Abstract
Lipopeptides (LPs) from B. tequilensis EA-CB0015 have antifungal activity against Fusarium species. Specifically, against F. oxysporum f. sp. cubense (Foc), the cause of Fusarium wilt of banana. Cinnamon (CN) extract is also known for its inhibitory activity against the Fusarium genus. The main goal of this research was to determine whether the effect of LPs and CN extract or their combination against a Foc isolate is related to an impairment of mitochondrial function. Our results show that biomass decreased by 74% (p < 0.0001) and 84% (p < 0.0001) when cultures were treated with 128 ppm LPs and 152.5 ppm CN extract, respectively. In parallel, we found a pronounced impairment of the bioenergetic response. That is, the routine oxygen consumption rate diminished by 55% (p = 0.0148) and 38% (p < 0.0001), respectively. Moreover, the ATP-linked respiratory rate decreased by 63% (p = 0.0461) and 44% (p = 0.0005), while the FCCP-simulated respiratory rate by 63% (p = 0.0255) and 45% (p = 0.0002). Therefore, our data suggest that the altered bioenergetic response observed in cultures of Foc treated with LPs or CN is mainly caused by an impairment of the activity of the respiratory complexes. On the other hand, biomass production was reduced by 80% (p < 0.0001) when cultures were treated with a mixture comprising only 10% LPs and 40% CN extract. Furthermore, ATP-linked and FCCP-stimulated respiratory rates decreased by 62% (p = 0.0024) and 68% (p < 0.0001), respectively under the same conditions. A potentially synergistic antifungal effect of cyclic LPs with a CN extract is suggested.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal plant diseases are a significant threat to agricultural production. They cause substantial crop yield losses, which, in turn, has a negative impact on the economy and global food security (Sharma & Manhas, 2020). Such is the case for Fusarium Wilt of Banana, a widely distributed disease caused by the fungus Fusarium oxysporum f. sp. cubense (Foc) (Mon et al., 2021; Ploetz, 2015). An eradication method for the Fusarium Wilt of Banana is not currently known (Ismaila et al., 2022); however, biological control is a very promising approach to protect banana crops against Foc (Abram et al., 2021; Dimkić et al., 2022; Köhl et al., 2019). In this regard, microbial secondary metabolites have proven to produce direct antibiosis against fungal pathogens (Köhl et al., 2019). Moreover, the Bacillus subtilis complex, which comprises Bacillus amyloliquefaciens, B. subtilis, B. velenzensis and B. tequilensis species, is known for its antifungal properties, due to metabolites such as polyketides (PKs), siderophores and non-ribosomal peptides (NRPs) (Caulier et al., 2019; Labiadh et al., 2021). Specifically, cyclic lipopeptides (LPs), a class of metabolites that comprises isoforms of fengycin, iturin and surfactin families, isolated from Bacillus tequilensis EA-CB0015, are biomolecules found to have prominent activity against Fusarium spp. (Arroyave-Toro et al., 2017). Despite the fact that LPs represent a promising alternative in the control of phytopathogens such as Fusarium spp., it is necessary to improve their efficacy and cost of production to compete with agrochemical methods. In this respect, an encouraging strategy is to look for combinations of biological compounds with a high potential to cause synergistic antifungal effects. Nevertheless, this approach needs additional research efforts in order to answer questions such as the underlying molecular or cellular events that determine their antifungal effect.
Plant-based extracts are another interesting approach against phytopathogens. These are composed of a broad range of organic bioactive compounds, which are known to have insecticidal, fungicidal, bactericidal, or anti-parasitic properties (Gonçalves et al., 2021). The antifungal effect of plant-based extracts has been reported to involve hydrophobic interactions with the membrane of the host organism, alterations of the cellular structure and affections of metabolic pathways (An et al., 2019; Bi et al., 2021; Feng et al., 2019; Roohinejad et al., 2017). Specifically, cinnamon (Cinnamomum zeylanicum) extract is known for its antifungal effect on the Fusarium genus, which is associated with increased production of reactive oxygen species (ROS), morphological alterations, and growth inhibition (Lee et al., 2020; Shreaz et al., 2016; Velluti, 2003; Xing et al., 2014a, 2014b). However, there is still a gap in knowledge as to the exact cellular targets of those extracts.
Some studies suggest that alterations of the mitochondrial function may be one underlying factor of the antifungal effect of bacterial LPs (Cárdenas-Monroy et al., 2017; de Zoysa et al., 2018; Patkar et al., 2012). Furthermore, there is evidence that Bacillus LPs decrease ATPase activity in fungal cells (Patkar et al., 2012), and that iturin A generates alterations in energy metabolism in Aspergillus carbonarus (Jiang et al., 2020). In addition, evidence from biophysical studies indicates that changes in the lipid milieu of the plasma membrane are among the fundamental factors that mediate fungal growth inhibition by bacterial lipopeptides. For instance, an alteration of the thermotropic phase transition of multilamellar vesicles of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was correlated to the action of fengyin C (González-Jaramillo et al., 2017).
Furthermore, cinnamon (CN) extract has been reported as inhibitor of secondary metabolism on Fusarium verticilloides (Velluti, 2003; Xing et al., 2014a), and compounds of cinnamon extract such as trans-cinnamaldehyde, neral, and geranial are reported to produce ROS generation and cell membrane disruption on Raffaelea quercus-mongolicae and Rhizoctonia solani (Lee et al., 2020). Taken together, these findings indicate the possibility of mitochondrial alterations caused by CN extract on fungi. However, it is completely unknown, to the best of our knowledge, whether a combination of bacterial LPs and CN extract may cause a synergistic antifungal effect against species of the Fusarium oxysporum complex, and whether alterations of the bioenergetic response may mediate such effect. Therefore, the aim of this study was to evaluate the effect of a methanolic CN extract and LPs, both as individual agents and as a mixture, against Foc strain IB (race1). Furthermore, we investigated the hypothesis that alterations of mitochondrial bioenergetics are part of the antifungal mechanism exhibited by these natural products. To this end, we used high resolution respirometry to determine changes in fungal oxygen consumption rates as a means to perform a bioenergetic discrimination of the effect of bacterial LPs and CN extract on fungal bioenergetics.
Materials and methods
Reagents
Oligomycin A, F1F0-ATPase inhibitor; carbonyl cyanide 4- trifluoromethoxy phenylhydrazone (FCCP), mitochondrial uncoupler; and antimycin A from Streptomyces sp., complex III inhibitor, were obtained from Sigma-Aldrich. Other reagents such as potassium chloride (KCl), magnesium chloride (MgCl2), and methanol were from Merck. Potassium phosphate dibasic (K2HPO4), dextrose, hepes and EGTA were from AMRESCO.
Microorganisms and culture conditions
Bacillus tequilensis EA-CB0015 (NCBI reference sequence NZ_CP048852.1), previously isolated from the phyllosphere of a banana plant in Urabá, Antioquia, Colombia (Ceballos et al., 2012) was activated from frozen cultures on 50% TSA (Tripticase Soy Agar, Merck) for 48 h at 30 °C before any experimental use. For identification of the Fusarium isolate, sequence analysis of the RNA polymerase II largest subunit (rpb1) and the RNA polymerase II second largest subunit (rpb2) were used. Nucleotide sequences were submitted to GenBank and were deposited with accession numbers OR698889 and OR698890, respectively. This isolate was identified as Foc IB, a pathogen on Gros Michel (AAA) (Maryani et al., 2019). Foc strain IB from Universidad EAFIT was kept at 4 ºC in folded filter paper during storage. Prior to their use for biomass production, the fungal strain was activated by plate culture on Potato Dextrose Agar (PDA, Oxoid) for 7-9 days at 30°C. Fusarium spores were resuspended with sterile water and were adjusted to 1.5 × 105 spores·mL−1.
On the other hand, additional experiments were conducted in the Laboratory of Phytopathology at the Wageningen University. To this end, strains of Foc IB, F. phialophorum CR1.1A (CR1.1A), and F. odoratissimum II5 (TR4-II5) were activated on PDA at 25°C for 7 days before growth assays.
Cyclic lipopeptides and cinnamon extract
Production, extraction and purification of cyclic LPs from B. tequilensis EA-CB0015 were performed as previously described (Mosquera et al., 2014, Villegas-Escobar et al., 2013). The mixture comprising iturin, fengycin, and surfactin isoforms was kept at 4 ºC until its use.
CN extract was obtained through hydro-distillation, following a previously described methodology (Roohinejad et al., 2017). Briefly, culinary cinnamon sticks (60 g) were immersed in a 350 mL ethanol bath (96%) and heated to boiling temperature. The extract decanted by cooling was evaporated to a solid residue, with a yield of 51 mg cinnamon extract/g cinnamon sticks. When needed, cinnamon extract was diluted in methanol and stored at 4 ºC until any experimental use.
Growth kinetics and culture conditions
Foc IB biomass was produced in liquid culture, using Sabouraud-2% Dextrose Broth (Merck). Briefly, fungal spores were collected from a Petri dish after 7 days of growth and adjusted to 1.5 × 105 spores·mL−1 using a Neubauer counting chamber. Erlenmeyer flasks containing 20 mL of liquid media were inoculated with 1 mL of the spore suspension and the corresponding treatment was added (lipopeptides, cinnamon extract or a combination of both). For biomass production assays, the following concentrations were used: 64, 80, 96, 128 and 256 ppm (LPs), and 122 and 152.5 ppm (CN extract). On the other hand, 128 ppm LPs and 152.5 ppm CN extract were the treatments used for respirometry assays. Also, these experiments were done using a mixture comprising 12.8 ppm LPs and 61 ppm CN extract. Treated cultures and the corresponding controls were incubated at 30 ± 0.5 ºC and 120 rpm for 96 h. In all cases, samples were taken every 4 h (for growth kinetics) or 24 h (for oxygen consumption assays). In addition, total biomass within each flask was harvested to determine its dry weight. Growth kinetics parameters were determined by fitting the data to the Gompertz model (Wilkins, 2022). A specific growth rate of 0.4211 h-1, 95% confidence interval [0.2611 h-1, 0.799 h-1], was determined for untreated cultures of Foc IB (R2 = 0.9639). All experiments were performed in triplicate.
In vitro inhibitory effect of LPs and CN extract on species of the Fusarium oxysporum complex
In order to contrast the inhibitory effect of LPs and CN extract on different species of the Fusarium oxysporum complex, which could be expected due to genetic diversity, growth of F. oxysporum IB, F. phialophorum CR1.1A, and F. odoratissimum II5 was evaluated through the dual plate technique, as previously described (Al-Rashdi et al., 2022). It is important to mention that those experiments were done thanks to a collaboration with Dr. Gert Kema, from the Laboratory of Phytopathology at Wageningen University (The Netherlands). This, as our laboratory is not legally allowed to manipulate Foc-TR4 due to high risk for banana plantations our Country.
High resolution respirometry assays
Respiratory characteristics of mycelium homogenates were determined by high resolution respirometry using an Oxygraph-2k (Oroboros Instruments) (Gnaiger, 2009; Pesta & Gnaiger, 2012; Porter et al., 2015). Briefly, fungal biomass was filtered using qualitative filter paper (Advantec No.1, diameter 110 mm), recovered in sterile 50 mL tubes, and resuspended in respiration buffer (20 mM Hepes, 5 mM K2HPO4, 135 mM KCl, 5 mM MgCl2, 1 mM EGTA and 10 mM dextrose, pH 7.0) (Robles-Martínez et al., 2014). The suspension was gently homogenized using a Dounce tissue grinder (Sigma-Aldrich), and mycelial homogenization was checked under the microscope. A volume of 2 mL of the suspension (40 mg of biomass) was added into each chamber of the Oxygraph-2k. Oxygen consumption rates were obtained using the O2k-software DatLab 7.4 (Oroboros Instruments). ATP-linked respiratory rate, FCCP-stimulated respiratory rate, H+ leak and non-mitochondrial respiration were determined by successive addition of 60 μM (total) oligomycin A, 6 μM FCCP and 8 μM antimycin A. Those concentrations were previously empirically established to be optimal.
Data analysis
Analysis of variance (ANOVA) was used to analyze each experiment in GraphPad Prism 9.3.0 (GraphPad Software Inc, California, USA). The normality assumption, homoscedasticity, and independence were also determined using the Shapiro-Wilks test, the Levenne’s test, and graphic residues vs. run order analysis, respectively. Tukey and Sidak's post hoc tests were applied to assess the significance level for multiple comparisons.
Results
Lipopeptides from Bacillus tequilensis EA-CB0015 and cinnamon extract cause growth inhibition of F. oxysporum f. sp. cubense both as individual agents and in combination.
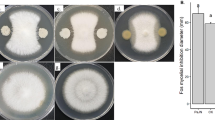
As shown in Fig. 1A, three growth phases (i.e. lag, exponential and stationary) are evident in liquid cultures of Foc IB. As previously stated, a specific growth rate of 0.4211 h-1, 95% confidence interval [0.2611 h-1, 0.7990 h-1], was determined for untreated cultures of Foc IB (R2 = 0.9639). In order to determine the effect of different treatments on fungal growth, biomass production was quantified upon 24 h, when cultures were in exponential phase. Analysis of growth kinetics was also done in cultures treated with LPs, CN extract of a mixture of both (data not shown). According to our results, bacterial LPs cause a concentration-dependent decrease in biomass, which was found to be 26% of the corresponding control in cultures treated with 128 ppm LPs (Fig. 1B). Similar results were obtained when fungal cultures were treated with an methanolic CN extract (Fig. 1C). Specifically, biomass production was observed to decline by 56% and 84% in cultures treated with 122 ppm and 152.5 ppm of CN extract, respectively.
Fungal biomass production decreases upon treatment of F. oxysporum f. sp. cubense IB liquid cultures with lipopeptides (LPs) from B. tequilensis EA-CB0015 and cinnamon (CN) extract. (A) Growth kinetics for control cultures was determined using dry biomass and fitting of the data was done according to the Gompertz model as described in Materials and Methods. Data are shown as the mean ± standard deviation for n = 3 independent experiments. (B-D) Effect of LPs, CN extract and the mix of both on biomass production after 24 h of growth. Results are shown as box and whisker plots. Different letters above boxes in panels B and C denote a statistically significant decrease (p < 0.0001, from a one-way ANOVA) in fungal biomass. The p value shown in panel D was obtained from a two-tailed student’s t-test. In all cases, symbols within boxes denote the number of independent experiments
On the other hand, when fungal cultures were treated with a mixture composed of 12.8 ppm LPs and 61 ppm CN extract, biomass production was reduced by 80%, relative to control cultures (Fig. 1D). Interestingly, the concentrations of both LPs and CN extract in the mixture were only 10% and 40%, respectively, of the concentrations to which those treatments, individually, caused growth inhibition to a similar extent. Moreover, as seen in the data shown in Table 1, Foc-TR4 is slightly less sensitive (44.9% vs 55.99%) to growth inhibition caused by LPs, relative to Foc IB. On the contrary, there is not significant difference in the inhibitory effect caused by CN extract or the combination of LPs with CN extract.
Treatment of F. oxysporum f. sp. cubense by lipopeptides from Bacillus tequilensis EA-CB0015 and cinnamon extract results in impaired mycelial bioenergetic response.
Our results show that Foc IB has a bioenergetic response very similar to that of mammalian cells, which is characterized for an O2 consumption pattern mostly associated to ATP synthesis (Fig. 2A). As also shown in Fig. 2A, ATP-linked respiratory rate, FCCP-stimulated respiratory rate, H+ leak and non-mitochondrial respiration can be determined by successive addition of oligomycin A, FCCP and antimycin A. Inhibition by antimycin A was used to determine non-mitochondrial O2 consumption (Fig. 2A).
Oxygen consumption and respiratory characteristics in samples of F. oxysporum IB untreated mycelium homogenate. (A) Samples were added to each chamber of the Oxygraph-2k system and O2 concentration (blue trace) and specific O2 consumption rate (red trace) were determined. As indicated, routine O2 consumption rate was determined in the closed chamber, following saturation of the respiration buffer. At the time points indicated, oligomycin A (60 μM total) and antimycin A (8 μM) were added as inhibitors of the mitochondrial F1F0-ATPase and the complex III, respectively. Also, FCCP (6 μM) was used to uncouple mitochondrial respiration. Traces are representative of at least sixteen independent experiments. All experiments were performed at 25 ºC. (B) Routine, oligomycin-sensitive, FCCP-stimulated, and antimycin A-sensitive O2 consumption rates determined in mycelium homogenate. Results are shown as box and whisker plots for cultures in exponential phase of growth. Numbers above boxes correspond to the p value obtained from a Tukey’s post-hoc test together with a one-way ANOVA (p < 0.0001). In all cases, symbols within boxes denote the number of independent experiments
Figure 2B shows routine, oligomycin-sensitive, FCCP-stimulated, and antimycin A-sensitive O2 consumption rates determined in mycelium homogenates from control cultures. According to our data, inhibition of the mitochondrial F1F0-ATPase by oligomycin A results, on average, in a 64% decrease of the O2 consumption rate. On the other hand, the uncoupler FCCP increased the oligomycin A-sensitive O2 consumption rate by a factor of 3.5, a clear indication of a functional ETC in Foc IB. To our surprise, no significant difference was observed between FCCP-stimulated and routine O2 consumption rates, suggesting the lack of bioenergetic reserve capacity in the exponential phase of fungal growth. In addition, inhibition of mitochondrial complex III by antimycin A was observed to decrease routine and FCCP-stimulated O2 consumption rates by 81% and 85%, respectively. Therefore, 19% of the routine O2 consumption rate, on average, appears to be related to non-mitochondrial processes.
With respect to the effect of bacterial LPs or CN extract, our results show a pronounced impairment of the bioenergetic response in all cases (Figs. 3, 4, 5). Based on our data, mycelial routine O2 consumption rate diminished by 55% (Fig. 3A) and 38% (Fig. 4A) in cultures treated with 128 ppm LPs and 152.5 ppm CN extract, respectively. The same treatments also decrease the FCCP-stimulated O2 consumption rate by 56% (Fig. 3A) and 40% (Fig. 4A). In order to discern the true extent to which mitochondrial bioenergetics is adversely affected in fungal mycelium treated with LPs or CN extract, changes in ATP-linked respiratory rate, FCCP-stimulated respiratory rate, and H+ leak were investigated. As shown in Figs. 3B and 4B, 128 ppm LPs and 152.5 ppm CN extract reduced the ATP-linked respiratory rate by 63% and 44%, respectively. Moreover, the same individual treatments of fungal cultures decreased the FCCP-simulated respiratory rate by 63% and 45%, respectively (Figs. 3C and 4C). Therefore, our data suggest that the altered bioenergetic response observed in cultures of Foc IB treated with LPs or CN is mainly caused by an impairment of the activity of the ETC components. One experimental result that supports this explanation is the lack of significant differences in the respiration rate corresponding to H+ leak, when comparing mycelium from treated cultures with their corresponding control (Figs. 3D and 4D). On average, H+ leak was determined to be 21% of the routine O2 consumption rate.
Bioenergetic response of F. oxysporum f. sp. cubense strain IB is adversely affected upon treatment of cultures with lipopeptides (LPs) from B. tequilensis EA-CB0015. (A) Routine, oligomycin-sensitive, FCCP-stimulated, and antimycin A-sensitive O2 consumption rates determined in both control and treated mycelium homogenates (n = 5). Results are shown as box and whisker plots for cultures in exponential phase of growth. Numbers above boxes correspond to the p value obtained from a Sidak’s multiple comparison test together with a two-way ANOVA analysis for the effect of lipopeptides (p = 0.0349). Analysis of ATP-linked respiratory rate (B), FCCP-stimulated respiratory rate (C), and H+ leak (D) (see Materials and methods). The p values shown in panels C-D were obtained from a two-tailed student’s t-test
Bioenergetic response of F. oxysporum f. sp. cubense strain IB is adversely affected upon treatment of cultures with cinnamon (CN) extract. (A) Routine, oligomycin-sensitive, FCCP-stimulated, and antimycin A-sensitive O2 consumption rates determined in both control and treated mycelium homogenates (n = 8). Results are shown as box and whisker plots for cultures in exponential phase of growth. Numbers above boxes correspond to the p value obtained from a Šidák’s multiple comparison test together with a two-way ANOVA analysis for the effect of lipopeptides (p < 0.0001). Analysis of ATP-linked respiratory rate (B), FCCP-stimulated respiratory rate (C), and H+ leak (D) (see Materials and methods). The p values shown in panels C-D were obtained from a two-tailed student’s t-test
Bioenergetic response of F. oxysporum f. sp. cubense strain IB is adversely affected upon treatment of cultures with a mix comprising 12.8 ppm LPs and 61 ppm CN extract. (A) Routine, oligomycin-sensitive, FCCP-stimulated, and antimycin A-sensitive O2 consumption rates determined in both control and treated mycelium homogenates (n = 6). Results are shown as box and whisker plots for cultures in exponential phase of growth. Numbers above boxes correspond to the p value obtained from a Šidák’s multiple comparison test together with a two-way ANOVA analysis for the effect of lipopeptides (p < 0.0001). Analysis of ATP-linked respiratory rate (B), FCCP-stimulated respiratory rate (C), and H+ leak (D) (see Materials and methods). The p values shown in panels C-D were obtained from a two-tailed student’s t-test
Because our analysis of mycelial biomass suggests that there may be a potential synergistic effect of the combination of bacterial LPs and CN extract (Fig. 1), we also investigated the bioenergetic response of mycelium from cultures treated with a mixture composed of 12.8 ppm LPs and 61 ppm. Similar to our results for cultures treated individually with these agents, our data show that, together, they decrease mycelial routine and FCCP-stimulated O2 consumption rates by 57% and 62%, respectively (Fig. 5A). Furthermore, our results indicate that such a combination diminishes ATP-linked and FCCP-stimulated respiratory rates by 62% and 68%, respectively (Fig. 5B and C). As highlighted in the previous section, interestingly, the concentrations of both LPs and CN extract in the mixture were only 10% and 40%, respectively, of the concentrations to which those treatments, individually, caused growth inhibition and decreased fungal bioenergetic capacity. Finally, although the respiratory rate corresponding to H+ leak in mycelium from cultures treated with the mixture was found to be significantly different from the corresponding control group (Fig. 5D), those values were so close (29% vs 25% of the routine O2 consumption rate) that does not seem reasonable to think on alterations of mitochondrial membrane integrity as the leading cause of the altered bioenergetic response observed.
Discussion
In this study, we shed light on the cellular events underlying the antifungal effect of natural products such as bacterial lipopeptides or the metabolites present in a methanolic cinnamon extract. Specifically, our data support the hypothesis that alterations to mitochondrial bioenergetics play a key role in the effect of these agents against the fungal phytopathogen F. oxysporum IB. This hypothesis is also supported by a recent study that demonstrates the key role cell energy status regulation plays in the mycelial development and pathogenicity of Arthrobotrys oligospora (Wang et al., 2022). Our detailed analysis of the fungal bioenergetic response reveals a significant decrease in the rate of oxygen consumption, which appears to be strictly related to a deterioration of the ETC components, when mycelium from treated cultures was compared to the corresponding controls. This is because ATP-linked and FCCP-stimulated respiratory rates were affected to a similar extent. In this regard, our results agree well with previous studies where a cinnamon aqueous extract was reported to cause significant inhibition of complex I activity in mitochondria isolated from rat liver (Usta et al., 2003, 2002). Furthermore, a decrease in the mitochondrial membrane potential was also reported by these authors. On the contrary, the activity of the F1F0-ATPase was stimulated when isolated mitochondria were incubated with the cinnamon extract in a concentration as high as 37 mM (Usta et al., 2003, 2002). Moreover, cinnamaldehyde, one of the main compounds found in cinnamon (Stevens & Allred, 2022; Tian et al., 2022) was recently reported to significantly inhibit the growth of the mold Penicillium expansum, a postharvest pathogen of fruits, at the same time that a diminished mitochondrial membrane potential was observed (Wang et al., 2018). Interestingly, significant downregulation of differentially expressed genes involved in energy-metabolism was also reported in the same study, consistent with an impaired bioenergetic response as on the leading factors mediating the antifungal capacity of cinnamaldehyde.
By determining the specific alterations to fungal bioenergetic response, our results add to previous efforts to discern the cellular events that mediate the antifungal effect of bacterial lipopeptides and cinnamon extract. In this regard, it is worth mentioning one pioneering study in which WH1fungin, a surfactin isolated from Bacillus amyloliquefaciens WH1, was found to increase ROS production and activity of caspase-like proteins, a characteristic feature of programmed cell death which involves mitochondrial dysfunction, as part of its activity against Rhizoctonia solani (Qi et al., 2010). In accordance with this, alterations in fungal cell ultrastructure, upon treatment with bacterial lipopeptides, have been extensively reported as well. For instance, hyphal morphology alteration in addition to an increase in the generation of Reactive Oxygen Species (ROS) was identified as the main effect of surfactin species CS30-1 and CS30-2 from Bacillus sp. CS30 on Magnaporthe grisea (Wu et al., 2019). In a similar study, chromatin condensation, ROS accumulation and lower mitochondrial membrane potential in hyphal cells of M. grisea were observed to be induced by fengycins isolated from Bacillus subtilis BS155 (Zhang & Sun, 2018).
Also, alteration of conidial and hyphal morphology, together with changes in cell surfaces and cellular contents, plasma membrane integrity and cell wall were reported in Fusarium graminearum treated with lipopeptides from B. amyloliquefaciens S76-3 (Gong et al., 2015). In addition, alterations of mycelial growth and intracellular ultrastructures were observed in Botrytis cinerea treated with lipopeptides from Bacillus XT1 CECT 8661 (Toral et al., 2018). On the other hand, similar mycelial alterations have been reported in the filamentous fungus Podospora anserina during aging (Osiewacz, 2002; Osiewacz et al., 2010). Therefore, our results could be indicating mitochondrial abnormalities that are reminiscent of fungal aging, as a consequence of bacterial lipopeptides and cinnamon extract. Specifically, alterations in mitochondrial dynamics were reported in P. anserina mutants lacking the dynamin-related protein 1 (Dnm1p), a protein key to regulate mitochondrial fission (Scheckhuber et al., 2007). Interestingly, those fungal mutants were resistant to induction of apoptosis and increased their life span. Thus, cell death and mitochondrial dynamics are intimately related in filamentous fungi.
Even though alterations of the lipid milieu of fungal biomembranes have been previously reported as the underlying factor of fungal inhibition by bacterial lipopeptides (Fiedler & Heerklotz, 2015; González-Jaramillo et al., 2017; Siahmoshteh et al., 2018), our data does not support that energy uncoupling, mediated by alterations in the inner membrane of mitochondria, plays a significant role in the loss of bioenergetic response. Moreover, our results indicate that lipopeptides from B. tequilensis EA-CB0015 and cinnamon extract significantly decrease oxidative capacity because an impairment of the ETC components, which could be hypothesized to be involved in alterations of mitochondrial dynamics.
Conclusion
The role mitochondrial function plays in fungal growth and pathogenicity has been a topic of interest during the last twenty years. While several studies have focused on fungi responsible by infections in humans, attention to mitochondria and energy metabolism in phytopathogens is only being paid more recently. Moreover, specific and detailed studies focused on establishing whether mitochondrial abnormalities are part of the mechanism by which bacterial and plant metabolites affect fungal phytopathogens are barely five years old. In this regard, this work represents, to the best of our knowledge, the first examination of alterations to mitochondrial bioenergetics in Foc related to the inhibitory effect of what appears to be a synergistic combination of lipopeptides from B. tequilensis EA-CB0015 with a methanolic cinnamon extract. Further work should be addressed in order to gain more insights into the potential applications of this combined treatment.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abram, P. K., Labbe, R. M., & Mason, P. G. (2021). Ranking the host range of biological control agents with quantitative metrics of taxonomic specificity. Biological Control, 152, 104427. https://doi.org/10.1016/j.biocontrol.2020.104427
Al-Rashdi, F. K. H., Al-Sadi, A. M., Al-Riyamy, B. Z., Maharachchikumbura, S. S. N., Al-Sabahi, J. N., & Velazhahan, R. (2022). Endophytic fungi from the medicinal plant Aloe dhufarensis Lavranos exhibit antagonistic potential against phytopathogenic fungi. South African Journal of Botany, 147, 1078–1085. https://doi.org/10.1016/j.sajb.2020.05.022
An, P., Yang, X., Yu, J., Qi, J., Ren, X., & Kong, Q. (2019). α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control, 98, 42–53. https://doi.org/10.1016/j.foodcont.2018.11.013
Arroyave-Toro, J. J., Mosquera, S., & Villegas-Escobar, V. (2017). Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biological Control, 114, 195–200. https://doi.org/10.1016/j.biocontrol.2017.08.014
Bi, Z., Zhao, Y., Morrell, J. J., Lei, Y., & Yan, L. (2021). The antifungal mechanism of konjac flying powder extract and its active compounds against wood decay fungi. Industrial Crops and Products, 164, 113406. https://doi.org/10.1016/j.indcrop.2021.113406
Cárdenas-Monroy, C. A., Pohlmann, T., Piñón-Zárate, G., Matus-Ortega, G., Guerra, G., Feldbrügge, M., & Pardo, J. P. (2017). The mitochondrial alternative oxidase Aox1 is needed to cope with respiratory stress but dispensable for pathogenic development in Ustilago maydis. PLoS One, 12, e0173389. https://doi.org/10.1371/journal.pone.0173389
Caulier, S., Nannan, C., Gillis, A., Licciardi, F., Bragard, C., & Mahillon, J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Frontiers in Microbiology, 10, 1–19. https://doi.org/10.3389/fmicb.2019.00302
Ceballos, I., Mosquera, S., Angulo, M., Mira, J. J., Argel, L. E., Uribe-Velez, D., Romero-Tabarez, M., Orduz-Peralta, S., & Villegas, V. (2012). Cultivable bacteria populations associated with leaves of banana and plantain plants and their antagonistic activity against Mycosphaerella fijiensis. Microbial Ecology, 64, 641–653. https://doi.org/10.1007/s00248-012-0052-8
da Costa Gonçalves, D., Ribeiro, W. R., Gonçalves, D. C., Menini, L., & Costa, H. (2021). Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Research International, 150, 110758. https://doi.org/10.1016/j.foodres.2021.110758
de Zoysa, G. H., Glossop, H. D., & Sarojini, V. (2018). Unexplored antifungal activity of linear battacin lipopeptides against planktonic and mature biofilms of C. albicans. European Journal of Medicinal Chemistry, 146, 344–353. https://doi.org/10.1016/j.ejmech.2018.01.023
Dimkić, I., Janakiev, T., Petrović, M., Degrassi, G., & Fira, D. (2022). Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms - A review. Physiological and Molecular Plant Pathology, 117, 101754. https://doi.org/10.1016/j.pmpp.2021.101754
Feng, L., Li, Y., Wang, Z., Qi, L., & Mo, H. (2019). Antifungal actions of glycinin basic peptide against Aspergillus niger through the collaborative damage to cell membrane and mitochondria. Food Biophysics, 14, 97–107. https://doi.org/10.1007/s11483-018-9561-4
Fiedler, S., & Heerklotz, H. (2015). Vesicle leakage reflects the target selectivity of antimicrobial lipopeptides from Bacillus subtilis. Biophysical Journal, 109, 2079–2089. https://doi.org/10.1016/j.bpj.2015.09.021
Ghoreishi, S. M., & Bataghva, E. (2011). Supercritical extraction of evening primrose oil: Experimental optimization via response surface methodology. AIChE Journal, 57, 3378–3384. https://doi.org/10.1002/aic.12532
Gnaiger, E. (2009). Capacity of oxidative phosphorylation in human skeletal muscle. The International Journal of Biochemistry & Cell Biology, 41, 1837–1845. https://doi.org/10.1016/j.biocel.2009.03.013
Gong, A.-D., Li, H.-P., Yuan, Q.-S., Song, X.-S., Yao, W., He, W.-J., Zhang, J.-B., & Liao, Y.-C. (2015). Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76–3 from Wheat Spikes against Fusarium graminearum. PLoS One, 10, e0116871. https://doi.org/10.1371/journal.pone.0116871
González-Jaramillo, L. M., Aranda, F. J., Teruel, J. A., Villegas-Escobar, V., & Ortiz, A. (2017). Antimycotic activity of fengycin C biosurfactant and its interaction with phosphatidylcholine model membranes. Colloids Surf B Biointerfaces, 156, 114–122. https://doi.org/10.1016/j.colsurfb.2017.05.021
Ismaila, A.A., Ahmad, K., Siddique, Y., Wahab, M.A.A., Kutawa, A.B., Abdullahi, A., Zobir, S.A.M., Abdu, A., Abdullah, S.N.A., 2022. Fusarium wilt of banana: Current update and sustainable disease control using classical and essential oils approaches. Horticultural Plant Journal.https://doi.org/10.1016/j.hpj.2022.02.004
Jiang, C., Li, Z., Shi, Y., Guo, D., Pang, B., Chen, X., Shao, D., Liu, Y., & Shi, J. (2020). Bacillus subtilis inhibits Aspergillus carbonarius by producing iturin A, which disturbs the transport, energy metabolism, and osmotic pressure of fungal cells as revealed by transcriptomics analysis. International Journal of Food Microbiology, 330, 108783. https://doi.org/10.1016/j.ijfoodmicro.2020.108783
Köhl, J., Kolnaar, R., Ravensberg, W.J., 2019. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Frontiers in Plant Science 10. https://doi.org/10.3389/fpls.2019.00845
Labiadh, M., Dhaouadi, S., Chollet, M., Chataigne, G., Tricot, C., Jacques, P., Flahaut, S., & Kallel, S. (2021). Antifungal lipopeptides from Bacillus strains isolated from rhizosphere of Citrus trees. Rhizosphere, 19, 100399. https://doi.org/10.1016/j.rhisph.2021.100399
Lee, J.-E., Seo, S.-M., Huh, M.-J., Lee, S.-C., & Park, I.-K. (2020). Reactive oxygen species mediated-antifungal activity of cinnamon bark (Cinnamomum verum) and lemongrass (Cymbopogon citratus) essential oils and their constituents against two phytopathogenic fungi. Pesticide Biochemistry and Physiology, 168, 104644. https://doi.org/10.1016/j.pestbp.2020.104644
Maryani, N., Lombard, L., Poerba, Y. S., Subandiyah, S., Crous, P. W., & Kema, G. H. J. (2019). Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Studies in Mycology, 92, 155–194. https://doi.org/10.1016/j.simyco.2018.06.003
Mon, Y. Y., Bidabadi, S. S., Oo, K. S., & Zheng, S.-J. (2021). The antagonistic mechanism of rhizosphere microbes and endophytes on the interaction between banana and Fusarium oxysporum f. sp. cubense. Physiological and Molecular Plant Pathology, 116, 101733. https://doi.org/10.1016/j.pmpp.2021.101733
Mosquera, S., González-Jaramillo, L. M., Orduz, S., & Villegas-Escobar, V. (2014). Multiple response optimization of Bacillus subtilis EA-CB0015 culture and identification of antifungal metabolites. Biocatalysis and Agricultural Biotechnology, 3, 378–385. https://doi.org/10.1016/j.bcab.2014.09.004
Osiewacz, H. D. (2002). Aging in fungi: Role of mitochondria in Podospora anserina. Mechanisms of Ageing and Development, 123, 755–764. https://doi.org/10.1016/S0047-6374(01)00421-3
Osiewacz, H. D., Brust, D., Hamann, A., Kunstmann, B., Luce, K., Müller-Ohldach, M., Scheckhuber, C. Q., Servos, J., & Strobel, I. (2010). Mitochondrial pathways governing stress resistance, life, and death in the fungal aging model Podospora anserina. Annals of the New York Academy of Sciences, 1197, 54–66. https://doi.org/10.1111/j.1749-6632.2010.05190.x
Patkar, R. N., Ramos-Pamplona, M., Gupta, A. P., Fan, Y., & Naqvi, N. I. (2012). Mitochondrial β-oxidation regulates organellar integrity and is necessary for conidial germination and invasive growth in Magnaporthe oryzae. Molecular Microbiology, 86, 1345–1363. https://doi.org/10.1111/mmi.12060
Pesta, D., & Gnaiger, E. (2012). High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods in Molecular Biology, 810, 25–58. https://doi.org/10.1007/978-1-61779-382-0_3
Ploetz, R. C. (2015). Fusarium Wilt of Banana. Phytopathology, 105, 1512–1521. https://doi.org/10.1094/PHYTO-04-15-0101-RVW
Porter, C., Hurren, N. M., Cotter, M. V., Bhattarai, N., Reidy, P. T., Dillon, E. L., Durham, W. J., Tuvdendorj, D., Sheffield-Moore, M., Volpi, E., Sidossis, L. S., Rasmussen, B. B., & Børsheim, E. (2015). Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism, 309, E224–E232. https://doi.org/10.1152/ajpendo.00125.2015
Qi, G., Zhu, F., Du, P., Yang, X., Qiu, D., Yu, Z., Chen, J., Zhao, X., 2010. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 31, 1978–1986. https://doi.org/10.1016/j.peptides.2010.08.003
Robles-Martínez, L., Guerra-Sánchez, M. G., Hernández-Lauzardo, A. N., Pardo, J. P., & Velázquez-del Valle, M. G. (2014). Effects of chitosan and oligochitosan on development and mitochondrial function of Rhizopus stolonifer. Journal of Basic Microbiology, 54, S42–S49. https://doi.org/10.1002/jobm.201300790
Roohinejad, S., Koubaa, M., Barba, F. J., Leong, S. Y., Khelfa, A., Greiner, R., & Chemat, F. (2017). Extraction Methods of Essential Oils From Herbs and Spices. Essential Oils in Food Processing (pp. 21–55). Wiley. https://doi.org/10.1002/9781119149392.ch2
Scheckhuber, C. Q., Erjavec, N., Tinazli, A., Hamann, A., Nyström, T., & Osiewacz, H. D. (2007). Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nature Cell Biology, 9, 99–105. https://doi.org/10.1038/ncb1524
Sharma, M., & Manhas, R. K. (2020). Purification and characterization of salvianolic acid B from Streptomyces sp. M4 possessing antifungal activity against fungal phytopathogens. Microbiological Research, 237, 126478. https://doi.org/10.1016/j.micres.2020.126478
Shreaz, S., Wani, W. A., Behbehani, J. M., Raja, V., Irshad, M., Karched, M., Ali, I., Siddiqi, W. A., & Hun, L. T. (2016). Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia, 112, 116–131. https://doi.org/10.1016/j.fitote.2016.05.016
Siahmoshteh, F., Hamidi-Esfahani, Z., Spadaro, D., Shams-Ghahfarokhi, M., & Razzaghi-Abyaneh, M. (2018). Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control, 89, 300–307. https://doi.org/10.1016/j.foodcont.2017.11.010
Stevens, N., Allred, K., 2022. Antidiabetic potential of volatile cinnamon oil: A review and exploration of mechanisms using in silico molecular docking simulations. Molecules 27. https://doi.org/10.3390/molecules27030853
Tian, F., Woo, S.Y., Lee, S.Y., Park, S.B., Zheng, Y., Chun, H.S., 2022. Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics 11. https://doi.org/10.3390/antibiotics11121727
Toral, L., Rodríguez, M., Béjar, V., & Sampedro, I. (2018). Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front Microbiol, 9, 1–12. https://doi.org/10.3389/fmicb.2018.01315
Usta, J., Kreydiyyeh, S., Bajakian, K., & Nakkash-Chmaisse, H. (2002). In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food and Chemical Toxicology, 40, 935–940. https://doi.org/10.1016/S0278-6915(02)00071-6
Usta, J., Kreydiyyeh, S., Barnabe, P., Bou-Moughlabay, Y., & Nakkash-Chmaisse, H. (2003). Comparative study on the effect if cinnamon and clove extracts and their main components on different types of ATPases. Human & Experimental Toxicology, 22, 355–362.
Velluti, A. (2003). Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. International Journal of Food Microbiology, 89, 145–154. https://doi.org/10.1016/S0168-1605(03)00116-8
Villegas-Escobar, V., Ceballos, I., Mira, J. J., Argel, L. E., Orduz Peralta, S., & Romero-Tabarez, M. (2013). Fengycin C Produced by Bacillus subtilis EA-CB0015. Journal of Natural Products, 76, 503–509. https://doi.org/10.1021/np300574v
Wang, W., Zhao, Y., Bai, N., Zhang, K.-Q., & Yang, J. (2022). AMPK Is involved in regulating the utilization of carbon sources, conidiation, pathogenicity, and stress response of the nematode-trapping fungus Arthrobotrys oligospora. Microbiology Spectrum, 10, e0222522. https://doi.org/10.1128/spectrum.02225-22
Wang, Y., Feng, K., Yang, H., Zhang, Z., Yuan, Y., & Yue, T. (2018). Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Frontiers in Microbiology, 9, 1–14. https://doi.org/10.3389/fmicb.2018.00597
Wilkins, M. R. (2022). Factors influencing microbial growth and its kinetics. Current Developments in Biotechnology and Bioengineering (pp. 373–395). Elsevier. https://doi.org/10.1016/B978-0-323-91167-2.00014-9
Wu, S., Liu, G., Zhou, S., Sha, Z., & Sun, C. (2019). Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Marine Drugs, 17, 199. https://doi.org/10.3390/md17040199
Xing, F., Hua, H., Selvaraj, J. N., Yuan, Y., Zhao, Y., Zhou, L., & Liu, Y. (2014). Degradation of fumonisin B1 by cinnamon essential oil. Food Control, 38, 37–40. https://doi.org/10.1016/j.foodcont.2013.09.045
Xing, F., Hua, H., Selvaraj, J. N., Zhao, Y., Zhou, L., Liu, X., & Liu, Y. (2014). Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control, 46, 343–350. https://doi.org/10.1016/j.foodcont.2014.04.037
Zhang, L., & Sun, C. (2018). Cyclic lipopeptides from marine Bacillus subtilis Strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing reactive oxygen species production and chromatin condensation. Applied and Environmental Microbiology, 84, e00445-18. https://doi.org/10.1128/AEM.00445-18
Acknowledgment
This research was done thanks to funding from Universidad EAFIT and Asociación de Bananeros de Colombia (AUGURA); and co-funding from Ministerio de Ciencia, Tecnología e Innovación de Colombia (MINCIENCIAS) [grant number 80740-467-2020]. Also, this research was posible thanks to a license granted by Ministerio de Medio Ambiente y Desarrollo Territorial [contracts 166 from 2017 and 139 from 2017] under the category “Contrato de acceso a recursos genéticos y productos derivados para la investigación científica”. Finally, we want to thank Dr. Liliana Hoyos-Carvajal (Universidad Nacional de Colombia) and Sebastián Zapata-Henao (CENIBANANO) for providing the genomic sequence data for identification of the F. oxysporum strain.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Contributions
Conceptualization (LAG, VVE, JMRM); Data curation (LAG, JMRM); Formal analysis (LAG, JMRM); Funding acquisition (LAG, VVE); Investigation (JMRM, LVR); Methodology (LAG, VVE, JMRM); Project administration (LAG, VVE); Resources (LAG, VVE); Supervision (LAG, VVE); Writing – original draft (LAG, JMRM); Writing – review & editing (LAG, VVE, JMRM). All the authors have read the paper and have agreed to be co-authors.
Corresponding author
Ethics declarations
Declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Highlights
• Cinnamon (CN) and cyclic lipopeptides (LP) inhibit growth of Fusarium spp.
• CN and LPs affect F. oxysporum f. sp. cubense (Foc) bioenergetic response.
• Foc bioenergetics reveals respiration is mostly associated to ATP synthesis.
• The combination of LPs and CN appears to synergistically inhibit Fusarium spp.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramírez-Mejía, J.M., Villegas-Escobar, V. & Gómez, L.A. Lipopeptides from Bacillus tequilensis EA-CB0015 and cinnamon extract decrease the bioenergetic response of Fusarium oxysporum f. sp. cubense. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02882-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02882-5