Abstract
High levels of mycotoxins are occasionally observed in Norwegian oat grain lots. Mycotoxins of primary concern in Norwegian oats are deoxynivalenol (DON) produced by Fusarium graminearum and HT2- and T2-toxins (HT2 + T2) produced by Fusarium langsethiae. Improved understanding of the epidemiology of Fusarium spp. is important for the development of measures to control mycotoxins. We studied the susceptibility to F. langsethiae after inoculation at early (booting, heading, flowering) or late (flowering, milk, dough) growth stages in three oat varieties in greenhouse experiments. The varieties had previously shown different levels of resistance to F. graminearum: Odal, Vinger (both moderately resistant), and Belinda (susceptible). The level of F. langsethiae DNA and HT2 + T2 were measured in harvested grain. In addition, we observed differences in aggressiveness (measured as the level of F. langsethiae DNA in grain) between F. langsethiae isolates after inoculation of oats at flowering. Substantial levels of F. langsethiae DNA (mean ≥ 138 pg per μg plant DNA) and HT2 + T2 (≥348 μg/kg) were detected in grain harvested from oats that were spray-inoculated at heading or later stages, but not at booting (mean ≤ 10 pg/μg and ≤ 25 μg/kg, respectively), suggesting that oats are susceptible to F. langsethiae from heading and onwards. Vinger was the most resistant variety to F. langsethiae/HT2 + T2, whereas Odal and Belinda were relatively susceptible. We observed that late inoculations yielded high levels of other trichothecene A metabolites (mean sum of metabolites of 35–1048 μg/kg) in addition to HT2 + T2, in harvested grain, an indication that infections close to harvest may pose a further risk to food and feed safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oats are an important crop for several countries of the northern hemisphere. Traditionally, oats have been an important raw material for feed. Lately oats have also gained increased use for human consumption due to their beneficial nutritional properties (Clemens & van Klinken, 2014). Oats are, however, susceptible to Fusarium Head Blight (FHB), one of the most important cereal diseases worldwide (Goswami & Kistler, 2004). The disease is caused by different Fusarium species that can infect and damage cereal heads and grains. Upon infection, the fusaria produce different mycotoxins (secondary metabolites) that are toxic to humans and animals upon intake of contaminated cereal or cereal based products. Because of this, Fusarium mycotoxins are one of the most important quality and safety risks of cereal grain for food and feed.
In northern and western Europe, deoxynivalenol (DON) and/or HT2- and T2- toxins (HT2 + T2) are the most predominant mycotoxins in oats, and the levels are sometimes high (Edwards, 2009; Fredlund et al., 2010; Hofgaard, Seehusen, et al., 2016; Schöneberg et al., 2018). The presence of DON has mainly been linked to F. graminearum, whereas HT2 + T2 in oats is predominantly associated with F. langsethiae (Edwards et al., 2012; Fredlund et al., 2010; Hofgaard, Seehusen, et al., 2016; Schöneberg et al., 2018). Fusarium langsethiae and its toxins are more common in oats than other cereals (Edwards et al., 2009; Hietaniemi et al., 2016; Hofgaard, Seehusen, et al., 2016). Opoku et al. (2017) showed that this is not only a result of differences in agronomy of the cereal species but that oats are more susceptible to F. langsethiae than barley and wheat. Divon et al. (2019) observed that the F. langsethiae infection proceeded faster and with denser mycelial growth in flowers of oats than in wheat. This study also showed that the fungus followed the same infection route as F. graminearum though at a slower pace. The presence of F. langsethiae or HT2 + T2 toxins in cereals has rarely been associated with any observation of disease symptoms (Divon et al., 2012; Torp & Adler, 2004).
The occurrence of F. graminearum and DON in naturally contaminated oats is not associated with the occurrence of F. langsethiae and its respective toxins (Brodal et al., 2020; Edwards, 2009; Fredlund et al., 2010; Hofgaard, Seehusen, et al., 2016; Kaukoranta et al., 2019), and it has been speculated whether this is linked to differences in the biology of these fungal species. Knowledge of the epidemiology of different Fusarium species is important for the development of control measures for FHB and mycotoxins, such as information on the host’s most susceptible growth stage and timing of the host infection. The epidemiology of FHB in wheat has been extensively studied, but less is known about the epidemiology of FHB in oats and in particular F. langsethiae and HT2 + T2. Field surveys have shown that the risk for HT2 + T2 in oats may increase in response to humidity or rain prior to heading and flowering (Hjelkrem et al., 2018; Kaukoranta et al., 2019; Xu et al., 2013). Opoku et al. (2013) observed F. langsethiae in oat heads collected from commercial oat fields at head emergence and that the levels increased until maturation. Spore dispersal of F. langsethiae, on the other hand, has been observed late in the season, close to crop maturity (Hofgaard, Aamot, et al., 2016).
Inoculation studies to elucidate different aspects of the F. langsethiae and oats interaction have proven to be challenging (Imathiu et al., 2013). No disease development was observed for seedlings emerging from naturally or artificially infected seed, seeds planted in inoculated soil, or spray inoculated seedlings, indicating that F. langsethiae is not a seedling blight pathogen (Brodal et al., 2018; Divon et al., 2012; Imathiu et al., 2010). Spray inoculations at different growth stages indicated that oat was more susceptible to F. langsethiae at mid-flowering than at heading or beginning of flowering (Schöneberg et al., 2019). Divon et al. (2012) observed that the levels of F. langsethiae DNA in harvested grain were equally high after spray inoculations at flowering and dough stage, and the levels were higher than that of grain from plants injected with a spore suspension into the boot during the booting stage. However, more information about susceptibility window for F. langsethiae in oats is needed.
The use of resistant varieties is critical to minimize the risk of Fusarium and mycotoxin in cereals. FHB resistance in oats has been less studied than for wheat. Recent work has shown that FHB resistance breeding in oats has provided varieties with reduced F. graminearum infection and DON accumulation (Hautsalo et al., 2020; Tekle et al., 2018). FHB breeding programs have focused on the most common FHB species F. graminearum or Fusarium culmorum, and in wheat it has been shown that the resistance of several Fusarium spp. are similar to that of F. graminearum (Mesterhazy et al., 2005). For oats, a recent study suggests that the ranking of varieties according to F. graminearum/DON and F. langsethiae/HT2 + T2 differs (Hofgaard et al., 2022).
In wheat, FHB resistance has been linked to the ability of the plant to convert DON to the less phytotoxic DON-3-O-glucoside (D3G), and relatively higher levels of D3G have been reported in resistant compared to susceptible wheat genotypes (Berthiller et al., 2013; Lemmens et al., 2016). The masked toxins are not toxic when intact but can contribute to toxicity after hydrolyzation (Gratz, 2017). Lattanzio et al. (2012) were the first to report the occurrence of glycosylated HT2 and T2 toxins (HT2- or T2-Glu) in naturally contaminated wheat and oats, and they observed levels of HT2-Glu/HT2 up to 7% and T2-Glu/T2 up to 4% in oats. It has been shown that Fusarium spp. may form glycosylated forms (Busman et al., 2011; Lysøe et al., 2016), though Busman et al. (2011) observed that this occurred at a relatively low degree. Whether glycosylation of toxins is linked to Fusarium resistance in oats is not known.
In addition to glycosylated HT2 and T2, several other modified forms of HT2 and T2 have also been identified (Lysøe et al., 2016; Nathanail et al., 2015; Uhlig et al., 2013). Modified forms of mycotoxins present in grain or food and feed commodities are generally believed to contribute to the overall toxicity (EFSA CONTAM panel, 2017a). However, knowledge on the occurrence and accurate toxicity of the different compounds is scarce, and they are not included in the current proposed EU regulation on the presence of HT2 + T2 in cereals and cereal products (EC, 2013).
After F. langsethiae was distinguished as a separate species in 2004, only a few studies of the genetic, phenotypic or pathogenic variability within this species have been performed. Yli-Mattila et al. (2004) observed genetic variability within F. langsethiae when sequencing the IGS region and distinguished two subgroups within the species. Gavrilova et al. (2017) identified the two IGS subgroups among a selection of isolates from northern Europe, and observed that they reflected differences in the nutrient requirement, growth rate, and in vitro toxin production. Variation in aggressiveness within F. langsethiae has been observed in detached leaf assays (Imathiu et al., 2009; Opoku et al., 2011). The variation was not associated with the cereal host from which they were isolated, suggesting no preferential host specialization for isolates within F. langsethiae. Medina and Magan (2010, 2011) observed an influence of temperature and water availability on the growth and toxin production that mainly was similar for isolates of different geographic origin (Norway, Finland, Sweden, and England).
Although effort has been made to understand the epidemiology of F. langsethiae, this is still not fully understood. In the present study, we aimed to increase our understanding of the F. langsethiae – oat interaction. To assess the susceptibility to F. langsethiae and to identify the most susceptible growth stage of oats, we performed inoculation studies in the greenhouse at early (booting, heading, flowering) and late (flowering, milk, dough) growth stages in three oat varieties that previously have shown different resistance to F. graminearum: Odal and Vinger (both moderately resistant), and Belinda (susceptible) (Tekle et al., 2018). We also studied the variation in aggressiveness among Norwegian isolates of F. langsethiae and identified isolates suitable for use in further inoculation studies. In addition, we studied the presence of the glycosylated form of HT2 and other trichothecene A metabolites in harvested grain after inoculations at late growth stages.
Material & Methods
Three greenhouse inoculation experiments (Exp.) were performed, one to assess the aggressiveness measured as fungal growth (fungal DNA in harvested grain) and toxin producing ability of a selection of Norwegian F. langsethiae isolates, another to assess the susceptibility of oats after inoculation at early growth stages (booting, heading, and flowering), and a third to assess the susceptibility of oats after inoculation at late growth stages (flowering, milk, and dough). Both aggressiveness and susceptibility were assessed in grains that were harvested at maturity and analysed for F. langsethiae DNA and mycotoxins.
Plants and growth conditions
Seeds were obtained from a seed testing laboratory (Kimen Seed Laboratory, Ski, Norway), and seed lots with the lowest possible level of Fusarium available at that time according to their routine seed health analysis (a blotter method modified after ISTA, 1966) were selected. Seeds were sown in pots (3.5 L volume, ten seeds per pot) containing peat soil mixture (10% soil, Degernes Go’ Jord, NORGRO). Normally six to ten plants emerged per pot. After emergence, plants were given 16 hours light day time using white light (Philips Master HPI-T plus 400w/645). Temperature settings for the individual experiments are described in Table 1. Plants were fertilized from 3 weeks after sowing, giving a total amount of N of about 14 kg/daa. The day before inoculation, all panicles that were at the desired growth stage were marked. Growth stage was determined according to Lancashire et al. (1991).
Inoculum preparation and inoculation procedure
Agar plugs with F. langsethiae mycelium were transferred from −80 °C to mung bean agar (MBA; Dill-Macky, 2003) plates and incubated for 10–14 days at 20–22 °C with 12 hours light (white and NUV by Osram L36–73 32 W). The plates were washed with 5–10 mL distilled sterile H2O (dsH2O). The spore concentrations were measured using Hycor glasstic slides with grids and adjusted to a concentration of 105 spores/mL. A volume of 0.5–1 mL of this spore suspension was transferred to new MBA plates and incubated as described above. New spore suspensions were made as described, but this time the final concertation was 106 spores/mL. The inoculum was stored at −20 °C. On the day of inoculation, spore suspensions were thawed in cold water and Tween20 (Sigma-Aldrich) was added to a final concentration of 0.1% (v/v). To verify the viability of the inoculum, 1 mL spore suspension was transferred to a Petri dish containing water agar, and the ratio of germinated spores were counted the following day.
On the day of inoculation, all panicles in a pot were spray inoculated using a paint sprayer connected to a compressor set to a pressure of 1 bar, which ensured that the inoculum was evenly distributed across the panicles. We used 1 mL of inoculum per panicle (equivalent to almost runoff). Treatment with dsH2O and Tween was used as an uninoculated control in all the experiments.
Harvesting and analyses of harvested grains
From each pot, the marked panicles (those that were at the desired growth stage at inoculation, which was six to ten panicles per pot) were harvested at maturity and dried for 48 hours at 22 °C in a drying chamber with airflow. Panicles were threshed using a laboratory thresher, and the grain milled in a ZM 200 mill fitted with a 1 mm sieve (Retch). Flour was stored at −20 °C upon analysis.
Q-PCR analysis of fusarium infection
According to the manufacturer’s protocol, DNA was extracted from 150 mg flour using the Fast DNA spin kit for soil (MP Biomedicals). The content of DNA from F. langsethiae and oat was analysed with qPCR according to Hofgaard, Aamot, et al. (2016). The primers and probes used are described in Table S1 (supplement ESM 1). Analysis of F. langsethiae and oat DNA was done in duplex qPCR reactions in a total volume of 25 μL, that consisted of 4 μL 10-fold diluted genomic DNA, 300 nM of each F. langsethiae primer, 75 nM of each plant primer,100 nM of each probe, and 1 × iQ™ Multiplex Powermix (BioRad). qPCR was conducted using a C1000 Touch Term Cycler combined with a CFX96TM Real-Time System (BioRad), and the following parameters: 95 °C for 3 min followed by 45 cycles of 95 °C for 10 sec and 60 °C for 30 sec.
Standard curves, based on five serial dilutions (range 1–4000 pg of DNA) of genomic DNA from a pure culture, were used to quantify DNA from F. langsethiae. DNA preparation from pure cultures was as described previously (Koga et al., 2020). For the quantification of host plant DNA, the serial dilution contained plant DNA in the range 0.01–40 ng. The amount of fungal DNA was normalized against the amount of plant DNA, and fungal content is presented as pg fungal DNA per μg plant DNA (pg/μg).
Analysis of mycotoxins
In Exp. 1 and 2, the quantification of HT2 + T2 was performed using AgraQuant® HT-2/T-2 (Assay 25/500) ELISA kits (Romer Labs) according to the manufacturer’s protocol. Absorbance was measured at 450 nm using a spectrophotometer (Spectra max190, Molecular devices). The data were analyzed using RomerLabs® software. Sample extractions were done by adding 25 mL of 70% methanol: distilled water (v/v) to 5 g of the ground sample. The mixture was shaken and centrifuged for 1 min at 1811×g, and the supernatant was diluted at 1:10 with distilled water. To obtain a limit of detection (LOD) of 150 μg kg−1 and a limit of quantification (LOQ) of 250 μg kg−1, an additional dilution of 1 + 1 with 7% methanol: distilled water (v/v) was made. Samples with toxin levels above the kit’s range were further diluted.
In Exp.3, the content of different trichothecene A mycotoxins was analysed using LC-MS/MS (Sulyok et al., 2020). In brief, 5 g of milled sample were extracted using 20 mL of extraction solvent (acetonitrile-water-acetic acid, 79:20:1, v/v/v) followed by a 1 + 1 dilution using acetonitrile-water-acetic acid, (20:79:1, v/v/v) and direct injection of 5 μL diluted extract. In case of concentrations exceeding the highest calibration level, the extract was further diluted 1:50 and re-analysed. Quantitative analysis of multiple fungal metabolites was performed with a QTrap 5500 LC-MS/MS System (Applied Biosystems) equipped with a Turbo Ion Spray electrospray ionization (ESI) source and a 1290 Series HPLC System (Agilent). Chromatographic separation was performed at 25 °C on a Gemini® C18-column, 150 × 4.6 mm i.d., 5 μm particle size, equipped with a C18 4 × 3 mm i.d. security guard cartridge (all from Phenomenex) using a methanol/water gradient containing 1% (v/v) acetic acid and 5 mM ammonium acetate. Quantification was based on external calibration using serial dilutions prepared in neat solvent. Results were corrected for apparent recoveries. The accuracy of the method is verified on a continuous basis by regular participation in proficiency testing schemes organized by BIPEA (Genneviliers, France). For oats, all results obtained so far in this proficiency testing scheme (42 samples) were in the satisfactory range (z-score between −2 and 2).
For statistical analyses, toxin values <LOD were adjusted to LOD/6.
The aggressiveness and toxin producing ability of F. langsethiae isolates (Exp. 1)
The aggressiveness, measured as level of F. langsethiae DNA per plant DNA, of 21 isolates of F. langsethiae (Table S2) was screened in the variety Belinda. Oat plants were grown as described above and spray inoculated at the beginning of heading (BBCH 51), or the beginning of flowering (BBCH 61, i.e., one week later). Plants were bagged for six days after inoculation and kept under the conditions described in Table 1 until mature. Panicles were harvested at maturity, and the samples prepared and the harvested grain analyzed for F. langsethiae DNA and HT2 + T2 as described above. The experiment included two replicates of each treatment; each replicate had two pots, each containing six to ten panicles per pot.
Inoculation with F. langsethiae at different growth stages (Exp. 2 and 3)
The three varieties Vinger, Odal, and Belinda, were spray inoculated with F. langsethiae at different growth stages: booting (BBCH 45), heading (BBCH 55), flowering (BBCH 65) in Exp. 2; and flowering (BBCH 65), milk (BBCH 73), and dough (BBCH 83) in Exp. 3. Panicles were harvested at maturity, and the samples prepared and analyzed as described above.
To obtain plants that were at the three desired growth stages at the same time, seeds were sown at three different time points (one-week intervals) in both experiments. When the plants reached the desired growth stages, they were spray inoculated with spore suspensions that contained a mixture of the two F. langsethiae isolates 201086 and 201058 (50% of each) in Exp. 2, and with F. langsethiae isolate 201058 in Exp. 3. The panicles were bagged for six days after inoculation and left to mature under the conditions described in Table 1. Panicles were harvested at maturity, and the samples were prepared and analyzed as described above. The experiments included four replicates of each treatment; each replicate had one pot, each containing six to ten panicles per pot.
In addition to the treatments mentioned above, Exp. 2 (early growth stages) also included injection with 200 μL of spore suspension (or water for the non-inoculated control) into the boot at booting. Exp. 3 (late growth stages) included a treatment with 3-day bagging after inoculation in addition to the 6-day bagging (used in the other experiments).
Statistical analysis
In Exp.1, the relationship between the response F. langsethiae DNA in harvested grain and isolate and growth stage of inoculation, or for inoculations at the beginning of flowering, the relationship between HT2 + T2 contamination and isolate was analyzed using the General Linear Model (GLM) and Tukey pairwise comparisons in Minitab 19 (Minitab, LLC., 2021). In Exp. 2 and 3, the effect of variety and growth stage for inoculation on the level of F. langsethiae DNA and HT2 + T2 in harvested grain was assessed using the same method. Simple linear regression was used to analyse the relationship between HT2 + T2 and F. langsethiae DNA in all experiments, and between the HT2-Glu and HT2 in Exp.3. The statistical analyses were done on the data from the F. langsethiae inoculated material (not including non-inoculated material).
For all analyses, the normality distribution of the data was assessed in the residual plots. If not fulfilled, normal distribution was obtained by Box-Cox transformation option optimal ʎ in the case of GLM or by log10 transformation in the case of linear regression. Since such data transformations cannot be performed on data that include 0, the value 1 was added to every value in datasets that contained 0 (i.e., F. langsethiae DNA [pg/μg +1] in the isolate aggressiveness dataset in Exp.1 and similarly in the early growth stages dataset in Exp. 2).
The full software output of all the models are shown in the supplement ESM 3.
Results
The aggressiveness and toxin producing ability of F. langsethiae isolates (Exp. 1)
The level of F. langsethiae DNA in harvested grain was influenced by plant growth stage at inoculation (p < 0.001, R2-adj of 30%, Model S1), and higher levels were observed in grain harvested from plants inoculated at the beginning of flowering than at the beginning of heading (mean of 306 at BBCH 61 vs. 99 pg/μg at BBCH 51 across 21 isolates in the Tukey comparison). This analysis did not reveal any significant effect of isolate or the interaction between isolate and growth stage (p > 0.05).
All the isolates that we screened were able to infect oats at the beginning of flowering (Fig. 1a), but not at the beginning of heading (Fig. S1 in supplement ESM 2). For inoculations at the beginning of flowering, differences in aggressiveness in terms of F. langsethiae DNA in harvested grain were revealed by GLM, which showed a significant effect of isolate (p = 0.014) on the level of F. langsethiae DNA in harvested grain (R2-adj of 48%, Model S2). Isolate 201058 produced the highest level of F. langsethiae DNA (1051 pg/μg), and according to Tukey pairwise comparisons, this was significantly higher than the levels that we observed for the six isolates that resulted in the lowest infection levels (111–206 pg/μg). The remaining 14 isolates had intermediate levels of F. langsethiae DNA (225–668 pg/μg). For inoculations at the beginning of heading, no significant differences were observed between isolates on the level of F. langsethiae DNA in harvested grain (p = 0.330, R2-adj of 8%, Model S3). The uninoculated controls had no F. langsethiae DNA.
Level of Fusarium langsethiae DNA (pg/μg plant DNA) (a) and HT2 + T2 toxins (μg/kg) (b) in grain harvested from oat inoculated at early flowering (BBCH 61). Plants (variety Belinda) were inoculated with different isolates of Fusarium langsethiae (n = 21). Fusarium langsethiae DNA levels varied between isolates, bars that do not share a letter are significantly different according to Tukey’s Pairwise Comparisons
An initial analysis of HT2 + T2 of ten grain samples (five of each of the inoculated growth stages) showed that also the toxin levels were higher in grain from plants inoculated at the beginning of flowering (409–1237 μg/kg) than at the beginning of heading (< LOQ). The samples represented grain from oat inoculated with the isolates 200869, 201058, 201059, 201085, and 201086 at both growth stages. Therefore, the analysis of HT2 + T2 was completed for samples from inoculations at beginning of flowering only (Fig. 1b). For three of the isolates, no HT2 + T2 were detected in the harvested grain. The mean HT2 + T2 contamination levels for the remaining isolates were in the range < LOQ-908 μg/kg. GLM analyses indicated a significant effect of both isolate and replicate on HT2 + T2 contamination in harvested grain (Model S4), however, since the residual plots indicated modeling issues, further comparisons were not performed.
In grains harvested from oats inoculated with different isolates of F. langsethiae at the beginning of flowering, the amount of HT2 + T2 was positively associated with F. langsethiae DNA (log10 [HT2 + T2] = − 1269 + 1387 log10 [F. langsethiae DNA +1]), R2-adj of 44% (Model S5).
Inoculation with F. langsethiae at early growth stages (Exp. 2)
The three varieties Vinger, Odal, and Belinda, were spray inoculated with F. langsethiae isolate 201086 and 201058 (50% of each) at early growth stages: booting (BBCH 45), heading (BBCH 55), flowering (BBCH 65), and incubated for six days. The level of F. langsethiae DNA and HT2 + T2 was measured in harvested grain.
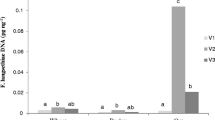
The contamination level in the grain varied with growth stage at inoculation. Inoculation at booting resulted in low or no levels of F. langsethiae DNA (mean level of 3, 10, 3 pg/μg, for Vinger, Odal, and Belinda, respectively) and HT2 + T2 (<LOD for all varieties) in harvested grain. Inoculations at heading and flowering yielded substantial F. langsethiae DNA and HT2 + T2 contamination (Fig. 2a and c, Table S3) and were further analyzed by GLM (Table 2). According to these analyses, the level of F. langsethiae DNA in harvested grain was associated with variety (p ≤ 0.001) and growth stage at inoculation (p = 0.013), but not their interaction (p = 0.953), in a model that explained 58% (R2-adj) of the variation (Table 2, and Model S6). Tukey pairwise comparisons revealed that inoculations at flowering resulted in higher levels of F. langsethiae DNA than heading (696 and 282 pg/μg plant DNA, respectively). Odal and Belinda had higher levels of F. langsethiae DNA than Vinger (967 and 704 vs. 128 pg/μg, respectively). Simple linear regression revealed a close relationship between F. langsethiae DNA and HT2 + T2 (p ≤ 0.001, R2-adj of 90%, Model S7). GLM showed that HT2 + T2 was significantly associated with variety (p = 0.003), but not growth stage or their interaction (p > 0.289, R2-adj 36% in Model S8). Tukey pairwise comparisons revealed that Belinda and Odal had significantly higher levels of HT2 + T2 than Vinger (1303 and 1136 vs. 487 μg/kg, respectively). For the Tukey comparisons, the values presented are the back-transformed means across varieties or growth stages.
Level of Fusarium langsethiae DNA (pg/μg plant DNA) (a and b) and HT2 + T2 (μg/kg) (c and d) in grain from plants of the three oat varieties Vinger, Odal and Belinda inoculated at growth stage (BBCH) booting (45), heading (55), and flowering (65) (Exp. 2; a and c), or at flowering (65), milk (73) and dough (83) (Exp. 3; b and d)
Low levels of F. langsethiae DNA were observed (mean of 0.5, 0.2, and 1.3 pg/μg plant DNA in Vinger, Odal and Belinda, respectively) and HT2 + T2 was not detected (<LOQ) in grain from non-inoculated controls at booting. Non-inoculated controls at heading and flowering were not analysed.
For plants that were inoculated by boot injection at booting stage, one replicate per variety was screened for the content of F. langsethiae DNA and HT2 + T2 in harvested grain. Since no HT2 + T2 and relatively low levels of F. langsethiae DNA were detected (6, 88, and 22 pg/μg plant DNA in Vinger, Odal, and Belinda, respectively), further analysis of this material was not performed.
Inoculation with F. langsethiae at late growth stages (Exp. 3)
The three varieties Vinger, Odal, and Belinda, were spray inoculated with F. langsethiae isolate 201058 at late growth stages: flowering (BBCH 65), milk (BBCH 73), and dough (BBCH 83), and incubated for six days. The level of F. langsethiae DNA and HT2 + T2 was measured in the harvested grain.
The inoculations at late growth stages resulted in substantial F. langsethiae DNA and HT2 + T2 contamination in the harvested grain (Fig. 2b and d, Table S4). According to GLM analyses (Table 2), the HT2 + T2 levels in harvested grain was influenced by variety and growth stage at inoculation (p ≤ 0.001), but not their interaction (p = 0.209 in a model that explained 69% [R2-adj] of the variation in Model S9). Tukey pairwise comparisons revealed that inoculations at dough resulted in the highest HT2 + T2 levels (2529 μg/kg), significantly higher than that after inoculations at flowering (997 μg/kg) and milk (619 μg/kg). Simple linear regression revealed a close relationship between F. langsethiae DNA and HT2 + T2 (p ≤ 0.001, R2-adj of 89% in Model S10). Fusarium langsethiae DNA levels were mostly influenced by variety (F = 35, p ≤ 0.001) and growth stage (F = 36, p ≤ 0.001), and only slightly by their interaction (F = 5, p = 0.002) in a model with R2-adj of 82% (Model S11). Tukey pairwise comparisons revealed that mean levels of F. langsethiae DNA were higher after the dough inoculation (4573 pg/μg) than after milk and flowering inoculations (941 and 876 pg/μg, respectively). For the Tukey comparisons, the values presented are the back-transformed means across varieties.
Further analysis of the contamination level of the different varieties was performed for data from flowering and milk inoculations only. These GLM analyses showed that both F. langsethiae DNA (Model S12) and HT2 + T2 (Model S13) was influenced by variety (p ≤ 0.001), but not growth stage or their interaction (p > 0.111), in models that explained 76% and 62% (R2-adj) of the variation in F. langsethiae DNA and HT2 + T2, respectively. According to Tukey comparisons, Belinda and Odal had significantly higher F. langsethiae DNA and HT2 + T2 levels than Vinger: For F. langsethiae DNA, the levels were 2609 and 1311 pg/μg in Belinda and Odal, respectively, vs. 219 pg/μg in Vinger. Accordingly, the level of HT2 + T2 were 1546 and 785 μg/kg in Belinda and Odal vs. 182 μg/kg in Vinger. The values represent the back-transformed means across growth stages in Tukey.
No or low levels of F. langsethiae DNA were observed (mean across growth stages for inoculation of 0, 0.3, and 0.8 pg/μg plant DNA in Vinger, Odal, and Belinda, respectively) and no HT2 + T2 was detected in the non-inoculated controls.
For explaining the variation in F. langsethiae DNA or HT2 + T2 in Exp. 2 and 3, we chose to use models that included variety and growth stage for inoculation, and their interaction as the sole explanatory factors. In comparison, GLMs that included replicate as a random factor in addition to the fixed factors variety and growth stage, and all possible interactions, showed that neither of these were significant (results not shown).
Trichothecene A metabolites other than HT2 + T2
In addition to HT2 + T2, several other trichothecene A metabolites (HT2-Glu, T2-tetraol, neosolaniol, monoacetoxyscirpenol, diacetoxyscirpenol, 4-deoxy HT2, deacetylneosolaniol, NT-2 toxin) were analysed in grain from oat plants inoculated at late growth stages (Exp. 3). The highest levels were observed for HT2-Glu (mean of 192 μg/kg across all samples) and T2-Tetraol (104 μg/kg), followed by deacetylneosolaniol (46 μg/kg) and NT-2 toxin (27 μg/kg) (Table S5). Low levels were detected for 4-deoxy-HT2, neosolaniol, monoacetoxyscirpenol, and diacetoxyscirpenol (mean across all samples of 20, 15, 9, and 5 μg/kg, respectively). Glycosylated T2 was not detected.
The mean relative level (percent of the total level) of the different trichothecene A metabolites (including HT2 and T2) in harvested grain for the three varieties inoculated at the three different growth stages are shown in Fig. 3. The results indicate that a higher percentage of metabolites other than HT2 + T2 were present in grain from plants inoculated at the milk or dough stage compared to flowering. The relative level of the other trichothecene A metabolites to HT2 + T2 (%SumTrichA) was calculated (Eq.1), and ranged from 15 to 34% depending on variety and growth stage for inoculation (Table 3).
Mean relative level (%) of different trichothecene A metabolites: HT2, T2, HT2-Glu, T2-tetraol, neosolaniol, monoacetoxyscirpenol, diacetoxyscirpenol, 4-deoxy HT2, deacetylneosolaniol, NT-2 toxin, in three oat varieties (Vinger, Odal, Belinda) inoculated with Fusarium langsethiae at different growth stages (BBCH); flowering (65), milk (73), or dough (83)
To obtain information on the level of glycosylation of the different varieties, we calculated the relative level of HT2-Glu to HT2 (%HT2-Glu; Eq. 2) for grain from the different varieties in Exp.3. The level of HT2-Glu varied with growth stage for inoculation and variety (Fig. 4). Simple linear regression revealed that the level of HT2-Glu was positively correlated to the level of HT2 (p ≤ 0.001, R2-adj of 74%, Model S14). The relative level of HT2-Glu to HT2 spanned from 10 to 50% (Table S5). The variation in %HT2-Glu for the three varieties inoculated at the different growth stages is shown in Fig. 4c. A GLM revealed a significant relationship between %HT-Glu and growth stage of inoculation (p ≤ 0.001, F = 19), and variety (p = 0.027, F = 4), but not their interaction (p = 0.643), in a model that explained 53% of the variation in %HT2-Glu (Model S15). Tukey pairwise comparison revealed that inoculations at milk resulted in significantly higher levels of %HT2-Glu (mean of 28%) than inoculations at dough (mean of 21%), and inoculation at flowering resulted in %HT2-Glu levels (mean of 15%) that were significantly lower than after dough inoculations. The effect of variety was lower (lower F-value, and less difference between means), but still significant, with Tukey pairwise comparisons indicating a significantly higher level of %HT2-Glu in Vinger (mean of 24%) vs. Odal (mean of 18%), and Belinda ranging in-between (mean of 20%).
Models that, in addition to growth stage and variety and their interaction, contained replicate and possible interactions, showed that neither were significant (not shown).
Effect of duration of incubation period on the level of F. langsethiae and HT2 + T2
In Exp. 3, where we spray inoculated three oat varieties with F. langsethiae at late growth stages, we also studied the effect of 3-day compared to the 6-day incubation that we normally used, on the level of F. langsethiae DNA and HT2 + T2 in the harvested grain.
GLM showed that incubation length, in addition to growth stage for inoculation, and variety impacted on the level of F. langsethiae DNA and HT2 + T2 in harvested grain (p ≤ 0.001, Fig. S2). The models explained 81% (R2-adj) of the variation in F. langsethiae DNA (Model S16) and 67% for HT2 + T2 (Model S17). Tukey pairwise comparisons revealed that the 6-day incubation resulted in significantly higher levels of F. langsethiae DNA and HT2 + T2 (about three times higher for 6-day than for the 3-day incubation). All possible interactions were tested in separate models but were not significant (results not shown).
Discussion
We observed significant amounts of F. langsethiae and HT2 + T2 in grain from three oat varieties that were spray inoculated at heading, flowering, milk, and dough stage, whereas no contamination was observed in grains from plants inoculated at booting. The highest contamination level was observed in grains from plants inoculated at dough. Rating of the varieties according to F. langsethiae/HT2 + T2 contamination level in harvested grain showed that Vinger was the most resistant variety, whereas Odal and Belinda were relatively susceptible.
Effect of growth stage at inoculation on the level of F. langsethiae and HT2 + T2 in oat grain
We observed substantial levels of HT2 + T2 in grains from oats that had been spray inoculated at heading or three successive stages, but not in grains from oats that had been spray inoculated at booting, suggesting that oat is susceptible to F. langsethiae and HT2 + T2 from heading and onwards. Previous studies have shown that it can be challenging to obtain successful infections by artificial inoculations of oats with F. langsethiae (reviewed in Imathiu et al., 2013), and the infection pathway and susceptibility window of oats to F. langsethiae has been relatively little studied in inoculation experiments. Divon et al. (2019) studied the infection pathway of F. langsethiae in flowers of wheat and oats, and observed that fungal growth and infection was assisted by the presence of pollen. In our case, pollen was not only present at flowering, but was still present at milk stage (though in different levels of decay). The presence of pollen may have contributed to infection, resulting in the higher levels of F. langsethiae DNA that we observed in grain from plants inoculated at flowering and milk, compared to heading. Plants inoculated at heading had probably started flowering at the end of the bagging period of six days, meaning that pollen just had started to emerge when the incubation was over. Schöneberg et al. (2019) studied the susceptibility of oats to F. langsethiae and HT2 + T2 infection at heading and flowering and observed that spray inoculation at mid-flowering resulted in grain with a higher contamination level than inoculations at heading or beginning of flowering. In comparison, we observed that the levels of HT2 + T2 in grain were high after inoculations at both heading and flowering. This can probably partly be explained by the difference in incubation lengths (6 days in ours vs. 4-12 h in Schöneberg et al., 2019), which means that the plants we inoculated at mid-heading probably reached beginning of flowering during the bagging period. Our finding that F. langsethiae can infest oats panicles already during heading is in line with observations from field surveys: F. langsethiae have been observed in oat panicles in naturally infected fields at head emergence (Opoku et al., 2013; Parikka et al., 2007). Sturz and Johnston (1983) observed two culture variants of Fusarium poae in barley and wheat flag leaf sheets collected during booting, where the most frequently encountered variant often had a powdery appearance and probably was F. langsethiae. The authors considered these fungi to be primary colonizers of cereals. Moreover, weather factors prior to flowering of oats have been shown to impact on the risk for HT2 + T2 contamination in the harvested grain, high humidity or rain during this period increased the risk (Hjelkrem et al., 2018; Kaukoranta et al., 2019; Xu et al., 2013). These findings support the hypothesis that F. langsethiae may infect oats prior to flowering.
We also observed high levels of F. langsethiae and mycotoxins in grain after inoculation at dough stage. Similarly, Divon et al. (2012) obtained high amounts of F. langsethiae DNA in grain from oat plants that were spray inoculated at dough stage as well as from plants inoculated at flowering. This suggests that oats are highly susceptible to F. langsethiae in the period close to harvest and that late infection can result in a high level of contamination in harvested oats. In Norway, dispersal of F. langsethiae spores have been observed in oat fields late in the season (Hofgaard, Aamot, et al., 2016), and humid conditions close to harvest have been associated with an increased risk of HT2 + T2 in harvested oats (Hjelkrem et al., 2018). It is also possible that infections that occurred at earlier stages might progress further during humid conditions. Together, these findings indicate that late developing infections may significantly incerase the level of F. langsethiae and HT2 + T2 in harvested oats.
Our finding that oat is susceptible to F. langsethiae and HT2 + T2 prior to flowering and onwards is in contrast to studies of the susceptibility window for F. graminearum in oats. Inoculation studies with F. graminearum have shown that oats are most susceptible at anthesis compared to earlier stages (Xue et al., 2015) and later stages (Tekle et al., 2012). These findings may imply that the susceptibility window of oats towards the different Fusarium species and their toxins could be slightly different: Oats is susceptible to F. langsethiae already before heading is complete and onwards, whereas, for F. graminearum, the most susceptible period is flowering. The wide susceptibility window that we observe for F. langsethiae/HT2 + T2 in oats implicate that control using fungicides, including determining the best timing of an application, could be challenging. No or limited effect of fungicide application on the level of HT2 + T2 in oats have been observed (Edwards & Anderson, 2011; Pettersson et al., 2008), but relatively little information is available on this topic, and as far as we know, information on the effect of relevant fungicides on different isolates of F. langsethiae is not available.
Resistance to F. langsethiae and HT2 + T2
In our study, Vinger was the variety that was most resistant towards F. langsethiae and HT2 + T2, whereas Odal and Belinda were relatively susceptible. This is mostly in line with variety screenings in field trials with natural Fusarium contamination, which showed that Vinger was of the most HT2 + T2 resistant varieties, and Odal and Belinda were of the most susceptible (Hofgaard et al., 2022). In a study of varieties of winter wheat that were inoculated with F. graminearum or F. culmorum it was observed that for DON, the relationship between different wheat genotypes varied with the timing of the infection (György et al., 2020). We observed that for the latest inoculation (dough stage) there were fewer differences between varieties in the level of contamination of F. langsethiae and HT2 + T2 in harvested grain. At this point plant head tissues were senescencing, and the lack of varietal difference might be linked to less active resistance responses in the plants. It is possible that in naturally infected fields, the infection may occur at multiple (or other) time points than in our experiments that may result in a slightly different variety ratings. Naturally contaminated fields with high F. langsethiae/HT2 + T2 contamination levels are sometimes scarce, depending on the season and location. Since our results mainly reflect variety resistance ranking observed by Hofgaard et al. (2022) under field conditions, this suggests that artificial inoculations can contribute with data that can be valuable in the resistance screening of oat varieties and breeding lines, ensuring a high level of F. langsethiae and HT2 + T2 that enables ranking of varieties according to contamination level. However, the possibility of an effect of timing of inoculation on toxin accumulation and variety rankings according to toxin contamination should be kept in mind.
The finding that Odal is relatively susceptible to HT2 + T2 (both in our greenhouse study and under natural field conditions as mentioned above) is in contrast to the ranking of these varieties according to DON contamination as observed by Tekle et al. (2018), where Odal (and Vinger) were of the most resistant varieties, and Belinda susceptible. This suggests that the resistance towards F. langsethiae/HT2 + T2 and F. graminearum/DON in oat is different. In wheat it has been shown that the resistance of several Fusarium spp. are similar, hence the ranking of wheat varieties will not depend on isolate or Fusarium species (Mesterhazy et al., 2005). If the FHB/mycotoxin resistance in oat is truly different between Fusarium species, this will have implications in Fusarium resistance breeding in oats and is a topic that needs further attention.
Aggressiveness and toxin producing ability of F. langsethiae isolates
We observed significant differences between isolates in the level of F. langsethiae DNA in harvested grain after inoculation, suggesting variation in aggressiveness in the Norwegian F. langsethiae population. Differences in aggressiveness have been observed within different Fusarium species, such as F. culmorum, F. poae, and F. graminearum (Aamot et al., 2015; Miedaner et al., 2021; Stenglein et al., 2014). For F. langsethiae, variations in aggressiveness have been observed among UK isolates in detached leaf assays, and the aggressiveness was independent of the host from which the isolates originated (Imathiu et al., 2009; Opoku et al., 2011). Most of our isolates originated from oats and therefore limited the opportunity to test the effect of host origin on aggressiveness. Differentiation of aggressiveness according to host or geographic origin is generally not expected for Fusarium species (Aamot et al., 2015; Dinolfo et al., 2010; Gale, 2003; Stenglein et al., 2014), and suggest that isolates for use as inoculum in variety screenings can be chosen regardless of origin if it has proven to be aggressive in the host of interest. It should be noted that isolate × host genotype interactions have been observed for some Fusarium species and cereal species combinations, but the impact of the interactions were limited compared to the main effects (György et al., 2020; Stenglein et al., 2014). Accordingly, Miedaner et al. (2021) observed no isolate × host genotype interactions for different F. culmorum isolates inoculated on several cereal species. Our study included one variety only. Isolate × host genotype interactions have, as far as we know, not been studied for F. langsethiae in oats, and if it exists, it could lead to different variety rankings depending on the pathogen genotype present.
In our study of aggressiveness of different F. langsethiae isolates, the relationship between HT2 + T2 concentration and the level of F. langsethiae DNA in harvested grain was relatively consistent, though with some exceptions: For three of the isolates, we observed F. langsethiae DNA, but no HT2 + T2, in the harvested grain. For one isolate, we observed a relatively high level of HT2 + T2 compared to the level of F. langsethiae DNA. This might indicate that HT2 + T2 is not directly correlated with the ability of the fungus to infect oats. In comparison, DON has proven to be a virulence factor for F. graminearum in wheat (facilitating the spread of the fungus from floret into the rachis, but not initial infection through the fruit coat), but not in barley, nor oats (Jansen et al., 2005; Langevin et al., 2004). Nishiuchi et al. (2006) showed that in Arabidopsis, the type A trichothecenes T2 and HT2 elicited cell death after leaf infiltrations, whereas the type B trichothecene DON was able to inhibit the plant translation process without eliciting cell death and suggested that the different types of trichothecenes had different roles in virulence of their producing fungi.
Glycosylated HT2
We observed a substantial level of HT2-Glu, and the amount was positively correlated to the level of HT2. This is similar to the glycosylation of DON, for which it has been observed that the level of glycosylated DON increased with DON concentration (Lemmens et al., 2016). Glucosyltransferases are responsible for the glycosylation process of mycotoxins in plants (Coleman et al., 1997). The ability for glycosylation of DON in wheat has been connected to FHB resistance as increased relative levels of glycosylated DON have been observed in resistant genotypes (Lemmens et al., 2016). Likewise, we observed that the % HT2-Glu to HT2 was significantly higher in the relatively resistant variety Vinger than in the more susceptible Odal, suggesting that also F. langsethiae/HT2 + T2 resistance in oats could in part be connected to toxin glycosylation. We observed, however, that %HT2-Glu was more strongly associated with the growth stage at inoculation than variety, suggesting that the ability for glycosylation is more dependent on the growth stage for infection than host genotype. The glycosylation process in cereals, and oats particularly, have been relatively little studied and should be further studied to elucidate the implications in FHB resistance.
The %HT2-Glu in our study was significantly higher in grain from oats inoculated at milk compared to flowering and dough. This is connected to the level of HT2 that was higher (though not significantly) in plants inoculated at flowering than milk, whereas the level of HT2-Glu showed the opposite trend. Hence, the ratio of glycosylated HT2 in grain from plants inoculated at flowering was lower than for milk inoculated, despite HT2 levels being higher and the time from inoculation until harvest longer in flower inoculated plants. Conversely, it may seem like the glycosylation process was more efficient in milk than in flowering inoculated plants, independent of the HT2 contamination level. This was somewhat surprising, since the plants inoculated at milk had a shorter time until physiological kernel maturation (when all physiological processes stop) than plants inoculated at flowering. Hence the duration of activity of the enzyme responsible for glycosylation was potentially shorter for plants inoculated at milk. Grains from oats inoculated at dough stage had the highest HT2 and HT2-Glu levels, and intermediate %HT2-Glu levels (lower than after milk inoculation, higher than after flowering inoculation). These plants were already senescing at the time of inocualtion, and had the shortest time until complete recedence of the metabolic activity. Seemingly, the glycosylation process is not only affected by HT2 levels, but also the age of the different plant tissues when initially infected may play a role, but as far as we know, this has not been studied.
Other Trichothecene A metabolites
In addition to HT2 + T2 and HT2-Glu, we measured a set of other trichothecene A metabolites in grain from oats inoculated at late growth stages. After HT2-Glu, T2-tetraol was the dominating trichothecene A metabolite. Modified forms of parent molecules as well as other metabolites produced by the same pathogen can co-occur in the grain and contribute to the overall toxicity (EFSA CONTAM panel, 2017b). We observed a significant rate of the other trichothecene A to HT2 + T2, suggesting that the overall toxicity of the grain could be higher than anticipated based on HT2 + T2 alone in grain from oats infected with F. langsethiae. Like for %HT2-Glu, we observed that the relative level of other trichothecene A metabolites was higher after inoculations at milk and dough than after inoculations at flowering. In light of these findings, we believe that late infections can occur and result in severe contamination of harvested oats, not only with HT2 + T2 and their masked forms, but also with several other trichothecene A metabolites that can increase the toxicity of harvested grain, but that are not included in the proposed EU legislation (EC, 2013). Further studies of the occurrence and toxicity of trichothecene A metabolites in oat grain infected with F. langsethiae is warranted.
Inoculation method
Lower levels of F. langsethiae and HT2 + T2 were observed in grain from plants that had received a shorter humidity incubation after inoculation (3 days instead of 6 days). Schöneberg et al. (2019) also obtained a higher level of HT2 + T2 in grain after increasing the incubation period (12- compared to 4- or 8-hours) in oats inoculated at flowering but observed no effect of prolonged incubation when inoculating at earlier growth stages. Our results are also in line with the study of Andersen (1948) of F. graminearum in wheat, which observed that prolonged moisture increased the initial infection. In vitro growth rate studies have shown that F. langsethiae strains were more sensitive to water stress than other fusaria (Kokkonen et al., 2012; Medina & Magan, 2010).
We did not obtain significant infection or HT2 + T2 in harvested grain after either spray nor boot injection at booting. In comparison, Divon et al. (2012) observed nearly equal levels of F. langsethiae and T2 in grain from oat plants inoculated by injection during booting with 1 mL of 106 spores/mL, compared to plants spray inoculated at anthesis or dough stage. In the same study, it was demonstrated that boot inoculations with low inoculum (0.5 and 0.1 mL), which is more like the volume we used (0.2 mL), resulted in substantially lower contamination levels. These amounts are probably more realistic compared to natural conditions, where we consider it highly unlikely that 1 mL of water dispersed spores would enter the boot. If the lower amount yielded stable infections, the method might have been useful in resistance screenings, but we consider it less suitable.
Conclusion
We observed a wide window of susceptibility of oats to F. langsethiae/HT2 + T2 (from heading and onwards), and inoculations of oats during grain filling and ripening resulted in high levels of HT2 + T2 in the harvested grain. This implicates that obtaining consistent control of F. langsethiae/HT2 + T2 in oats could be challenging, since potential effects of agricultural control measures applied earlier in the season (such as fungicide applications during flowering) can readily be overcome by late infections. Moreover, late infections also resulted in a relatively high amount of several other trichothecene A metabolites that likely contribute to the overall toxicity and represent a threat to food and feed safety, however, they are not included in the current proposed EU regulations. We also observed that the three oat varieties we tested varied in their resistance to F. langsethiae and HT2 + T2 and we identified the variety Vinger as relatively resistant; information that can be utilised in integrated pest management as resistant varieties are of great importance in reducing mycotoxin contamination in harvested grain. We also observed the variety Odal as relatively susceptible to F. langsethiae/HT2 + T2, which is in contrast to previous rankings of oat cultivars according to DON, where Odal has been ranked as relatively resistant. This finding has implications for resistance breeding, and the resistance of oat varieties to F. graminearum/DON and F. langsethiae/HT2 + T2 should be further studied.
References
Aamot, H., Ward, T., Brodal, G., Vrålstad, T., Larsen, G., Klemsdal, S., Elameen, A., Uhlig, S., & Hofgaard, I. (2015). Genetic and phenotypic diversity within the fusarium graminearum species complex in Norway. European Journal of Plant Pathology, 142, 501–519. https://doi.org/10.1007/s10658-015-0629-4
Andersen, A. (1948). The development of Gibberella zeae headblight of wheat. Phytopathology, 38, 595–611.
Berthiller, F., Crews, C., Dall'Asta, C., Saeger, S. D., Haesaert, G., Karlovsky, P., Oswald, I. P., Seefelder, W., Speijers, G., & Stroka, J. (2013). Masked mycotoxins: A review. Molecular Nutrition & Food Research, 57, 165–186. https://doi.org/10.1002/mnfr.201100764
Brodal, G., Aamot, H., Isaksen, B., Grieu, C., & Hofgaard, I. (2018). Fusarium langsethiae in oats seeds in Norway - occurrence and possible role as primary inoculum. In "8th ISTA seed health symposium, 4–7 September", Poznan, Poland.
Brodal, G., Aamot, H. U., Almvik, M., & Hofgaard, I. S. (2020). Removal of small kernels reduces the content of fusarium mycotoxins in oat grain. Toxins, 12, 346. https://doi.org/10.3390/toxins12050346
Busman, M., Poling, S. M., & Maragos, C. M. (2011). Observation of T-2 toxin and HT-2 toxin glucosides from fusarium sporotrichioides by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Toxins, 3, 1554–1568. https://doi.org/10.3390/toxins3121554colem
Clemens, R., & van Klinken, B. J.-W. (2014). Oats, more than just a whole grain: An introduction. British Journal of Nutrition, 112, S1–S3. https://doi.org/10.1017/S0007114514002712
Coleman, J., Blake-Kalff, M., & Davies, E. (1997). Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends in Plant Science, 2, 144–151. https://doi.org/10.1016/S1360-1385(97)01019-4
Dill-Macky, R. (2003). Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp.184-210). The American Phytopathological society
Dinolfo, M. I., Stenglein, S. A., Moreno, M. V., Nicholson, P., Jennings, P., & Salerno, G. L. (2010). ISSR markers detect high genetic variation among Fusarium poae isolates from Argentina and England. European Journal of Plant Pathology, 127, 483–491. https://doi.org/10.1007/s10658-010-9613-1
Divon, H. H., Bøe, L., Tveit, M. M. N., & Klemsdal, S. S. (2019). Infection pathways and penetration modes of fusarium langsethiae. European Journal of Plant Pathology, 154, 259–271. https://doi.org/10.1007/s10658-018-01653-3
Divon, H. H., Razzaghian, J., Udnes-Aamot, H., & Klemsdal, S. S. (2012). Fusarium langsethiae (Torp and Nirenberg), investigation of alternative infection routes in oats. European Journal of Plant Pathology, 132, 147–161. https://doi.org/10.1007/s10658-011-9858-3
EC (2013). Commission recommendation (2013/165/EC) of march 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Vol. 2013/165/EU. Official Journal of the European Union Brussels.
Edwards, S., & Anderson, E. (2011). Impact of agronomy on HT-2 and T-2 toxin content of oats. Plant Breeding and Seed Science, 63, 49–57. https://doi.org/10.2478/v10129-011-0015-0
Edwards, S., Barrier-Guillot, B., Clasen, P., Hietaniemi, V., & Pettersson, H. (2009). Emerging issues of HT-2 and T-2 toxins in European cereal production. World Mycotoxin Journal, 2, 173–179. https://doi.org/10.3920/WMJ2008.1126
Edwards, S. G. (2009). Fusarium mycotoxin content of UK organic and conventional oats. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure & Risk Assessment, 26, 1063–1069. https://doi.org/10.1080/02652030902788953
Edwards, S. G., Imathiu, S. M., Ray, R. V., Back, M., & Hare, M. C. (2012). Molecular studies to identify the fusarium species responsible for HT-2 and T-2 mycotoxins in UK oats. International Journal of Food Microbiology, 156, 168–175. https://doi.org/10.1016/j.ijfoodmicro.2012.03.020
EFSA CONTAM panel (EFSA Panel on Contaminants in the Food Chain), Knutsen, H. K., Alexander, J., Barregåard, L., Bignami, M., Brüschweiler, B., Ceccatelli, S., Cottrill, B., Dinovi, M., Grasl-Kraupp, B., & Hogstrand, C. (2017a). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal, 15, e04718. https://doi.org/10.2903/j.efsa.2017.4718
EFSA CONTAM panel (EFSA Panel on Contaminants in the Food Chain), Knutsen, H.-K., Barregård, L., Bignami, M., Brüschweiler, B., Ceccatelli, S., Cottrill, B., Dinovi, M., Edler, L., Grasl-Kraupp, B., Hogstrand, C., Hoogenboom, L., Nebbia, C. S., Oswald, I., Petersen, A., Rose, M., Roudot, A.-C., Schwerdtle, T., Vleminckx, C., … Alexander, J. (2017b). Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA Journal, 15, e04655. https://doi.org/10.2903/j.efsa.2017.4655
Fredlund, E., Gidlund, A., Pettersson, H., Olsen, M., & Börjesson, T. (2010). Real-time PCR detection of fusarium species in Swedish oats and correlation to T-2 and HT-2 toxin content. World Mycotoxin Journal, 3, 77–88. https://doi.org/10.3920/WMJ2009.1179
Gale, L. R. (2003). Population biology of Fusarium species causing head blight crops. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp. 120–143). The American Phytopathological society.
Gavrilova, O., Skritnika, A., & Gagkaeva, T. (2017). Identification and characterization of spontaneous auxotrophic mutants in Fusarium langsethiae. Microorganisms, 5, 14. https://doi.org/10.3390/microorganisms5020014
Goswami, R. S., & Kistler, H. C. (2004). Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology, 5, 515–525. https://doi.org/10.1111/j.1364-3703.2004.00252.x
Gratz, S. W. (2017). Do plant-bound masked mycotoxins contribute to toxicity? Toxins, 9, 85. https://doi.org/10.3390/toxins9030085
György, A., Tóth, B., Varga, M., & Mesterhazy, A. (2020). Methodical considerations and resistance evaluation against Fusarium graminearum and F. culmorum head blight in wheat. Part 3. Susceptibility window and resistance expression. Microorganisms, 8, 627. https://doi.org/10.3390/microorganisms8050627
Hautsalo, J., Jauhiainen, L., Hannukkala, A., Manninen, O., Veteläinen, M., Pietilä, L., Peltoniemi, K., & Jalli, M. (2020). Resistance to Fusarium head blight in oats based on analyses of multiple field and greenhouse studies. European Journal of Plant Pathology, 158, 15–33. https://doi.org/10.1007/s10658-020-02039-0
Hietaniemi, V., Rämö, S., Yli-Mattila, T., Jestoi, M., Peltonen, S., Kartio, M., Sieviläinen, E., Koivisto, T., & Parikka, P. (2016). Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Additives & Contaminants: Part A, 33, 831–848. https://doi.org/10.1080/19440049.2016.1162112
Hjelkrem, A.-G. R., Aamot, H. U., Brodal, G., Strand, E. C., Torp, T., Edwards, S. G., Dill-Macky, R., & Hofgaard, I. S. (2018). HT-2 and T-2 toxins in Norwegian oat grains related to weather conditions at different growth stages. European Journal of Plant Pathology, 151, 501–514. https://doi.org/10.1007/s10658-017-1394-3
Hofgaard, I., Aamot, H., Torp, T., Jestoi, M., Lattanzio, V., Klemsdal, S., Waalwijk, C., Van der Lee, T., & Brodal, G. (2016). Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin Journal, 9, 365–378. https://doi.org/10.3920/WMJ2015.2003
Hofgaard I.S., Brodal G., Almvik M., Lillemo M., Russenes A.L., Edwards S.G,. Aamot H.U. (2022). Different resistance to DON versus HT2+ T2 producers in Nordic oat varieties. Toxins 14 (5):313. https://doi.org/10.3390/toxins14050313
Hofgaard, I. S., Seehusen, T., Aamot, H. U., Riley, H., Razzaghian, J., Le, V. H., Hjelkrem, A.-G. R., Dill-Macky, R., & Brodal, G. (2016). Inoculum potential of Fusarium spp. relates to tillage and straw management in Norwegian fields of spring oats. Frontiers in Microbiology, 7, 556. https://doi.org/10.3389/fmicb.2016.00556
Imathiu, S. M., Edwards, S. G., Ray, R. V., & Back, M. A. (2013). Fusarium langsethiae – A HT-2 and T-2 toxins producer that needs more attention. Journal of Phytopathology, 161, 1–10. https://doi.org/10.1111/jph.12036
Imathiu, S. M., Hare, M. C., Ray, R. V., Back, M., & Edwards, S. G. (2010). Evaluation of pathogenicity and aggressiveness of F. langsethiae on oat and wheat seedlings relative to known seedling blight pathogens. European Journal of Plant Pathology, 126, 203–216. https://doi.org/10.1007/s10658-009-9533-0
Imathiu, S. M., Ray, R. V., Back, M., Hare, M. C., & Edwards, S. G. (2009). Fusarium langsethiae pathogenicity and aggressiveness towards oats and wheat in wounded and unwounded in vitro detached leaf assays. European Journal of Plant Pathology, 124, 117–126. https://doi.org/10.1007/s10658-008-9398-7
ISTA. (1966). Handbook of seed health testing. Series 3. Working sheet S.3.No.1, Avena sativa, fusarium nivale. International Seed Testing Association.
Jansen, C., von Wettstein, D., Schafer, W., Kogel, K. H., Felk, A., & Maier, F. J. (2005). Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proceedings of the National Academy of Sciences of the United States of America, 102, 16892–16897. https://doi.org/10.1073/pnas.0508467102
Kaukoranta, T., Hietaniemi, V., Rämö, S., Koivisto, T., & Parikka, P. (2019). Contrasting responses of T-2, HT-2 and DON mycotoxins and Fusarium species in oat to climate, weather, tillage and cereal intensity. European Journal of Plant Pathology, 155, 1–18. https://doi.org/10.1007/s10658-019-01752-9
Koga, S., Aamot, H. U., Uhlen, A. K., Seehusen, T., Veiseth-Kent, E., Hofgaard, I. S., Moldestad, A., & Böcker, U. (2020). Environmental factors associated with glutenin polymer assembly during grain maturation. Journal of Cereal Science, 91, 102865. https://doi.org/10.1016/j.jcs.2019.102865
Kokkonen, M., Medina, A., & Magan, N. (2012). Comparative study of water and temperature relations of growth and T-2/HT-2 toxin production by strains of Fusarium sporotrichioides and Fusarium langsethiae. World Mycotoxin Journal, 5, 365–372. https://doi.org/10.3920/WMJ2012.1406
Lancashire, P., Bleiholder, H., Boom, T., Langelüddeke, P., Stauss, R., Weber, E., & Witzenberger, A. (1991). A uniform decimal code for growth stages of crops and weeds. Annals of Applied Biology, 119, 561–601. https://doi.org/10.1111/j.1744-7348.1991.tb04895.x
Langevin, F., Eudes, F., & Comeau, A. (2004). Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. European Journal of Plant Pathology, 110, 735–746. https://doi.org/10.1023/B:EJPP.0000041568.31778.ad
Lattanzio, V. M., Visconti, A., Haidukowski, M., & Pascale, M. (2012). Identification and characterization of new Fusarium masked mycotoxins, T2 and HT2 glycosyl derivatives, in naturally contaminated wheat and oats by liquid chromatography–high-resolution mass spectrometry. Journal of Mass Spectrometry, 47, 466–475. https://doi.org/10.1002/jms.2980
Lemmens, M., Steiner, B., Sulyok, M., Nicholson, P., Mesterhazy, A., & Buerstmayr, H. (2016). Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin Journal, 9, 741–754. https://doi.org/10.3920/WMJ2015.2029
Lysøe, E., Frandsen, R. J., Divon, H. H., Terzi, V., Orrù, L., Lamontanara, A., Kolseth, A.-K., Nielsen, K. F., & Thrane, U. (2016). Draft genome sequence and chemical profiling of Fusarium langsethiae, an emerging producer of type A trichothecenes. International Journal of Food Microbiology, 221, 29–36. https://doi.org/10.1016/j.ijfoodmicro.2016.01.008
Medina, A., & Magan, N. (2010). Comparisons of water activity and temperature impacts on growth of Fusarium langsethiae strains from northern Europe on oat-based media. International Journal of Food Microbiology, 142, 365–369. https://doi.org/10.1016/j.ijfoodmicro.2010.07.021
Medina, A., & Magan, N. (2011). Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiology, 28, 392–398. https://doi.org/10.1016/j.fm.2010.09.012
Mesterhazy, A., Bartok, T., Kaszonyi, G., Varga, M., Toth, B., & Varga, J. (2005). Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. European Journal of Plant Pathology, 112, 267–281. https://doi.org/10.1007/s10658-005-2853-9
Miedaner, T., Lieberherr, B., & Gaikpa, D. S. (2021). Aggressiveness of Fusarium culmorum isolates for head blight symptoms is highly stable across four cereal crops. Journal of Phytopathology, 169, 387–392. https://doi.org/10.1111/jph.12995
Minitab, LLC. (2021). Minitab 19 Statistical Software. Retrieved from https://www.minitab.com
Nathanail, A. V., Varga, E., Meng-Reiterer, J., Bueschl, C., Michlmayr, H., Malachova, A., Fruhmann, P., Jestoi, M., Peltonen, K., & Adam, G. (2015). Metabolism of the Fusarium mycotoxins T-2 toxin and HT-2 toxin in wheat. Journal of Agricultural and Food Chemistry, 63, 7862–7872. https://doi.org/10.1021/acs.jafc.5b02697
Nishiuchi, T., Masuda, D., Nakashita, H., Ichimura, K., Shinozaki, K., Yoshida, S., Kimura, M., Yamaguchi, I., & Yamaguchi, K. (2006). Fusarium phytotoxin trichothecenes have an elicitor-like activity in Arabidopsis thaliana, but the activity differed significantly among their molecular species. Molecular Plant-Microbe Interactions, 19, 512–520. https://doi.org/10.1094/mpmi-19-0512
Opoku, N., Back, M., & Edwards, S. (2011). Aggressiveness of Fusarium langsethiae isolates towards wheat, barley and oats in an in vitro leaf assay. Plant Breeding and Seed Science, 64, 55–63 http://ojs.ihar.edu.pl/index.php/pbss/article/view/342
Opoku, N., Back, M., & Edwards, S. (2013). Development of Fusarium langsethiae in commercial cereal production. European Journal of Plant Pathology, 136, 159–170. https://doi.org/10.1007/s10658-012-0151-x
Opoku, N., Back, M., & Edwards, S. (2017). Susceptibility of cereal species to Fusarium langsethiae under identical field conditions. European Journal of Plant Pathology, 1–11. https://doi.org/10.1007/s10658-017-1329-z
Parikka, P., Hietaniemi, V., Rämö, S., & Jalli, H. (2007). The effect of cultivation practices on Fusarium langsethiae infection of oats and barley. In: "Fusarium workshop: Fusarium diseases in cereals–potential impact from sustainable cropping systems", pp. 15.
Pettersson, H., Börjesson, T., Persson, L., Lerenius, C., Berg, G., & Gustafsson, G. (2008). T-2 and HT-2 toxins in oats grown in northern Europe. Cereal Research Communications, 36, 591–592.
Schöneberg, T., Jenny, E., Wettstein, F. E., Bucheli, T. D., Mascher, F., Bertossa, M., Musa, T., Seifert, K., Gräfenhan, T., & Keller, B. (2018). Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. European Journal of Agronomy, 92, 123–132. https://doi.org/10.1016/j.eja.2017.09.004
Schöneberg, T., Kibler, K., Wettstein, F., Bucheli, T., Forrer, H., Musa, T., Mascher, F., Bertossa, M., Keller, B., & Vogelgsang, S. (2019). Influence of temperature, humidity duration and growth stage on the infection and mycotoxin production by Fusarium langsethiae and Fusarium poae in oats. Plant Pathology, 68, 173–184. https://doi.org/10.1111/ppa.12922
Stenglein, S. A., Dinolfo, M. I., Barros, G., Bongiorno, F., Chulze, S. N., & Moreno, M. V. (2014). Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant Disease, 98, 1733–1738. https://doi.org/10.1094/PDIS-02-14-0182-RE
Sturz, A. V., & Johnston, H. W. (1983). Early colonization of ears of wheat and barley by Fusarium poae. Canadian Journal of Plant Pathology, 5, 107–110.
Sulyok, M., Stadler, D., Steiner, D., & Krska, R. (2020). Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Analytical and Bioanalytical Chemistry, 412, 2607. https://doi.org/10.1007/s00216-020-02489-9
Tekle, S., Dill-Macky, R., Skinnes, H., Tronsmo, A. M., & Bjørnstad, Å. (2012). Infection process of Fusarium graminearum in oats (Avena sativa L.). European Journal of Plant Pathology, 132, 431–442. https://doi.org/10.1007/s10658-011-9888-x
Tekle, S., Lillemo, M., Skinnes, H., Reitan, L., Buraas, T., & Bjørnstad, Å. (2018). Screening of oat accessions for Fusarium head blight resistance using spawn-inoculated field experiments. Crop Science, 58, 143–151. https://doi.org/10.2135/cropsci2017.04.0264
Torp, M., & Adler, A. (2004). The European Sporotrichiella project: A polyphasic approach to the biology of a new Fusarium species. International Journal of Food Microbiology, 95, 241–245. https://doi.org/10.1016/j.Ijfoodmicro.2003.12.015
Uhlig, S., Eriksen, G. S., Hofgaard, I. S., Krska, R., Beltrán, E., & Sulyok, M. (2013). Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins, 5, 1682–1697. https://doi.org/10.3390/toxins5101682
Xu, X., Madden, L. V., & Edwards, S. (2013). Modelling the effects of environmental conditions on HT2 and T2 toxin accumulation in field oat grains. Phytopathology, 104, 57–66. https://doi.org/10.1094/phyto-03-13-0070-r
Xue, A. G., Chen, Y., Marchand, G., Guo, W., Ren, C., Savard, M., & McElroy, A. R. (2015). Timing of inoculation and Fusarium species affect the severity of fusarium head blight on oat. Canadian Journal of Plant Science, 95, 517–524. https://doi.org/10.4141/cjps-2014-300
Yli-Mattila, T., Mach, R. L., Alekhina, I. A., Bulat, S. A., Koskinen, S., Kullnig-Gradinger, C. M., Kubicek, C. P., & Klemsdal, S. S. (2004). Phylogenetic relationship of Fusarium langsethiae to Fusarium poae and Fusarium sporotrichioides as inferred by IGS, ITS, beta-tubulin sequences and UP-PCR hybridization analysis. International Journal of Food Microbiology, 95, 267–285. https://doi.org/10.1016/j.ijfoodmicro.2003.12.006
Acknowledgements
The authors would like to thank Andrew Dobson and Chloè Grieu for participating in the experimental work, and Torfinn Torp for statistical assistance. This research was funded by The Agriculture and Food Industry Research Funds—FFL/JA (Project SafeOats, The Research Council of Norway grant number 254751), Graminor AS, Lantmännen Corporate R&D, Felleskjøpet Agri SA, Felleskjøpet Rogaland Agder, Fiskå Mølle Moss AS, Strand Unikorn AS, Norgesmøllene AS, and Kimen Seed Laboratory AS.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aamot, H.U., Mousavi, H., Razzaghian, J. et al. Fusarium langsethiae and mycotoxin contamination in oat grain differed with growth stage at inoculation. Eur J Plant Pathol 164, 59–78 (2022). https://doi.org/10.1007/s10658-022-02539-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02539-1