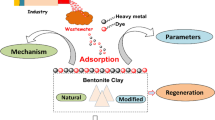
Abstract
Water treatment is of paramount importance to ensure the availability of clean and safe drinking water. In recent years, clay-based materials have gained significant attention as promising adsorbents for water treatment applications. This review provides a comprehensive analysis of different clay types and their surface adsorption properties for water treatment. This review begins by introducing the diverse types of clays commonly used in water treatment, including kaolin, montmorillonite, bentonite, and others. Each clay type is examined in terms of its unique mineral composition, surface properties, and structural characteristics. Subsequently, the adsorption mechanisms of clay surfaces are explored, shedding light on the intricate interactions between contaminants and the active sites on clay surfaces. The factors influencing the adsorption process, such as pH, temperature, contact time, and initial concentration of contaminants, are discussed in detail. Furthermore, the review highlights the adsorption capacity and efficiency of different clay types for the removal of various contaminants from water. These contaminants encompass heavy metals, organic pollutants, dyes, and emerging contaminants. The role of surface modification techniques, such as cation exchange, functionalization, and composite formation, in enhancing the adsorption performance of clays is also elucidated. Moreover, the review addresses the challenges and limitations associated with clay-based adsorbents, including issues related to regeneration, disposal, and cost-effectiveness. Strategies for overcoming these challenges and potential future directions in the field of clay-based water treatment are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Excessive and uncontrolled anthropogenic activities in different areas such as transport, industry, agriculture and urbanization lead to adverse environmental conditions globally. Day by day improvement of living standards and development of higher consumer demand have resulted in increase in the air pollution for instance NOx, SOx and greenhouse gases including CO2. Water pollution has been caused by excessive utilization of chemicals, leachates, disposable goods, non-biodegradable, oil spills, sludge, and pesticide spreading along with improper facilities of waste disposal [1]. The production, distribution and release of synthetic chemicals into environment have been exceeding with unexpectedly high rate that is greater than any other factor responsible for global change in ecosystem [2]. A statistical report published in 2013 by EURO-STAT, clearly inferred that 50% of the total compounds produced from the year 2002 to 2011 represents the class of environmentally hazardous compounds. Sever environmental consequences are caused by above 70% of these chemicals [1]. Worldwide textile dyeing industry is considered as a major source of environmental pollution. Inorganic salts, alkalis, surfactants and dyes from these textile industries are discharged excessively to the effluents [3].
Water is the key element for life. Only 3% of the total water present on earth is categorized as fresh water and of this 3% only 0.01% is available to fulfill human needs. Availability of safe and healthy drinking water is the basic need and right of mankind throughout the world. Due to excessive use of water for drinking purpose as well as its usage as a universal solvent makes it a source of infectious diseases. According to a report of World Health Organization (WHO) waterborne diseases accounts for 80% of the diseases in developing countries [4]. WHO describes “safe drinking water” as the water that does not pose any serious risk to health over the period of its usage, with various sensitivities that may happen between its different life stages [5]. Surface and ground water have been considered as separate resources but now it is evident that surface water after seeping through soil become ground water.
Industrialization is a key to success for each country so Pakistan being a developing country has established many industrial units, this rapid industrialization has negative impact on the environment. The most threatening situation is that most of the industrial units in many cities of Pakistan are without any proper wastewater treatment plant [6]. Different anthropogenic activities have been found to be responsible for water pollution. The sources of water pollution can be classified into two types; point sources and diffuse sources. Pipes draining into river are example of point sources while crop production is an example of diffuse source of pesticides and nutrients in river [7].
Being major source of drinkable water ground water reserve has major importance for human being. It was considered that ground water is purer form of water than any other form as it is not directly contaminated by organisms and wastes. But recent researches indicate that rapid industrialization and urbanization have resulted in accumulation of hazardous substances and consequently deterioration of water quality. Consumption of contaminated water resulted in accumulation of toxic metals in the body of humans. Industrial wastes and emissions directly or indirectly contaminates the underground water of industrial areas of Pakistan [8]. By-products of different industries including cement, fertilizers, leather, dyeing chemicals, sugar, steel, energy and power, food processing, engineering, mining and many others are the main source of pollution of both surface and groundwater [9]. Industrial waste, municipal sewage and waste of urban and rural areas are drained to river and canals thus increases the water pollution. This exceedingly high pollution have resulted in higher chemical oxygen demand (COD), biological oxygen demand (BOD), total suspended solids (TSS), total dissolved substances (TDS), fecal coliform and toxic metals like Cd, Cr, Pb and Ni in river and canal water. In developing countries safe drinking water is not in the access of about 60% of the population [10].
Those dyes and pigments which are easily soluble in water, are considered as pollutants with accommodate in the wrong place and wrong time, also some of them are responsible for increasing the toxicity of water. The number of commercially available dyes has been increased up to one hundred thousand and these are easily visible with the naked eye even at very limited concentration in contaminated water [11]. Globally 10,000 different types of dyes are produced and textile industries consume about 8 × 105 tons of synthetic dyes [12]. Color is one of the most toxic organic pollutants that must be removed during wastewater treatment. Out of all types of dyes azo dyes are preferably used for dyeing in the textile industries due to their ability to form colloidal dispersion and having low solubility in water. Azo dyes are further classified as direct, acidic, basic, vat, disperse and reactive dyes etc. Disperse dyes contain azo chromophores combined with aromatic groups. These dyes are used for coloring a wide variety of synthetic fibers such as nylon, polyester and cellulose etc. Due to their stable and complex molecular structures these dyes are difficult to be degraded by traditional technologies. These are highly stable towards light, temperature and microbial attack [13]. Most of industries including textile industries, pharmaceutical, paper and pulp etc. have been found to use reactive dyes most frequently. Reactive dyes are non-biodegradable dyes and require new technologies for their removal from polluted water [11].
Presence of heavy metals in water up to toxic level also pose a great threat to the human health, animal and plants [14]. Heavy metals enter the open water system through a variety of ways such as erosion of rocks, weathering, volcanic eruption and manly activities for example mining, smelting, industrial and agricultural. These heavy metals include copper (Cu2+), iron (Fe), sodium (Na+), magnesium (Mg2+), chromium (Cr), zinc (Zn), mercury (Hg), nickel (Ni) and lead (Pb) [15].
Waste water of coloring industries is considered as the most polluted water across the world and it has become the potential source of harm for all creatures including human beings due to deleterious consequences of the dyes present in it. Some dyes have been proved to have mutagenic and carcinogenic effects. Other effects include improper functioning of liver, kidney, reproductive system, brain and central nervous system [16]. In textile industry dyeing process results in about 10–50% loss of coloring material to the environment. In addition to the toxicity and mutagenicity dyes are believe to reduce the penetration of light through water thus results in overall reduction of photosynthetic activity and oxygen deficiency. This polluted water cannot be used for drinking purpose, recreation and irrigation [17]. Toxic effects caused by heavy metals include abdominal pain, irritability headache, kidney damage, skeletal damage, nerve damage and cancer [15].
Traditional waste water treatment techniques include biological oxidation, chemical oxidation, advanced oxidation, adsorption and coagulation. But these techniques are not very effective if applied individually and these are not applicable to all types of dyes for example disperse dyes can be easily decolorized by coagulation process but this process is ineffective for soluble dyes [18]. All technologies of water treatment can be divide into three main categories namely biological, physical and chemical. All methods have their own pros and cons.
Biological methods are used in four ways for adsorption such as utilization of white rot fungi, utilization of microbial culture, utilization of living or dead microbial biomass and anaerobic bioremediation system. White rot fungi have ability to dissolve dye using enzymes but at the same time it has always been considered unreliable. Mixed bacterial cultures can decolorize dye solution in 24 to 30 h but these are unable to metabolize azo dyes in aerobic conditions. Some dyes can bind effectively with living or dead microbial biomass but does not work well for all dyes. Under anaerobic conditions all water-soluble dyes including azo dyes are decolorized but it results in production of hydrogen sulfide and methane.
Chemical methods for adsorption can be performed by following ways; oxidative process, fenton’s reagent, ozonation, photochemical, sodium hypochlorite (NaOCl) and electrochemical destruction. Oxidative process is simpler process but some methods are required for the activation of H2O agent. Fenton’s reagent is also an important method but it gives large amount of sludge. Ozonation is an effective method as ozone gas can be used in its gaseous form and there is limited amount of sludge production but it has short half -life of about 20 min. Photochemical method has advantage of no sludge production and reduced production of foul smell but it involves synthesis of by-products. Sodium hypochlorite accelerate the cleavage of azo bond but this reaction is accompanied by the release of aromatic amines. Electrochemical destruction method does not utilize any chemical and also there is no sludge production but it has problem of relatively high flow rate that decrease the overall removal of dye.
Physical methods for waste water treatment include adsorption by activated carbon, membrane filtration, ion exchange, irradiation and electrokinetic coagulation. Adsorption process utilizing activated carbon as an adsorbent is very efficient method for the removal of dye but it is expensive. Membrane filtration can remove all types of dyes but produce sludge. In ion exchange process no adsorbent is lost but it is not equally effective for large number of dyes. Irradiation involves lab scale irradiation but, in this process, dissolved oxygen is required in large amount. Electrokinetic coagulation is an economically friendly process but it involves production of large amount of sludge [19].
Adsorption is the most appropriate method for the removal of dyes as it is low cost, has flexible design and does not involve production of any by-product. Chemical and biological methods have their own disadvantages as chemical methods results in the production of foul smell and harmful by-products while biological methods are very time consuming. Adsorption is also an effective method as compared to other physical processes as in ion exchange process high amount of sludge is produced. In addition, adsorption is easy to use, cheap and best suited for the elimination of nearly all types of dyes. So it is most desirable method for the removal of dyes, pigments, heavy metals and other contaminants from waste water [16]. Adsorption process is of two types i.e., physiosorption or physical adsorption and chemiosorption or chemical adsorption. Physiosorption involves Van der Waals forces, static interactions, pi–pi interaction and dipole–dipole forces and other weak intermolecular forces between adsorbent and adsorbate. Chemiosorption involves formation of bond by electron exchange between adsorbent and adsorbate [20].
The adsorption process requires adsorbent that is cheap and have high surface area. High surface area may results in increase in adsorption potential [21]. A variety of materials have been used as adsorbent such as activated carbon, agricultural solid wastes, agricultural solid waste based on activated carbon, clay and minerals [22]. Activated carbon is the most effective adsorbent used for dye removal due to its various properties such as micro-pore structure, high degree of surface reactivity, large surface area and good adsorption capacity. But despite of these advantages activated carbon cannot be employed as an adsorbent because it is very costly and after being used its regeneration is very costly [23].
Agricultural wastes are the chief adsorbent for the process of dye removal due to some beneficial properties such as they are easily available in excess amount, renewable and very economic friendly. Examples of agricultural wastes that have been used as adsorbent are coconut shell, bamboo dust, rice husk, and straw [24]. The attempts to derive activated carbon from agricultural wastes such as bamboo, olive stone, corn cob and many others have been done as this would be a cheap source of activated carbon. Agricultural wastes are comparatively less costly and renewable source of activated carbon. In order to be a precursor of activated carbon any material should be cheap, must be rich in carbon and its inorganic content should be low. Activated carbon is obtained from agricultural waste through pyrolysis in controlled conditions. Pyrolysis may or may not require chemical activating agent [25].
Needle like and sheet like structured clay material have been used as a cheaper adsorbent because of their high specific surface area. Clay has layered structure and this layered structure give the basis of classification of clay material into some classes like sepiolite, serpentine, kalonite and vermiculite. Clays are the natural, widely used and environmentally friendly adsorbent possessing very good specific surface area. These material have strong ability to bind with heteroatomic dyes of both cationic and anionic nature [26].
Clay is hydrous aluminosilicate material chiefly composed of two repeating units: a tetrahedron of silicon and oxygen (Si2O5)2− and an octahedron of aluminium also termed as gibbsite sheet. In tetrahedral sheets every three out of four oxygen atoms is shared by each tetrahedron. These tetrahedron arranged in a pattern forming a hexagon in which basal oxygens are linked while the apical oxygens are pointing up and down. In octahedral sheets each octahedron shares edges with nearby octahedron. These edges consist of oxygen atoms and groups of hydroxyl anions together with Mg2+, Fe2+ and Al3+ as coordinating cations. Hexagonal structure is formed by these octahedrons. Both naturally occurring and modified clay materials can be used as adsorbent. Clay is divided into three or four major groups namely Kaolinite, illite, montmorillonite, chlorite and smectite (Fig. 1).
Clay is a versatile adsorbent material that can be used in various applications to remove contaminants and impurities from liquids and gases. The adsorption process involves attracting and holding molecules of the adsorbate (contaminant) onto the surface of the adsorbent (clay). There are different types of clay, and each type exhibits unique adsorption properties. Some of the common types of clay used as adsorbents are [27]: kaolin clay also known as china clay, kaolin clay is a white or light-colored clay that has a high adsorption capacity for organic compounds. It is commonly used in water purification processes and wastewater treatment to remove pollutants such as heavy metals, organic dyes, and oils [28]. Bentonite is a type of clay composed mainly of montmorillonite. It has a high surface area and a strong affinity for organic compounds and certain heavy metals. Bentonite clay is widely used in the removal of contaminants from water and in the purification of edible oils and wines [29]. Montmorillonite is a type of clay with excellent swelling properties and a large surface area. It is used as an adsorbent in various applications, including the removal of heavy metals, organic compounds, and radioactive materials from water and soil [30]. These are just a few examples of the many types of clay that can serve as effective adsorbents. The choice of clay depends on the specific application and the type of contaminants to be removed. Each type of clay has its own unique adsorption properties and may require specific preparation and activation methods to optimize its adsorption capacity.
Illite is the only common mineral and chlorites are sometimes considered as a distinct group of phyllosilicate materials. Almost thirty various types of clays are present in these groups but natural clay is actually mixture of these clay types and weathered minerals [31]. Negatively charged silicate groups determine the adsorption capacity of clay minerals. These negative charges bind to and neutralize by the cationic dyes. Montmorillonite is the type of clay with highest adsorption capacity. The clay can be modified to improve its adsorption efficiency [32]. 1:1 layer structure consists of one tetrahedral and one octahedral sheet, each octahedral sheet is connected with tetrahedral sheets through its apical O2− ions. 2:1 layer structure is made up of two tetrahedral sheets and one octahedral sheet, one of the tetrahedral sheet is linked to octahedral sheet on each side.
Two types of charges exist in clay minerals namely structural charge and surface charge. Structural charge on the clay mineral is due to substitution of ions and it is permanent charge originating within the interior of layers while the surface charge keeps on changing depending on the pH and in clay minerals with 1:1 layer type both tetrahedral and octahedral sheets contributes in surface charge while in 2:1 layer type surface charges appear on the tetrahedral sheet at their basal surface. In both layer type i.e. 1:1 and 2:1 surface charges appear on the edges of sheet. Surface charge is produced by the hydrolysis of Si–OH or Al–OH bonds present throughout the clay lattices. Hence the net surface charge depends on the structure of silica and pH of the solution. Anion exchange capacity develop in clay when pH is less than the pH at point zero charge or pH (pzc) while the cation exchange ability develop in clay when pH is greater than pH at point zero charge or pH (pzc). Point zero charge (pHpzc) denotes the pH value at which total net charge is zero (Eqs. 1, 2).
Surface charge contributes to different extent in case of both 1:1 and 2:1 layered type. In the case of 1:1 layer type maximum portion of the total charge is contributed by the surface charge while in the case of 2:1 layer type less than 1% of the total charge is contributed by the surface charge [33].
Alginates are the naturally occurring polysaccharides obtained from bacteria and brown algae. It is a linear co-polymer that is formed by polymerization of two subunits α-l-guluronic acid and β-d-mannuronic acid [34]. Alginates are derived from alginic acid, a carboxyl group carrying biopolymer having strong ability to form metal ions containing complexes. Hydrogel formation is an important property of alginates. By the addition of metallic cations most appropriately divalent cations for example Ca2+, its aqueous solution is converted to hydrogel. Microbial cultures containing immobilized calcium alginate have been studied as an adsorbent for dye and metals ions [35]. Alginates have some different useful properties such as it is hydrophilic in nature, nontoxic and biocompatible. The property of gel formation in sodium alginate depends on exchange of sodium ions already present in guluronic acid residue with various divalent cations for example Ca2+, Ba2+ and Sr2+, etc. This exchange of cations results in a three dimensional network [36].
1.1 Water pollution and treatment
Water is a crucial and an indispensable component of earth, it has high importance as all life forms depend on it, pure water is used for drinking purpose as well as for the completion of processes crucial in life of an organism [37]. Pakistan is widely known for its abundant natural resources water. But with the passage of time quality and quantity of that water is deteriorating. Pakistan is one of those thirty countries which are on the brink of water deficiency and the situation is becoming worse [38]. Water is considered pure when it has no color smell and taste. Water is becoming impure due to presence of inorganic chemicals or organic compounds, micro-organisms, industrial wastes domestic wastes and hazardous chemical compounds. Water purity can be determined by both either physical like smell, taste, color, particles, pH, temperature etc. or by chemical routes [39]. Presence and absence of chemical substances define purity of water [40]. Generally, the amount contaminants like calcium bicarbonates and calcium chlorides increased upon down streaming due to water–rock interactions [41].
One of the most significant environmental problems endangering the viability of healthy ecological processes in semi-arid and arid regions is the salinity hazard of agricultural land [42]. The leachate leaking through the permeable Cenomanian marly limestone generates the high mineralization. The accumulation of Cd, Pb, Cu, Zn, Fe, and Mg elements in sediments was also the reason for the water quality degradation [43]. It was studied, that vulnerability mapping appears to be a useful method for managing water resources [44, 45].
Municipal, industrial and agricultural wastes are being discharged in open water system and these are major causes of water pollution. In every 24 h 2000 million gallon of the effluents are being expelled in the water bodies. Bigger industrial units such as leather pesticides paint, chemical textile paper and pulp, plastic, poultry, pharmaceuticals, dairy, leather and tanneries are major source of pollution of both surface and ground water [46]. Fertilizers and pesticides on excessive usage become contributor of water pollution as they seeps through soil and become part of ground water [47].
1.2 Dyes as pollutant
Dyes are molecules of organic nature having highly complicated structures and give color to the substrate by binding with its surface. Dyes have applications in various fields like plastics, food processing, paper, leather, textile tanning, printing and cosmetics and to fulfill their purpose in these areas these are made to remain unaffected by numerous things such as detergents. When waste water of these industries is discharged into natural water resources than unusual color develop in the natural water and penetration of light through it decreases which ultimately results in reduced biological and photochemical reactions. About more than 100,000 commercial dyes are produced at the rate of about 7 × 105 tons per year and textile dyes consumed about 10,000 tons of these dyes annually. Annually about 100 tons of dyes are discharged in natural water resources [19].
1.3 Classification of dyes
1.3.1 Natural dyes
In early times dyes were obtained from natural resources most commonly from vegetables. Such dyes were in common use of Egyptians who may have acquired this knowledge of dyes from Chinese. Properties of some dyes are given in Table 1 [48].
1.3.2 Synthetic dyes
The origin of synthetic dyes can be drawn back to nineteenth century when an 18-year-old chemist Perkin working with August William von Hofmann for the synthesis of quinine for the treatment of malaria by chance discovered bright purple color dye. It can dye silk fabric to bright purple and was named “mauve”. The name mauve was derived from the French name for the mallow flower but later it was changed to “mauveine”. Later a variety of dyes has been synthesized for example azo dyes, direct dyes, indigo dyes and many more [48]. Various classes of such type of dyes their application and examples are given in Table 2.
1.4 Impact of dyes on human health
In terms of quantity and manufacturing, azo dyes make up 60 to 70 percent of all organic dyes manufactured by industries across the world. The aryl amine derivatives produced by the reductive biotransformation of the azo-bond, along with the azo compounds themselves, are very poisonous. As soon as they reach the body, intestinal microbes create the enzymes azoreductase and nitroreductase, which catalyse the breaking of the azo bond and the nitro group, respectively. N-hydroxylamines are often produced as a result of this sort of bond breaking, which might harm DNA [17, 49, 50]. Aromatic amines produce explosive, extremely poisonous, and carcinogenic aromatic amines, which are formed by azo dyes. One of the most frequent carcinogens in dyes is benzidine, which needs to be eliminated from water. Additionally, metals and other additives used in the production of dyes are present in waste water. To keep the purity of the water, these impurities must be eliminated [51].
1.5 Techniques used for water treatment
Presence of synthetic dyes in wastewater is very dangerous for environment. These dyes must be removed by adopting separation techniques to avoid their harmful effect on environment as well as on health of human. Dye separation techniques are categorized into three types namely biological, physiochemical method and chemical. These techniques are adopted by analyzing various facts like efficiency, design, and overall cost Dawood and Sen, 2014). Broadly waster water treatment strategies are classified into three major brancher chemical treatement, biologhical treatement and physiochemical treatment see Fig. 2.
1.6 Adsorption
Adsorption means up taking or immobilization of contaminants by the adsorbents. This immobilization occurs by different mechanisms such as structural incorporation, surface precipitation, partition, surface adsorption (Fig. 3a–f). Surface precipitation refers to the phenomenon that involves adsorption of contaminants on the adsorbent surface and then precipitates formed by saturation of cations and anions in large amount [52, 53]. For example, co-adsorption of cations and oxyanions on the surface of metal (hydr) oxides. Partition involves distribution of contaminants between two phases and penetration of contaminants into the entire bulk phase rather than being concentrated on the surface of adsorbent. Soil organic carbon materials can enhance the fertility upon transformation between phases [54]. Incorporation of ions or free radicals on the surface of adsorbent or stationary phase is one of the structure incorporation process, which can be used as a tool for water treatment. For example, we can use mineral crystal structures for incorporation of amorphous metal ions, this incorporation or adsorption may be physical or chemical [55, 56].
Uptake mechanism of various absorbents. a Surface precipitation: Cd+2 and phosphate precipitation on the surface of metal(hydr)oxide, b Partition: uptake of HOC to soil organic matter (SOM) or partition process, c structural incorporation: Ni+2 and bimessite up taking by structure incorporations, d adsorption process, e ligand exchange, f ionic exchange or up taking of cations, green color shows the cation that should be replaced by incoming cation in purple)
The contaminations of particles on the surface of adsorbent via weak intermolecular forces like van der wall forces are considered as physical adsorption, while contamination because of ionic or covalent bonding are called chemisorption. For example, the contaminants such as adsorbed HOCs on the surface of activated carbon is a type of physical adsorption [57] while that of metal ions on metal (hydr) oxides are type of chemisorption [58]. The contaminants suspended in water can easily exchange with the metal ions adsorbed on the adsorbent surface when attached via electrostatic interactions. An illustration of electrostatic interaction is the adsorption of heavy metal cations by montmorillonite [59].
1.7 Adsorbents
Adsorbent selectivity is influenced by its overall cost, usability, surface area, and adsorption capability. For the removal of dye during waste water treatment, a variety of adsorbents including commercially available activated carbon, solid wastes of agriculture, clay and minerals can be employed [22]. Dyes are removed due to different types of interaction with these functional groups present on the cell surface. There are many factors that affect the interaction between dyes and functional groups on the cell surface and these are pH, temperature, contact time and properties of both adsorbate and adsorbent [60].
1.7.1 Clay as adsorbent
The clay’s lamellar structure provides a larger specific surface area, leading to increased adsorption capacity. When compared to activated carbon at the same temperature and pH, clay demonstrates an even higher adsorption capacity [61]. Clays are naturally occurring aluminosilicate materials that contain small amounts of organic compounds and metallic ions. They exist as colloidal fractions in water, soil, rocks, and sediments [62]. Clays are great adsorbents because they have a lot of surface area, are chemically and mechanically stable, have a layered structure, and have strong cation exchange abilities. Moreover, compared to other adsorbents, clays are widely accessible and inexpensive. Natural clays have negative charges, which makes it possible for them to successfully remove cationic colors like methylene blue. Clay's surface charge can be changed to positively charged by surfactants, which improves the adsorption of anionic dyes. Metal ions and textile dyes have both been removed using various types of clays. For instance, sodium bentonite has been utilized for congo red, kaolin for zinc ions, and montmorillonite clay for vibrant green dye [22].
1.7.1.1 Clay structure
The coordination of metal ions[63] to the fixed charge sites depends critically on the structure of clay and the charge on its layers, and this interaction differs depending on the particular clay type utilized (see Fig. 4). Although many physical interactions help too much, in layer formation but hydrogen bonding is very important for interlayer connections [64]. As a result, different clay minerals produce unique configurations of silanol and aluminum surface hydroxyl sites. Clay has the ability to adsorb due to the negative charge present in the silicate structure, which is balanced by cationic dyes. The considerable surface area of clay, which equals about 800 m2/g, is also directly related to its high adsorption capacity [32].
Structure of clay (2:1) Adopted from Ref. [65]
1.7.1.2 Calcination of clay
Upon calcination, the structure, composition, and physiochemical properties of clay undergo significant alterations. The extent of these changes is contingent upon factors such as the clay type, particle size, and heating conditions. As the temperature increases, reaching the dehydration threshold, the clay loses adsorbed water, resulting in modifications to its porosity and a decline in adsorption potential. The destruction of interlayer spaces leads to a reduction in cation exchange capacity. Additionally, there is an increase in the hydrophilicity of the clay due to the partial release of hydration and adsorbed water. At the culmination of the dehydration stage, the weight of the clay diminishes, while the overall surface area available for adsorption expands. Calcination serves to stabilize the clay and maintain its useful permanent properties. Subsequent to dehydroxylation, further heating induces alterations in the clay's structure and its surface functional groups, following dihydroxylation. [66].
1.7.1.3 Natural and modified clay
Montmorillonite clays have the highest adsorption capacity when compared to other forms of clay. Clay may be altered, nevertheless, to increase its capacity for purging pollutants from wastewater. Clay Type, its modification, type of dye removed and adsorption efficiency are given in Table 3 [32].
1.7.2 Alginates as adsorbent
A the polysaccharides known as alginates are derived from specific types of brown algae, such as Laminaria hyperborea and lessonia, which are found worldwide in coastal waters [76]. Alginates are also discovered to be produced by soil bacteria, such as Pseudomonas species and Azotobacter vinelandii. Alginates are homopolymeric linear copolymers made of 1,4-linked homopolymeric blocks of -d-mannuronic acid and -l-guluronic acid. Alginate received from diverse sources is first washed, then macerated, and finally is extracted with sodium carbonate to produce sodium or calcium alginate. Following filtering, calcium or sodium chlorides are added to the filtrate, converting alginate to calcium alginate or sodium alginate, respectively [77]. See Table 4 where properties of alginate are given.
Due to its capacity to filter out impurities, alginate, a naturally occurring carbohydrate polymer, has been used to remove colours from waste water. Alginate is derived from many kinds of Brown algae. An essential property of alginate is the capacity to produce hydrogels. Aqueous alginate is easily transformed to hydrogel when metallic divalent cations are introduced. Calcium alginate beads containing immobilized activated carbons have been utilized for the removal of dyes, better efficiency [78]. Alginates possess distinctive characteristics in term of affordability, resources, hydrophilicity, biocompatibility, which enable them to suitable candidate for coating on the surface of nanoparticles [79,80,81].
1.7.3 Alginate hybrid and clay
To enhance adsorption capacity and increased surface area hybrid materials containing alginates and clay are synthesized [90]. An alginate clay hybrid refers to a composite material that combines alginate, a natural polymer derived from seaweed, with clay particles. Adsorption capacity or dye removal efficiency of alginate clay hybrid is shown in Table 5. This hybrid material combines the desirable properties of both components, offering improved functionality and performance in various applications. When it comes to dye removal, alginate clay hybrids have been studied for their adsorption capabilities. The clay component provides a high surface area and porous structure, which can effectively adsorb dyes through noncovalent interactions like intermolecular hydrogen bonding, electrostatic interactions, hydrophobic interactions and Van der Waals force [64]. Alginate, on the other hand, acts as a binder and stabilizer, enhancing the mechanical properties and stability of the hybrid material.
1.8 Factors effecting adsorption
1.8.1 Effect of pH
The adsorption process is dependent on the pH up to greater extent as it can change the dye chemistry and functional group of adsorbents. Low pH causes increase in the possibility of adsorption of anionic dye to the surface of negatively charged adsorbent, at the same time higher pH supports adsorption of cationic dyes to the negatively charged adsorbent’s surface and at this higher pH adsorption of anionic dyes becomes limited. Maximum adsorption can be achieved by optimizing pH on the basis of various factors such as clay type, its modification and dye nature [32].
The effect of pH can be explained for alginates such as adsorption of malachite green dye on the alginate coated with Fe3O4 nanoparticles increased accordingly as the pH of dye increased because pH has a great influence on the interactions found between dye molecule and adsorbent. Ionization of carboxyl and hydroxyl groups present on sodium alginate increased with increase in pH, this will in turn increase the interaction of alginate with cationic dye. Adsorption of cationic dye decreased with decrease in pH because at lower pH carboxylic group would be protonated. But at the same time due to enhanced repulsion of carboxylate groups, volume of polymer chains would increase and it causes penetration of cationic dyes into the alginate [79].
1.8.2 Effect of temperature
The rate of dye adsorption is impacted by temperature change, in both before and after equilibrium, which is one of two ways that temperature variation affects the adsorption process. For a certain adsorbate, equilibrium temperature impacts the adsorption equilibrium of the adsorbent [70]. Dye adsorption processes can be endothermic or exothermic; for endothermic processes, a rise in temperature increases adsorption capacity; for exothermic processes, an increase in temperature decreases adsorption capacity. For instance, temperature rise favours the adsorption of Congo red dye onto modified hectorite clay while decreasing the adsorption of methylene blue onto montmorillonite clay [22].
1.8.3 Initial dye concentration and contact time effect
The amount of dye that is adsorbed (mg/g) and the percentage of dye that is removed are strongly influenced by the initial dye concentration. All adsorption sites get saturated when the original dye concentration is raised, which lowers the effectiveness of dye removal. With increasing contact duration and starting dye concentration, the amount of adsorbed dye (mg/g) rises. The driving force produced by the initial dye concentration is used to overcome resistance in the mass transfer of dye between solution and adsorbent [22].
1.8.4 Effect of adsorbent dosage
As the dosage of the combination is increased, the efficiency of colour removal increases. The increase in surface area and the number of active adsorption sites are responsible for this. However, for some adsorbents, such clay and calcium hydroxide combination dosage of around 1 g L−1 produce dye removal effectiveness of about 99% of Congo red as achieved from 8–23 g L−1 of other inexpensive adsorbents. Because of the cheap cost and little sludge creation, utilising a small amount of adsorbent with the same dye removal effectiveness as a big amount of adsorbent is advantageous [67].
1.9 Electrocoagulation
It is an efficient method for the treatment of waste water obtained from various sources including textile industry. In this method direct current is supplied by a DC source connected externally to the electrode or an assembly of electrodes. On passage of direct current electrodes get dissolved in the effluents. At a definite pH metal ion will form coagulated species and hydroxide that form the aggregation with particles and dissolved contaminants are adsorbed on these. Commonly used electrode materials are stainless steel, aluminum, iron and graphite. These electrode materials are nontoxic, cheap and easily available (Khandegar and Saroha 2013).
In electrocoagulation process anode material is dissolved continuously on passage of current and release cations in wastewater. The particles of contaminants are charged due to their surface potential cations thus attract the dissolved cations. This process results in increased agglomeration and unstable suspension. Bubbles are formed as the result of hydrolysis and these bubbles help in floatation of coagulated agglomerates. Three parameters that determine the principle of electrocoagulation are particulate size, spatial density of vapor or droplet and particulate size. Three stages are involved in formation of charged agglomerates: (a) electrolytic disintegration of anode results in formation of coagulant, (b) suspension is destabilized and (c) formation of floating material or flocs by aggregation. It is a useful process in removal of organic ligands [92], dyes, heavy metals, suspended particles, heavy metals, oil and grease from effluents of industries. Hydroxides and oxyhydroxides formed during electrolysis results in an increased surface area which is essential for interaction of contaminants.
1.9.1 Mechanism of electrocoagulation
Electrocoagulation is completed in following steps;
(1) Interaction of anodic cations with charged particles of contaminants
Cations produced when electric current is passed through anode and these cations attracted towards and react with charged particles of contaminant.
(2) Neutral coagulation
Dissolution of anode resulted in production of large amount of counterions and these counterions interact with and neutralize the charged species. At the end van der waals interactions develop and stable coagulates will be formed.
(3) Floatation by gas bubbles
Hydrolysis resulted in production of gas bubbles and colloidal particles will be entrapped in these bubbles. Floatation produced by this phenomenon helps in gravity separation.
Reactions occurring at anode and cathode
The reactions occurring at aluminum or iron anode electrodes are shown in Eqs. 3, 4.
(4) Coagulation
Metal hydroxide or oxyhydroxides formed by the hydrolysis of cations (produced from sacrificial anode) act as coagulating agent. Interaction of negatively charged particles of contaminants with cations resulted in coagulation. In this step electrocoagulation differ from chemical coagulation in which the chemical precipitation is added externally while in electrocoagulation it is produced within the reaction solution. Mechanism of electrocoagulation is shown in Fig. 5.
Mechanism of electrocoagulation [93]
(5) Release of oxygen at anode
Hydrolysis of water in side reaction resulted in production of oxygen bubbles at anode this oxygen flocculates the coagulated contaminant species to the surface by buoyancy effect [93].
1.9.2 Assembly of an electrocoagulation unit
Following considerations must be kept in mind while building an electrochemical cell for the purpose of electrocoagulation in dye separation:
-
a)
There must be minimum resistance between electrodes to avoid potential drop.
-
b)
There should be negligible resistance to mass transfer in the region between electrodes.
-
c)
There should be limited accumulation of bubbles of oxygen and hydrogen gas at the surface of electrodes. Excessive evolution of gaseous bubbles at the electrodes results in increase in the internal resistance hence decrease in electrical conductivity [93].
1.9.3 Dyes removal by electrocoagulation
During decolorization of dye by electrocoagulation, adsorption and precipitation occur depending on pH range. It can be explained for Al electrodes as follows: Precipitation and Adsorption followed routes according to following Eqs. 5–8.
The presence of Al(OH)4– can be noted at pH higher than 9. Freshly formed Al (OH) 3 are amorphous flocs having large surface area which promotes adsorption of solubilized organic compounds and colloidal particles trapping. The polymerization of these flocs occurs according to Eq. 9. These polymers can be easily separated from aqueous solution.
1.10 Techniques for analysis
For measurement of dye concentration in the samples UV-spectroscopy are utilized, as each [49] chromophore or dye have their own absorption maxima, so that the reason UV spectra assist us in analysis of exact concentration. For the determination of various functional groups FTIR spectra are taken normally from 400 to 4000 cm−1 range. FTIR spectra of surface before and after absorption give us very important information about adsorption process [94]. Similarly SEM, TEM, and EDX are also used for determination of surface morphology [95].
2 Recommendations and outlook perspectives
Clay minerals have garnered considerable attention in the realm of water treatment due to their distinctive properties and pronounced adsorption capacity. Various clay types, encompassing montmorillonite, kaolin, bentonite, and others, have been exhaustively studied for their efficacy in eliminating dyes and other contaminants from water. These clay minerals, endowed with substantial surface areas, layered structures, and cation exchange capabilities, serve as exemplary adsorbents. Particularly in the context of wastewater from textile and dyeing industries, the efficient removal of dyes assumes environmental significance due to their potential toxicity and persistence. Clay minerals have demonstrated remarkable potential in extracting dyes from water, as they adeptly adsorb and eliminate these contaminants. This adsorption mechanism hinges on the interaction between the negatively charged clay surface and the positively charged dye molecules, culminating in their immobilization and subsequent removal from the water.
Distinct types of clay minerals have showcased commendable efficiency in the realm of dye removal from water. The unique attributes of clay, marked by their expansive surface areas, layered structures, and cation exchange abilities, substantiate their robust adsorption capacity and render them effective agents for dye extraction. The adsorption process orchestrating the interplay between clay and dyes presents a promising and sustainable avenue for water treatment. It beckons further research and development to optimize adsorption processes employing clay minerals. This could involve the exploration of modification techniques aimed at augmenting adsorption capacity and selectivity concerning distinct dye molecules. Equally pivotal is the exploration of regeneration and reusability in the context of clay adsorbents. This inquiry is essential for ensuring their cost-effectiveness and practical viability in large-scale water treatment systems. In summation, the application of clay minerals for dye removal in water treatment holds immense potential. It offers an eco-friendly and efficient solution to grapple with the challenges posed by dye-laden wastewater, thereby contributing to the enhancement of cleaner and safer water resources.
3 Conclusion
Water treatment assumes a critical role in ensuring the availability of clean and safe drinking water. In recent times, clay-based materials have gained prominence as promising adsorbents to meet the demands of water treatment. This review conducts a comprehensive analysis of diverse clay types, investigating their surface adsorption properties and emphasizing their practical utility within water treatment domains.
The exploration begins by examining prevalent clay types used in water treatment, including kaolin, montmorillonite, and bentonite. This scrutiny delves into their distinct mineral compositions, surface characteristics, and inherent structural attributes. Subsequent sections embark on an exploration of the intricate adsorption mechanisms manifesting on clay surfaces, revealing the nuanced interplay between contaminants and the active sites present on these surfaces. Furthermore, the review meticulously dissects the influence wielded by pivotal factors such as pH, temperature, contact time, and initial contaminant concentration.
The review highlights the adsorption efficiency and capacity of diverse clay types in effectively removing an array of contaminants, ranging from heavy metals to organic pollutants and dyes. By accentuating the cardinal role played by surface modification techniques, the investigation critically evaluates cation exchange, functionalization, and composite formation for their roles in bolstering adsorption efficacy.
Transitioning beyond the enumeration of virtues, the review forthrightly addresses challenges linked with clay-based adsorbents, encapsulating issues spanning regeneration, disposal, and cost-effectiveness. The narrative culminates by unveiling strategies poised to surmount these challenges, while concurrently outlining potential trajectories to drive advancements in the realm of clay-based water treatment.
Data availability
Not applicable.
References
Gavrilescu M, Demnerova K, Aamand J, Agathos S, Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol 32(1):147–156
Bernhardt ES, Rosi EJ, Gessner MO (2017) Synthetic chemicals as agents of global change. Front Ecol Environ 15(2):84–90
Khatri A, Peerzada MH, Mohsin M, White M (2015) A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J Clean Prod 87:50–57
Khan N, Hussain ST, Saboor A, Jamila N, Kim KS (2019) Physicochemical investigation of the drinking water sources from Mardan, Khyber Pakhtunkhwa, Pakistan. IJPS 8(33):1661–1671
Khwaja MA, Aslam A (2018) Comparative assessment of Pakistan national drinking water quality standards with selected Asian countries and World Health Organization. SDPI 1:1–12. https://sdpi.org
Khan S, Haq F, Saeed K (2012) Pollution load in industrial effluent and ground water due to marble industries in District Buner, Khyber Pakhtunkhwa, Pakistan. Int J Recent Sci Res 3(5):366–368
Strokal M, Spanier JE, Kroeze C, Koelmans AA, Flörke M, Franssen W, Hofstra N, Langan S, Tang T, van Vliet MT (2019) Global multi-pollutant modelling of water quality: scientific challenges and future directions. Curr Opin Environ Sustain 36:116–125
Tariq SR, Shah MH, Shaheen N, Jaffar M, Khalique A (2008) Statistical source identification of metals in groundwater exposed to industrial contamination. Environ Monit Assess 138(1–3):159–165
Hamed Y, Hadji R, Redhaounia B, Zighmi K, Bâali F, El Gayar A (2018) Climate impact on surface and groundwater in North Africa: a global synthesis of findings and recommendations. Euro-Mediterr J Environ Integr 3:1–15
Tariq M, Ali M, Shah Z (2006) Characteristics of industrial effluents and their possible impacts on quality of underground water. Soil Environ 25(1):64–69
MUNEERak M, Adeel S, Bhatti IA, Jamal MA., Rehman FU (2015) Removal of reactive orange P3r dye by oxidative pathway. Oxid Commun 38(2):808–817
Gupta V, Khamparia S, Tyagi I, Jaspal D, Malviya A (2015) Decolorization of mixture of dyes: a critical review. Glob J Environ Sci Manag 1(1):71–94
Bhatti HN, Noreen S, Tahir N, Ilyas S, Siddiqua UH (2015) Equilibrium, thermodynamic and kinetic studies for biosorption of Terasil Brown 2RFL from contaminated water using economical biomaterial. Mediterr J Chem 4(5):239–251
Kallel A, Ksibi M, Dhia HB, Khélifi N (2018) Recent advances in environmental science from the Euro-Mediterranean and surrounding regions. In: Proceedings of Euro-Mediterranean conference for environmental integration (EMCEI-1), Tunisia 2017. Springer
Rasool A, Xiao T, Farooqi A, Shafeeque M, Masood S, Ali S, Fahad S, Nasim W (2016) Arsenic and heavy metal contaminations in the tube well water of Punjab, Pakistan and risk assessment: a case study. Ecol Eng 95:90–100
Kaykhaii M, Sasani M, Marghzari S (2018) Removal of dyes from the environment by adsorption process. Chem Mater Eng 6(2):31–35
Chequer FMD, de Oliveira GAR, Ferraz ERA, Cardoso JC, Zanoni MVB, de Oliveira DP (2013) Textile dyes: dyeing process and environmental impact. In: Eco-friendly textile dyeing and finishing, IntechOpen 6(6):151–176
Bazrafshan E, Alipour MR, Mahvi AH (2016) Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin Water Treat 57(20):9203–9215
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Coll Interface Sci 209:172–184
Ngulube T, Gumbo JR, Masindi V, Maity A (2017) An update on synthetic dyes adsorption onto clay based minerals: a state-of-art review. J Environ Manag 191:35–57
Bhatnagar A, Jain A (2005) A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci 281(1):49–55
Dawood S, Sen T (2014) Review on dye removal from its aqueous solution into alternative cost effective and non-conventional adsorbents. J Chem Process Eng 1(104):1–11
Bharathi K, Ramesh S (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3(4):773–790
Oliveira LS, Franca AS, Alves TM, Rocha SD (2008) Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J Hazard Mater 155(3):507–512
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235
Sharma P, Kaur H, Sharma M, Sahore V (2011) A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ Monit Assess 183(1–4):151–195
Gil A, Santamaría L, Korili S, Vicente M, Barbosa L, De Souza S, Marçal L, De Faria E, Ciuffi K (2021) A review of organic-inorganic hybrid clay based adsorbents for contaminants removal: Synthesis, perspectives and applications. J Environ Chem Eng 9(5):105808
Reeves C (1963) Geology of API Project 49 kaolin clay reference locality, Mesa Alta, New Mexico. Econ Geol 58(2):237–249
Borah D, Nath H, Saikia H (2022) Modification of bentonite clay and its applications: a review. Rev Inorg Chem 42(3):265–282
Baek M, Lee J-A, Choi S-J (2012) Toxicological effects of a cationic clay, montmorillonite in vitro and in vivo. Mol Cell Toxicol 8:95–101
Adeyemo AA, Adeoye IO, Bello OS (2017) Adsorption of dyes using different types of clay: a review. Appl Water Sci 7(2):543–568
Kausar A, Iqbal M, Javed A, Aftab K, Bhatti HN, Nouren S (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Ismadji S, Soetaredjo FE, Ayucitra A (2015) Natural clay minerals as environmental cleaning agents. In: Clay Materials for Environmental Remediation. SpringerBriefs in Molecular Science. Springer, Cham. https://doi.org/10.1007/978-3-319-16712-1_2
Belhouchat N, Zaghouane-Boudiaf H, Viseras C (2017) Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl Clay Sci 135:9–15
Aravindhan R, Fathima NN, Rao JR, Nair BU (2007) Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads. Colloids Surf A 299(1–3):232–238
Hassan A, Abdel-Mohsen A, Fouda MM (2014) Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr Polym 102:192–198
Haseena M, Malik MF, Javed A, Arshad S, Asif N, Zulfiqar S, Hanif J (2017) Water pollution and human health. Environ Risk Assess Remediat 1(3):16–19
Arshad N, Imran S (2017) Assessment of arsenic, fluoride, bacteria, and other contaminants in drinking water sources for rural communities of Kasur and other districts in Punjab, Pakistan. Environ Sci Pollut Res 24(3):2449–2463
Benmarce K, Hadji R, Zahri F, Khanchoul K, Chouabi A, Zighmi K, Hamed Y (2021) Hydrochemical and geothermometry characterization for a geothermal system in semiarid dry climate: the case study of Hamma spring (Northeast Algeria). J Afr Earth Sci 182:104285
Praveen PK, Ganguly S, Kumar K, Kumari K (2016) Water pollution and its hazardous effects to human health: a review on safety measures for adoption. Int J Sci Environ Tech 5(3):1559–1563
Benmarce K, Hadji R, Hamed Y, Zahri F, Zighmi K, Hamad A, Gentilucci M, Ncibi K, Besser H (2023) Hydrogeological and water quality analysis of thermal springs in the Guelma region of North-Eastern Algeria: a study using hydrochemical, statistical, and isotopic approaches. J Afr Earth Sci 205:105011
Besser H, Dhaouadi L, Hadji R, Hamed Y, Jemmali H (2021) Ecologic and economic perspectives for sustainable irrigated agriculture under arid climate conditions: an analysis based on environmental indicators for southern Tunisia. J Afr Earth Sci 177:104134
Brahmi S, Baali F, Hadji R, Brahmi S, Hamad A, Rahal O, Zerrouki H, Saadali B, Hamed Y (2021) Assessment of groundwater and soil pollution by leachate using electrical resistivity and induced polarization imaging survey, case of Tebessa municipal landfill, NE Algeria. Arab J Geosci 14:1–13
Hamad A, Abdeslam I, Fehdi C, Badreddine S, Mokadem N, Legrioui R, Djebassi T, Rahal O, Hadji R, Hamed Y (2022) Vulnerability characterization for multi-carbonate aquifer systems in semiarid climate, case of Algerian-Tunisian transboundary basin. Int J Energy Water Resour 6(1):67–80
Hamad A, Baali F, Hadji R, Zerrouki H, Besser H, Mokadem N, Legrioui R, Hamed Y (2018) Hydrogeochemical characterization of water mineralization in Tebessa-Kasserine karst system (Tuniso-Algerian Transboundry basin). Euro-Mediterr J Environ Integr 3:1–15
Hamed Y, Hadji R, Ahmadi R, Ayadi Y, Shuhab K, Pulido-Bosch A (2023) Hydrogeological investigation of karst aquifers using an integrated geomorphological, geochemical, GIS, and remote sensing techniques (Southern Mediterranean Basin—Tunisia). Environ Dev Sustain. https://doi.org/10.1007/s10668-023-02994-8
Jabeen A, Huang X, Aamir M (2015) The challenges of water pollution, threat to public health, flaws of water laws and policies in Pakistan. J Water Resour Prot 7(17):1516
Abel A (2012) 24 - The history of dyes and pigments: from natural dyes to high performance pigments. In: Janet Best (ed) Colour design. Woodhead Publishing, Elsevier, pp 557–587. https://doi.org/10.1016/B978-0-08-101270-3.00024-2
Khan S, Rahman FU, Zahoor M, Haq AU, Shah AB, Rahman MU, Rahman HU (2023) The DNA threat probing of some chromophores using UV/VIS spectroscopy. World J Biol Biotechnol 8(2):19–22
Ullah A, Liu J, Khan AU, Khan QU, Guo F, Nazir S, Quan Z, Wang X, Alosaimi AM, Hussein MA (2021) Diversification and design of novel aniline-pyrimidines via Sonogashira/Suzuki cross coupling reactions catalyzed by novel CLPN-Pd. ChemistrySelect 6(47):13551–13558
Ahmad A, Mohd-Setapar SH, Chuong CS, Khatoon A, Wani WA, Kumar R, Rafatullah M (2015) Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv 5(39):30801–30818
Ncibi K, Hadji R, Hajji S, Besser H, Hajlaoui H, Hamad A, Mokadem N, Ben Saad A, Hamdi M, Hamed Y (2021) Spatial variation of groundwater vulnerability to nitrate pollution under excessive fertilization using index overlay method in central Tunisia (Sidi Bouzid basin). Irrig Drain 70(5):1209–1226
Ncibi K, Mastrocicco M, Colombani N, Busico G, Hadji R, Hamed Y, Shuhab K (2022) Differentiating nitrate origins and fate in a semi-arid basin (Tunisia) via geostatistical analyses and groundwater modelling. Water 14(24):4124
Bagwan WA, Gavali RS, Maity A (2023) Quantifying soil organic carbon (SOC) density and stock in the Urmodi River watershed of Maharashtra, India: implications for sustainable land management. J Umm Al-Qura Univ Appl Sci 9(2):1–17. https://doi.org/10.1007/s43994-023-00064-3
da Silva Veiga PA, Schultz J, da Silva Matos TT, Fornari MR, Costa TG, Meurer L, Mangrich AS (2020) Production of high-performance biochar using a simple and low-cost method: optimization of pyrolysis parameters and evaluation for water treatment. J Anal Appl Pyrol 148:104823
Aguilar-Carrillo J, Herrera-García L, Reyes-Domínguez IA, Gutiérrez EJ (2020) Thallium(I) sequestration by jarosite and birnessite: structural incorporation vs surface adsorption. Environ Pollut 257:113492
Bandosz T (2006) Chapter 5 Desulfurization on activated carbons. In: Interface science and technology, Chap 5. Elsevier, pp 231–292. https://doi.org/10.1016/S1573-4285(06)80014-1
Zhang N, Blowers P, Farrell J (2005) Evaluation of density functional theory methods for studying chemisorption of arsenite on ferric hydroxides. Environ Sci Technol 39(13):4816–4822
Zhu R, Chen Q, Zhou Q, Xi Y, Zhu J, He H (2016) Adsorbents based on montmorillonite for contaminant removal from water: a review. Appl Clay Sci 123:239–258
Srinivasan A, Viraraghavan T (2010) Decolorization of dye wastewaters by biosorbents: a review. J Environ Manag 91(10):1915–1929
Elmoubarki R, Mahjoubi F, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, El Albani A, Barka N (2015) Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour Ind 9:16–29
Taib H, Hadji R, Hamed Y, Bensalem MS, Amamria S (2023) Exploring neotectonic activity in a semiarid basin: a case study of the Ain Zerga watershed. J Umm Al-Qura Univ Appl Sci 3:1–14. https://doi.org/10.1007/s43994-023-00072-3
Gul Z, Khan S, Ullah S, Ullah H, Khan MU, Ullah M, Altaf AA (2022) Recent development in coordination compounds as a sensor for cyanide ions in biological and environmental segments. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2022.2085027
Khan S, Zahoor M, Rahman MU, Gul Z (2023) Cocrystals; basic concepts, properties and formation strategies. Z Phys Chem 237(3):273–332
Gitari MW, Mudzielwana R (2018) Mineralogical and chemical characteristics of raw and modified clays and their application in arsenic and fluoride removal. In: Zoveidavianpoor M (ed) Current topics in the utilization of clay in industrial and medical applications. IntechOpen, London, UK. https://doi.org/10.5772/intechopen.74474
Chukwujike IC, Igwe IO (2016) Extender properties of some Nigerian clays. J Miner Mater Charact Eng 4(5):1–13
Vimonses V, Jin B, Chow CW, Saint C (2009) Enhancing removal efficiency of anionic dye by combination and calcination of clay materials and calcium hydroxide. J Hazard Mater 171(1–3):941–947
Tehrani-Bagha A, Nikkar H, Mahmoodi N, Markazi M, Menger F (2011) The sorption of cationic dyes onto kaolin: kinetic, isotherm and thermodynamic studies. Desalination 266(1–3):274–280
Auta M, Hameed B (2013) Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J Ind Eng Chem 19(4):1153–1161
Cottet L, Almeida C, Naidek N, Viante M, Lopes M, Debacher N (2014) Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Appl Clay Sci 95:25–31
Chaari I, Moussi B, Jamoussi F (2015) Interactions of the dye, CI direct orange 34 with natural clay. J Alloy Compd 647:720–727
Hajjaji W, Pullar R, Labrincha J, Rocha F (2016) Aqueous acid orange 7 dye removal by clay and red mud mixes. Appl Clay Sci 126:197–206
Santos SC, Boaventura RA (2016) Adsorption of cationic and anionic azo dyes on sepiolite clay: equilibrium and kinetic studies in batch mode. J Environ Chem Eng 4(2):1473–1483
Bessaha H, Bouraada M, de Ménorval LC (2017) Removal of an acid dye from water using calcined and uncalcined ZnAl-r anionic clay. Water Environ Res 89(9):783–790
Chaari I, Fakhfakh E, Medhioub M, Jamoussi F (2019) Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J Mol Struct 1179:672–677
Augst AD, Kong HJ, Mooney DJ (2006) Alginate hydrogels as biomaterials. Macromol Biosci 6(8):623–633
Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym Int 57(3):397–430
Mahmoodi NM (2011) Equilibrium, kinetics, and thermodynamics of dye removal using alginate in binary systems. J Chem Eng Data 56(6):2802–2811
Mohammadi A, Daemi H, Barikani M (2014) Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int J Biol Macromol 69:447–455
Saleh EAM, Khan AU, Tahir K, Almehmadi SJ, Al-Abdulkarim HA, Alqarni S, Muhammad N, Dawsari AMA, Nazir S, Ullah A (2021) Phytoassisted synthesis and characterization of palladium nanoparticles (PdNPs); with enhanced antibacterial, antioxidant and hemolytic activities. Photodiagn Photodyn Ther 36:102542
Wanwu D, Khan SU, Khan QU, Khan S, Alam A, Ullah A, Ullah H (2022) In-situ phase transformation and microstructure of reinforced aluminium matrix composites (AA-6061 T6+ NICR NP) with and without TIC nanoparticles. Publ Eur J Mater Sci Eng 7(1):28–36
Rocher V, Siaugue J-M, Cabuil V, Bee A (2008) Removal of organic dyes by magnetic alginate beads. Water Res 42(4–5):1290–1298
Rocher V, Bee A, Siaugue J-M, Cabuil V (2010) Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin. J Hazard Mater 178(1–3):434–439
Lu T, Xiang T, Huang X-L, Li C, Zhao W-F, Zhang Q, Zhao C-S (2015) Post-crosslinking towards stimuli-responsive sodium alginate beads for the removal of dye and heavy metals. Carbohydr Polym 133:587–595
Sun L, Fugetsu B (2014) Graphene oxide captured for green use: influence on the structures of calcium alginate and macroporous alginic beads and their application to aqueous removal of acridine orange. Chem Eng J 240:565–573
Mohammed N, Grishkewich N, Berry RM, Tam KC (2015) Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22(6):3725–3738
Mohammed N, Grishkewich N, Waeijen HA, Berry RM, Tam KC (2016) Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr Polym 136:1194–1202
Semeraro P, Fini P, D’Addabbo M, Rizzi V, Cosma P (2017) Removal from wastewater and recycling of azo textile dyes by alginate-chitosan beads. Int J Environ Agric Biotechnol 2(4):1835–1850
Ong S-T (2018) Application of experimental design for dyes removal in aqueous environment by using sodium alginate-TiO2 thin film. Chem Data Collect 15:32–40
Uyar G, Kaygusuz H, Erim FB (2016) Methylene blue removal by alginate–clay quasi-cryogel beads. React Funct Polym 106:1–7
Auta M, Hameed B (2012) Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem Eng J 198:219–227
Gul Z, Salman M, Khan S, Shehzad A, Ullah H, Irshad M, Zeeshan M, Batool S, Ahmed M, Altaf AA (2023) Single organic ligands act as a bifunctional sensor for subsequent detection of metal and cyanide ions, a statistical approach toward coordination and sensitivity. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2023.2186165
Mondal S, Purkait MK, De S (2018) Electrocoagulation. In: Advances in dye removal technologies, vol 1. Springer, pp 289–312. https://doi.org/10.1007/978-981-10-6293-3
Anirudhan T, Ramachandran M (2015) Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): kinetic and competitive adsorption isotherm. Process Saf Environ Prot 95:215–225
Edathil AA, Pal P, Banat F (2018) Alginate clay hybrid composite adsorbents for the reclamation of industrial lean methyldiethanolamine solutions. Appl Clay Sci 156:213–223
Acknowledgements
Strongly acknowlege Dr. Zarif Gul for their help and support throughout the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Mr. SK design scheme of the current study, collect date about soil, interpret data, formal analysis, verification of data, improve the manuscript condition for journal. Ms SA collect data included in this review and prepare initial draft. Mr. TH validate the data, wrote the electrocoagulation section and MUR improved the figures qualities, helped in revisions and evaluated the recommendations and outlook perspectives section.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, S., Ajmal, S., Hussain, T. et al. Clay-based materials for enhanced water treatment: adsorption mechanisms, challenges, and future directions. J.Umm Al-Qura Univ. Appll. Sci. (2023). https://doi.org/10.1007/s43994-023-00083-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-023-00083-0