Abstract
In this study, nanomuscovite adsorbents were prepared by intercalation with various organic intercalates (DTAB-TTAB-DTPA-PA-PN) and used to remove Cd2+ and Pb2+ from polluted water. The best nanomuscovite was prepared using DTPA and muscovite (Muc/DTPA) and characterized by XRD, TEM, EDX, FTIR, and BET surface area. The developed nanoadsorbent was used to remove Cd2+ and Pb2+ from polluted water. The effect of various factors, including contact time, adsorbent dosage, solution pH, and temperature, was investigated. The results reveal that the maximum adsorption of Cd2+ and Pb2+ was 91.5% and 97%, respectively, at the initial metal concentration 50 ppm, adsorbent dosage 0.2 g, contact time 60 min, solution temperature 25 °C, and pH 6 for Pb2+ and pH 7 for Cd2+. Adsorption isotherm models (Freundlich, Langmuir, Dubunin–Radushkevich, and Temkin isotherm models) as well as kinetic models (pseudo-first-order, pseudo-second-order, Elovich, and intra-particle diffusion models) were employed to evaluate the experimental results. The adsorption of Cd2+ and Pb2+ on Muc/DTPA fitted well within the Langmuir isotherm model and followed pseudo-second-order kinetics. Thermodynamics parameters of metal adsorption indicated exothermic and spontaneous processes. Results were applied to the real wastewater that showed high Cd2+ and Pb2+ removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals removal from different water sources (natural water and ground drinking water) and wastewater have attracted considerable attention from scientists. Several methods have been modified for treating wastewater from heavy metals which include coagulation, precipitation, reduction, flotation, ion exchange, adsorption, electrochemical, membrane technology electrolysis, and membrane processes (Rashed et al., 2020; Mahdavi et al., 2013). Among these technologies, adsorption has a widely application, with high advantages of an efficient and cost-effective alternative to conventional contaminated water treatment facilities. Adsorption also does not result in the formation of harmful substances (Gürses et al., 2014; Khataee et al., 2013).
Low-cost adsorbents include natural substances such as clays, zeolites, and chitosan, and some industrial wastes (including fly ash, coal, and oxides) were used for wastewater treatment (Cheira et al., 2019; Rashed et al., 2019). One of the clay minerals in the mica family is muscovite. Natural 2M1 muscovite is a 2:1 phyllosilicate silicate mineral with the formula KAl2(Si3AlO10)(OH)2.
Muscovite is a hydrated magnesium aluminum silicate mineral resulting from iron-bearing phlogopite micas and weathering biotite. Its advantages include high availability, low-cost adsorbent, easy handling, and selectivity over other natural adsorbents (Katsou et al., 2011). The octahedral Al(O/OH)6 sheet and two tetrahedral SiO4 sheets make up the mica group minerals (Muazu et al., 2020).
As the results of the applity of their charge can change and cation exchange, layered silicates have been widely studied and applied for preparation of nanocomposite materials. It is advantageous to occupy the interlayer area while creating layered silicate nanocomposite materials. Muscovite is a layered silicate mineral that is widely used in industrial applications (absorbents, automobiles, packaging, and electrical appliances (Bao et al., 2020). Yuan etr al. (2018) synthesized 20–45 nm muscovite at 250 °C for 18–72 h using K-feldspar.
Clay minerals consist of interlayer space that has the can introduce intercalate agents, so some organic agents were intercalated into clay minerals, and the resultant intercalates exhibit fascinating properties and have numerous uses, including adsorbents, organic synthesis, polymer–clay nanocomposites, catalysis, photofunctional materials, and drug carrier in the pharmaceutical area. Several variables, including the degree of exchange, host–guest and guest–guest interactions, layer charge density, and alkyl chain length, influence the intercalation of organic compounds into clay (Lagaly et al., 2013).
The additional positive charges in the interlamellar domain are balanced by the intercalated hydrated anions. Naturally occurring anionic clays with highly organized 2D structures and superior catalytic capabilities are known as layered double hydroxides (LDHs). Enhancing the surface characteristics of the adsorbent and increasing the adsorption capacity can be accomplished by modifying the LDHs components with additional materials (Karim et al. 2022; Lan et al., 2014).
Two-dimensional (2D) nanomaterials with sheet-like structures, such as transition metal dichalcogenides (TMDs), graphene, layered double hydroxides (LDHs), metal–organic frameworks (MOFs), metal carbides and nitrides (MXenes), and covalent organic frameworks (COFs), have spurred an increased interest in adsorption and catalytic fields due to their diverse desirable properties. The narrow thickness and double-sided surface structure increased the specific surface area while also producing a significant amount of exposed active sites, which are crucial for catalytic processes. LDHs are an emerging and adaptable class of 2D inorganic layered nanomaterials, together with naturally occurring or artificial anionic clay minerals, which can be included among these 2D nanomaterials (Xie et al., 2019, Karim et al. 2022).
Various natural materials, including zeolite, natural polymers, silica, carbonaceous nanomaterials, metals, and metal oxides, were used to create nanomaterial adsorbents (Ethaib et al., 2022). Yu et al. (2006) and Yu (2007) prepared muscovite nanocomposites via the intercalation of muscovite using the inorganic–organic ion exchange steps. HafizahChe et al. (2017) prepared nanomuscovite by an alkaline salt and modified it with various concentrations of cetyltrimethylammonium bromide. El-Sheikh et al. (2020) prepared nanokaolinite photocatalyst using hydrazine hydrate as intercalate agent and used it for the removal of P-nitrophenol under UV irradiation.
Tettch et al. (2022) prepared kaolinite/muscovite composite by activation with H2SO4 and NaOH and used it to remove Pb2+ ions from aqueous media. Due to their distinctive properties, silica-based nanomaterials were used for adsorption of metal ions due to their highly adsorptive properties (surface area, and pore size). Ashrafi et al. (2017) prepared nanoparticle of Fe3O4@MnO2 MNPs with a core–shell structure and used as an effective adsorbent for removal of lead ions from aqueous solution.
The innovation of this study is to find suitable nanomaterials from abundant ores with low-cost that can be used for treatment polluted water. So, the main objective of the present study is the preparation of novel nanomoscovite from the activated muscovite by intercalation with different intercalates (DTAB-TTAB-DTPA-PA-PN). The developed adsorbents will be used for the removal of Cd and Pb ions from wastewater.
Nanosilica is an environmentally non-toxic and acceptable adsorbent. Li et al. (2019) modified silica gel with nitrilotriacetic acid and sued it for removal of Cd2+, Cu2+, and Pb2+ within 2 to 20 min. Kotsyuda et al. (2017) studied the removal of Cu2+ ions using amino-functionalized (3-aminopropyl and phenyl groups) silica nanospheres and found that the adsorption capacity increased linearly with the concentration of amino groups. Liu et al. (2018) studied Cd2+ ions adsorption from solutions using nano-montmorillonite and found the maximum adsorption of Cd ions was estimated to be 17.61 mg/g.
Materials and methods
Chemicals and reagents
A high analytical grade of these chemicals was used: lead nitrate, Pb(NO3)2, M. wt 331.21, assay (65%), Sigma-Aldrich; cadmium nitrate, Cd(NO3)2, M. wt 308.477, assay (65%), Sigma-Aldrich; TTAB (1-tetradecyl) trimethyl ammonium bromide.
(CH3(CH2)13N-(CH3)3Br, M.wt 336.40, assay (98%), Alfa Aesar DTAB (1-dodecyl) trimethyl ammonium bromide (CH3(CH2)11N(CH3)3Br, M.wt 308.35, assay (99%), Alfa Aesar DTPA diethylene tri-amine penta-acetic acid (C14H23N3O10), M.wt 393.35, assay (100%), Alfa Aesar.
Instruments and tools
All the batch experiments were carried out in Pyrex conical beaker (100 ml) at room temperature under mechanical stirring (150 rmp); the solution pH was adjusted with (1N) NaOH or HCl. The concentrations of the metals were determined by AAS (flame atomic absorption spectrophotometry, GBC 932AA).
Preparation of working and standard solutions
Cadmium and lead standard solution (1000 ppm) was prepared: cadmium stock solution, 2,744 g Cd (NO3)2 in 0.5 M HNO3,Cd2+ = 1000 ± 0.002 ppm; lead stock solution, 1.599 g Pb(NO3)2 in HNO3 0.5 M, concentration of Pb2+ = 1000 ± 0.002 ppm.
Characterization of nanomuscovite adsorbent
Morphology of nanomuscovite adsorbent was examined using SEM, XRD, and FTIR. To measure the surface area and porosity of nanomuscovite adsorbent samples, the Micromeritics Tristar 3000 (Georgia, USA) device was employed.
Sample collection
Bulk sample (5 kg) of muscovite was collected from Southeast desert, Egypt. The samples were crushed and ground to powder with agate mortar. The silica particles were removed from muscovite samples by contacting the powdered muscovite with deionized water for one day. The suspension solution was filtered, dried at 60 °C for 24 h, and sieved to 565 μm.
Preparation of nanomuscovite via intercalation method
Intercalated muscovite complexes were prepared using different intercalating organic agents (DTAB-TTAB-DTPA-PA-PN), and then, deintercalation process was used to have nanomuscovite particles.
Potassium acetate intercalated into muscovite (Mus /PA)
5 g activated muscovite (Mus) was added to a 250 mL in a plastic flask, containing 1.6157 g of potassium acetate (PA) dissolved in 100 mL deionized water. The solution was filtered and the precipitate was washed with sodium hydroxide (0.1 M) until pH 7, followed by deionized water, and dried at 100 °C for 10 h (Qian et al., 2011).
Potassium nitrate intercalated into muscovite (Mus /PN)
1.6645 g potassium nitrate (PN) was added to 100 mL of deionized water and mixed with 5 g of activated muscovite for 3 days at 70 °C. The solution was filtered, and the precipitate was washed with sodium hydroxide (0.1 M) until pH 7 and then repeatedly rinsed with deionized water before being dried at 100 °C for 10 h.
DTAB intercalated into muscovite (Mus /DTAB)
1.6645 g DTAB was added to 100 mL deionized water and mixed with 5 g of activated muscovite for 3 days at 70 °C. The solution was filtered and the precipitate was repeatedly rinsed with deionized water to remove any excess bromide anions (confirmed by silver nitrate test) and then dried at 100 °C for 10 h.
TTAB intercalated into muscovite (Mus /TTAB)
5 g activated muscovite was added to 100 mL deionized water containing 1.6645 g TTAB and mixed for 3 days at 70 °C. The solution was filtered and the precipitate was repeatedly rinsed with deionized water to remove any excess bromide anions (confirmed by silver nitrate test) and then dried at 100 °C for 10 h.
DTPA intercalated into muscovite (Mus /DTPA)
5 g activated muscovite was added to a 250-mL plastic flask, containing 6.475 g of DTPA dissolved in 100 mL deionized water. The mixture was mixed for 3 days at 70 °C and filtered and the precipitate was washed with sodium hydroxide (0.1 M) until pH 7, followed by deionized water several times and then dried at 100 °C for 10 h.
Optimal parameters for the maximum adsorption of Pb2+ and Cd2+ by the prepared nanomuscovite
Solution pH effect
Solution pH effect on Pb2+ and Cd2+ removal efficiency by nanomuscovite was studied by stirring 0.1 g of nanomuscovite with 50 mL of 50 ppm Pb2+ and Cd2+ at various pH (2, 4, 5, 6, 7, 8, and 10). The mixture was filtrated by filter paper Whitman No 42, and the concentration of Pb2+ and Cd2+ in the filtrate was measured by atomic absorption spectrophotometer (AAS).
Adsorbent dose effect
Nanomuscovite dosage ranging from 0.05 to 1.0 g was stirred with 50 mL of 50 ppm Pb2+ and Cd2+ at pH 6 for Pb2+ and pH 7 for Cd2+. After filtration, the concentrations of Pb2+ and Cd2+ were measured using AAS.
Effect of initial metal concentration
0.2 g of nanomuscovite was stirred with 50 ml of Pb2+ and Cd2+ solutions with initial concentrations (10, 20, 30, 50, 75 and 100 ppm) and pH 6 for Pb2+ and pH 7 for Cd2+. The mixture was filtrated by filter paper Whitman No 42, and the metal concentration was measured using AAS.
Effect of contact time
0.2 g nanomuscovite was stirred with 50 mL 50 ppm Pb2+ and Cd2+ solutions at contact times (30, 60, 120 and 240 min) and pH 6 for Pb2+ and pH 7 for Cd2+. After filtration, the concentrations of Pb2+ and Cd2+ were measured using AAS.
Effect of temperature
0.2 g nanomuscovite was stirred with 50 mL 50 ppm Pb2+ and Cd2+ solutions for 60 min. at different temperatures (25, 35, 45, and 55 °C) keeping pH6 for Pb2+ and pH7 for Cd2+. The mixture was filtrated by filter paper Whitman No 42, and the metal concentration was measured using AAS.
Results and discussion
Characterization of nanomuscovite
XRD analysis
XRD provides information about the degree of the exfoliation of the organoclay. Activated muscovite shows two intense diffraction peaks at 2θ values of 8.9° and 26.8°, less intense peaks at 2θ of 17.8°, 26.6°, 35.9°and at 2θ 45.3°. The main peak at 8.87° corresponds to the interplanar spacing of 9.98 Å, which are all associated with muscovite as determined by JCPDS data (JCPDS: 00–058-2016). Crystallite size was determined by Scherer's equation (Scherrer P. (1918) D = (Kλ)/ (βcos θ), where D is the particle diameter size, λ is the wavelength of the X-ray radiation (λ = 0.154056 nm), K is the Scherer constant (K = 0.891), θ is the characteristic angle of diffraction (2θ = 8.9º), and β is the full width at half maximum of the (001) plane after correcting with instrumental broadening using the Warren function. The XRD pattern for nanomuscovite samples prepared in the presence of DTPA, PA, PN, TTAB, and DTAB is obtained in Fig. 1. From this figure, it was noticed that the nanomuscovite samples display the same characteristic peaks as activated muscovite, suggesting the maintenance of the original crystallinity for the inorganic matrix when the reactions take place, where it shows two intense peaks at 8.9° and 26.8°, less intense peaks at 2θ of 17.8°, 26.6°, 35.9°, and at 2θ 45.3° as determined by JCPDS data (JCPDS: 00-058-2016). But in the case of TTAB-treated muscovite, a new form of rectorite (JCPDS: 00-014-0183) has appeared. It was observed that the crystalline size became smaller, which indicates that all intercalating agents reduced the crystallite size as summarized in Table 1. The high intensity and smallest crystallite size were obtained for the sample muscovite/DTPA.
TEM examination
Figure 2 shows the TEM image of Mus/DTPA nanomuscovite sample. The face size of the majority of the particles is less than 100 nm, demonstrating the plate-like form of the muscovite particles and the compactness of the aggregated plates from the margins. Strong intermolecular forces cause the muscovite layers to be tightly packed together, and the smaller size of nanomuscovite particles than that of activated muscovite suggests that organic molecules may be intercalated between the muscovite layers.
From Table 2 and Fig. 3, EDX spectra reflect the muscovite purity and end to that the particle is composed of O, Mg, Si, Al, and K.
FTIR analysis
Figure 4 shows the FTIR spectra of all nanomuscovite samples prepared by different intercalating agents between 4000 and 400 cm−1. The spectrum exhibits the typical appearance, where a peak at 1016 cm−1 corresponds to the stretching of the Si–O bond and the bands at 2922 and 2855 cm−1 correspond to the stretching vibration modes of the alkyl chain from the quaternary ammonium modifier. The peak at 440 cm−1 corresponds to the bending of the Mg–O bond. The bands of Al–O bond bending are observed at 918 cm−1. The peaks at 1635 and 3298 cm−1 are assigned to hydration HOH and –OH vibration groups between the tetrahedral and octahedral sheets (Nooshabadi et al., 2014). The band at ∼793 cm−1 is for the OH bending vibrations with respect to the silicate plate in the muscovite (Chen et al., 2020). The bands at 1403 cm−1 and 1542 cm−1 are corresponding to the symmetric and asymmetric stretching vibration of the CH3COO- group, respectively. The 1395–1440 cm−1 band is attributed to the formation of coupling between C–O vibration and deformation vibration mode of the O–H groups (Ding et al., 2011). In the case of PN/muscovite, not all peaks appear and have a new band around 1385 cm−1 which is attributed to N–O stretching.
Surface area, BET, and BJH analysis
The surface area of the prepared samples was also estimated by the BET method, and it was found that surface area values of prepared samples have the following order: Muc/DTPA (195.15 m2g−1) > Muc/DTAB (190.97 m2g−1) > Muc/TTAB (182.46 m2g−1) > Muc/PA(178.45 m2 g−1) > Muc/PN (172.62 m2 g−1) > AM(137.659 m2 g−1). Table 3 shows that the pore size of Muc/DTPA was smaller one due to decreasing the particles of muscovite.
A comparative study of the prepared nanomuscovite samples for Cd2+ and Pb2+ removal efficiency
For comparison between the prepared nanomuscovite samples by adsorption of cadmium and lead (Table 4) it is clear that Muc/DTPA is the best one for the removal of cadmium and lead and these results have been confirmed previously. So, the Muc/DTPA will be used in all the other experiments.
Optimal parameters for the adsorption of Cd2+ and Pb2+ on nanomuscovite (Muc/DTPA)
Effect of pH on metals adsorption
One of the key factors affecting how metal ions adsorb is the pH of the system, which can alter the metal forms in solution and have an impact on the surface characteristics of the adsorbent in terms of the dissociation of functional groups and surface charges. The outcomes demonstrated that as the pH rose, the amount of heavy metal ions that could be removed by adsorption on nanomuscovite (Muc/DTPA) increased (Fig. 5). The adsorption removal percentage increased from 49 to 84% when the pH increased from 2 to 7 for Cd2+ and increased from 52 to 89.7% when the pH increased from 2 to 6 for Pb2+. At a low pH, the competition at the binding surfaces between heavy metal ions and H+ ion occurs which results in a lower repulsion of the adsorbed metal (Sdiri et al., 2012). With the increase in solution pH, the deprotonation of the nanomuscovite surface occurs, resulting in an increase in the number of negative charge sites and so resulting in the high adsorption of metal ions on nanomuscovite (Zhu et al., 2013).
Liu et al., (2018) found that by using nano-montmorillonite (NMMT) at pH 7, maximum Cd ions adsorption from aqueous solutions occurs. Eloussaief and Benzina (2010) investigated the efficiency of removing Pb2+from aqueous solutions using natural and acid-activated clays and discovered that the Pb2+ ions adsorption increased with pH up to a neutral pH (7.0). Yin et al. (2018) revealed that Pb(II) was effectively adsorbed in solution at pH 4 using a nanoillite/smectite clay as adsorbent. Al-a'qarbeh et al., (2020) investigated the Pb2+ ions adsorption from aqueous solution using the nanoplatelets client and found the maximum adsorption capacity of Pb2+ ions at pH 7. Using Saudi Arabian clay, Ahmad et al. (2014) investigated the removal of Pb2+ and discovered that pH 6 had the highest adsorption capability.
Effect of adsorbent dosage on heavy metal adsorption
To investigate the effect of varying nanomuscovite adsorbent doses on the uptake of Cd2+ and Pb2+ from aqueous solution, different nanomuscovite doses (0.05, 0.1, 0.2, 0.3, 0.5, and 1.0 g) were studied with constant parameters (initial metal concentration, pH, contact time, temperature). The results (Fig. 6) show that increase in adsorbent dose from 0.05 to 0.2 g increased the adsorption percentage of the metal ions from 82 to 97.1% for Pb2+ and from 80.2 to 96.1% for Cd2+; as a result of increased negative charge on the surface and the electrostatic potential decrease near the solid surface, it led to sorbent–solute interaction.
Cadmium and lead removal percentages, therefore, dropped when the effects of accumulation on the nanomuscovite adsorbent occurred with increases in its dose of more than 0.2 g, and increases in active sites with higher surface area also had little effect after equilibrium was reached (Gerçel & Gerçel, 2007). Similar results to our study have been reported by Unuabonah et al. (2007) for the adsorption of Cd and Pb ions from solutions by adsorbent (tripolyphosphate-impregnated kaolinite clay).
Effect of heavy metals concentration on the adsorption
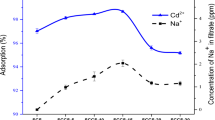
The initial metal concentration effect on the removal of Cd2+ and Pb2+ by the adsorbent is represented in Fig. 7. The data evidence that the removal percentage increases from 76 to 97.1% for Pb2+ and from 74 to 96% for Cd2+, as the metal initial concentration increases from 10 to 50 ppm. This might be because Cd2+ and Pb2+ are more mobile on the surface of nanomuscovite due to the concentration differential between the adsorbate and its outer surface, increasing the driving force (Zhu et al., 2013). After then, the metal removal efficiency decreases with increased metal concentration; the reason is that when the metal concentration rises, the active surface sites of the adsorbent become saturated, which lowers the percentage of metal removal. Additionally, additional adsorption sites are needed when Cd2+ and Pb2+ are present in the solution at greater starting concentrations (Masindi & Gitari, 2016). The final result refers to that the best concentration of Cd2+ and Pb2+ was 50 ppm for the adsorption on nanomuscovite adsorbent.
Liu et al. (2018) studied the cadmium ions adsorption from aqueous solutions using nano-montmorillonite (NMMT) at an initial Cd ion concentration (22.4–224 mg/L) and found that the maximum Cd adsorption (17.61 mg/g) was at 22.4 mg/L Cd2+ concentration. David et al. (2016) prepared intercalated clay nanoparticles using cetylpyridinium chloride (CPC) and tetradecyltrimethylammonium bromide (TTAB). The resulted nanoparticles C-TTAB and C-CPC were applied to remove Cd, Pb and pentachlorophenol (PCP) through batch process. They reported the initial concentration for removal of Pb by CNP was 80 ppm and 50 ppm for Cd.
Effect of contact time on heavy metals adsorption
The results of contact time effects on the efficiency of Cd and Pb ion removal are presented in Fig. 8. The results revealed that the adsorption of Cd2+ and Pb2+ increased rapidly with time up to 60 min in which it reached the metal removal 95%; after then, the metal removal rate decreased as the result of the Cd2+ and Pb2+ accumulation on the vacant sites of nanomuscovite. The rapid increase in metal adsorption with time resulted a large number of vacant sites.
Effect of temperature on heavy metals adsorption
The temperature effect on the adsorption of Cd2+ and Pb2+ on nanomuscovite had been studied with different temperatures (25 °C, 35 °C, 45 °C, and 60° C). The results (Fig. 9) showed that the removal of Cd2+ and Pb2+ on nanomuscovite was high at room temperature (25 °C); after then, it decreased gradually. The adsorption effectiveness decreased from 99 to 81% for Pb and from 97.2 to 79% for Cd when the temperature was raised to 55 °C. Since the van der Waals bonds break down with increased temperature, the active sites are reduced (Negm et al., 2022). Because of this, Pb and Cd ions can be better adsorbed by nanomuscovite at room temperature (25 °C) than at any other temperature.
Adsorption isotherm study
Studies of adsorption isotherms are helpful to understand how ions move from the adsorbents during adsorption process. Characterization of Pb and Cd adsorption on the nanomuscovite approach was done using Freundlich, Langmuir, Temkin, and Dubinin–Raduskevich (D–R) isotherm models.
The experimental data ( solution temperature 25 °C, initial metals concentration 50 ppm /50 ml, 0.2 g adsorbent dosage, and 60 min contact time) are presented in Table 5.
Langmuir isotherm
Langmuir isotherm model described the saturated monolayer adsorption on the homogenous surface at constant energy. Langmuir equation can be quantified by equation:
Ce is the equilibrium concentration of heavy metals (mg/L), Qo, the adsorbent monolayer adsorption capacity of (mg/g), qe, the metal equilibrium capacity at adsorbent (mg/g), and b, the Langmuir binding energy coefficient (L/mg).
Calculating the Qo and b1 from Eq. 1 requires graphing Ce/qe against Ce and determining the intercept and slope (Table 6).
From the data in Table 7, the correlation coefficients (R2) of Cd2+ and Pb2+ were found as 0.986 and 0.982 for nanomuscovite adsorbent, respectively, indicating that the adsorption mechanism of Cd2+ and Pb2+ ions onto the nanomuscovite surface is carried out via monolayer. The maximum monolayer adsorption capacities (Qo) of Cd2+ and Pb2+ were found to be 26.6 and 25.2 mg g−1, respectively, and close to the experimental capacities.
Also, from the results bL < 1 for the studied metal adsorption reveals that the adsorption of Cd2+ and Pb2+ ions on nanomuscovite is favorable according to Langmuir isotherm. So, the Langmuir model best fits the experimental data.
Freundlich isotherm
The Freundlich isotherm is applied to determine the applicability of heterogeneous surface energy in the adsorption process. The convenient formula for Freundlich is:
The constants Kf, and n are the adsorption capacity and the adsorption intensity, respectively. So, when 1/n values are < 1, it indicates normal adsorption with a strong interaction between adsorbent and the metal, while if 1/n < 1, it indicates cooperation adsorption.
From Eq. 2, the intercept and slope of the linear plot of log qe against log Ce can be used to compute the Kf and n.
From Eq. 2, the Kf and n can be calculated from the intercept and slope of the linear plot of log qe against log Ce and are tabulated in Table 7. The results obtained of Kf and 1/n were 0.36 and 2.09 for Cd2+ and 0.26 and 2.29 for Pb2+, respectively. The resulted 1/n values indicate that the adsorption of Cd and Pb ions onto nanomuscovite was favorable at studied conditions. The Freundlich isotherm model did not provide a better fitting than the Langmuir isotherm, as indicated by the lower correlation coefficient (R2) values (0.805 and 0.773 for Cd2+ and Pb2+, respectively) than that in the Langmuir model.
The Temkin isotherm
The Temkin isotherm model suggests that the population of surface binding adsorption will have a uniform distribution of binding energies.
The Temkin equation's linear form is written as:
B = RT/b, where b is the heat of sorption-related Temkin constant. A plot of qe versus ln Ce allows one to estimate the constants B, which is related to the heat of sorption (J/mol), and K, which is the Temkin isotherm equilibrium binding constant.
The values KT = 4.3 and 8.9 L g−1, R2 = 0.98 and 0.96, and B = 5.44 and 4.62 J mol−1 for Cd2+and Pb2+, respectively, were estimated from the Temkin plot (Table 7). The heat of sorption (bT) indicates a physical adsorption process as the values of bT are less than 8 kJ/mol. This means that the adsorption of cadmium and lead on nanomuscovite follows the Temkin model.
Dubinin–Radushkevich isotherm model
On a heterogeneous surface, the Dubinin–Radushkevich isotherm is typically used to express the adsorption mechanism with Gaussian energy distribution. It is used to differentiate between metal adsorption which is physical and chemical.
The linearized D-R equation:
By graphing ln qe versus E2 using Eq. 4, it can calculate the β values from the slope and qm from the intercept.
Information regarding chemical and physical adsorption is provided by the mean adsorption energy (E). The obtained values of the mean free energy (E) for cadmium and lead, respectively, are 0.32 kJ/mol and 0.5 kJ/mol, respectively (Table 7).
E value for Cd2+ and Pb2+ is less than the 8–16 kJ /mol range of the adsorption reaction. Therefore, physical adsorption was used to describe adsorption of Cd2+ and Pb2+ on nanomuscovite.
From Table 7, the adsorption of Cd2+ and Pb2+ on nanomuscovite fitted well Langmuir isotherm due to the high correlation factor (R2) than the other isotherm models.
The R2 values of Cd2+ and Pb2+ using the evaluated isotherms confirm the following sequence Langmuir (best fit) > Temkin > Dubinin–Radushkevich > Freundlich.
Adsorption kinetic studies
Kinetic models have been proposed to identify the adsorption process' mechanism. These models offer important data that can improve adsorption effectiveness and process scale viability (Eftekhari et al., 2010). The pseudo-first-order, pseudo-second-order, Elovich models, and intra-particle diffusion were applied to examine the adsorption kinetics that characterize the effectiveness of Pb and Cd adsorption on nanomuscovite. All adsorption steps, including external film diffusion, internal particle diffusion, and adsorption, are included in these models. Tables 8 and 9 show the kinetic information for Pb and Cd adsorption on nanomuscovite.
Pseudo-first-order model
The following is a representation of the pseudo-first-order kinetic model:
K1 is the rate constant of the pseudo-first order and qe and qt (mg/g) are the adsorption capacities at equilibrium and time (min), respectively.
The pseudo-first-order kinetic constants for the adsorption of Cd2+ and Pb2+ on nanomuscovite were calculated from Eq. 5 using the slope and intercept of the linear relation between time (t) and Log (qe –qt). The correlation coefficient (R2) values were 0.843 and 0.854 for Cd2+ and Pb2+, respectively. The k1values were 0.02 and 5.8 × 10−3 min−1 for Cd2+ and Pb2+, respectively (Table 10). The pseudo-first-order model's R2 value for Cd2+ and Pb2+ is lower than the R2 values in the other models, indicating that the model is irrelevant. This indicates that pseudo-first-order equation might not be adequate to construe the mechanism of Cd2+ and Pb2+ onto nanomuscovite interactions.
Pseudo-second-order model
It is confirmed that the pseudo-second-order model is the rate-limiting stage in the formation of the van der Walls bond between Cd2+ and Pb2+ via electron sharing or exchange. The following is a representation of the pseudo-second-order kinetic model:
K2 is the rate constant of pseudo-second-order adsorption (g/mg/ min).
From Eq. 6, the plots of t/qt vs time (t) for the adsorption of Cd2+ and Pb2+ are derived using the pseudo-second-order model. Two parameters (qe and K2) were determined using the intercept and slope, respectively. High correlation coefficient (R2) values were obtained for Cd2+ and Pb2+ ions (0.996 and 0.997, respectively) (Table 10). For Cd2+ and Pb2+, the calculated equilibrium adsorption capacity (qe) values were 12 and 12.1 mgg−1, respectively. The adsorption of Cd2+ and Pb2+ followed pseudo-second-order model, as seen by the high conformity between the qe, cal and qe exp values.
Intra-particle diffusion model
The solute transfer for solid–liquid adsorption is often characterized by an intra-particle diffusion model, which was used to determine the adsorption mechanism:
where C (mgg−1) indicates the boundary layer effect and Ki (mg g−1 min−1/2) is the rate constant of the intra-particle diffusion model.
The slop and intercept of the linear plot of qt versus t1/2 allow for the determination of the Ki and C from Eq. 7 (Fig. 10). Table 10 contains the estimated and collected intraparticle diffusion constants for adsorption of Cd2+ and Pb2+ onto nanomuscovite.
The multilinearity forms shown in Fig. 10 indicate that there may be two or more additional steps. The first shows that the line's sharper portion can be attributable to the boundary stratum diffusion of solutions to the diffusion of divalent ions (Cd2+ and Pb2+) from the solution to the surface of nanomuscovite. The progressive adsorption stage is described in the second section, with intraparticle diffusion serving as the rate-limiting step (Bany-Aiesh et al., 2015; Dawodu & Akpomie, 2014; Srivastava & Hasan, 2011). Kid values for Cd2+ and Pb2+ were 0.19 and 0.17 mg g−1 min−1/2, while R2 equal 0.83 and 0.88, respectively. C (10.2 and 10.4 mgg−1 for Cd2+ and Pb2+, respectively) values provide information regarding the boundary layer's thickness. The impact of the boundary strata increases with increasing intercept (Bany-Aiesh et al., 2015). The variation in the rate of mass transfer at the first and final stages of adsorption may cause the straight lines' aberration from the origin. Furthermore, pore dispersion is not the only rate-controlling step indicated by such a divergence of the straight lines from the origin (Srivastava & Hasan, 2011). Furthermore, there may be two distinct stages involved in the adsorption of two divalent ions onto nanomuscovite. The surface adsorption or rapid external diffusion stage was considered to be the first linear fraction. The piecemeal adsorption process, which is managed by intra-particle-diffusion, forms the basis of the second linear portion (Dawodu & Akpomie, 2014). Due to low R2 values, the sorption of Cd2+ and Pb2+ on nanomuscovite did not follow the intraparticle diffusion equation.
Elovich model
Elovich equation is one of the best models for characterizing these activated chemisorptions and described as:
α is the initial adsorption, and β, the adsorption coefficient
In Eq. 8, when qe is plotted against ln (t), graphs are created from which the constants α, β and correlation coefficient (R2) are computed (Table 10). The rate-controlling step (RCS) of the Elovich model is the chemical reaction of Cd2+ and Pb2+ with the adsorbent surface, and the removal process involves multilayer adsorption (Hosseini-Bandegharaei et al., 2016).
The results reveal that the initial Cd2+ and Pb2+ adsorption rates onto nanomuscovite were studied, and the Elovich values (α) were determined to be 2.1 and 2.4 mg min−1 Cd2+ and Pb2+, respectively. The values of β, which represent the number of adsorption sites, were 1.1 and 0.1.3 for Cd2+ and Pb2+, respectively. The adsorption of Cd2+ and Pb2+ onto nanomuscovite did not follow the Elovich equation because of low R2 values.
Last but not least, Table 10 shows that all four kinetic models used for Cd2+ and Pb2+ adsorption on nanomuscovite follow a pseudo-second-order kinetic model.
Thermodynamics of adsorption
The actual indications for particle applications are thermodynamic variables such (ΔG°), entropy (ΔS°), and enthalpy (ΔH°). Thermodynamics of adsorption was assessed in relation to various temperatures (298, 313, and 333 K).
The following equation (Ghosal & Gupta, 2017) was used to compute the thermodynamic parameters:
R is temperature (K) and T is the gas constant (8.314 J/mol K).Table 11 lists the estimated thermodynamic parameters.
The findings demonstrate that the characteristic sign of the ΔG° values was negative at all temperatures, demonstrating the spontaneous nature of these adsorptions. Additionally, it was found that the ΔG° values drop as T rises, suggesting that there is less driving force for adsorption at higher T. The negative sign of ΔH° indicates that the adsorption processes are exothermic. The results show the ΔH° values for adsorption of Cd2+ and Pb2+ ions are smaller than 20.9 kJ/mol which implies that Cd2+ and Pb2+ ions are physically adsorbing to nanomuscovite. The ΔH° value in the range of 2.1–20.9 kJ/mol indicates physical adsorption and 80–200 kJ/mol chemical adsorption processes. The organization of the adsorbed ions becomes less random, and the negative ΔS° values reflect a drop in ion concentration at the solid solution interface, indicating an increase in divalent ions adsorption on the nanocrosslinked solid phase.
Conclusion
In this study, effective nanomuscovite adsorbent was prepared by intercalation muscovite with intercalates (DTAB-TTAB-DTPA-PA-PN).
The results of the batch adsorption of Pb and Cd on the nanomuscovite revealed high adsorption dependent on pH, contact time, adsorbent dose, initial metal concentration, and solution temperature. The adsorption kinetics of lead and cadmium ions followed the pseudo-second-order kinetic model.
In addition, the adsorption isotherm was analyzed by Langmuir, Freundlich, Dubinin, and Temkin isotherm models. The Langmuir model was fitted well to the well-explained Pb and Cd adsorption on the nanomuscovite with maximum adsorption 25.2 mg/g for Pb and 26.6 mg/g for Cd. The thermodynamic parameters values such as ΔG°, ΔH°, and ΔS° were calculated for the nanomuscovite adsorbent and ended that the ΔG° value was negative, indicating the adsorption was spontaneous.
The easy-to-use, environmentally safe, and effective procedure of this adsorbent would offer a crucial technique for removing industrial wastewater.
Data availability
Not applicable.
References
Ahmad, F., Fouad, E., & Ahmad, N. (2014). Removal of lead from wastewater by adsorption Using Saudi Arabian Clay. International Journal of Chemical and Environmental Engineering, 5(2), 65–68.
Al-a’qarbeh, M. M., Shammout, M. W., & Awwad, A. M. (2020). Nano platelets kaolinite for the adsorption of toxic metal ions in the environment. Chemistry International, 6(2), 49–55.
Ashrafi, A., Rahbar-Kelishami, A., & Shayesteh, H. (2017). Highly efficient simultaneous ultrasonic assisted adsorption of Pb (II) by Fe3O4@MnO2 core-shell magnetic nanoparticles: Synthesis and characterization, kinetic, equilibrium, and thermodynamic studies. Journal of Molecular Structure, 1147, 40–47. https://doi.org/10.1016/j.molstruc.2017.06.083
Bany-Aiesh, H., Banat, R., & Al-Sou’od, K. (2015). Kinetics and adsorption isotherm of ibuprofen onto grafted β-CD/Chitosan polymer. American Journal of Appllied Science, 12, 917.
Bao, T., Damtie, M. M., Hosseinzadeh, A., Frost, R. L., Yu, Z. M., Jin, J., & Wu, K. (2020). Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: Synthesis, characterization, and mechanism studies. Applied Clay Science, 195, 105735.
Cheira, M. F., Rashed, M. N., Mohamed, A. E., Zidan, I. H., & Awadallah, M. A. (2019). The performance of Alizarin impregnated bentonite for the displacement of some heavy metal ions from the wet phosphoric acid. Separation Science and Technology. https://doi.org/10.1080/01496395.2019.1675701
Chen, S., Chen, F., Di, Y., Han, S., & Zhu, X. (2020). Preparation and characterisation of exfoliated muscovite/poly (2,3-dimethyl-aniline) nanocomposites with an enhanced anticorrosive performance. Micro & Nano Letters, 15(8), 509–513.
David, M., Harrison, W., & Erastus, G. (2016). Novel Materials from Clay and Functionalized Clay Nanoparticles: Application in Remediation of Lead, Cadmium and Pentachlorophenol from Water. International Journal of Scientific & Engineering Research, 7(10), 914–929.
Dawodu, F. A., & Akpomie, K. G. (2014). Kinetic, equilibrium, and thermodynamic studies on the adsorption of cadmium(II) ions using “Aloji Kaolinite” mineral. The Pacific Journal of Science and Technology., 15, 268–276.
Ding, S. L., Xu, B. H., Liu, Q. F., & Sun, Y. Z. (2011). Preparation of nano-kaolinite and mechanism. Advanced Materials Research, 204–210, 1217–1220.
Eftekhari, S., Habibi-Yangjeh, A., & Sohrabnezhad, S. (2010). Application of AlMCM-41 for competitive adsorption of methylene blue and Rhodamine B: Thermodynamic and kinetic studies. Journal of Hazard Materials., 178, 349–355.
Eloussaief, M., & Benzina, M. (2010). Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions. Journal of Hazard Materials., 178, 753–757.
El-Sheikh, S. M., Shawky, A., Abdo, S. M., Rashed, M. N., & El-Dosoqy, T. I. (2020). Preparation and characterisation of nanokaolinite photocatalyst for removal of P-nitrophenol under UV irradiation. International Journal Nanomanufacturing, 16(3), 232–242.
Ethaib, S., Al-Qutaifia, S., Al-Ansari, N., & Zubaidi, S. L. (2022). Function of nanomaterials in removing heavy metals for water and wastewater remediation: A review. Environments, 9, 123. https://doi.org/10.3390/environments9100123
Gerçel, Ö., & Gerçel, F. H. (2007). Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chemical Engneering. Journal, 132(1–3), 289–297.
Ghosal, P. S., & Gupta, A. K. (2017). Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. Journal of Molecular Liquids, 225, 137–146.
Gürses, A., Hassani, A., Kıranşan, M., Açışlı, Ö., & Karaca, S. (2014). Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: Kinetic, thermodynamic and equilibrium approach. Journal of Water Process Engineering., 2, 10–21.
HafizahChe, I. N., Bakhtiar, N. S. A., & Akil, H. (2017). Effects of cetyltrimethylammonium bromide (CTAB) on the structural characteristic of non-expandable muscovite. Materials Chemistry and Physics, 196, 324–332.
Hosseini-Bandegharaei, A., Khamirchi, R., Hekmat-Shoar, R., et al. (2016). Sorption efficiency of three novel extractant-impregnated resins containing vesuvin towards Pb(II) ion: Effect of nitrate and amine functionalization of resin backbone. Colloids Surface: A Physicochem Engneering Asp, 504, 62–74.
Karim, A. V., Hassani, A., Eghbali, P., & Nidheesh, P. V. (2022). Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Current Opinion in Solid State & Materials Science, 26, 100965.
Katsou, E., Malamis, S., Tzanoudaki, M., Haralambous, K. J., & Loizidou, M. (2011). Regeneration of natural zeolite polluted by lead and zinc in wastewater treatment systems. Journal of Hazard Materials, 189, 773–786.
Khataee, A., Leila, A., & Aydin, H. (2013). Response Surface Analysis of Removal of a Dye by a Turkish Coal Powder. Advances in Environmental Research, 2(4), 291–308.
Kotsyuda, S. S., Tomina, V., Zub, Y., Furtat, I., Lebed-Phothisane, A., Vaclavikova, M., & Melnyk, I. (2017). Bifunctional silica nanospheres with 3-aminopropyl and phenyl groups. Synthesis approach and prospects of their applications. Applied Surface Science, 420, 782–791.
Lagaly, G., Ogawa, M., & Dékány, I. (2013). Clay mineral–organic interactions. In F. Bergaya & G. Lagaly (Eds.), Handbook of Clay Science developments in Clay Science (pp. 435–505). Amsterdam: Elsevier.
Lan, M., Fan, G., Yang, L., & Li, F. (2014). Significantly Enhanced Visible-Light-Induced Photocatalytic Performance of Hybrid Zn Cr Layered Double Hydroxide/Graphene Nanocomposite and the Mechanism Study. Industrial & Engineering Chemistry Research., 53, 12943–12952.
Li, Y., He, J., Zhang, K., Liu, T., Hu, Y., Chen, X., Huang, X., Kong, L., & Liu, J. (2019). Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Advances., 9, 397–407.
Liu, W., Zhao, C., Wang, S., Niu, L., Wang, Y., Liang, S., & Cui, Z. (2018). Adsorption of cadmium ions from aqueous solutions using nano-montmorillonite: Kinetics, isotherm and mechanism evaluations. Research on Chemical Intermediates, 44, 1441–1458.
Mahdavi, S., Jalali, M., & Afkhami, A. (2013). Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chemical Engineering Communications., 200, 448–470.
Masindi, V., & Gitari, W. M. (2016). Simultaneous removal of metal species from acidic aqueous solutions using cryptocrystalline magnesite/bentonite clay composite: An experimental and modelling approach. Journal Cleaner Production, 112, 1077–1085.
Matˇejka, V., Kˇrístková, M. S., Kratošová, G., & Valášková, M. (2006). Preparation of Mg-vermiculite nanoparticles using potassium persulfate treatment. Journal of Nanoscience and Nanotechnology, 6, 1–5.
Muazu, N. D., Bukhari, A., & Munef, K. (2020). Effect of montmorillonite content in natural Saudi Arabian clay on its adsorptive performance for single aqueous uptake of Cu(II) and Ni(II). Journal of King Saud University Science, 32, 412–422.
Negm, S. H., Abd El-Magied, M. O., El Maadawy, W. M., Abdel Aal, M. M., Abd El Dayem, S. M., Taher, M. A., Abd El-Rahem, K. A., Rashed, M. N., & Cheira, M. F. (2022). Appreciatively Efficient Sorption Achievement to U(VI) from the El Sela Area by ZrO2/Chitosan. Separations, 9, 311. https://doi.org/10.3390/separations9100311
Nooshabadi, M. S., Ghoreishi, S. M., Jafari, Y., & Kashanizadeh, N. (2014). Electrodeposition of polyaniline-montmorrilonite nanocomposite coatings on 316L stainless steel for corrosion prevention. Journal Polymer Research, 21, 416–417.
Qian, Y., Lindsay, C. I., Macosko, C., & Stein, A. (2011). Synthesis and properties of vermiculite-reinforced polyurethane nanoco-mposites. Applied Material Interfaces, 3, 3709–3717.
Rashed, M. N., Gad, A.-E., & Fathy, N. M. (2019). Adsorption of Cd (II) and Pb (II) Using Physically Pretreated Camel Bone Biochar. Advanced Journal of Chemistry-Section A, 2(4), 347–364.
Rashed, M. N., Arfien, A. A., & El-Dowy, F. A. (2020). Adsorption of Heavy Metals on Chemically Modified Muscovite. Aswan University Journal of Environmental Studies, 1(2), 183–203.
Scherrer, P. (1918). Bestimmung der Grösse und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften, Göttingen. Mathematisch-Physikalische Klasse, 2(1918), 98–100.
Sdiri, A., Higashi, T., Jamoussi, F., Bouaziz, S., Hatta, T., Jamoussi, F., Tase, N., & Bouaziz, S. (2012). Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J. Environ. Manag., 93, 171–179.
Srivastava, P., & Hasan, S. H. (2011). Biomass of Mucor heimalis for the biosorption of cadmium from aqueous solutions: Equilibrium and kinetic studies. BioResourses, 6, 3656–3675.
Tetteh, S., Ofori, A., Quashie, A., Jääskeläinen, S., & Suvanto, S. (2022). Modification of kaolinite/muscovite clay for the removal of Pb(II) ions from aqueous media. Physical Sciences Reviews. https://doi.org/10.1515/psr-2021-0145
Unuabonah, E. I., Olu-Owolabi, B. I., Adebowale, K. O., & Ofomaja, A. E. (2007). Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated kaolinite clay. Colloids and Surfaces a: Physicochemical Engineering Aspects, 292, 202–211.
Xie, W., Song, Y., Li, S., Shao, M., & Wei, M. (2019). Integrated Nanostructural Electrodes Based on Layered Double Hydroxides. Energy Environ. Mater., 2, 158–171. https://doi.org/10.1002/eem2.12033
Yin, J., Deng, C., Yu, Z., Wang, X., & Xu, G. (2018). Effective removal of lead ions from aqueous solution using nano illite/smectite clay: isotherm, kinetic, and thermodynamic modeling of adsorption. Water, 10(210), 1–14.
Yu, X. (2007). The preparation and characterization of cetyltrimethyl-ammonium intercalated muscovite. Microporous and Mesoporous Materials, 98, 70–79.
Yu, X., Zhaoa, L., Gaob, X., Zhang, X., & Wu, N. (2006). The intercalation of cetyltrimethylammonium cations into muscovite by a two-step process: II. The intercalation of cetyltrimethylammonium cations into Li-muscovite. Journal of Solid State Chemistry, 179, 1525–1535.
Jiangyan, Y., JingYang, H. M., Shuangqing, S., Chang, Q., & Komarneni, S. (2018). Green synthesis of nano-muscovite and niter from feldspar through accelerated geomimicking process. Applied Clay Science, 165, 71.
Zhu, Z., Gao, C., Wu, Y. L., Sun, L. F., Huang, X. L., Ran, W., & Shen, Q. R. (2013). Removal of heavy metals from aqueous solution by lipopeptides and lipopeptides modified Na-montmorillonite. Bioresource Technology Reports, 147, 378–386.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by FAE. The first draft of the manuscript was written by MNR and AAA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial ornon-financial interests to disclose.
Ethical approval
No ethical issues.
Consent to participate
Consent.
Consent for publication
Consent.
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashed, M.N., Arifien, A.E. & El-Dowy, F.A. Preparation and characterization of nanomuscovite by intercalation method for adsorption of heavy metals from polluted water. Environ Geochem Health 45, 5127–5144 (2023). https://doi.org/10.1007/s10653-023-01545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01545-4