Abstract
Water quality deterioration hinders economic and social development in developing countries that are facing freshwater security and shortages. Based on the collection of 29 water samples, this study focused on the relationship between sewage treatment plant and groundwater system surrounding it using multidisciplinary approach that combines the characterization of groundwater system and its connection with surrounding canal and drains, using chemical and isotopic characterization revealing that there is a direct relation between the surface water system and surrounding groundwater system. About 58% of the groundwater samples and all surface water samples in the investigated area are threatened by high concentrations of trace elements. The multivariate statistical analysis elucidates that anthropogenic effect and fertilizers sewage contamination are the main causes of groundwater pollution. Nearly, 31% and 11.5% of groundwater samples were posing oral chronic non-carcinogenic health risk and dermal chronic risk for adult, respectively, while all surface water samples were posing oral chronic non-carcinogenic health risk, with no dermal hazard. The uncharged species of Fe and Al are expected to be more mobile in groundwater because they would not be attracted to the surface charge of minerals. Inorganic ligands (HCO3−, SO42−, Cl−, and NO3−) act as nucleation centers that were linked with those trace elements creating new species with higher solubility degree in water that are transported away randomly for long distances in the water path.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deterioration of water quality is considered as a major challenge that hinders economic and social development in developing countries where freshwater security and shortages are problematic issues. Raising water demands and the rapid and continuous increment of population together with the climatic change put stresses on freshwater resources quality and quantity. Human health risk assessment of groundwater is mandatory and essential issue that correlates and quantifies the environmental pollution loads on human being. This can be done through the estimation of potential risk sources indices, by estimating the values of risk indices, in addition to determining the health consequences of exposure.
Although water is considered to be a survival factor for human life-sustaining and its related ecosystem, every year more than 3.4 million people around the world die suffering from diseases originated from using polluted water (WHO, 2017). Recently, many studies discussed the sources of groundwater pollution and their influences on ecosystems components. They revealed that the pollution of the ecosystem by heavy metals harms the living organisms and human health (Aguirre et al., 2019; Lupi et al., 2019). The geochemical characteristics of the water system, ecotoxicology of the heavy metals, and high pollution level related play a vital role in controlling the stability/mobility and availability that affects the pathways of trace metals in water bodies (Ali et al., 2016; Zhao et al., 2021). Hence, the full understanding and explanation of the source, fate, and potential effects of these parameters are the main factor in environmental protection management as it might give more details on systematically alteration monitoring process, and risk assessment to preserve this valuable ecological component and avoiding any potential risk for human water related diseases and infections(Mirzaei et al., 2020).
Chemical species of trace elements have a direct effect on their environmental chemistry concerning their mobility, potential toxicity to living organism. The geochemistry of trace metals is strongly influenced by its speciation. As it exhibits a variety of aqueous and particulate species through the association with some ligands such as Cl−,OH−, SO42−,F−, and HCO3−to form complexes.
The sewage treatment plant especially in developing countries might add some constrains and threats on the surrounding environmental component. A special focus on Cairo city as the increment in the population in this capital increases the risk of groundwater quality deterioration. The combined effects of the applications of fertilizer, pesticides, in addition to, the use sewage water for irrigation, which might have a diverse impact on groundwater quality in this area might lead to serious health problems for its residents.
Aim of the work
The present work aimed to characterize the groundwater resources in Shoubra area and determination of its quality in the vicinity of sewage treatment plant in the study area. In this paper, several combined tools have been used to achieve this aim; hydrochemistry, environmental stable isotopes, and microbiology of collected samples were integrated with statistical analysis for complete characterization of the groundwater system and its realtion with surface water, in addition to that geochemical modeling has been functioned as the major ions and trace elements levels and their speciation in the groundwater, as it would be beneficial prior to remediation and mitigation actions. This might help in water resources protection and management issues, through the recognition of the metals pollution trends and its sources in water their influence on environmental systems.
Study area
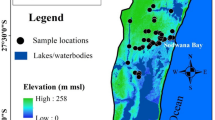
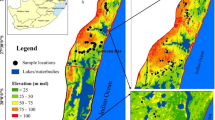
The study area, Shoubra area, is characterized by a lot of industrial activities considered one of the main four big cities in Egypt. It is located in Qalyubia governorate is in the Nile Delta, north of Cairo, and extended between latitudes 30°07′30″ to 30°32′92″ N and longitudes 31°3′30″ to 31°34′30″ E, with a total area of 270 km2 and a population of 1,073,000.
The study area is dominated by Holocene Nile deposits consisting of Nile silt, sand, and sandy clay deposits, with thickness ranging from 0 to 20 m. It is underlain by the Pleistocene sediments which are formed mainly of sand and gravels with intercalations of clay lenses. These sediments have a variable thickness at the eastern parts of the Nile Delta, ranging from zero in the south to nearly 600 m, while at the northwestern parts they may reach 900 m (Khalil et al., 1988; Mansour, 2020). To the east of the area, the oldest Tertiary rocks (Pliocene, Miocene, Oligocene, and Eocene sediments) appear on the surface. The Oligocene basalt appears on the surface as small exposure in the southern parts of the study area at Abu Zaabal region shown as Fig. 1. The Oligocene rocks are dominated by sands, gravels, and fractured basalt. The thickness of these rocks varies from 30 to 100 m and acts as a deep aquifer below the basaltic sheets. Capping basalt may (about 30 m) overlie the fluviatile sands and gravels. The Miocene sediments are present in some places of the study area and composed of alternating sandy limestone and sandy marls of shallow marine origin. Faulting is the dominating structural element and is mainly vertical and facilitates both the downward passage of water and up flow from deep aquifers (Elawadi et al., 2006; Farag & Ismail, 1955; Sultan et al., 2008).
Geologic map of the study area and its vicinities (CONOCO, 1987)
Hydrogeology
There are two main aquifers in the study area, Quaternary and Miocene aquifer. The Quaternary aquifer is the main bearing formation in the study area formed of heterogeneous materials of yellowish brown, medium to coarse-grained sands with occasional soft and brown clay lenses and few amounts of gravels and calcareous materials Fig. 1. It has a variable thickness at the eastern parts of the Nile Delta, ranging from zero in the south to nearly 600 m, while at the northwestern parts the thickness may reach 900 m (Khalil et al., 1988; Mansour, 2020). The Quaternary aquifer is overlain by a semi-permeable Nile silty layer (aquitard) of Holocene age, rendering the aquifer under semi-confining conditions. The Nile silt layer is composed of heterogeneous and anisotropic materials (silt, clay, and sand). It has a thickness that ranges from 15 m adjacent to Ismailia Canal due east to 5 m in the western part of the study area. The depth of water below the land surface is shallow. The groundwater levels range from about 12.8 m above mean sea level (MSL) under the cultivated lands to more than 14.9 m (MSL) under the westwards and 16.3 m (MSL) southwestwards in the urban areas, (Farid, 1985; Nofal et al., 2015; RIGW, 1989; Sherif et al., 2012; Taha et al., 1997; Yehia, 2000). The groundwater approximately moves from all directions toward the cultivated lands. The Miocene sediments, which are made of overlapping sandy dolomite and sandy marls of deep marine origin, may be found in several areas of the study area. The Ismailia freshwater canal, which is located on the southern outskirts of the Eastern region of Nile Delta and has a higher level of water than groundwater, is the principal source of groundwater refill in the Eastern region of Nile Delta. (Nofal et al., 2015).
Materials and methods
For this study, 29 water samples were collected from Shoubra area, complete physico-chemical analyses of the collected water samples were conducted according to the Standards Methods for Examination of Water and Wastewater. The pH was measured at 25°C in the field using pH meter, (Model: Cole Parmer date meter). Electrical Conductivity (EC) was measured in the field using EC meter (Model: Cole Parmer date meter CON 410 series). Major ions (Na+, K+, Ca2+, Mg2+, HCO3−, Cl− and SO42−) as well as trace elements(Al, Ba, Fe, Mn, Sr, Zn, Cr, V)were measured using ICP (Inductive Coupled Plasma) at Egyptian Desert Research Center. The stable isotopes (Oxygen -18 and Deuterium) were measured according to the standard methods using Laser spectroscopy Piccaro (Model 2120i) in the Central laboratory of Isotope Hydrology in Egypt. Microbiological analyses(Total Coliform, Fecal Coliform, Salmonella and Shigella, and Total Count)were carried out according to APHA (2017) at Agricultural Research Center (ARC).
Suitability of groundwater for human uses mainly depends on groundwater geochemistry; hence, each groundwater system has its unique chemical composition that might be altered as a result of many factors. Recently, a new technique adapted that combines several effects of distinct water quality parameters on the whole quality of water at certain location and time known as water quality index (Canadian Environmental Quality Guidelines, 2001). This index (WQI) is characterized by lower score indicating better water quality, and higher score indicating worse water quality, providing a rapid unique score of groundwater status for non-specialized decision-maker, Table 1.
The Canadian water quality index (Rocchini & Swain, 1995) that consists of three factors (Scope F1, frequency F2, and amplitude F3) was utilized in this study according to the method adopted by Canadian Environmental Quality Guidelines, 2001. Fourteen hydrochemical parameters were used as variables from the data set of hydrochemical analyses for all samples of groundwater and surface water. Once the variables and the objectives have been defined, three factors (F1, F2, and F3) for each sample were used to calculate the water quality Index (CCME WQI). The calculation of each factor and the overall quality index described as follows.
F1 (Scope) represents the percentage of variables that do not meet their objectives at least once during the time period under consideration (“failed variables”), relative to the total number of variables measured for only one sample Eq. 1:
F2 (Frequency) represents the percentage of individual tests that do not meet objectives (“failed tests”) and can be calculated as follows Eq. 2:
F3 (Amplitude) represents the amount by which failed test values do not meet their objectives. F3 is calculated in three steps.
i) The number of times by which an individual concentration is greater than (or less than, when the objective is a minimum) the objective is termed an “excursion” and is expressed as follows in Eq. 3 (When the test value must not exceed the objective):
ii) The collective amount by which individual tests are out of compliance is calculated by summing the excursions of individual tests from their objectives and dividing by the total number of tests (both those meeting objectives and those not meeting objectives). This variable, referred to as the normalized sum of excursions, or nse, is calculated as in:Eq. 4
iii) F3 is then calculated by an asymptotic function that scales the normalized sum of the excursions from objectives (nse) to yield a range between 0 and 100, Eq. 5:
Once the F1, F2 and F3 factors have been determined, the overall water quality index CCMEWQI can be calculated by summing the three factors as if they were vectors according to the following equation Eq. 6.
3. Results and discussion
3.1 Isotopic characterization of groundwater samples
Oxygen-18 (18O) and Deuterium (D) are commonly used as natural tracers in hydrology for elucidation groundwater recharge sources and its flow paths, as well as, determining the hydrological processes that affecting the chemical reactions, evaporation and mixing processes that occur along hydrological pathways. The isotopic composition of collected groundwater and surface water samples from Canal is statistically described in Table 2; it was found that δ18O values range from -0.75 to 4.52‰, and δD values range from 2.59 to 32.96‰, respectively.
The diagram of δ 18O and δD is plotted in Fig. 2, with three possible end members that might contribute the aquifer recharge are illustrated in, Recent Nile (δD = 29‰ and δ18O = 3.1‰), Old Nile Before High Dam Construction (δD = 4.3‰ and δ18O = 0.6‰) and return Irrigation Water (δD = 31‰ and δ18O = 4.8‰). The regression line for δ18O and δD has an equation that is defined by (δD = 5.89 δ18O + 6.26), the location of the samples below Global Meteoric Water Nile with slope of 5.89 revealing evaporation process before infiltration in less permeable zones as a results of the existence of silt component in the aquifer matrices. The plotted canal samples were isotopically enriched more than Recent Nile indicating more evaporation occurring in canals. All ground water samples fall between recent Nile component (represented by Ismailia El-Sharkaweya Canals) and old Nile. Groundwater samples are discretized into two main groups, group (A) that is mainly recharged from Recent Nile component that comprises about 80% of the samples; group (B) comprises 20% of the collected samples and mainly recharged from Old Nile buried channels in the study area, revealing that the Old Nile component and Recent Nile are two main components of recharge in this localities. The majority of samples (80%) are recharged mainly from Nile system (canals and drains).
The relationship between δ18O and TDS have been plotted to give more declaration about the geochemical mechanism of groundwater caused by the evaporation or water–aquifer matrices interaction. The same groups appear in Fig. 3, and both groups show a limited increment in the isotopic contents accompanied by wide observed increment in TDS values indicating water–rock interaction between feeding water and aquifer matrices.
The results of tritium analysis conducted on a selected number of groundwater samples in the study area are summarized and shown in \* MERGEFORMAT Table 2. The tritium content of only one sample is either undetectable or varying in the range of the lower limit of detection confirming the presence of paleo-recharge source of more than 45 years; no upper limits on this age can be estimated from the tritium data of this sample revealing the contribution of old Nile buried branches effect. On the other hand, the rest of the analyzed samples have tritium content in the range from (0.1TU to 4.55 TU), suggesting a contribution from recently infiltrated Nile water (adjacent Ismailia and El-Sharkaweya Canals) to this groundwater from recently infiltrated Nile water that seeps to adjacent Ismailia and El-Sharkaweya Canals. The relationship between tritium content in groundwater against δ18O is shown as Fig. 4 with proportional trend (Tritium = 1.121 δ18O-0.0654) indicating the effect of recent Nile contribution through Ismailia and El-Sharkaweya Canals to the aquifer.
3.2 Water quality assessment
Nearly about 23% of the groundwater samples were ranged in categorization of fair water quality (occasionally threatened), while it was 42% as good water quality revealing that they have a minor degree of threat; the rest of samples (35%) ranged from marginal to unsuitable revealing direct continuous threaten. In case of surface water, 67% showed good water quality around Ismailia and El-Sharkaweya canals, while the rest 33% was marginal; Table 3.
3.3 Causes of water pollution using statistical approach
The multivariate statistical analysis has been utilized in this study as a trial to correlate and relate the variables for comparing them within the whole samples domain to elucidate the causes of groundwater pollution in the studied area. An attempt has been done by choosing the most threatened variable (physicochemical variables as: Hardness, pH, TDS; and chemical variables as: SO4, NO3, Al, Ba, Fe, Mn, Sr; in addition to microbial variables as: Total Coliform, Fecal Coliform, Salmonella and Shigella, Total Count) to groundwater quality in the study area. Hierarchical cluster Analysis (HCA) and Principal Components Analysis (PCA) using SPSS.22 statistical software have been used for fourteen variables chosen from the measured items in the study to trace and detect the pollution sources in groundwater, through classifying them into groups and components representing the features and variations. The hierarchical cluster analysis (HCA) classified the water samples into two groups, based on the combination of interval based on multiple parameters from different samples and according to their similarity to each other. The dendrogram, shown as Fig. 5, classified the majority of samples (88%) according to the proximity of the water quality parameters into two major groups (A: 76% and B: 24%). Group (A) comprises two clusters: cluster (1) where majority of groundwater samples are included (59%), and cluster (2) comprises 7% of the samples (one groundwater sample and one surface water). On the other hand, group B includes two clusters: cluster (3) that included only 7% of the samples (one groundwater sample and one surface water sample), and cluster (4) for about 17% of samples including both groundwater samples and surface water samples. Principal Components Analysis (PCA) technique was used as it has the large data reduction ability for the fourteen variables into a set of variables called Principal Components, based on their variances and eigenvalues relations shown in Table 4. Five components representing about 82.8% of the samples (Eigen values > 1) were considered to be representative for the overall characteristics of the water quality data set. The first principal component accounts for 36% of the variance in the data set and combines only chemical and trace elements variables Hardness, TDS, SO4,NO3, Al, Sr, Fe, and Ba; the presence of trace elements and nitrate in this group reflecting anthropogenic effect and application of fertilizers.
The second component represents about 17.7% of the variance and combines Microbial contamination variables (Total Coliform, Fecal Coliform, Salmonella and Shigella, and Total Count) indicating a direct contamination from sewage plant to the surrounding groundwater. On the other hand, Ba and NO3 combine in the third group with variance 11.4% with negative correlation and association and combination of Fe and Mn, revealing that this samples might be affected by fertilizers. The fourth group of variances 9.8% includes Ba and Al with negative association of microbial contamination variables indicating industrial pollution or dissolution of aquifer matrices rather than sewage pollution. Finally, the fifth group with variance 7.8% is influenced only by pH with some association of Al.
3.4 Health risk exposure assessment
In this study, the assessment of the human health risks of groundwater is used to correlate and link environmental contamination with human health (Çelebi et al., 2014; Zhang et al., 2019; Tirkey et al., 2017; Singh et al., 2018). The potential risk sources indices have been determined by estimating the values of risk indices then calculated and estimating the health consequences of exposure for adults via both the direct ingestion of the water and dermal contact using the method applied in EPA (Hagagg et al., 2020; Ji et al., 2020; Li et al., 2017). Chronic non-carcinogenic health risk for adults via exposure of both oral and dermal routes is summarized in Table 5 for groundwater samples. In case of oral exposure, nearly about 31% of groundwater samples and 11.5% of samples were imposing oral and dermal chronic risk for adult (> 1), respectively. On the other hand, all surface water samples were posing oral Chronic non-carcinogenic health risk, with no dermal hazard.
Geochemical modeling and its role in mitigation actions
The correlation between TDS and Sr, Mn, Fe, Al, Ba, and Cr has been calculated to be 0.78, 0.61, 0.57, -0.02, 0.30, and 0.33, respectively. Sr,Mnand Feshowed a positive higher correlations with TDS concentrations revealing that Sr interacts with mineral surfaces through relatively weak outer-sphere coordination (McBride, 1997), while Mn and Fe adsorption could beoccur through outer- and inner-sphere coordination (Goldberg, 2005; Peak et al., 2003).This might explain the lower correlation between them and TDS as compared to that of Sr. On the other hand, concentrations of Al, Ba, Cr were not/ only slightly, affected by TDS as those elements seem to interact with mineral surfaces through inner-sphere coordination.
The positive correlation between TDS and Trace elements indicates the continuous dissolution of aquifer matrices minerals and amorphous phases; hence, this mineral dissolution releases trace elements to the aqueous phase accompanied by increasing the TDS concentration of groundwater. Visual MINTAQ geochemical modeling program was used in this work for calculating the distribution of groundwater aqueous species and the saturation degree of groundwater relative aquifer matrices for each sample to indicate their effect on the distribution of trace element in groundwater. Table 6 confirms the assumption of continuous dissolution of minerals (under-saturation) within the aquifer matrices, Gibbsite and Barite that was supersaturated.
Trace elements in solution mainly exist as complexes with halides, sulfate, phosphate, hydroxides, carbonates, DOC, or as free ions. Chemical speciation shows the distribution of a chemical element between different molecular ionic forms in water (Apostoli, 2006; Zhao et al., 2021).
By modeling, the chemical species of these elements will demarcate the behavior of these elements in the surrounding environment. This would give more insights about their mobility and their bioavailability to living organism, which might help in determining their potential toxicity.
Metal speciation studies for the samples were characterized, Table 7, to determine the un-complexed form of Ba, Mn, Zn, and Sr existed as free radical ion in the hazardous samples and were ranged from 85.74 to 96.5%, 55.41% to 80.7%, 75.59% to 91.5%, and 79.88 to 91.5%, respectively. It suggests that those elements would be available for further sorption process and can be mitigated through sorption processes. The mobility of those elements will not increase in our case as results of absence of acidic conditions and low dissolved organic matter content. A small extent of these elements existed as inorganic ligands. Nevertheless, the uncharged species of Fe and Al are expected to be more mobile in groundwater because they would not be attracted to the surface charge of minerals. Inorganic ligands (HCO3−, SO42−, Cl−, and NO3−) act as nucleation centers that were attached and linked with those trace elements creating new species with higher solubility degree in water that are transported away randomly for long distances in the water path. Predominant ionic species of Fe and Al in groundwater samples were exist as metal hydroxides and they might show toxic effects in their inorganic forms.
A monitoring program is recommended for groundwater quality monitoring to ensure and inhibit water contamination, as mitigation action step toward imminent trace metal poisoning.
Reduction/oxidation processes and their influence on mitigation actions
Redox conditions play an important role in several geochemical processes that affect groundwater quality such as ion exchange, degradation, sorption, complexation, and mineral dissolution/precipitation (Böhlke, 2002; McMahon et al., 2019; Welch et al., 2000). Those processes have a direct effect on influencing the toxicity, mobility, and transport of anthropogenic contaminants (e.g., arsenic, manganese, and nitrate).
Although Iron is one of most survival vital elements for growth of all living organisms, if it exceeds permissible limits, it greatly affects the benthic invertebrates and fish diversity in canal causing hindering the respiration of fishes (Vuori, 1995; EPA, 2016). Also, aquatic plants as rice will be affected by iron toxicity as results of irrigation with this water leading to leaves acropetal translocation, also bronzing of rice leaves and yield loss (Becker & Asch, 2005; Phippen et al., 2008).
Unlike Iron, Aluminum has no biological role and a toxic nonessential metal to microorganisms. It inhibits the functions of some enzymes since it has a greater affinity to DNA and RNA. It affects the living organism metabolic pathways that involve iron metabolism, phosphorous, fluorine, and calcium, (Olaniran et al., 2013).
Recommendation toward mitigation actions
Groundwater is vital and critical source for all kind of life species (humans, animals, and plants) especially in arid and semiarid regions of developing countries. The results of this study indicated that the majority of water samples in the study region posing some adverse health consequences for humans in case of using it in drinking or domestic uses. Therefore, some mitigation action should be recommended as follows:
-
Minimizing or avoiding the direct spilling of industrial, agricultural, and domestic wastes in water ways as canals or drains, hence there is a direct infiltration to the groundwater system. This can be done proper treatment of hazardous waste and recycling it.
-
Adapting new practices in agriculture as organic farming and integrated pest management for decreasing the use of fertilizers in the study area.
-
Raising the awareness of the residents for protecting the water sources should be carried out through governments and non-governmental organizations.
-
A monitoring program is recommended to ensure and inhibit water contamination, as mitigation action step toward imminent trace metal poisoning and as a step toward the improving of the groundwater quality.
Conclusion
Groundwater is vital resource as being a life-sustaining factor influences the health of many ecosystems. This study focused on the relationship between sewage treatment plant and groundwater system surrounding it using multidisciplinary approach that combines the characterization of groundwater system and its connection with surrounding canal and drains using chemical and isotopic characterization. In addition, the multivariate statistical analysis has been utilized as a trial to correlate and relate the variables within the whole samples domain by choosing the most threatened variable (physicochemical, chemical, microbial parameters) to elucidate the causes of groundwater pollution in the study area. The potentially toxic trace elements exposure for adults as general group through the direct ingestion and dermal contact exposure pathways were estimated. Visual MINTAQ geochemical modeling program was used for determining the groundwater aqueous species distribution to indicate their effect on the distribution of trace element in groundwater, revealing that Ba, Mn, Zn, and Sr existed as free radical ion in the hazardous samples; illustrating that those elements would be matigated through sorption processes.
The results indicated that the Nile system is the main source of recharge in the groundwater system. Water quality index (WQI) was calculated to assess the drinking water quality in the study area reveling that 58% of the samples are unsuitable for drinking. The contamination consequences on human health were determined through and chronic non-carcinogenic health risk for adults via exposure of both oral and dermal routes. The positive correlation between TDS and Trace elements indicate the continuous dissolution of aquifer matrices minerals and amorphous phases, which was confirmed from Visual MINTAQ geochemical modeling program; continuous dissolution of minerals within the aquifer matrices, except Gibbsite and Barite that was supersaturated. The metal speciation studies of Ba, Mn, Zn, and Sr for the samples existed as free radical ion, and small extent of these elements existed as inorganic ligands in the hazardous samples suggests that those elements would be available for further sorption process and can be mitigated through sorption processes. The mobility of those elements will not increase in our case as results of absence of acidic conditions and low dissolved organic matter content.
Data availability
All data and materials as well as software application or custom code support our published claims and comply with field standards.
References
Aguirre, B. P., Masachessi, G., Ferreyra, L. J., Biganzoli, P., Grumelli, Y., Panero, M. D., et al. (2019). Searching variables to assess recreational water quality: The presence of infectious human enterovirus and its correlation with the main variables of water pollution by multivariate statistical approach in Córdoba. Argentina Environmental Science Pollution Research, 26(7), 6586–6601. https://doi.org/10.1007/s11356-019-04124-2
Ali, M. M., Ali, M. L., Islam, M. S., & Rahman, M. Z. (2016). Preliminary assessment of heavy metals in water and sediment of karnaphuli river, Bangladesh. Environmental Nanotechnology, Monitoring Management, 5, 27–35. https://doi.org/10.1016/j.enmm.2016.01.002
Apostoli, P. (2006). Elemental speciation in human health risk assessment. World Health Organization. 23
Becker, M., & Asch, F. (2005). Iron toxicity in rice—conditions and management concepts. Journal Plant Nutrition Soil Science, 168(4), 558–573. https://doi.org/10.1002/jpln.200520504
Böhlke, J.-K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, 10(1), 153–179. https://doi.org/10.1007/s10040-001-0183-3
Çelebi, A., Şengörür, B., & Kløve, B. (2014). Human health risk assessment of dissolved metals in groundwater and surface waters in the melen watershed, Turkey. Journal Environmental Science Health, Part A, 49(2), 153–161. https://doi.org/10.1080/10934529.2013.838842
CONOCO, (1987). Geological map of Egypt 1: 500,000. In: Klitzsch, E., List, F.K., Pohlmann, G. (Eds.), Map Sheets NH36NW and NH35NE.
Elawadi, E., El-Qady, G., Nigm, A., Shaaban, F., & Ushijima, K. (2006). Integrated Geophysical Survey for Site Investigation at a New Dwelling Area. Egypt. Journal of Environmental and Engineering Geophysics, 11(4), 249–259. https://doi.org/10.2113/JEEG11.4.249
US EPA. (2016). Drinking Water Standards and Health Advisories. Available online: https://www.epa.gov/dwstandardsregulations/drinking-water-standards-and-health-advisory-tables (accessed on 30 April 2016).
Farag, I. A. M., & Ismail, M. M. (1955). On structure of Wadi Hof area north-east of Helwan. Bull. Inst. Desert Egypt, 1(5), 179–192.
Farid, M.S., (1985). Management of Groundwater System in the Nile Delta. (Ph.D. Thesis, Cairo Univ. Egypt. 286 p).
Goldberg, S. (2005). Inconsistency in the triple layer model description of ionic strength dependent boron adsorption. Journal of Colloid and Interface Science, 285(2), 509–517. https://doi.org/10.1016/j.jcis.2004.12.002
Hagagg, K., Hosny, M., Hussien, R., & Rahman, M. A. (2020). Evaluation of environmental pollution and microbial treatment of shallow groundwater in El Omayed area Egypt. Integrated Environmental Assessment Management, 16(4), 461–471. https://doi.org/10.1002/ieam.4259
Ji, Y., Wu, J., Wang, Y., Elumalai, V., & Subramani, T. (2020). Seasonal variation of drinking water quality and human health risk assessment in hancheng city of Guanzhong plain. China. Exposure and Health, 12(3), 469–485. https://doi.org/10.1007/s12403-020-00357-6
Khalil, J. B., Atta, S. A., & Diab, M. S. H. (1988). Hydrogeological studies on the groundwater aquifer of the eastern part of the Nile Delta, Egypt. Water Science 4th issue. 79–90.
Li, P., Tian, R., Xue, C., & Wu, J. (2017). Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environmental Science Pollution Research, 24(15), 13224–13234. https://doi.org/10.1007/s11356-017-8753-7
Lupi, L., Bertrand, L., Monferrán, M. V., Amé, M. V., del Diaz, M., & P. (2019). Multilevel and structural equation modeling approach to identify spatiotemporal patterns and source characterization of metals and metalloids in surface water and sediment of the ctalamochita river in Pampa region, Argentina. Journal Hydrology, 572, 403–413. https://doi.org/10.1016/j.jhydrol.2019.03.019
Mansour, N. M. (2020). Hydrochemical studies and evaluation of groundwater quality of the quaternary aquifer at faquss, Al sharqiya governorate. Egypt. Sustainable Water Resources Management, 6(2), 19. https://doi.org/10.1007/s40899-020-00374-y
McBride, M. B. (1997). A critique of diffuse double layer models applied to colloid and surface chemistry. Clays Clay Minerals, 45(4), 598–608. https://doi.org/10.1346/CCMN.1997.0450412
McMahon, P. B., Belitz, K., Reddy, J. E., & Johnson, T. D. (2019). Elevated manganese concentrations in united states groundwater, role of land surface–soil–aquifer connections. Environmental Science Technology, 53(1), 29–38. https://doi.org/10.1021/acs.est.8b04055
Mirzaei, M., Jafari, A., Gholamalifard, M., Azadi, H., Shooshtari, S. J., Moghaddam, S. M., et al. (2020). Mitigating environmental risks: Modeling the interaction of water quality parameters and land use cover. Land Use Policy, 95, 103766. https://doi.org/10.1016/j.landusepol.2018.12.014
Nofal, E. R., Amer, M. A., El-Didy, S. M., & Fekry, A. M. (2015). Delineation and modeling of seawater intrusion into the nile delta aquifer: A new perspective. Water Science, 29(2), 156–166. https://doi.org/10.1016/j.wsj.2015.11.003
Olaniran, A., Balgobind, A., & Pillay, B. (2013). Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. International Journal Molecular Sciences, 14(5), 10197–10228. https://doi.org/10.3390/ijms140510197
Peak, D., Luther, G. W., & Sparks, D. L. (2003). ATR-FTIR spectroscopic studies of boric acid adsorption on hydrous ferric oxide. Geochimica Et Cosmochimica Acta, 67(14), 2551–2560. https://doi.org/10.1016/S0016-7037(03)00096-6
Phippen, B., Horvath, C., Nordin, R., & Nagpal, N. (2008). Ambient water quality guidelines for iron: Overview. ministry of environment province of britishcolumbia. British Columbia. P. 2–10.
RIGW, (1989). Hydrological Map of Egypt-Nile Delta. 1: 500 000 -Research Institute Groundwater. El Kanater, Cairo. TN 70.120–88–03.
Rocchini, R., & Swain, L. G. (1995). The british columbia water quality index. water quality branch, EP Department, BC, Ministry of Environment, Land and Park, Victoria, BC, Canada, 13.
Sherif, M., Sefelnasr, A., & Javadi, A. (2012). Incorporating the concept of equivalent freshwater head in successive horizontal simulations of seawater intrusion in the nile delta aquifer. Egypt. Journal of Hydrology, 464–465, 186–198. https://doi.org/10.1016/j.jhydrol.2012.07.007
Singh, B. K., Singh, A. K., & Singh, V. K. (2018). Exposure assessment of traffic-related air pollution on human health - a case study of a metropolitan city. Environmental Engineering Management Journal, 17(2), 335–342.
Sultan, S. A., Mansour, S. A., & Santos, F. A. M. (2008). A hydrogeophysical investigation of the ain mousa area, near Cairo. Egypt. Bulletin Engineering Geology Environment, 67(1), 111–117. https://doi.org/10.1007/s10064-007-0107-0
Taha, A. A., Serage, H. M., El Din, A., & El Haddad, I. M. (1997). Hyrogeological situation of the area between Ismailia canal and Cairo-ismailia desert road, faculty of science, Mansoura University. Journal Environment Science 14.
Tirkey, P., Bhattacharya, T., Chakraborty, S., & Baraik, S. (2017). Assessment of groundwater quality and associated health risks: A case STUDY of Ranchi city, jharkhand, India. Groundwater Sustainable Development, 5, 85–100. https://doi.org/10.1016/j.gsd.2017.05.002
Vuori, K.-M. (1995). Direct and indirect effects of iron on river ecosystems. Annales Zoologici Fennici, 32(3), 317–329.
Welch, A. H., Westjohn, D. B., Helsel, D. R., & Wanty, R. B. (2000). Arsenic in ground water of the United States: Occurrence and geochemistry. Ground Water, 38(4), 589–604. https://doi.org/10.1111/j.1745-6584.2000.tb00251.x
World Health Organization. (2017). Guidelines for Drinking‑water Quality Fourth edition incorporating the first addendum. WHO Library Cataloguing-in-Publication Data Guidelines. https://doi.org/10.5005/jp/books/11431_8
Yehia, M. M. (2000). Environmental impacts of sewage irrigation water on groundwater quality of northeast Cairo Egypt. Engineering Research Journal, 72, 176–193.
Zhang, Q., Xu, P., & Qian, H. (2019). Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the central-western guanzhong basin, China. International Journal of Environmental Research Public Health, 16(21), 4246. https://doi.org/10.3390/ijerph16214246
Zhao, H., Song, F., Su, F., Shen, Y., & Li, P. (2021). Removal of cadmium from contaminated groundwater using a novel silicon/aluminum nanomaterial: An experimental study. Archives Environmental Contamination Toxicology, 80(1), 234–247. https://doi.org/10.1007/s00244-020-00784-1
Acknowledgements
The authors acknowledge the Egyptian Academy of Scientific Research and Technology (ASRT) for their financial support and, also, acknowledge the support provided by Egyptian Atomic Energy Authority (Nuclear and Radiation Safety Research Center) as well as Egyptian Nuclear and Radiological Regulatory Authority (ENNRA).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was financially supported by the Egyptian Academy of Scientific Research and Technology (ASRT) Scientists for Next Generation Program (SNG) Scholarship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Amany Ahmed, all authors write the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among authors.
Ethical Approval
Not applicable.
Consent to participate
All authors consent to participate in this manuscript.
Consent to publish
All authors consent to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kandil, A.T., Haggag, K., Gamal, A.A. et al. Adverse health and environmental outcomes of sewage treatment plant on surrounding groundwater with emphasis on some mitigation recommendations. Environ Geochem Health 45, 3345–3359 (2023). https://doi.org/10.1007/s10653-022-01413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01413-7