Abstract
Adsorption-based water treatment technology is a sustainable strategy for health and environmental wellness, as well as mineral recovery and resource conservation. Extended studies on the Cd2+ adsorption characteristics of the cross-linked/phosphorylated carboxymethyl starch (SCCS) derivatives produced by treating a Type-C starch with anionic precursors, including sodium trimetaphosphate (STMP) and sodium monochloroacetate (SMCA) were carried out. The optimum product was subjected to surface area studies using the Brunauer–Emmett–Teller (BET) method, and then Fourier transformed infrared (FTIR), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) before and after adsorption of Cd2+. The BET results showed that the derivative is mesoporous (pore size: 3.5–6.4 m3/g), while the FTIR results indicated that the adsorption of Cd2+ can be attributed to interactions with the hydroxyl, carbonyl, and phosphoryl functional groups on the SCCS platform. Adsorption equilibrium, kinetics, isotherms, thermodynamics, and recovery/regeneration were extensively studied using various models and experimental conditions. The results showed that Cd2+ was efficiently adsorbed (≈ 99%) at equilibrium, and the data fitness for multiple models indicated that the adsorption process is based on a combination of physisorption and chemisorption processes that are thermodynamically feasible and reversible for economic utilization of the adsorbent. The adsorbent was used in the treatment of mine tailing, and the result showed that the removal of minerals from the tailings was very efficient (≈ 100%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the fast-growing industrialization, urbanization, and global population, water security has continued to face diverse challenges that are life-threatening [1]. Water pollution from anthropogenic sources has continued to be a major challenge both to water security and resource conservation (loss of valuable minerals) [2]. Toxic chemical pollutants in water are emerging as chemical substances with complex speciation are being continuously studied by scientists [3]. When toxic chemical pollutants enter the biological systems, they disrupt the endocrine system and other biochemical processes, leading to various disorders and physiological breakdowns in humans and animals [1, 4]. Toxic heavy metals are non-biodegradable; hence they can accumulate to dangerous levels over time [4]. Cadmium is a toxic heavy metal, and the history of its devastating pollution of water as well as its health and environmental degradation is not unpopular [5]. Adsorption-based water treatment technology has been efficiently used in the removal of cadmium and other toxic heavy metals from water. The adsorption-based water treatment technology is technically simple, and the process is typically cost- and energy-efficient [4, 6, 7]. For these reasons, the adsorption technique has its own unique advantages over other complex and cost-intensive water treatment techniques such as ion exchange, electrochemical separation, chemical precipitation, membrane filtration, reverse osmosis, oxidation, solvent extraction, microbial degradation, etc. [1, 6]. Current research on adsorption-based water treatment is focused on the preparation and development of renewable, low-cost, non-toxic, and environment-friendly materials into efficient adsorbents. The characteristic efficiency of the adsorbent is also desired to be relative to factors such as adsorbent-adsorbate interactions under variable conditions (temperature, pH, concentration/amounts, etc.), adsorbent structural characteristics and surface area, as well as adsorbent stability, which are very important factors in the adsorption process [6]. The entire technoeconomic attributes of the developments in adsorption-based water treatment technology will continue to toll the path of sustainability in water treatment and management. Based on these attributes, polysaccharides are favorites in the selection of pristine materials with versatile chemical functionality and structural characteristics that potentially facilitates adsorption, and that are compatible with the environment (biodegradable and renewable).. Polysaccharides such as starch, cellulose, chitin/chitosan, and natural gums are biopolymers, which are renewable and eco-friendly materials with intrinsic physicochemical properties that potentially select them as natural adsorbents [1, 7]. These biopolymers are also tailorable chemically and physically to achieve enhanced and/or specific adsorption characteristics [5, 8, 9].
Modifications of starches as well as their composites have presented a versatile technique for the development of varieties of starch derivative adsorbents for water treatments [5, 8, 9]. Starch is relatively easier to extract from its native sources, and the native starches from different sources often provide natural alternatives (in physicochemical characteristics) for the development of starch derivative materials [8]. Among the other modification methods that have been used in the development of starch-based adsorbents, the dual incorporation of the carboxymethyl and phosphate functional groups onto the starch platform has been justified to increase the adsorption characteristics of the derivatives produced [8, 10, 11]. Specific literature available (outside our previous studies) on the development of starch derivatives that incorporated 3D network structures (cross-linked), carboxymethyl, and phosphate functional groups has been synthesized based on native starches obtained from corn [12] and bananas [10], and the fundamental review of these studies have concluded that the variations in the adsorption efficiency of the derivatives can be attributed to the different botanical sources of the native starches [8, 13].
The novelty in the course of this research lies in the incorporation of Type-C starches in the development of adsorbents for efficient removal of heavy metal ions from water. Type-C starch has unique physicochemical properties that may impact unique adsorption characteristics [13]. The typical Type-C starch content of wild, non-staple, and underutilized legume plants can be valorized in non-food applications. These renewable agricultural products will also diversify the sourcing of starch for functional applications and potentially reduce starch production costs. In our previous study, sodium trimetaphosphate (STMP) cross-linked carboxymethyl starch (SCCS) derivatives were developed based on starch from an underutilized legume and studied for the removal of lead (Pb2+) from water [13]. According to Pearson’s hard and soft acids and bases theory, Pb2+ can interact as a borderline acid, while Cd2+ is a soft acid. It is therefore essential to carry out further studies on the adsorption characteristics of starch derivatives with soft acids, which is the category within which many heavy metals also falls [14]. Based on this knowledge gap and others, the current study is extended to surface area studies, the characterizations for Cd2+ adsorption and the mechanisms of the adsorption process, as well as the application of the SCCS derivative in the treatment of mine tailings. This extended study provides relevant findings that will further establish the versatility and potential of the SCCS derivative adsorbent in water and wastewater treatment and management.
2 Materials and method
2.1 Materials
The chemicals used include sodium monochloroacetate (SMCA), sodium trimetaphosphate (STMP), acetone, n-propanol, methanol, ethanol (99%), cadmium nitrate, hydrochloric acid, nitric acid, potassium iodide, and sodium hydroxide. The chemicals are synthesis/analytical grade products supplied from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Kidney beans were supplied by variety legume farmers in Plateau State, Nigeria. Wastewater/tailings samples were collected from a laboratory scale galena process, a crude oil mining site (produced water) at Forcardos Terminal, Ogulagha, Burutu Local Government, Delta State, and a gold processing site at Nassarawa-Kanji, Kashegu Local Government, Niger State, Nigeria.
2.2 Starch extraction, modification and analysis
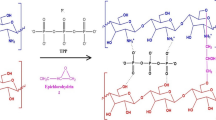
The extraction of starch from the beans, modification of the starch via cross-linking using STMP, and carboxymethylation using SMCA were carried out using the procedures reported in the previous study [13]. In brief, after alkaline treatment (100 g in 500 ml of 0.125 M NaOH) of raw beans for 12 h, the coat was removed and the bean was thoroughly cleaned with tap water. The beans were then ground gently in a blender using a cold (∼1 °C) NaOH (0.25 M) solution to enhance smooth slurry blends. The slurry was then filtered through muslin cloths (100 and then 200 mesh) into a bowl of distilled water, and the pH of the suspension was adjusted to 8. After 2 h, the supernatant was removed and the residue was centrifuged to efficiently separate the starch from water. The starch was then washed with ethanol (95%) and thrice with double distilled water before drying to a constant weight at 40 °C. Starch purity was 93% (carbohydrate content). STMP cross-linking starch (SCS) was prepared by mixing the starch (20 g, dry basis dispersed in deionized water) with NaCl (2 g) and then STMP (0.2 g dissolved in 20 ml of dionized water). The pH of the mixture was adjusted to 11.5, and it was setup to react under reflux at 40 °C with continuous stirring for 5 h. The crosslinked starch was neutralized, centrifuged, washed, and dried to a constant weight at 40 °C. The phosphorus content of SCS was 0.08%. The carboxymethyl derivative (SCCS) was synthesized by dispersing SCS (20 g, dry basis) in 300 ml of N-propanol and adding drops of 50% NaOH solution (8 g) while stirring over 1 h at room temperature (27 ± 2 °C). Afterwards, the temperature was raised to 50 °C, SMCA was added, and the reaction continued while stirring under reflux for 3 h. The SCCS was neutralized, washed, and dried to a constant weight at 60 °C. Carboxymethylation was repeated for 5—30% SMCA w/w of starch in serial batches. The degree of carboxymethyl group substitution (DS) and phosphorous content (Pc) of the SCCS derivatives falls within 0.015–0.075 and 0.08–0.06% respectively, showing that DS increased while Pc decreased when the amount of SMCA was increased in the synthesis of the derivatives [13].
2.3 Characterization of starch derivative
The surface area characteristic was carried out using the Brunauer–Emmett–Teller (BET) method, which is based on nitrogen adsorption in a Quantachrome Autosorb. The PerkinElmer Fourier transformed infrared (FTIR) spectrophotometer was used to elucidate chemical functionalities. Microstructure and elemental composition were analyzed using the Philips XL-30 FEG, which is a scanning electron microscope equipped with the energy dispersive X-ray technique (SEM–EDX).
2.4 Adsorption studies
Batch adsorption methods were used in all the experiments by shaking the conical flask containing the adsorbent in an aqueous solution at 100 rpm on the orbital shaker. Aqueous solutions of Cd2+ were prepared using double-distilled water and cadmium nitrate and through the appropriate serial dilutions. The initial and final concentrations of Cd2+ in solutions before and after adsorption experiments respectively, were determined using the Buck Scientific 210 VGP atomic adsorption spectrometer (AAS). Adsorption efficiency and the amount adsorbed were calculated using Eqs. 1 and 2, respectively. A Jenway PFP7 flame emission spectrometer was used to determine Na+ concentrations. The conditions used in each of the adsorption processes for the equilibrium studies are summarized in Table 1.
where C0 = initial concentration, Ce = equilibrium/final concentration, V = volume of aqueous solution in cm3 and W = weight of adsorbent in gram.
Prior to adsorption experiments, the wastewater samples (100 ml) were filtered through filter paper and digested using 9 ml of a HNO3/HCl mixture (2: 1) at 90 °C for 2 h. The final solution was filtered again, and the pH was adjusted to 6. The concentrations of some selected heavy metals (Pb, Cd, Cr, Zn, Cu, and Fe) tailings in the wastewaters were determined using AAS before and after treatment with 1% (w/v) of SCCS. Adsorption processes were carried out in triplicate.
2.4.1 Adsorption kinetics studies
The fitness of adsorption data obtained from the rate equilibrium studies to pseudo-first, pseudo-second, and third order was evaluated using the linear Eqs. 3, 4, and 5 respectively [15]. Equation 6 was used to test the Elovich model [16, 17]. Equations 7 and 8 were used for the intra-particle and external diffusion fitness tests, respectively [18].
where k1, k2 and k3 are the respective rate constants, qt (mg/g) is the amount of adsorbate on the adsorbent at time t (min) and qe (mg/g) is the amount of adsorbate on the adsorbent at equilibrium.
a is the initial adsorption rate (mg/g min) and b is related to the extent of surface coverage, the desorption constant, and the activation energy for chemisorption (g/mg).
kid is the intra-particle diffusion rate constant, and I (mg/g) is proportional to the layer boundary effect.
ked (min−1) is the effective diffusion coefficient of adsorbate in the adsorbent phase. F(t) is the fractional attainment of equilibrium at time t \(({q}_{t}/{q}_{e})\).
2.4.2 Adsorption isotherm studies
The fitness of adsorption data from the initial concentration studies was studied using the linear and derivative equations relative to the Langmuir (Eqs. 9 and 10), Freundlich (Eq. 11), Temkin (Eq. 12), Halsey (Eq. 13), and Dubinin and Radushkevich (D-R) (Eqs. 14 and 15) isotherm models [15, 19].
where qe (mg/g) is the amount of adsorbate on the adsorbent and Ce (mg/L) is the adsorbate concentration in solution at equilibrium, kl (L/mg) is the Langmuir constant, which correlates with variation in the active surface area and porosity on the adsorbent, and qm (mg/g) is the maximum adsorption capacity of the monolayer formed on the adsorbent. RL is a dimensionless constant called the separation factor.
KF (mg/g) and n are the Freundlich isotherm constants indicating the maximum adsorption capacity of metal ions and adsorption intensity, respectively.
where the Temkin isotherm binding constant B = RT / bT (bT is the heat transfer or heat of adsorption (J/mol), R is the universal gas constant (R = 8.314 J/mol K), T (K) is the temperature). KT is the Temkin isotherm constant corresponding to the maximum binding energy.
KH and n are Halsey isotherm constant
where ɛ is the Polanyi potential expressed as \(RT\text{ln}(1+\frac{1}{{C}_{e}})\). E (kJ/mol) is the mean free energy of adsorption per adsorbate molecule.
2.4.3 Adsorption thermodynamic studies
The vant Hoff’s linear equation (Eq. 16) was used to determine ΔS and ΔH from the equilibrium temperature studies, and the parameters were substituted into the thermodynamic equation (Eq. 17) to determine ΔG.
where kc (equilibrium constant) = Q/Ce, Q (mg/g) is the equilibrium solid-phase concentration Ce (mg/L) is the equilibrium solution concentration, R is the gas constant (8.314 J mol−1 K−1) and T (K) is the temperature. ΔS, ΔH, and ΔG are entropy, enthalpy, and Gibb’s free energy, respectively.
2.5 Recovery/regeneration studies
Cd2+ recovery and adsorbent regeneration were carried out by modifying the method reported by Kim and Lim, [12]. The adsorbent (1 g) loaded with the maximum amount of Cd2+ after the adsorption experiments was re-dispersed in 40 ml of deionized water. HNO3 (1 M) was added to the suspension until the pH was stable at 2.0. The suspension was then stirred for 2 min using a magnetic stirrer, filtered, washed twice with 2 ml of deionized water (as eluent), adjusted to pH 8.0 and dried at 40 °C to obtain a regenerated adsorbent. Cd2+ concentration in the filtrate and eluent mixture was determined using AAS. The experiment was repeated, where the stirring time was increased to 5, 10, 15, and 20 min. Recovery efficiency was calculated using Eq. 16. The reusability experiment was carried out over five cycles.
where C0 = initial concentration, Csorption = concentration after sorption, and Cdesorption = concentration after desorption.
Adsorption and desorption experiments were carried out in duplicate, where the mean and standard error were recorded.
3 Results and discussion
3.1 Preliminary adsorption experiments
A preliminary Cd2+ adsorption experiment was carried out using all the starch derivatives produced (Fig. 1). The efficency of Cd2+ adsorption on the native starch was 26.29 ± 3.94%. Cross-linking increased the adsorption efficency to 97 ± 0.44%, and the highest efficiency (98 ± 0.02%) was obtained on SCCS-15 (DS: 0.068). The results indicated that adsorption stability is remarkably increased (lower error value) on the derivative with carboxymethyl DS of 0.068. Adsorption efficiency decreased on derivatives with higher DS of the carboxymethyl group. This suggested that the anionic functional groups on the starch derivative (-PO43−, CO−, and COO−) created structural characteristics and frontier molecular orbitals (FMOs) that synergetically enhanced the adsorption of Cd2+ until the optimum DS on SCCS-15 [20]. Figure 1 also shows the concentration of sodium infiltrated/released into the treated aqueous solution, where it followed a similar trend as the Cd2+ removal efficiency. This indicated that ion-exchange (Na+ replacements expectedly) on the carboxymethyl groups may contribute to the overall adsorption process [4, 13]. SCCS-15 was selected for further studies.
3.2 Brunauer-emmett- teller (BET) isotherm analysis
The BET analysis presents the nitrogen (N2) adsorption–desorption curve for SCCS-15 (Fig. 2). The curve showed a Type IV adsorption–desorption (multilayer) isotherm with a hysteresis loop accompanied by a broad capillary condensation and evaporation profile (≈0.16–0.99). This indicated the presence of the typical mesoporous structures of starch derivatives [1]. Table 2 presents the other structural parameters of the pores on SCCS-15. The parameters indicated that the material is relatively less porous compared to conventional adsorbents such as activated carbon (≈168–2090 m3/g) [21]. However, this characteristic will favor the functional groups (interaction with metal ions) on the surface to play a major role in the adsorption process [22].
3.3 Fourier transformed infrared (FTIR) analysis
The IR spectra presented in Fig. 3 compare the spectra of the pristine (SCCS-15) and the Cd2+-loaded derivative (SCCS-Cd) after the adsorption process. The functional groups that are characteristic of the peaks exhibited on the SCCS-15 spectra have also been identified in Fig. 3. The –OH, –C–H, –C–O–C–, C–OH, and C–C are intrinsic functional groups of starch, while the OH–P = O, P = O, P–O–C, and P-O-Na are chemical functionalities introduced onto the starch by the cross-linking/phosphorylating agent (sodium trimetaphosphate STMP) [13]. The carbonyl (C = O) and carboxylate (–COO−Na+) functional groups are impacted onto the derivative by the carboxymeylating agent (SMCA). After the adsorption process, (1) the intensity of the O–H band (3378 cm−1) decreased, (2) the O = P–OH band (2356 cm−1) disappeared, and (3) the carbonyl (C = O) and phosphoryl (P-O-C) bands were shifted to lower wavelengths (blue shift). A general reduction in FTIR peak intensity is a typical aftermath effect of adsorption [6]. The reduction in O–H intensity is due to the multilayer adsorption of Cd2+ on the O–H sites. The disappearance of the O = P–OH band may be due to the valency interactions of Cd2+ on the functional group. The shift in the C = O and P–O–C bands can also be attributed to valency interactions with Cd2+. Valency interactions that create charge induced polarization on carbonyl groups are expected to exhibit blue-shifts [23]. Based on the FTIR analysis, scheme 1 presents the proposed Cd2+ adsorption interactions on the starch derivative.
3.4 Scanning electron microscope-energy dispersive X-ray (SEM–EDX) analysis
Figure 4 shows the micrograph images of the SCCS-15 adsorbent before (Fig. 4a and a*) and after (SCCS-Cd) the adsorption process (Fig. 4b and b*). The SCCS-15 granule exhibits surface cracks and fissures, which depict the mesopores elucidated by the BET isotherm curves (Fig. 2). The pores on the surface of the adsorbent disappeared after the adsorption process. This suggested that the adsorption process impacted granular swelling and/or mass transfer to the surface of the granules. Similar findings were reported by Feng and Wen, [24] for cross-linked starch xanthate used in the removal of Pb2+ and Cd2+, as well as the adsorption of Pb2+ on the SCCS derivative in our previous study [13]. Figures 4c and 4d present the EDX spectra of the adsorbent before and after the adsorption process, respectively. The result showed expected changes in the elemental composition of the derivative after adsorption, where Cd2+ was loaded appreciably (≈18% weight) on the derivative.
3.5 Adsorption equilibrium studies
Adsorption capacity and efficiency are typically influenced by some experimental factors or conditions. The effects of these experimental conditions on the equilibrium of Cd2+ adsorption onto SCCS-15 were studied, and the results are presented in Fig. 5. The effect of pH (Fig. 5a) was studied up to pH 7 to avoid ion precipitation. The result shows that the adsorption efficiency increased and reached equilibrium (˃ 90%) above pH 4. This indicated that the adsorption of Cd2+ is driven by the deprotonation (removal of H3O+) of the anionic sites on SCCS-15 as the pH of the aqueous solution was increased [25]. This phenomenon further suggests that the adsorption process is controlled by electrostatic interaction between deprotonated anionic sites and the Cd2+ (cation) in the aqueous solution [26]. Figure 5b showed that the adsorption of Cd2+ reached equilibrium (˃ 90%) within 10 min. This rapid adsorption can be attributed to strong electrostatic interactions due to the multiple anionic functional groups (–PO43−, CO−, and COO−) on the adsorbent. Rapid adsorption can also be attributed to rapid mass transfer enhanced by the porous surface of SCCS-15, as shown by the surface cracks and fissures on the micrograph of the adsorbent (Figs. 4a and a*). The rate of adsorption recorded in this study is comparable to the rates (10–20 min) reported by Zhang et al. [27]. Rapid adsorption processes are of significant benefit in continuous (membrane) adsorption processes. Figure 5c showed that the absorption efficiency of Cd2+ was reduced remarkably in aqueous solutions with initial concentrations higher than 50 ppm. This is due to the saturation of the adsorption sites on the adsorbent in the aqueous solutions with higher concentrations [13]. This also indicated the limitations of the active sites available for the stable and efficient adsorption of Cd2+ on the SCCS-15 adsorbent. The high adsorption efficiency (˃ 95%) recorded in this study will be of significant benefit in typical environmental applications as well as in the treatment of tailings, where mineral concentrations in water are typically low. Figure 5d showed that the efficiency of Cd2+ adsorption decreased as the temperature of the aqueous solution increased. This decrease in efficiency (especially between 30 and 40 °C) can be attributed to the exothermic nature of the adsorption process [28]. The decrease in adsorption efficiency may also be attributed to dissolution and other hydrothermal degradation of the polysaccharide-based adsorbent, leading to loss of adsorption sites. Some starch-based adsorbents have been observed to exhibit poor affinity at high temperatures (> 40 °C) [9]. Using the 200 ppm aqueous solution, Fig. 5e showed that the adsorption of Cd2+ increased as the adsorbent dosage was increased until it reached a constant efficiency (≈100%). This indicated that the adsorption site and the surface area of the adsorbent also increased as the bulk/mass of the adsorbent increased. Adsorbent dosage is a critical factor in the optimization of the adsorption equilibrium, and in the technoeconomic evaluation of adsorption-based processes. Figure 5f showed that the adsorption of Cd2+ on SCCS-15 decreased as the concentration of co-/competitive ion (mixed with 50 ppm Cd2+ solution at equal volumes) increased. There was a larger reduction in the adsorption of Cd2+ with the divalent co-ion (Ca2+) than the monovalent co-ion (Na+). This indicated that the adsorption sites on the adsorbent may not be strongly selective for cadmium ions.
3.5.1 Adsorption kinetic studies
To establish the kinetics of the adsorption process, some relevant models were used to investigate the experimental adsorption data obtained from the rate of adsorption of Cd2+ on SCCS-15 (Fig. 5a). Figure 6 presents some kinetic and diffusion model curves, while Table 3 presents the parameters of the models for the adsorption of Cd2+ on the SCCS-15 adsorbent. The data showed that the adsorption of Cd2+ is best fitted (R2 = 0.9999) to pseudo-first order (Fig. 6a). The parameters of the pseudo-second order model (Fig. 6b) also show appreciable fitness (R2 = 0.9976) of the experimental data. These therefore indicated that both physisorption (molecular interactions relative to pseudo-first order) and chemisorption (valency interactions relative to pseudo-second order) processes were involved in the overall process of adsorption of Cd2+ on SCCS-15. The third-order kinetic model (Fig. 6c) did not show fitness (R2 = 0.6065) with the experimental data. This may imply that the chemisorption process is less significant in the rate-determining step(s) of the overall adsorption process [29]. The data in this study does not show appreciable fitness (R2 = 0.8696) to the Elovich model (Fig. 6d). This implies that the overall adsorption process is controlled by the chemisorption process. Adsorption data fitness for the Elovich model has been adequately used to elucidate chemisorption adsorption processes (chemical interactions between the adsorbate and the heterogeneous active sites on the adsorbent) [17]. The intra-particle diffusion model (Fig. 6e) showed two diffusion steps, which depict mass transfer due to macropores (shown in Fig. 4a) and mesopores (depicted in Fig. 1) structures [16]. The derived Kid1 (1.345 mg g−1 min−0.5) is higher than Kid2 (0.03 mg g−1 min−0.5) (Table 3). This implies that the intra-macropores diffusion is more significant in the diffusion of Cd2+ on the adsorbent [16], while supporting the observed disappearance of the macropores on the surface of the adsorbent as shown in Fig. 4b. The intra-particle diffusion model curve does not pass through the origin (I ≠ 0) due to variations in the initial and final rates of mass transfer in the adsorption process [30]. This also implies that intra-particle diffusion cannot be regarded as the adsorption rate-determining step [30]. Figure 6f showed that the Cd2+ adsorption data fit (R2 = 0.9984) the external diffusion model. This infers that the diffusion of Cd2+ through the external surface layer of the SCCS-15 adsorbent contributes significantly to the rate of Cd2+ adsorption [31].
3.5.2 Adsorption isotherm studies
Adsorption mechanisms can be established at equilibrium from the distribution of adsorbate on the adsorbent surface as the initial concentration (C0) changes (Fig. 5c). This can be predicted by investigation using some isotherm models presented in Fig. 7, and the comprehensive parameters of the models are presented in Table 4. The adsorption data of Cd2+ showed relative fitness to both Langmuir (R2 = 0.9803) and Freundlich (R2 = 0.9509) isotherm models, as shown in Figs. 7a and 7b, respectively, while the RL values range (0 < RL < 1) and the n value range (1–10) from the Langmuir and Freundlich models (Table 4) also showed that the adsorption process is favorable. This implies a distribution of monolayer and heterogeneous multilayer adsorption of Cd2+ on SCCS-15 adsorbent. A complex combination of monolayer and multilayer adsorption has also been reported for polysaccharides and their derivative adsorbents [8, 11, 15]. The parameters (R2 = 0.9462; B = 2.2949 mg/L and KT = 12.751 L/mg) obtained from the Temkin isotherm model (Fig. 7c) imply that the energy distribution on the active sites on SCCS-15 is favorable for the adsorption of Cd2+ [19]. The Halsey isotherm model (Fig. 7d) depicts parameters (R2 = 0.9471) that attest to multilayer formation on the adsorption sites in agreement with the deduction from the BET analysis (Fig. 2). Parameters from the D-R isotherm (Fig. 7e) showed that the model cannot be used to describe the overall adsorption of Cd2+ at equilibrium. However, the E value (3.6662 kJ/mol) calculated using the model falls within the range ( < 8 kJ/mol) that indicated involvement of physisorption process in the overall adsorption process [18].
3.5.3 Adsorption thermodynamic studies
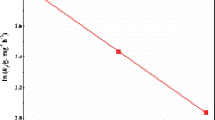
The thermodynamic model based on vant Hoff’s equation was presented in Fig. 8, and the thermodynamic parameters calculated using the thermodynamic equations were presented in Table 5. ΔH value (− 52.82 kJ.mol−1) is negative, indicating that the adsorption process is exothermic. The value also falls within the (± 40– ± 200 kJ.mol−1) range that corresponds to chemisorption processes [18]. ΔS values are negative, indicating the diffusion of Cd2+ from the aqueous solution (higher randomness) to an ordered arrangement on the surface of the adsorbent [18]. ΔG values transited from negative to positive values as the temperature increased. This indicated that the adsorption of Cd2+ is feasible and spontaneous at lower temperatures, whereas this diminishes at higher temperatures.
The complex adsorption mechanism reported in this study is in agreement with several studies on starch-based adsorbents, where different combinations of mechanisms such as chelation, ion-exchange, electrostatic interactions, and the physical trapping/ultrafiltration of adsorbates on the polymer matrix have been reported for the adsorption process [5].
3.6 Cd2+ recovery, adsorbent regeneration and reusability studies
Recovery permits the conservation of valuable minerals and avoids secondary pollution by the adsorbent at disposal. The recovery of Cd2+ and the regeneration of SCCS-Cd (amount adsorbed at 16.98 mg/g) presented in Fig. 9a showed that equilibrium recovery (≈ 99%) was attained in 15 min. The recovery process (using 1 M HNO3) indicated that the adsorption of Cd2+ was reversible, and the reverse process was based on the protonation of the adsorption sites by the acid (H+) [32]. The regenerated adsorbent was reused in adsorption-regeneration processes over five cycles (Fig. 9b), where it retains an adsorption efficiency ˃ 80%. This indicated the appreciable stability of the adsorption sites on the adsorbent. The reusability efficiency in this study is comparable with the results recorded for Cd2+ in the study on starch phosphate adsorbents by Bashir et al. [4].
3.7 Comparison of SCCS with other starch based absorbents
The comparison of the SCCS with other starch-based adsorbents for the removal of Cd2+ from water is presented in the table below. The table indicated that the SCCS adsorbent exhibits rapid adsorption (relatively low adsorption equilibrium time) and remarkable stability after several reuse cycles. However, the adsorption capacity of the SCCS adsorbent was relatively low at high initial concentrations of aqueous ions. The SCCS adsorbent therefore has potential for the efficient treatment of wastewaters with low metal ion concentration (especially from treatment tanks), which are typically released in large volumes that requires rapid treatment.
Adsorbent | Adsorption capacity | Adsorption equilibrium time (min) | Reuse efficiency (%) after 3 cycles | Reference | |
|---|---|---|---|---|---|
Efficiency (%) | Amount (mg.g−1) | ||||
SCCS | 99 | 16.98 | < 15 | 95.99 | This study |
magnetic CNT/Starch/Fe3O4, nanocomposite | 98.76 | ≈ 44.72 | 50 | ND | [33] |
Magnetic cassava starch microsphere | 81.02 | 210.68 | 300 | < 80 | [34] |
Potato starch phosphate | ≈ 80 | 91.84 | 90 | < 80 | [4] |
Cassava starch/ montmorillonite composite | 96.7 | 4.2 | 120 | ND | [35] |
Dibenzo crown ether grafted corn starch | 92 | ND | 120 | 88 | [32] |
Vinyl imidazole grafted maize starch | 32.30 | 5.92 | 1440 | ND | [36] |
Semi- and di-Starch ester | 15.12 | 7.57 | 1440 | ND | [20] |
Starch-based magnetic nanocomposite hydrogel | 73.40 | 53.20 | 1440 | < 70 | [37] |
Amination and glycidyl methacrylate grafted cassava starch | 98 | ≈ 134.9 | 100 | 98 | [38] |
Carboxymethyl and cross-linked (using electron beam) | 46.4 | 4.59 | 30 | ND | [39] |
Cross-linked carboxymethyl banana starch | 95.6 | ≈ 2.39 | 10 | ND | [9] |
3.8 Application of adsorbent in the treatment of mine tailings
The results obtained from the application of SCCS-15 in the removal of minerals from mine tailings are presented in Table 6. Major minerals determined in the tailings were no longer detected after the batch adsorption treatment of the tailings with the SCCS-15 adsorbent. The results indicated that the SCCS-15 adsorbent can be potentially used in the removal of toxic metal ions from environmental and portable water, as well as in the recovery of valuable minerals from mine tailings and industrial wastewater.
4 Conclusion
The advanced studies on the SCCS derivative in this work have precisely shown that the adsorbent possesses surface characteristics that support adsorption characteristics. The evaluation of the changes in the chemical functionalities before and after Cd2+ loading/adsorption on the derivative confirmed the interactions between Cd2+ and the functional groups on the starch derivative adsorbent. The changes observed in microstructure and chemical composition also support the successful adsorption of Cd2+ by the adsorbent. Under the established process conditions, the adsorbent demonstrated effective (about 99%) removal of Cd2+ from aqueous solutions via complex mechanisms that may involve chelation, ion-exchange, and electrostatic interaction, as well as physical trapping on porous sites. Additionally, the process was exothermic and thermodynamically feasible. The adsorption process was efficiently reversible to recover Cd2+ and regenerate the adsorbent for efficient (˃ 80%) reuse up to five cycles of adsorption–desorption. When the adsorbent was used to treat mine tailings, it was discovered that the main minerals had been totally eliminated (beyond the instrument detection limit). The findings from this study demonstrate the potential of SCCS derivatives in the versatile removal of heavy metals from water as well as the economic recovery of minerals. In view of the current findings, SCCS may also exhibit remarkable adsorption efficiency for cation dyes and serve as a platform for the stable incorporation of metal and metal oxide nanoparticles in the development of composites with a unique combination of functional characteristics such as adsorption, photo-oxidative degradation, and magnetic properties for more advanced water treatment.
Data availability
Data is provided within the manuscript. Any additional data will be provided on request.
References
Gunawardene OH, Gunathilake CA, Amaraweera AP, Fernando NM, Manipura A, Manamperi WA, Kulatunga KM, Rajapaksha SM, Gamage A, Dassanayake RS, Weerasekara BG. Removal of Pb (II) ions from aqueous solution using modified starch. J Compos Sci. 2021;5(2):46. https://doi.org/10.3390/jcs5020046.
Nakhjiri AT, Sanaeepur H, Amooghin AE, Shirazi MM. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: a critical review. Desalination. 2022;527:11551. https://doi.org/10.1016/j.desal.2021.115510.
Teramoto EH, Gemeiner H, Zanatta MB, Menegário AA, Chang HK. Metal speciation of the Paraopeba river after the Brumadinho dam failure. Sci Total Environ. 2021;25(757):143917. https://doi.org/10.1016/j.scitotenv.2020.143917.
Bashir A, Manzoor T, Malik LA, Qureashi A, Pandith AH. Enhanced and selective adsorption of Zn (II), Pb (II), Cd (II), and Hg (II) ions by a dumbbell-and flower-shaped potato starch phosphate polymer: a combined experimental and DFT calculation study. ACS Omega. 2020;5(10):4853–67. https://doi.org/10.1021/acsomega.9b03607.
Akinterinwa A, Reuben U, Atiku JU, Adamu M. Focus on the removal of lead and cadmium ions from aqueous solutions using starch derivatives: a review. Carbohyd Polym. 2022;15(290):119463. https://doi.org/10.1016/j.carbpol.2022.119463.
Keskin ZS, Şenol ZM, Kaya S, Şimşek S. Prunus mahaleb shell as a sustainable bioresource for carminic acid removal from aqueous solution: Experimental and theoretical studies. J Mol Struct. 2023;1275:134618. https://doi.org/10.1016/j.molstruc.2022.134618.
Şen NE, Şenol ZM. Effective removal of Allura red food dye from water using cross-linked chitosan-diatomite composite beads. Int J Biol Macromol. 2023;2023(253):126632. https://doi.org/10.1016/j.ijbiomac.2023.126632.
Akinterinwa A, Oladele E, Adebayo A, Gurgur E, Iyanu OO, Ajayi O. Cross-linked-substituted (esterified/etherified) starch derivatives as aqueous heavy metal ion adsorbent: a review. Water Sci Technol. 2020;82(1):1–26. https://doi.org/10.2166/wst.2020.332.
Gupta AD, Rawat KP, Bhadauria V, Singh H. Recent trends in the application of modified starch in the adsorption of heavy metals from water: a review. Carbohyd Polym. 2021;269:117763. https://doi.org/10.1016/j.carbpol.2021.117763.
Carmona-Garcia R, Aguirre-Cruz A, Yee-Madeira H, Bello-Pérez LA. Dual modification of banana Starch: partial characterization. Starch-Stärke. 2009;61(11):656–64. https://doi.org/10.1002/star.200900152.
Musarurwa H, Tavengwa NT. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohyd Polym. 2020;237:116142. https://doi.org/10.1016/j.carbpol.2020.116142.
Kim BS, Lim ST. Removal of heavy metal ions from water by cross-linked carboxymethyl corn starch. Carbohyd Polym. 1999;39(3):217–23. https://doi.org/10.1016/S0144-8617(99)00011-9.
Akinterinwa A, Oladele E, Adebayo A, Adamu M. Characterization of aqueous Pb2+ adsorption onto cross-linked-carboxymethyl legume starch phosphate using FTIR and SEM-EDX. Biomass Conv Bioref. 2023. https://doi.org/10.1007/s13399-023-03971-x.
Reis DT, de Aguiar Filho SQ, Grotto CGL, Bihain MFR, Pereira DH. Carboxymethylcellulose and cellulose xanthate matrices as potential adsorbent material for potentially toxic Cr3+, Cu2+ and Cd2+ metal ions: a theoretical study. Theoret Chem Acc. 2020;139:1–8. https://doi.org/10.1007/s00214-020-02610-2.
Madala S, Nadavala SK, Vudagandla S, Boddu VM, Abburi K. Equilibrium, kinetics and thermodynamics of Cadmium (II) biosorption on to composite chitosan biosorbent. Arab J Chem. 2017;10:S1883–93. https://doi.org/10.1016/j.arabjc.2013.07.017.
Lin H, Xu J, Dong Y, Wang L, Xu W, Zhou Y. Adsorption of heavy metal cadmium (II) ions using chemically modified corncob: mechanism, kinetics, and thermodynamics. Desalin Water Treat. 2016;57(39):18537–50. https://doi.org/10.1080/19443994.2015.1088898.
Brandani S. Kinetics of liquid phase batch adsorption experiments. Adsorption. 2021;27(3):353–68. https://doi.org/10.1007/s10450-020-00258-9.
Chen YX, Zhong BH, Fang WM. Adsorption characterization of lead (II) and cadmium (II) on crosslinked carboxymethyl starch. J Appl Polymer Sci. 2012;124(6):5010–20. https://doi.org/10.1002/app.35607.
Adewuyi A, Pereira FV. Nitrilotriacetic acid functionalized adansonia digitata biosorbent: preparation, characterization and sorption of Pb (II) and Cu (II) pollutants from aqueous solution. J Adv Res. 2016;7(6):947–59. https://doi.org/10.1016/j.jare.2016.10.001.
Soto D, Urdaneta J, Pernía K, León O, Muñoz-Bonilla A, Fernandez-García M. Removal of heavy metal ions in water by starch esters. Starch-Stärke. 2016;68(1–2):37–46. https://doi.org/10.1002/star.201500155.
Jawad AH, Abdulhameed AS, Wilson LD, Syed-Hassan SS, ALOthman ZA, Khan MR. High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: optimization and mechanism study. Chinese J Chem Eng. 2021;32:281–90. https://doi.org/10.1016/j.cjche.2020.09.070.
Zheng Y, He Y, Liu C, Song L, Zhang H. Preparation of citric acid/porous starch composite adsorbents and their adsorption studies. Mater Adv. 2023;4(15):3356–68. https://doi.org/10.1039/D3MA00228D.
Maslowsky E Jr. Vibrational and computational data for homoleptic main-group element carbonyl complexes. Coord Chem Rev. 2023;486:215151. https://doi.org/10.1016/j.ccr.2023.215151.
Feng K, Wen G. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate. Int J Polymer Sci. 2017;2017:3. https://doi.org/10.1155/2017/6470306.
Shi Y, Song G, Li A, Wang J, Wang H, Sun Y, Ding G. Graphene oxide-chitosan composite aerogel for adsorption of methyl orange and methylene blue: effect of pH in single and binary systems. Colloids Surf A. 2022;20(641):128595. https://doi.org/10.1016/j.colsurfa.2022.128595.
Aniagor CO, Elshkankery M, Fletcher AJ, Morsy OM, Abdel-Halim ES, Hashem A. Equilibrium and kinetic modelling of aqueous cadmium ion and activated carbon adsorption system. Water Conserv Sci Eng. 2021;6(2):95–104. https://doi.org/10.1007/s41101-021-00107-y.
Zhang M, Yin Q, Ji X, Wang F, Gao X, Zhao M. High and fast adsorption of Cd (II) and Pb (II) ions from aqueous solutions by a waste biomass based hydrogel. Sci Rep. 2020;10(1):3285. https://doi.org/10.1038/s41598-020-60160-w.
Priyadarshini B, Patra T, Sahoo TR. An efficient and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies. J Magnesium Alloys. 2021;9(2):478–88. https://doi.org/10.1016/j.jma.2020.09.004.
Xiang B, Fan W, Yi X, Wang Z, Gao F, Li Y, Gu H. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohyd Polym. 2016;136:30–7. https://doi.org/10.1016/j.carbpol.2015.08.065.
Ahmad R, Haseeb S. Adsorption of Pb (II) on Mentha piperita carbon (MTC) in single and quaternary systems. Arab J Chem. 2017;10:S412–21. https://doi.org/10.1016/j.arabjc.2012.09.013.
Girish CR, Murty VR. Mass transfer studies on adsorption of phenol from wastewater using Lantana camara, forest waste. Int J Chem Eng. 2016. https://doi.org/10.1155/2016/5809505.
Ibrahim BM, Fakhre NA. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int J Biol Macromol. 2019;123:70–80. https://doi.org/10.1016/j.ijbiomac.2018.11.058.
Zhang M. Lead and cadmium decontamination from water media by CNT/Starch/Fe3O4, as reclaimable magnetic nanocomposite. Biomass Convers Bioref. 2024. https://doi.org/10.1007/s13399-024-05594-2.
Xie X, Zhao X, Luo X, Zhang Y, Qin Z, Ji H. Characterization of modified mechanically activated cassava starch magnetic porous microspheres and its adsorption for Cd (II) Ions. Nanomaterials. 2023;13(3):513. https://doi.org/10.3390/nano13030513.
Van HN, Van HC, Hoang TL, Nguyen DKV, Thuc CNH. The starch modified montmorillonite for the removal of Pb (II), Cd (II) and Ni (II) ions from aqueous solutions. Arab J Chem. 2020;13(9):7212–23. https://doi.org/10.1016/j.arabjc.2020.08.003.
Sabaa MW, Magid EHA, Mohamed RR. Maize Starch-g-poly (n-vinylimidazole) Synthesis and its application in sewage water treatment. Res Rev J Chem. 2017;6:55–67.
Pour ZS, Ghaemy M. Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly (vinyl alcohol)/carboxymethyl starch-g-poly (vinyl imidazole). RSC Adv. 2015;5(79):64106–18. https://doi.org/10.1039/C5RA08025H.
Xie G, Shang X, Liu R, Hu J, Liao S. Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohyd Polym. 2011;84:430–8. https://doi.org/10.1016/j.carbpol.2010.12.003.
Pant BR, Jeon HJ, Park CI, Lee BC, Park JH, Song HH. Radiation-modified carboxymethyl starch derivative as metal scavenger in aqueous solutions. Starch-Stärke. 2010;62(1):11–7. https://doi.org/10.1002/star.200900175.
Acknowledgements
Authors acknowledge the rich and professional contributions by Prof. Olubode Ajayi and Prof. Albert Adebayo of the Department of Chemistry, Federal University of Technology, Akure. Prof. I. Nkafamiya, Prof. P. Dass, and U. Onyebuchi who are members of our current project team are also acknowledged.
Funding
This work was supported by the Modibbo Adama University of Yola TETFUND institution-based research grants (IBR 7th Batch, 2023).
Author information
Authors and Affiliations
Contributions
A.A. handled sample collection and purchase of chemicals and other materials, as well as the laboratory experiments, characterization and drafting of the manuscript, and he was assisted by D.M. and A.M,H., while E.O. carried out final editing of the paper for both technical and grammatical improvements.
Corresponding author
Ethics declarations
Competing interests
The authors declare that no competing interests exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akinterinwa, A., Oladele, E., Hammed, A.M. et al. Studies on the adsorption of aqueous cadmium and the treatment of mine tailings using anionic Type-C starch. Discov. Chem. 1, 2 (2024). https://doi.org/10.1007/s44371-024-00001-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44371-024-00001-1