluten and rheological properties of modern wheats (Ciaffi et al. 1992; Liu and Shepherd 1996). Liu and Shepherd (1996) showed that the T. dicoccoides lines from different countries had two to five B subunit bands. Compared to durum wheat, there were similar bands with only minor differences in the intensity and mobility that resembled the − 43.2 gliadin in durum wheat.
Another classification for the LMW-GS is based on their first amino acid residue (D’Ovidio and Masci 2004), with three types: LMW-GS serine (s), LMW-GS methionine (m), and LMW-GS isoleucine (i) (Glu 3 loci), and a new class reported by Huang et al. (2018) from Aegilops comosa, called LMW-leucine (l). The high variability of LMW-GS at the Glu 3 loci in T. dicoccoides has also been described (Ciaffi et al. 1993), making it an important source to identify new alleles or genes to improve modern wheat quality. Qin et al. (2015) amplified DNA sequence of the LMW-GS genes usingthe know Glu 3 alleles of wheat and related grasses and identified five novel LMW-GS (i) genes from T. dicoccoides named emmer-1 to emmer-5. Among them, the coding region of emmer-1 had 1128 bp and was the longest and may have some potential to improve the breadmaking quality of bread wheat. Masci et al. (2000) showed that the larger the coding region the better the wheat dough quality because of the improvement of the viscoelastic properties. In a more recent study, Xiang et al. (2019) evaluated the LMW-GS of two advanced lines obtained from the cross of bread wheat ‘CN19’ with T. dicoccum ‘D97’. The advanced lines ‘BAd7-209’ and ‘BAd7-213’ had the LMW-GS C and D types at the Glu-A3 locus from the wild emmer ‘D97’ parent. Both had a higher protein content, higher SDS-sedimentation value, wet gluten content, better dough development time and stability time than the parent ‘CN19’. Of the two lines, ‘Bad7-209’ had better dough stability time and development time than ‘BAd7-213’ which was attributed to the presence of the novel D-type LMW-GS at Glu-A3 present in ‘Bad7-209’.
Many QTL associated with the GPC have been located on chromosomes of tetraploid wheats (Table 3), and some have been successfully introgressed into modern wheat cultivars using the marker-assisted selection (Kumar et al. 2018). For example, Fatiukha et al. (2020a) identified a total of 8 stable GPC-related QTL under five different environments on chromosomes 1A, 2A, 3A, 4A, 4B, 5A, 6B, and 7B in T. dicoccoides ‘Y12-3’, which explained between 0.6 and 24.4% of the phenotypic variation for GPC. Among all the QTL detected for GPC, the most important one was Gpc-B1, originally detected by Joppa and Cantrell (1990) on chromosome 6B in T. dicoccoides lines ‘FA-15-3’ and ‘F-28-8-3’ from Israel. It significantly increased the the GPC relative to the recurrent durum parent ‘Langdon’ [LDN] (Joppa et al. 1991). Joppa et al. (1997) mapped a QTL for GPC on chromosome arm 6BS in a RIL population derived from LDN and CASL ‘LDN (DIC6B)’, which explained 66% of the phenotypic variation for GPC. Olmos et al. (2003) mapped this QTL as a simple Mendelian locus, Gpc-B1, located within a 0.3 cM interval flanked by DNA markers Xucw71 and Xucw79. Gpc-B1 encodes for a NO APICAL MERISTEM-B1 (NAM-B1), which is a NAC transcription factor that accelerates senescence and increases nutrient remobilization from leaves to developing grains, hence affecting the final GPC (Uauy et al. 2006; Avni et al. 2014). This transcription factor is nonfunctional in modern wheat. Uauy et al. (2006), through positional cloning, identified a functional NAM-B1allele present mainly in wild wheats and landraces. The presence of Gpc-B1 has been associated with reductions in yield and other agronomic parameters in different environments (Brevis and Dubcovsky 2010; Tabbita et al. 2013). However, as suggested by Eagles et al. (2014), introgression of the functional allele into wheat cultivars with large grain size may increase GPC and the development of high-yielding cultivars carrying functional GPC-B1 allele. Some approaches for conventional wheat breeding focused on increasing the grain protein content without losing grain yield, or such quality parameters as the test weight. This can be done by introgression of alien genes from related species and finding the QTL for grain protein with a less negative effect on grain yield and other related traits (Liu et al. 2019). Lines without Gpc-B1 showed an improvement in TKW and grain protein content in a study made by Liu et al. (2019), who regions on chromosome arms 2BS and 7BL with no negative effect on TKW which can be useful for grain protein content as well. Introgression of the functional Gpc-B1 allele from wild emmer increased grain protein content in several elite and commercial cultivars of common/durum wheat and in different environments (Mishra et al. 2015; Vishwakarma et al. 2016), with only marginally negative impacts on yields (Tabbita et al. 2017). Specifically, the wild Gpc-B1 allele had positive effects on the semolina protein content (increased 16 g kg−1), wet gluten (increased 50 g kg−1), mixing time and peak height (increased 0.65 min and 1.3 cm, respectively), cooked firmness (increased 0.9 g cm), and cooking loss (decreased 5 g kg−1) compared to the ‘UC1113’ and ‘Kronos’ NILs (Brevis and Dubcovsky 2010; Tabbita et al. 2017).
Nutritional value through Zinc and Iron content
Zinc and iron content and their bioavailability
According to the World Health Organization (WHO 2002), the iron (Fe) deficiency ranks 6th, while the zinc (Zn) deficiency is 5th among the 20 most important nutritional ailments in the world. This is related to "hidden hunger," a consequence of a nutrient-poor diet because of the low intake and absorption of these micronutrients. This is of concern in both developed and developing countries (Lowe 2021). Iron deficiency can cause problems such as anemia, deficiencies in cognitive development, maternal mortality, premature births, and low energy; zinc deficiency is related to abnormalities in growth development (stunting) and a weak immune system (Santos et al. 2017).
Cereals not only have low levels of Fe and Zn but also contain compounds such as phytate or phytic acid (PA) (myoinositol-1,2,3,4,5,6-hexakisphosphate) and fiber that limit Fe and Zn bioavailability in the human body (Cakmak et al. 2002; Welch and Graham 2004). This happens because phytate generates a strong chelation with Fe and Zn, reducing their bioavailability (Coudray et al. 2001). Bioavailability is considered high when the molar ratios of PA:Zn are < 5, moderate 5–15, and low > 15 (Magallanes-López et al. 2017); for PA:Fe, the molar ratio should be < 1 for high Fe bioavailability (Magallanes-López et al. 2017). One of the strategies to mitigate this problem is through the biofortification of crops through plant breeding programs that target high grain mineral concentrations, which have been signaled as one of the most economical and environmentally safe approaches to solving malnutrition (Cakmak et al. 2002, 2010; Welch and Graham 2004; Bouis et al. 2011). Bioavailability is the capacity to absorb theminerals in the digestive tract (Beasley et al. 2019). Some important criteria to consider when developing a biofortified crop are the bioavailability of the nutrients, retention of the nutrients after processing, nutrients requirements and consumption in a population (Cakmak et al. 2010). To improve the micronutrient concentration in cereals through breeding, a breeding program must consider: (1) a large variation in the content of micronutrients in the seeds reflecting large genetic variation, and the phytic acid content (Cakmak et al. 2002, 2010); (2) agronomical practices such as fertilization, water supply, and soil nutrient concentrations and associations with mycorrhizal fungi (Tran et al. 2021); (3) the genotype x environment interaction for a quantitative trait (Blair et al. 2009).
Durum wheat, and all modern cultivated wheats in general, show narrow micronutrient variation, this limits their use as a genetic source to increase Fe and Zn content (Cakmak et al. 2000, 2010; Ficco et al. 2009; Rachón et al. 2012; Hakki et al. 2014). Magallanes-López et al. (2017) tested 46 durum wheat cultivars from the main durum growing areas for the bioavailability of Fe and Zn. The variation ranged from 25.7 to 40.5 mg kg−1 for Fe and from 24.8 to 48.8 mg kg−1 for Zn, with the PA acid content ranging from 4.6 to 9.5 mg g−1. The values reported by Ficco et al. (2009) for 84 durum wheat cultivars from Italy fluctuated between 33.6–65.6 mg kg−1 for Fe, 28.5–46.3 mg kg−1 for Zn,, and 4.6–7.6 mg g−1 for PA Based on the existing information, it is clear that the genetic variation available in durum wheat is insufficient for the development of cultivars rich in Fe and Zn; other genetic resources must be accessed in the breeding process. The most promising resources for the improvement of grain Zn and Fe concentrations are wild relatives, ancient wheats, and landraces (Monasterio and Graham 2000; Cakmak et al. 2010). T. dicoccum (Monasterio and Graham 2000) and T. dicoccoides have been shown to have high micronutrient concentrations (Cakmak et al. 2000, 2004, 2010; Distelfeld et al. 2007; Gomez-Becerra et al. 2010). High concentrations of Fe and Zn has been detected in T. dicoccoides accessions in a greenhouse experiment (Cakmak et al. 2004) with the levels of Fe reaching up to 109 mg kg−1 (with a minimum value of 14 mg kg−1), with up to 190 mg kg−1 for Zn (minimum of 15 mg kg−1). Similarly large variation was also detected by Guzman et al. (2014). Peleg et al. (2008) identified the ‘MM 5/4’ and ‘24/39’ T. dicoccoides accessions as promising micronutrient sources with 139 mg kg−1 of Zn and 88 mg kg−1 of Fe and adequate agronomic performance. Among a wide range of wheat germplasm surveyed at CIMMYT, T. dicoccum had the highest Fe and Zn concentrations (Welch and Graham 2004).
As described before, the wild wheat ancestors have the highest Fe and Zn concentrations (Table 1) and generally they have lower grain yields compared to modern wheat cultivars. In sense there is a negative correlation between grain yield and mineral accumulation, especially with Zn (McDonald et al. 2008; Ficco et al. 2009; Liu et al. 2014; Velu et al. 2017). Modern wheats with higher grain yields appear to dilute micronutrient concentrations by increasing starch and grain density (McDonald et al. 2008; Magallanez–López et al. 2017). However, some studies did not find such negative correlations (Welch and Graham 2004; Peleg et al. 2008) (Table 4). This opens an important question why modern wheats have lower mineral accumulation per grain weight unit. Currently, it is difficult to breed for high mineral concentration and high grain yield, especially for Zn, and breeding programs prioritize other traits, not related to micronutrients. Another interesting fact reported by Zhao et al. (2009), Gomez-Becerra et al. (2010), and Cakmak et al. (2010) is a strong positive correlation between Fe and Zn concentration and protein content, which can be explained by shared transporters between both nutrients (Kobayashi and Nishizawa 2012). Overall, the improvement of the nutritional value of durum cultivars may lead to an indirect increase in technological and industrial quality, which can be simultaneously improved by breeding.
Genetic architecture of Fe and Zn
The physiological and genetic bases underlying the accumulation of Zn and Fe are poorly understood. The International Maize and Wheat Improvement Center (CIMMYT) and the Consultative Group on International Agricultural Research (CGIAR) are two important entities involved in genetic screening for Fe and Zn. In 2013, CGIAR created HarvestPlus, a program to enhance nutritional quality via biofortification. Due to the importance of the topic, several reviews have been made on the genetics of wheat germplasm micronutrients; among the most recent are Gupta et al. (2020) who reviewed wheat bioavailability and biofortification of Zn, Fe, and Selenium (Se), including the genetics and physiology of macronutrients, Saini et al. (2020) who collated information from wheat studies related to QTL associated with the biofortification traits including MAS and genomic selection; Sharma et al. (2021) reviewed wheat quality changes due to domestication (green revolution), including changes in the wild wheat ancestors and the enhancement of Fe and Zn, as well as the application of genetic engineering focused on transgenic-based Fe and Zn-enriched wheat. Here we only focus on the genetic diversity offered by tetraploid wheat species for the Fe and Zn improvement.
T. dicoccoides has a high genetic diversity for the mineral nutrient concentrations (Cakmak et al. 2004; Peleg et al. 2008). Cakmak et al. (2004) evaluated two sets of T. dicoccoides substitution lines, the first was the bread wheat cv. ‘Chinese Spring’-T. dicoccoides and the second was the durum cv. ‘Langdon’-T. dicoccoides suggested that the major genes affecting the accumulation of these micronutrients are located on chromosome 6B of T. dicoccoides, while 5B and 6A also appeared to be involved the Chinese Spring–T. dicoccoides. Several QTLs have been identified for grain Fe and Zn content in tetraploid wheats (Table 5); these could be introduced into elite genetic backgrounds to improve the micronutrient content. In fact, a total of 5 cultivars of durum wheat rich in either Fe, Zn, or both, have been developed and released in India (Wani et al. 2022). Peleg et al. (2009a), using a RIL population derived from a cross between durum wheat (‘Langdon’) and T. dicoccoides (‘G18-16’), identified 38 stable QTL associated with the wild alleles from ‘G18-16’, which explained between 0.7 and 19.2% of the grain mineral nutrient concentration variation, including Zn and Fe. The T. dicoccoides gene Gpc-B1 described by Uayu et al. (2006) and Distelfeld et al. (2007), which has been associated with GPC increases (see above), is also related to Zn and Fe grain concentration increases (Tabbita et al. 2017). This allele was evaluated for its effects on grain micronutrient concentrations in recombinant chromosome substitution lines (RSLs) developed from the cross ‘DIC-6B’ x ‘Langdon’ (Joppa et al. 1997). RSLs carrying the wild Gpc-B1 allele had, on average, 12%, 18%, and 29% higher concentrations of Zn, Fe, and Mn in the grain, respectively, as compared to RSLs with the allele from Langdon (Distelfeld et al. 2007). The problem was in the association of the Gpc-B1 allele with reductions in grain weight and yield (Uauy et al. 2006; Brevis and Dubcovsky 2010; Tabbita et al. 2013). However, as discussed above, it is important to note that high-yielding wheat cultivars carry the GPC-B1 allele, therefore, more research can be done regarding this gene (Eagles et al. 2014). Besides, new genomic regions need to be explored (Liu et al. 2021). Liu et al. (2021) characterized Fe and Zn concentrations in 161 advanced lines derived from T. dicoccoides through GWAS. They identified six markers associated with grain Fe concentration, on chromosomes 3B, 4A, 4B, 5A, and 7B, and three markers associated with Zn concentration on chromosomes 1A and 2A. Some lines with the markers associated with high grain Fe and Zn concentrations showed no reduction in TKW. The Zn concentration markers on chromosome 2A were in the same interval (139.9–145.2 cM) as those of Peleg et al. (2009a), who identified a wild emmer GZnC-QTL (77.4–147.4 cM). Fatiukha et al. (2020b) identified nine QTLs related to grain Fe content (LOD: 2.2–16.4 cM; PVE: 0.6–22.3%) and 10 QTLs for grain Zn content in the ‘G18-16’ accession of T. dicoccoides.
As mentioned earlier, the bioavailability of Zn and Fe depends on several factors which need to be considered in theimprovement of the nutritional value of modern wheats. The PA is a critical component limiting bioavailability. Reducing PA is one strategy for durum breeding programs; however, there is no defined minimum value that would not have a negative effect on the crop (Ram and Govindan 2020). Considering that the main form of storage for P is PA (Raboy et al. 1991), there is a negative correlation between Zn/Fe and P (Fatiukha et al. 2020b). Peleg et al. (2009a) identified a QTL on chromosome 5B of ‘G18-16’ (T. dicoccoides) for grain P that co-localized with a QTL for GPC. This is important as selection for increased GPC is associated with increased grain PA. The wild-type allele associated with high GPC and low P, making it a future alternative for increasing grain protein content without increasing PA. Genes involved in the biosynthesis pathway of PA are known for multiple crops, such as corn and soybean, but not for durum and/or other tetraploid wheat species. The closest identification of these types of genes was made in common wheat by Bhati et al. (2014), who identified six genes potentially involved in the ultimate phases of the PA biosynthesis with four of those six genes encoding for inositol tetraphosphate kinases (TaITPK1, TaITPK2, TaITPK3, and TaITPK4) and the remaining encoding for the inositol triphosphate kinase (TaIPK2) and the inositol pentakisphosphate kinase (TaIPK1). The study of the genes related to PA biosynthesis may help in the development of new cultivars with low grain PA content.
Conclusions and future prospects
The information gathered here may offer a better understanding of the general qualities and genetics of T. dicoccum and T. dicoccoides relative to durum wheat. A comparison of ancestral wheats with durum shows differences in the grain physical qualities, GPC, protein quality, and the Fe/Zn content. Durum wheat is superior in grain yield, TW, TKW, and VK but its GPC and Fe/Zn concentrations are lower. protein quiality is better in durum than in ancestral wheats, but some promising alleles were detected in T. dicoccum for the B-type LMW-GS (Oak et al. 2002; Degaonkar et al. 2005) and in T. dicoccoides (Qin et al. 2015; Xiang et al. 2019). These may improve viscoelastic properties of the dough made from modern wheats. Lower agronomical performance (biomass and grain yield) of ancestral wheats had been attributed to less selection over time, and hence small grain and associated proportionately higher nutrient and protein content, and cultivation issues associated with hulled, difficult-to-thresh wheats compared to durum wheat.
Better adaptation to abiotic and biotic stressors and the genetic potential of T. dicoccum and, especially, T. dicoccoides as sources of important genes/alleles for the improvement of quality parameters in modern wheats, such as protein and mineral concentrations, are important considerations for future research. The T. dicoccoides gene Gpc-B1 on chromosome 6B, first described by Joppa and Cantrell (1990), has been a well-studied source for the improvement of GPC and Fe and Zn contents. The functional allele from wild emmer eas successfully introgressed into several elite and commercial wheat cultivars, for various environments. However, the presence of Gpc-B1 is also associated with a reduction in grain yield and some other agronomic parameters in different environments. More research is required to identify new genes for GPC and mineral concentration without affecting agronomic qualities.
The negative phenotypic and genetic correlations between grain yield and grain protein quality, besides the positive correlations among GPC, Zn, and Fe, have been reported in various studies. In the future, it is important to understand more deeply the association between agronomical parameters, protein qualities, and mineral concentrations. This would facilitate selection through breeding. Furthermore, a study of genes related to the accumulation and transport of specific minerals and components like PA should be assessed.
Finally, all information summarized in this review points to the importance of ancestral genetic resources for further studies, as alternative ways to address the grain protein content and mineral Zn and Fe concentrations in durum wheat. Keeping in mind that the primary goal for breeding, for food-related industries, and for growers, is the grain yield increase, further studies should focus on a combination of techniques such as conventional breeding, MAS, GWAS and gene editing. Major QTLs that are expressed in several environments can be used for MAS in breeding and for positional cloning, which may lead to parallel improvement of multiple traits, such as GPC and mineral content, without the grain yield penalty, making this a promising strategy for developing new durum cultivars with improved chemical qualities while retaining agronomic qualities.
References
AAFC (Agriculture and Agri-Food Canada) (2022) Canada: outlook for principal field crops. https://agriculture.canada.ca/en/canadas-agriculture-sectors/crops/reports-and-statistics-data-canadian-principal-field-crops/canada-outlook-principal-field-crops-2021-10-20. Accessed 14 Aug 2022
Ahmadi H, Nazarian F (2007) The inheritance and chromosomal location of morphological traits in wild wheat. Triticum Turgidum l Ssp Dicoccoides Euphytica 158:103–108
Akman H, Karaduman Y (2021) Evaluating technological quality of cultivated Triticum species, interspecific, and intergeneric hybrids for wheat-based products and breeding programs. J Cereal Sci 99:103–188. https://doi.org/10.1016/j.jcs.2021.103188
Anuarbek S, Abugalieva S, Pecchioni N, Laidò G, Maccaferri M, Tuberosa R, Turuspekov Y (2020) Quantitative trait loci for agronomic traits in tetraploid wheat for enhancing grain yield in Kazakhstan environments. PLoS One 15:e0234863. https://doi.org/10.1371/journal.pone.0234863
Araya-Flores J, Guzmán C, Matus I, Parada R, Jarpa G, Costa de Camargo A, Shahidi F, Schwember AR (2020) new findings in the amino acid profile and gene expression in contrasting durum wheat gluten strength genotypes during grain filling. J Agric Food Chem 68:5521–5528. https://doi.org/10.1021/acs.jafc.9b07842
Arriagada O, Marcotuli I, Gadaleta A, Schwember AR (2020) Molecular mapping and genomics of grain yield in durum wheat: a review. Int J Mol Sci 21:7021. https://doi.org/10.3390/ijms21197021
Atar B, Kara B (2017) Comparison of grain yield and some characteristics of hulled, durum and bread wheat genotypes varieties. Turkish J Agricul Food Sci Technol. https://doi.org/10.24925/turjaf.v5i2.159-163.1028
Avni R, Nave M, Eilam T, Sela H, Alekperov C, Peleg Z, Dvorak J, Korol A, Distelfeld A (2014) Ultra-dense genetic map of durum wheat × wild emmer wheat developed using the 90K iSelect SNP genotyping assay. Mol Breed 34:1549–1562. https://doi.org/10.1007/s11032-014-0176-2
Avni R, Oren L, Shabtay G, Assili S, Pozniak C, Hale L, Ben-David R, Peleg Z, Distelfeld A (2018) Genome based meta-QTL analysis of grain weight in tetraploid wheat identifies rare alleles of GRF4 associated with larger grains. Genes 9:636. https://doi.org/10.3390/genes9120636
Axford DWE, McDermott EE, Redman DG (1978) Note on sodium dodecyl sulfate test of breadmaking quality: comparison with Pelshenke and Zeleny tests. Cereal Chem 56:582–584
Beasley JT, Bonneau JP, Sánchez-Palacios JT, Moreno-Moyano LT, Callahan DL, Tako E, Glahn R, Lombi E, Johnson A (2019) Metabolic engineering of bread wheat improves grain iron concentration and bioavailability. Plant Biotechnol J 17:1514–1526. https://doi.org/10.1111/pbi.13074
Bellil I, Hamdi O, Khelifi D (2014) Diversity of five glutenin loci within durum wheat (Triticum turgidum L ssp. durum (Desf.) Husn.) germplasm grown in Algeria. Plant Breed 133:179–183. https://doi.org/10.1111/pbr.12156
Bhati KK, Aggarwal S, Sharma S, Mantri S, Singh SP, Bhalla S, Kaur J, Tiwari S, Roy JK, Tuli R, Pandey A (2014) Differential expression of structural genes for the late phase of phytic acid biosynthesis in developing seeds of wheat (Triticum aestivum L.). Plant Sci 224:74–85. https://doi.org/10.1016/j.plantsci.2014.04.009
Biel W, Jaroszewska A, Stankowski S, Sobolewska M, Kępińska-Pacelik J (2021) Comparison of yield, chemical composition and farinograph properties of common and ancient wheat grains. Eur Food Res Technol 247:1525–1538. https://doi.org/10.1007/s00217-021-03729-7
Bilgin O, Baser I, Korkut K, Genctan T (2009) Variations for grain yield and milling value of durum wheat landraces and obsolete cultivars. Philipp Agric Sci 92:1–8
Biradar SS, Yashavanthakumar KJ, Navathe S, Reddy UG, Baviskar VS, Gopalareddy K, Lamani K, Desai SA (2022) Dicoccum wheat: Current status and future perspectives. New Horizons in Wheat and Barley Research. https://doi.org/10.1007/978-981-16-4449-8_21
Blair MW, Sandoval TA, Caldas GV, Beebe SE, Paez MI (2009) Quantitative trait locus analysis of seed phosphorus and seed phytate content in a recombinant inbred line population of common bean. Crop Sci 49:237–246. https://doi.org/10.2135/cropsci2008.05.0246
Blanco A, Mangini G, Giancaspro A, Give S, Colasuonno P, Simeone R, Signorile A, De Vita P, Mastrangelo AM, Cattivelli L, Gadaleta A (2012) Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol Breed 30:79–92. https://doi.org/10.1007/s11032-011-9600-z
Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH (2011) Biofortification: a new tool to reduce micronutrient malnutrition. Food Nut Bull 32:31–40. https://doi.org/10.1177/15648265110321S105
Brevis JC, Dubcovsky J (2010) Effects of the chromosome region including the Gpc-B1 locus on wheat grain and protein yield. Crop Sci 50:93–104. https://doi.org/10.2135/cropsci2009.02.0057
Buvaneshwari G, Yenagi NB, Hanchinal RR, Naik RK (2003) Glycaemic responses to dicoccum products in the dietary management of diabetes. Indian J Nutr Dietet 40:363–368
Cabas-Lühmann P, Manthey F (2020) Effect of hydration on physical grain quality of durum wheat. Cereal Chem 97:877–887. https://doi.org/10.1002/cche.10311
Cakmak I, Özkan H, Braun HJ, Welch RM, Romheld V (2000) Zinc and iron concentrations in seeds of wild, primitive and modern wheats. Food Nutr Bull 21:401–403. https://doi.org/10.1177/156482650002100411
Cakmak I, Torun A, Millet E, Feldman M, Fahima T, Korol A, Nevo E, Braun HJ, Özkan H (2004) Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci Plant Nutr 50:1047–1054. https://doi.org/10.1080/00380768.2004.10408573
Cakmak I, Pfeiffer WH, McClafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 12:452. https://doi.org/10.5601/jelem.2016.21.4.1336
Cakmak I, Graham R, Welch RM (2002) Agricultural and molecular genetic approaches to improving nutrition and preventing micronutrient malnutrition globally. In: Cakmak I, Welch RM (eds) Encyclopedia of life support systems. Oxford, pp 1075–1099
Canadian Cereals (2022) https://canadiancereals.ca/wp-content/uploads/2022/01/2021CropData.pdf. Accessed 4 Aug 2022
Chacón EA, Vázquez FJ, Giraldo P, Carrillo JM, Benavente E, Rodríguez-Quijano M (2020) Allelic variation for prolamins in spanish durum wheat landraces and its relationship with quality traits. Agronomy 10:136. https://doi.org/10.3390/agronomy10010136
Chaieb N, Rezguia M, Ayedb S, Bahria H, Cheikh H, M’hameda MR, Annabia M (2020) Effects of tillage and crop rotation on yield and quality parameters of durum wheat in Tunisia. J Anim Plant Sci 44:7654–7676. https://doi.org/10.35759/JAnmPlSci.v44-2.7
Chee PW, Elias EM, Anderson JA, Kianian SF (2001) Evaluation of a high grain protein QTL from Triticum turgidum L. var. dicoccoides in an adapted durum wheat background. Crop Sci 44. https://doi.org/10.2135/cropsci2001.412295x
Ciaffi M, Dominici L, Lafiandra D, Porceddu E (1992) Seed storage proteins of wild wheat progenitors and their relationships with technological properties. Hereditas 116:315–322. https://doi.org/10.1111/j.1601-5223.1992.tb00162.x
Ciaffi M, Lafiandra D, Porceddu E, Benedettelli S (1993) Storage-protein variation in wild emmer wheat (Triticum turgidum ssp. dicoccoides) from Jordan and Turkey. I. Electrophoretic characterization of genotypes. Theor Appl Genet 86:474–480
Colasuonno P, Marcotuli I, Blanco A, Maccaferri M, Condorelli GE, Tuberosa R, Parada R, de Camargo AC, Schwember AR, Gadaleta A (2019) Carotenoid pigment content in durum wheat (Triticum turgidum L. var durum): an overview of quantitative trait loci and candidate genes. Front Plant Sci 10:1347. https://doi.org/10.3389/fpls.2019.01347
Colasuonno P, Marcotuli I, Gadaleta A, Soriano JM (2021) From genetic maps to QTL cloning: an overview for durum wheat. Plants 10:315. https://doi.org/10.3390/plants10020315
Coudray C, Levrat-Verny MA, Tressol JC, Feillet-Coudray C, Horcajada-Molteni NM, Demigné C, Rayssiguier Y, Rémésy C (2001) Mineral supplementation of white wheat flour is necessary to maintain adequate mineral status and bone characteristics in rats. J Trace Elem Med Biol 15:131–137. https://doi.org/10.1016/S0946-672X(01)80056-0
D’Ovidio R, Masci S (2004) The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci 39:321–339. https://doi.org/10.1016/j.jcs.2003.12.002
De Vita P, Riefolo C, Codianni P, Cattivelli L, Fares C (2006) Agronomic and qualitative traits of T. turgidum ssp dicoccum genotypes cultivated in Italy. Euphytica 150:195–205. https://doi.org/10.1007/s10681-006-9107-6
De Vita P, Mastrangelo AM, Codianni P, Fornara M, Palumbo M, Cattivelli L (2007) Bio-agronomic evaluation of old and modern wheat, spelt and emmer genotypes for low-input farming in mediterranean environment. Ital J Agron Riv Agron 3:291–302. https://doi.org/10.4081/ija.2007.291
De Vita P, Mastrangelo A, Matteu L, Mazzucotelli E, Virzì N, Palumboc M, Lo Storto M, Rizza F, Cattivelli L (2010) Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. Field Crops Res 119:68–77. https://doi.org/10.1016/j.fcr.2010.06.016
Degaonkar AM, Tamhankar SA, Rao VS (2005) An assessment of cultivated emmer germplasm for gluten proteins polymorphism of gluten proteins in cultivated emmer germplasm. Euphytica 145:49–55
Delcour JA, Hoseney RC (2010) Principles of cereal science and technology, 3rd edn. AACCI, St. Paul, MN
Desheva GN, Kyosev BN, Stoyanova SD, Sabeva MD (2016) Grain quality of emmer germplasm (Triticum dicoccon) from the National Collection of Bulgaria. Phytol Balcan 22:223–232
Dexter JE, Marchylo BA (2001) Recent trends in durum wheat milling and pasta processing: impact on durum wheat quality requirements. In: Pierre F (ed) Proceedings of the international workshop on durum wheat, semolina and pasta quality: recent achievements and new trends. Institut National de la Recherche, Montpellier, France, pp 139–164
Dick JW, Quick JS (1983) A modified screening test for rapid estimation of gluten strength in early-generation durum wheat breeding lines. Cereal Chem 60:315–318
Distelfeld A, Uauy C, Fahima T, Dubcovsky J (2006) Physical map of the wheat high-grain protein content gene Gpc-B1 and development of a high-throughput molecular marker. New Phytol 169:753–763. https://doi.org/10.1111/j.1469-8137.2005.01627.x
Distelfeld A, Cakmak I, Peleg Z, Ozturk L, Yazici AM, Budak H, Saranga Y, Fahima T (2007) Multiple QTL effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol Plantarum 129:635–643. https://doi.org/10.1111/j.1399-3054.2006.00841.x
Dvorák J, Terlizzi P, Zhang HB, Resta P (1993) The evolution of polyploid wheats: identification of the a genome donor species. Genome 36:21–31. https://doi.org/10.1139/g93-004
Eagles HA, McLean R, Eastwood RF, Appelbee M-J, Cane K, Martin PJ, Wallwork H (2014) High-yielding lines of wheat carrying Gpc-B1 adapted to Mediterranean-type environments of the south and west of Australia. Crop Pasture Sci 65:854. https://doi.org/10.1071/cp14106
EC (European Commision) (2022) Cereals statistics. https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/overviews/market-observatories/crops/cereals-statistics_en. Accessed 21 Aug 2022
Edwards NM, Mulvaney SJ, Scanlon MG, Dexter JE (2003) Role of gluten and its components in determining durum semolina dough viscoelastic proprieties. Cereal Chem 80:755–763. https://doi.org/10.1094/CCHEM.2003.80.6.755
Edwards NM, Gianibelli MC, McCaig TN, Clarke JM, Ames NP, Larroque OR, Dexter JE (2007) Relationships between dough strength, polymeric protein quantity and composition for diverse durum wheat genotypes. J Cereal Sci 45:140–149. https://doi.org/10.1016/j.jcs.2006.07.012
Ehsanzadeh P, Vaghar M, Roushanzamir V (2021) Persistent indifference of emmer wheats grain yield and physiological functions to nitrogen supply: evidence from two irrigation regimes and dryland conditions. Int J Plant Prod, 15:391–405. https://doi-org.pucdechile.idm.oclc.org/https://doi.org/10.1007/s42106-021-00143-7
EUROSTAT (2022) https://ec.europa.eu/eurostat/data/database. Accessed 21 Aug 2022
Fatiukha A, Filler N, Lupo I, Lidzbarsky G, Klymiuk V, Korol AB, Pozniak C, Fahima T, Krugman T (2020a) Grain protein content and thousand kernel weight QTLs identified in a durum × wild emmer wheat mapping population tested in five environments. Theor Appl Genet 133:119–131. https://doi.org/10.1007/s00122-019-03444-8
Fatiukha A, Klymiuk V, Peleg Z, Saranga Y, Cakmak I, Krugman T, Korol AB, Fahima T (2020) Variation in phosphorus and sulfur content shapes the genetic architecture and phenotypic associations within the wheat grain ionome. Plant J 101:555–572. https://doi.org/10.1111/tpj.14554
Ficco DBM, Riefolo C, Nicastro G, De Simone V, Di Gesù AM, Beleggia R, Platani C, Cattivelli L, De Vita P (2009) Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crops Res 111:235–242. https://doi.org/10.1016/j.fcr.2008.12.010
Fu BX, Wang K, Dupuis B, Taylor D, Nam S (2018) Kernel vitreousness and protein content: Relationship, interaction and synergistic effects on durum wheat quality. J Cereal Sci 79:210–217. https://doi.org/10.1016/j.jcs.2017.09.003
Geisslitz S, Wieser H, Scherf K, Koehler P (2018) Gluten protein composition and aggregation properties as predictors for bread volume of common wheat, spelt, durum wheat, emmer and einkorn. J Cereal Sci 83:204–212. https://doi.org/10.1016/j.jcs.2018.08.012
Giacintucci V, Guardeño L, Puig A, Hernando I, Sacchetti G, Pittia P (2014) Composition, protein contents, and microstructural characterisation of grains and flours of emmer wheats (Triticum turgidum ssp. dicoccum) of the Central Italy Type. Czech J Food Sci 32:115–121
Giuliani A, Karagöz A, Zencirc N (2009) Emmer (Triticum dicoccon) production and market potential in marginal mountainous areas of Turkey. Mt Res Dev 29:220–229
Golan G, Oksenberg A, Peleg Z (2015) Genetic evidence for differential selection of grain and embryo weight during wheat evolution under domestication. J Exp Bot 66:5703–5711. https://doi.org/10.1093/jxb/erv249
Golan G, Ayalon I, Perry A, Zimran G, Ade-Ajayi T, Mosquna A, Distelfeld A, Peleg Z (2019) GNI-A1 mediates trade-off between grain number and grain weight in tetraploid wheat. Theor Appl Genet 132:2353–2365. https://doi.org/10.1007/s00122-019-03358-5
Gomez-Becerra HF, Yazici A, Ozturk L, Budak H, Peleg Z, Morgounov A, Fahima T, Saranga Y, Cakmak I (2010) Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica 171:39–52. https://doi.org/10.1007/s10681-009-9987-3
Gupta PK, Balyan HS, Sharma S, Kumar R (2020) Biofortification and bioavailability of Zn, Fe and Se in wheat: present status and future prospects. Theor Appl Genet 134:1–35. https://doi.org/10.1007/s00122-020-03709-7
Guzmán C, Medina-Larqué AS, Velu G, González-Santoyo H, Singh RP, Huerta-Espino J, Ortiz-Monasterio I, Peña RJ (2014) Use of wheat genetic resources to develop biofortified wheat with enhanced grain zinc and iron concentrations and desirable processing quality. J Cereal Sci 60:617–622. https://doi.org/10.1016/j.jcs.2014.07.006
Hakki EE, Dograr N, Pandey A, Khan MK, Hamurcu M, Kayis SA, Gezgin S, Ölmez F, Akkaya M (2014) Molecular and elemental characterization of selected Turkish durum wheat varieties. Not Bot Horti Agrobot Cluj-Nap 42:431–439
Hassan A, Heneidak S, Gowayed SMH (2007) Comparative studies of some Triticum species by grain protein and amino acids analyses. J Agron 6:286–293
Hassan M, Mohamed E, El-Rawy M, Amein K (2016) Evaluating interspecific wheat hybrids based on heat and drought stress tolerance. J Crop Sci Biotechnol 19:85–98. https://doi.org/10.1007/s12892-015-0085-x
Huang L, He Y, Jin YR, Wang F, He JS, Feng LH, Liu DC, Wu BH (2018) Characterization of novel LMW glutenin subunit genes at the Glu-M3 locus from Aegilops comosa. 3 Biotech 8:379. https://doi.org/10.1007/s13205-018-1407-8
Joppa LR, Cantrell RG (1990) Chromosomal location of genes for grain protein content of wild tetraploid wheat. Crop Sci 30:1059–1064. https://doi.org/10.2135/cropsci1990.0011183X003000050021x
Joppa LR, Hareland GA, Cantrell RG (1991) Quality characteristics of the Langdon durum-dicoccoides chromosome substitution lines. Crop Sci 31:1513–1517. https://doi.org/10.2135/cropsci1991.0011183X003100060024x
Joppa LR, Du C, Hart G, Hareland G (1997) Mapping gene(s) for grain protein in tetraploid wheat (Triticum turgidum l.) using a population of recombinant inbred chromosome lines. Crop Sci 37:1586–1589. https://doi.org/10.2135/cropsci1997.0011183X003700050030x
Klymiuk V, Fatiukha A, Huang L, Wei ZZ, Kis-Papo T, Saranga Y, Krugman T, Fahima T (2019) Durum wheat as a bridge between wild emmer wheat genetic resources and bread wheat. In: Applications of genetic and genomic research in cereals. Elsevier, pp 201–230. https://doi.org/10.1016/B978-0-08-102163-7.00010-7
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152. https://doi.org/10.1146/annurev-arplant-042811-105522
Konvalina P, Capouchová I, Stehno Z (2012) Agronomically important traits of emmer wheat. Plant Soil Environ 58:341–346
Kulathunga J, Reuhs BL, Zwinger S, Simsek S (2021) Comparative study on kernel quality and chemical composition of ancient and modern wheat species: einkorn, emmer, spelt and hard red spring wheat. Foods 10:761. https://doi.org/10.3390/foods10040761
Kumar A, Jain S, Elias EM, Ibrahim M, Sharma LK (2018) An overview of QTL identification and marker-assisted selection for grain protein content in wheat. In: Sengar R, Singh A (eds) Eco-friendly agro-biological techniques for enhancing crop productivity. Springer, Singapore, pp 245–274. https://doi.org/10.1007/978-981-10-6934-5_11
Kuznetsova E, Shayapova L, Klimova E, Nasrullaeva G, Brindza J, Stolyarov M, Zomiteva G, Bychkova T, Gavrilina V, Kuznetsova E (2019) Composition, quality characteristics and microstructure of the grain Triticum dicoccum. Slovak J Food Sci 13:933–940. https://doi.org/10.5219/1174
Lacko-Bartošová M, Čurná V (2015) Nutritional characteristics of emmer wheat varieties. J Microbiol Biotech Food Sci 4:95–98. https://doi.org/10.15414/jmbfs.2015.4.special3.95-98
Liu CY, Shepherd KW (1996) Variation of B subunits of glutenin in durum, wild and less-widely cultivated tetraploid wheats. Plant Breed 115:172–178. https://doi.org/10.1111/j.1439-0523.1996.tb00896.x
Liu H, Wang ZH, Li F, Li K, Yang N, Yang Y, Huang D, Liang D, Zhao H, Mao H, Liu J, Qiu W (2014) Grain iron and zinc concentrations of wheat and their relationships to yield in major wheat production areas in China. Field Crops Res 156:151–160. https://doi.org/10.1016/j.fcr.2013.11.011
Liu J, Huang L, Wang C, Liu Y, Yan Z, Wang Z, Zhong X, Gong F, Zheng Y, Liu D, Wu B (2019) Genome-wide association study reveals novel genomic regions associated with high grain protein content in wheat lines derived from wild emmer wheat. Front Plant Sci 10:464. https://doi.org/10.3389/fpls.2019.00464
Liu J, Huang L, Li T, Liu Y, Yan Z, Tang G, Zheng Y, Liu D, Wu B (2021) Genome-wide association study for grain micronutrient concentrations in wheat advanced lines derived from wild emmer. Front Plant Sci 12:651283. https://doi.org/10.3389/fpls.2021.651283
Longin CFH, Ziegler J, Schweiggert R, Koehler P, Carle R, Würschum T (2016) Comparative study of hulled (einkorn, emmer, and spelt) and naked wheats (durum and bread wheat): agronomic performance and quality traits. Crop Sci 56:302–311. https://doi.org/10.2135/cropsci2015.04.0242
Lowe N (2021) The global challenge of hidden hunger: perspectives from the field. Proc Nutr Soc 80:283–289. https://doi.org/10.1017/S0029665121000902
Lu F, Chen M, Zhao Y, Wu S, Yasir M, Zhang H, Hu C, Rong J (2022) Genetic mapping and candidate gene prediction of a QTL related to early heading on wild emmer chromosome 7BS in the genetic background of common wheat. Agronomy 12:1089. https://doi.org/10.3390/agronomy12051089
Magallanes-López A, Hernandez-Espinosa N, Velu G, Posadas-Romano G, Ordoñez-Villegas G, Crossa J, Ammar K, Guzmán C (2017) Variability in iron, zinc and phytic acid content in a worldwide collection of commercial durum wheat cultivars and the effect of reduced irrigation on these traits. Food Chem 237:499–505. https://doi.org/10.1016/j.foodchem.2017.05.110
Marcotuli I, Colasuonno P, Hsieh YSY, Fincher GB, Gadaleta A (2020) Non-starch polysaccharides in durum wheat: a review. Int J Mol Sci 21:2933. https://doi.org/10.3390/ijms21082933
Marino S, Tognetti R, Alvino A (2009) Crop yield and grain quality of emmer populations grown in central Italy, as affected by nitrogen fertilization. Eur J Agron 31:233–240. https://doi.org/10.1016/j.eja.2009.08.002
Marino S, Cocozza C, Tognetti R, Alvino A (2016) Nitrogen supply effect on emmer (Triticum dicoccum Schübler) ecophysiological and yield performance. Int J Plant Prod 10:457–468
Masci R, D’Ovidio D, Lafiandra D, Kasarda D (2000) A 1B-coded low-molecular-weight glutenin subunit associated with quality in durum wheats shows strong similarity to a subunit present in some bread wheat cultivars. Theor Appl Genet 100:396–400. https://doi.org/10.1007/s001220050052
McDonald GK, Genc Y, Graham RD (2008) A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil 306:49–55. https://doi.org/10.1007/s11104-008-9555-y
Merchuk-Ovnat L, Barak V, Fahima T, Ordon F, Lidzbarsky GA, Krugman T, Saranga Y (2016a) Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front Plant Sci 7:452. https://doi.org/10.3389/fpls.2016.00452
Merchuk-Ovnat L, Fahima T, Krugman T, Saranga Y (2016b) Ancestral QTL alleles from wild emmer wheat improve grain yield, biomass and photosynthesis across environments in modern wheat. Plant Sci 251:23–34. https://doi.org/10.1016/j.plantsci.2016.05.003
Merchuk-Ovnat L, Fahima T, Ephrath JE, Krugman T, Saranga Y (2017) Ancestral QTL alleles from wild emmer wheat enhance root development under drought in modern wheat. Front Plant Sci 8:703. https://doi.org/10.3389/fpls.2017.00703
Mishra VK, Gupta PK, Balasubramaniam A, Chand R, Vasistha NK, Vishwakarma MK, Singh P, Joshi A (2015) Introgression of a gene for high grain protein content (Gpc-B1) into two leading cultivars of wheat in Eastern Gangetic Plains of India through marker assisted backcross breeding. J Plant Breed Crop Sci 7:292–300
Mohan BH, Malleshi NG (2006) Characteristics of native and enzymatically hydrolyzed common wheat (Triticum aestivum) and dicoccum wheat (Triticum dicoccum) starches. Eur Food Res Technol 223:355–361. https://doi.org/10.1007/s00217-005-0212-x
Monasterio I, Graham R (2000) Breeding for trace minerals in wheat. Food and Nutr Bull 21:392–396. https://doi.org/10.1177/156482650002100409
Mondini L, Grausgruber H, Pagnotta M (2014) Evaluation of European emmer wheat germplasm for agro-morphological, grain quality traits and molecular traits. Genet Resour Crop Evol 61:69–87. https://doi.org/10.1007/s10722-013-0016-y
Nevo E, Grama A, Beiles A, Golenberg EM (1986) Resources of high-protein genotypes in wild wheat, Triticum dicoccoides in Israel: Predictive method by ecology and allozyme markers. Genetica 68:215–227. https://doi.org/10.1007/BF02424445
Oak MD, Tamhankar SA, Rao VS, Bhosale SB (2002) Polymorphism of gluten proteins in Indian dicoccum wheat (Triticum turgidum ssp. dicoccum) revealed by SDS and acid-page. J Genet Breed 56:245–250
Oak MD, Tamhankar SA, Rao VS, Misra SC (2011) Milling and pasta making potential of cultivated dicoccum wheat (Triticum turgidum ssp. dicoccum). Cereal Res Commun 39:426–435
ODEPA (2022) Estadísticas de cultivos. https://www.odepa.gob.cl/estadisticas-del-sector/estadisticas-productivas. Accessed 14 Dec 2022
Olmos S, Diestelfeld A, Chicaiza O, Schatter AR, Fahima T, Echenique V, Dubcovsky J (2003) Precise mapping of a locus affecting grain protein content in durum wheat. Theor Appl Genet 107:1243–1251. https://doi.org/10.1007/s00122-003-1377-y
Oury FX, Lasme P, Michelet C, Rousset M, Abecassis J, Lullien-Pellerin V (2015) Relationships between wheat grain physical characteristics studied through near-isogenic lines with distinct puroindoline-b allele. Theor Appl Genet 128:913–929. https://doi.org/10.1007/s00122-015-2479-z
Özberk I (2018) Durum wheat in Turkey; yesterday, today and tomorrow. In: Royo C, Nachit MN, Difonzo N, Araus JL, Pfeiffer WH, Slafer GA (eds) Durum wheat breeding: current aproaches and future strategies. The Howard Press İnc, USA, p 1049
Özkan H, Brandolini A, Pozzi C, Effgen S, Wunder J, Salamini F (2005) A reconsideration of the domestication geography of tetraploid wheats. Theor Appl Genet 110:1052–1060. https://doi.org/10.1007/s00122-005-1925-8
Özkan C, Altuğ N, Yüksek N, Kaya A, Akgül Y (2011) Assessment of electrocardiographic findings, serum nitric oxide, cardiac troponins and some enzymes in calves with hyperkaliemia related to neonatal diarrhoea. Revue Méd Vét 162:171–176
Pagnotta MA, Mondini L, Codianni P, Fares C (2009) Agronomical, quality, and molecular characterization of twenty Italian emmer wheat (Triticum dicoccon) accessions. Genet Resour Crop Evol 56:299–310. https://doi.org/10.1007/s10722-008-9364-4
Peleg Z, Saranga Y, Yazici A, Fahima T, Ozturk L, Cakmak I (2008) Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 306:57–67. https://doi.org/10.1007/s11104-007-9417-z
Peleg Z, Cakmak I, Ozturk L, Yazici A, Jun Y, Budak H, Korol A, Fahima T, Saranga Y (2009a) Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor Appl Genet 119:353–369. https://doi.org/10.1007/s00122-009-1044-z
Peleg Z, Fahima T, Krugman T, Abbo S, Yakir DAN, Korol AB, Saranga Y (2009b) Genomic dissection of drought resistance in durum wheat× wild emmer wheat recombinant inbreed line population. Plant Cell Environ 32:758–779. https://doi.org/10.1111/j.1365-3040.2009.01956.x
Peleg Z, Fahima T, Korol A, Abbo S, Saranga Y (2011) Genetic analysis of wheat domestication and evolution under domestication. J Exp Bot 62: 5051–5061. https://doi.org/10.1093/jxb/err206
Peña RJ, Trethowan R, Pfeiffer WH, Van Ginkel M (2003) Quality (end-use) improvement in wheat. Compositional, genetic, and environmental factors. J Crop Prod 5:1–37. https://doi.org/10.1300/J144v05n01_02
Peng J, Sun D, Nevo E (2011) Wild emmer wheat, Triticum dicoccoides, occupies a pivotal position in wheat domestication process. Aust J Crop Sci 5:1127–1143
Petrova I (2009) Criteria and indices for durum wheat technological quality. Agric Sci 43:23–32
Qin L, Liang Y, Yang D, Dun L, Xia G, Liu S (2015) Novel LMW glutenin subunit genes from wild emmer wheat (Triticum turgidum ssp. dicoccoides) in relation to Glu-3 evolution. Dev Genes Evol 225:31–37. https://doi.org/10.1007/s00427-014-0484-x
Raboy V, Noaman MW, Taylor GA, Pickett SG (1991) Grain phytic acid and protein are highly correlated in winter wheat. Crop Sci 31:631–635. https://doi.org/10.2135/cropsci1991.0011183X003100030017x
Rachoń L, Pałys E, Szumiło G (2012) Comparison of the chemical composition of spring durum wheat grain (Triticum durum) and common wheat grain (Triticum aestivum ssp. Vulgare). J Elem 17(1):105–114. https://doi.org/10.5601/jelem.2012.17.1.10
Rachoń L, Bobryk-Mamczarz A, Kiełtyka-Dadasiewicz A (2020) Hulled wheat productivity and quality in modern. Agriculture 10:275. https://doi.org/10.3390/agriculture10070275
Ram S, Govindan V (2020) Improving wheat nutritional quality through biofortifcation. In: Igrejas G, Ikeda TM, Guzmán C (eds) Wheat quality for improving processing and human health. Springer Nature, Switzerland, pp 205–224
Roncallo PF, Guzmán C, Larsen AO, Achilli AL, Dreisigacker S, Molfese E, Astiz V, Echenique V (2021) Allelic variation at glutenin loci (Glu-1, Glu-2 and Glu-3) in a worldwide durum wheat collection and its effect on quality attributes. Foods 10:2845. https://doi.org/10.3390/foods10112845
Ruiz M, Bernard G, Giraldo P (2018) An update of low molecular weight glutenin subunits in durum wheat relevant to breeding for quality. J Cereal Sci 83:236–244. https://doi.org/10.1016/j.jcs.2018.09.005
Rustgi S, Shewry P, Brouns F, Lomme J, Delcour J (2019) Wheat seed proteins: factors influencing their content, composition, and technological properties, and strategies to reduce adverse reactions. Compr Rev Food Sci Food Saf 18:1751–1769. https://doi.org/10.1111/1541-4337.12493
Saini DK, Devi P, Kaushik P (2020) Advances in genomic interventions for wheat biofortification: a review. Agronomy 10:62. https://doi.org/10.3390/agronomy10010062
Santos CA, Fonseca J, Lopes MT, Carolino E, Guerreiro AS (2017) Serum zinc evolution in dysphagic patients that underwent endoscopic gastrostomy for long term enteral feeding. Asia Pac J Clin Nutr 26:227–233
Sayaslan A, Koyuncu M, Yildirim A, Güleç T, Sönmezoğlu Ö, Kandemir N (2012) Some quality characteristics of selected durum wheat (Triticum durum) landraces. Turk J Agric for 36:14. https://doi.org/10.3906/tar-1201-69
Sharma JS, Running KLD, Xu SS, Zhang Q, Peters H, Sharma S, McClean PE, Faris JD (2019) Genetic analysis of threshability and other spike traits in the evolution of cultivated emmer to fully domesticated durum wheat. Mol Genet Genom 294:757–771. https://doi.org/10.1007/s00438-019-01544-0
Sharma V, Choudhary M, Kumar P, Choudhary JR, Khokhar JS, Kaushik P, Goli S (2021) Harnessing the wild relatives and landraces for fe and zn biofortification in wheat through genetic interventions—a review. Sustainability 13:12975. https://doi.org/10.3390/su132312975
Shavrukov Y, Kurishbayev A, Jatayev S, Shvidchenko V, Zotova L, Koekemoer F, Groot S, Soole K, Langridge P (2017) Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front Plant Sci 8:1950. https://doi.org/10.3389/fpls.2017.01950
Shewry PR, Tatham AS, Forde J, Kreis M, Miflin BJ (1986) The classification and nomenclature of wheat gluten proteins: a reassessment. J Cereal Sci 4:97–106. https://doi.org/10.1016/s0733-5210(86)80012-1
Shewry PR, Tatham AS, Barro F, Barcelo P, Lazzeri P (1995) Biotechnology of breadmaking: unraveling and manipulating the multi-protein gluten complex. Nat Biotechnol 13:1185–1190. https://doi.org/10.1038/nbt1195-1185
Sieber A-N, Würschum T, Longin F (2015) Vitreosity, its stability and relationship to protein content in durum wheat. J Cereal Sci 61:71–77. https://doi.org/10.1016/j.jcs.2014.10.008
Singh B, Jasrotia P, Crespo-Herrera L (2022) Breeding for aphid resistance in wheat: status and future prospects. New Horizons Wheat Barley Res. https://doi.org/10.1007/978-981-16-4449-8_16
Sissons M, Kadkol G, Taylor J (2020) Genotype by environment effects on durum wheat quality and yield-implications for breeding. Crop Breed Genet Genom 2:e200018. https://doi.org/10.20900/cbgg20200018
Stehno Z (2007) Emmer wheat rudico can extend the spectra of cultivated plants. Czech J Plant Breed 43:113–115
Subira J, Peña RJ, Álvaro F, Ammar K, Ramdani A, Royo C (2014) Breeding progress in the pasta–making quality of durum wheat cultivars released in Italy and Spain during the 20th Century. Crop Pasture Sci 65:16–26. https://doi.org/10.1071/CP13238
Suchowilska E, Wiwart M, Kandler W, Krska R (2012) A comparison of macro- and microelement concentrations in the whole grain of four Triticum species. Plant Soil Environ 58(3):141–147. https://doi.org/10.17221/688/2011-PSE
Swamy BM, Marathi B, Ribeiro-Barros AI, Ricachenevsky FK (2021) Development of healthy and nutritious cereals: recent insights on molecular advances in breeding. Front Genet 12:635006. https://doi.org/10.3389/fgene.2021.635006
Tabbita F, Lewis S, Vouilloz JP, Ortega MA, Kade M, Abbate PE, Barneix A (2013) Effects of the Gpc-B1 locus on high grain protein content introgressed into argentinean wheat germplasm. Plant Breed 132:48–52. https://doi.org/10.1111/pbr.12011
Tabbita F, Pearce S, Barneix AJ (2017) Breeding for increased grain protein and micronutrient content in wheat: ten years of the GPC-B1 gene. J Cereal Sci 73:183–191. https://doi.org/10.1016/j.jcs.2017.01.003
Taneva K, Bozhanova V, Hadzhiivanova B (2015) Study of emmer (Triticum dicoccum (Schrank) Shuebl.) accessions for traits related to spike productivity and grain quality in connection to durum wheat improvement. Agri Sci Technol 7:199–203
Tonk F, Ilker E, Tosun M (2010) A study to incorporate high protein content from tetraploid wheat (T. turgidum dicoccoides) to hexaploid wheat (T. aestivum vulgare). Turk J Field Crops 15:69–72
Tran B, Cavagnaro T, Able J, Watts-Williams S (2021) Bioavailability of zinc and iron in durum wheat: a trade-off between grain weight and nutrition? Plants People Planet 3:627–639. https://doi.org/10.1002/ppp3.10151
Troccoli A, Codianni P (2005) Appropriate seeding rate for einkorn, emmer, and spelt grown under rainfed condition in southern Italy. Eur J Agron 22:293–300. https://doi.org/10.1016/j.eja.2004.04.003
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Uppal SK, Bhise S (2018) Light golden chapatti from durum wheat-nutritionally superior and better shelf life. MOJ Curr Res Rev 1:143–146
US Wheat Commission (2022) https://www.uswheat.org/crop-quality/durum/. Accessed 21 Aug 2022
USDA (United States Department of Agriculture) (2022) Small grains 2021 summary. https://www.nass.usda.gov/Publications/Todays_Reports/reports/smgr0921.pdf. Accessed 21 Aug 2022
Vecherska LA, Liubych VV, Relina LI, Golik OV, Suchkova VM, Bohuslavskyi RL (2021) Starting material for breeding spring emmer (Triticum dicoccum shrank.) of groats use. Agric Sci Pract 8:2312–3370. https://doi.org/10.15407/agrisp8.02.062
Velu G, Tutus Y, Gomez-Becerra HF, Hao Y, Demir L, Kara R, Crespo-Herrera LA, Orhan S, Yazici A, Singh R, Cakmak I (2017) QTL mapping for grain zinc and iron concentrations and zinc efficiency in a tetraploid and hexaploid wheat mapping populations. Plant Soil 411:81–99. https://doi.org/10.1007/s11104-016-3025-8
Villegas D, Alfaro C, Ammar K, Cátedra MM, Crossa J, García del Moral LF, Royo C (2016) Daylength, temperature and solar radiation effects on the phenology and yield formation of spring durum wheat. J Agron Crop Sci 202:203–216. https://doi.org/10.1111/jac.12146
Vishwakarma MK, Arun B, Mishra VK, Yadav PS, Kumar H, Joshi AK (2016) Marker-assisted improvement of grain protein content and grain weight in Indian bread wheat. Euphytica 208:313–321. https://doi.org/10.1007/s10681-015-1598-6
Wang K, Fu B (2020) Inter-relationships between test weight, thousand kernel weight, kernel size distribution and their effects on durum wheat milling, semolina composition and pasta processing quality. Foods 9:1308. https://doi.org/10.3390/foods9091308
Wani SH, Gaikwad K, Razzaq A, Samantara K, Kumar M, Govindan V (2022) Improving zinc and iron biofortification in wheat through genomics approaches. Mol Biol Rep 49:8007–8023. https://doi.org/10.1007/s11033-022-07326-z
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55:353–364. https://doi.org/10.1093/jxb/erh064
WHO (World Health Organization) (2002) Ten leading causes of illness and disease in low income countries. The world health report 2002 geneva: WHO. http://www.who.int/mediacentre/factsheets/fs310_ 2008.pdf. Accessed 21 Aug 2022
Xiang L, Huang L, Gong F, Liu J, Wang Y, Jin Y, He Y, He J, Jiang G, Zheng Y, Liu D, Wu B (2019) Enriching LMW-GS alleles and strengthening gluten properties of common wheat through wide hybridization with wild emmer. 3 Biotech 9:355. https://doi.org/10.1007/s13205-019-1887-
Zhang W, Chao S, Manthey F, Chicaiza O, Brevis JC, Echenique V (2008) QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theor Appl Genet 117:1361–1377. https://doi.org/10.1007/s00122-008-0869-1
Zhao FJ, Su YH, Dunham SJ, Rakszegi M, Bedo Z, McGrath SP, Shewry PR (2009) Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J Cereal Sci 49:290–295. https://doi.org/10.1016/j.jcs.2008.11.007
Zhen S, Han C, Ma C, Gu A, Zhang M, Shen X, Li X, Yan Y (2014) Deletion of the low-molecular-weight glutenin subunit allele Glu-A3a of wheat (Triticum aestivum L.) significantly reduces dough strength and breadmaking quality. BMC Plant Biol 14:367. https://doi.org/10.1186/s12870-014-0367-3
Zhou W, Wu S, Ding M, Li J, Shi Z, Wei W, Guo J, Zhang H, Jiang Y, Rong J (2016) Mapping of Ppd-B1, a major candidate gene for late heading on wild emmer chromosome arm 2BS and assessment of its interactions with early heading QTLs on 3AL. PLoS One 11:e0147377. https://doi.org/10.1371/journal.pone.0147377
Acknowledgements
To the Agencia Nacional de Investigación y Desarrollo de Chile (ANID), Fondecyt Postdoctorado number 3220020 and Fondecyt Regular 1210092 for the support in the publication of this review.
Funding
This work was supported by the Agencia Nacional de Investigación y Desarrollo de Chile (ANID) [Fondecyt Postdoctorado grant number 3220020, 2022].
Author information
Authors and Affiliations
Contributions
The first draft was written by PC-L and the rest of the authors commented and made suggestions which were corrected, read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no competing interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cabas-Lühmann, P., Arriagada, O., Matus, I. et al. Comparison of durum with ancient tetraploid wheats from an agronomical, chemical, nutritional, and genetic standpoints: a review. Euphytica 219, 61 (2023). https://doi.org/10.1007/s10681-023-03188-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-023-03188-z