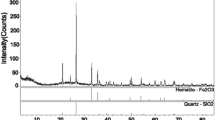
Abstract
Cadmium (Cd) is a harmful element to human health and biodiversity. The removal of Cd from groundwater is of great significance to maintain the environmental sustainability and biodiversity. In this work, a novel low-temperature roasting associated with alkali was applied to synthesize an eco-friendly adsorbent using coal fly ash. Scanning electron microscopy, Fourier transform infrared spectroscopy, X-ray fluorescence, and X-ray photoelectron spectroscopy were applied to analyze the physical and chemical characteristics of the adsorbent. The experiments show that a significant improvement in specific surface area and activity of adsorbent was observed in this study. The functional groups of Na–O and Fe–O were verified to be beneficial in the removal of Cd2+. The material capacity to adsorb Cd2+ was considerably improved, and the maximum uptake capacity was 61.8 mg g−1 for Cd2+ at 25 °C. Furthermore, pH and ionic strength play critical roles in the adsorption process. The Langmuir and pseudo-second-order models can appropriately describe the adsorption behavior, and the enhanced adsorption ability of Cd2+ by modified coal fly ash was attributed to ion-exchange, co-precipitation, and complexation. Higher sorption efficiency was maintained after two regeneration cycles. These results offer valuable insights to develop high-performance adsorbent for Cd2+ removal.






Similar content being viewed by others
References
Astuti W, Martiani W, Any IKN (2017) Competitive adsorption of Pb2+ and Zn2+ ions from aqueous solutions by modified coal fly ash. AIP Conf Proc 1818:020007
An CJ, Yang SQ, Huang GH, Zhao S, Yao Y (2016) Removal of sulfonated humic acid from aqueous phase by modified coal fly ash waste: equilibrium and kinetic adsorption studies. Fuel 165:264–271
Agarwal S, Rani A (2016) Adsorption of resorcinol from aqueous solution onto CTAB/NaOH/fly ash composites: equilibrium, kinetics and thermodynamics. J Environ Chem Eng 5(1):526–538
Abdelwahab O, Fouad YO, Amin NK, Mandor H (2015) Kinetic and thermodynamic aspects of cadmium adsorption onto raw and activated guava (Psidium guajava) leaves. Environ Prog Sustain Energy 34(2):351–358
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) Adsorption of cadmium from aqueous solution onto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J Hazard Mater 184(1–3):126–134
Bukhari SS, Benin J, Kazemian H, Rohani S (2015) Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: a review. Fuel 140(15):250–266
Borhade AV, Dholi AG, Wakchaure SG, Tope DR (2012) Chemical modification of coal fly ash into iodate sodalite and its use for the removal of Cd2+, Pb2+, and Zn2+ from their aqueous solutions. Desalin Water Treat 50(1–3):157–169
Chen G, Shah KJ, Shi L, Chiang PC (2017) Removal of Cd(II) and Pb(II) ions from aqueous solutions by synthetic mineral adsorbent: performance and mechanisms. Appl Surf Sci 409:296–305
Demangeat E, Pédrot M, Dia A, Bouhnik-Le-Coz M, Grasset F, Hanna K, Kamagate M, Cabello-Hurtado F (2018) Colloidal and chemical stabilities of iron oxide nanoparticles in aqueous solutions: the interplay of structural, chemical and environmental drivers. Environ Sci Nano 5(4):992–1001
Deng X, Qi L, Zhang Y (2018) Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash. Water Air Soil Pollut 229(18):1–6
Deng YX, Zhang T, Sharma B, Nie HY (2019) Optimization and mechanism studies on cell disruption and phosphorus recovery from microalgae with magnesium modified hydrochar in assisted hydrothermal system. Sci Total Environ 646:1140–1154
Elshkaki A, Shen L (2019) Energy-material nexus: the impacts of national and international energy scenarios on critical metals use in China up to 2050 and their global implications. Energy 180:903–917
Elumalai V, Nethononda VG, Manivannan V, Rajmohan N, Li PY, Elango L (2020) Groundwater quality assessment and application of multivariate statistical analysis in Luvuvhu catchment, Limpopo. South Africa J Afr Earth Sci 171:103967
Elumalai V, Brindha K, Elango L (2017) Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: a case study from South Africa. Water 9(4):234
Feng Y, Liu P, Wang YX, Finfrock YZ, Xie XJ, Su CL, Liu N, Yang YY, Xu Y (2020) Distribution and speciation of iron in Fe-modified biochars and its application in removal of As(V), As(III), Cr(VI), and Hg(II): An X-ray absorption study. J Hazard Mater 384:121342
Forouzan A, Zadeh ZHRL, Omid S, Mohammad K, Ezzatollah N (2019) Novel Cd(II) ion imprinted polymer coated on multiwall carbon nanotubes as a highly selective sorbent for cadmium determination in food samples. J AOAC Int 97(1):174–178
Fongsatitkul P, Elefsiniotis P, Khuhasawan N, Jindal R (2009) Use of power plant ash to remove and solidify heavy metals from a metal-finishing wastewater. Water Air Soil Pollut 203(1–4):147–154
Guo JY, Yang CP, Zeng GM (2013) Treatment of swine wastewater using chemically modified zeolite and bioflocculant from activated sludge. Bioresour Technol 143:289–297
Han Y, Hu Y, Li A, Cheng R (2016) pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem Eng J 284:1397–1405
He X, Li P (2020) Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): occurrence, sources and health risks. Expo Health 12(3):385–401. https://doi.org/10.1007/s12403-020-00344-x
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He K, Chen Y, Tang Z, Hu Y (2015) Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ Sci Pollut Res 23(2):2778–2788
He SR, Li YT, Weng LP, Wang JJ, He JX, Liu YL, Zhang K, Wu QH, Zhang YL, Zhang Z (2018) Competitive adsorption of Cd2+, Pb2+ and Ni2+ onto Fe3+-modified argillaceous limestone: Influence of pH, ionic strength and natural organic matters. Sci Total Environ 637–638:69–78
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
He YH, Lin H, Luo MK, Liu JF, Dong YB, Li B (2020) Highly efficient remediation of groundwater co-contaminated with Cr(VI) and nitrate by using nano-Fe/Pd bimetal-loaded zeolite: process product and interaction mechanism. Environ Pollut 263:114479
Hasri M (2018) Adsorption of Cd(II) metal ion on adsorbent beads from biomass saccharomycess cereviceae-chitosan. J Phys Conf Series 954(1):012020
Hirokawa K, Danzaki Y (2010) Analytical application of XPS for surface characterization of coal fly-ash and coal. Surf Interface Anal 6(4):193–195
Holler H, Wirsching U (1985) Zeolite formation from fly ash. Fortschr Miner 63:21–43
Hu XJ, Wang JS, Liu YG, Li X, Zeng GM, Bao ZL, Zeng XX, Chen AW, Long F (2011) Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J Hazard Mater 185(1):306–314
Huang XR, Zhao HH, Hu XF, Liu FH, Wang L, Zhao X, Gao PC, Ji PH (2020) Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+. J Hazard Mater 392:122461
Huang XR, Zhao HH, Zhang GB, Li JT, Yang Y, Ji PH (2020) Potential of removing Cd(II) and Pb(II) from contaminated water using a newly modified fly ash. Chemosphere 242:125148
Iwona W, Pacewska B (2019) Comparative investigation of reactivity of different kinds of fly ash in alkaline media. J Therm Anal Calorim 138:3857–3872
Ji Y, Wu J, Wang Y, Elumalai V, Subramani T (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain. China Expo Health 12(3):469–485. https://doi.org/10.1007/s12403-020-00357-6
Jia TT, Zhang BL, Huang LH, Wang SG, Xu CH (2019) Enhanced sequestration of Cr(VI) by copper doped sulfidated zerovalent iron (SZVI-Cu): characterization, performance, and mechanisms. Chem Eng J 366:200–207
Javadian H, Ghorbani F, Tayebi HA, Asl SMH (2015) Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab J Chem 247(8):837–849
Kong JJ, Gu R, Yuan JN, Liu WQ, Yue QY (2018) Adsorption behavior of Ni(II) onto activated carbons from hide waste and high-pressure steaming hide waste. Ecotox Environ Safe 156:294–300
Li P (2020) To make the water safer. Expo Health 12(3):337–342. https://doi.org/10.1007/s12403-020-00370-9
Li P, Wu J (2019a) Sustainable living with risks: meeting the challenges. Hum Ecol Risk Assess 25(1–2):1–10. https://doi.org/10.1080/10807039.2019.1584030
Li P, Wu J (2019b) Drinking water quality and public health. Expo Health 11(2):73–79. https://doi.org/10.1007/s12403-019-00299-8
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert. Northwest China Expo Health 8(3):331–348. https://doi.org/10.1007/s12403-016-0193-y
Li JH, Zheng LR, Wang SL, Wu ZP, Wu WD, Niazi NK, Shaheen SM, Rinklebe J, Bolan N, Ok YS, Wang HL (2019) Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: spectroscopic investigation. Sci Total Environ 672:572–582
Li HQ, Huang GH, An C, Hu JT, Yang SQ (2013) Removal of tannin from aqueous solution by adsorption onto treated coal fly ash: kinetic, equilibrium, and thermodynamic studies. Ind Eng Chem Res 52(45):15923–15931
Liang YZ, Tian L, Lu Y, Peng LF, Shi ZQ (2018) Kinetics of Cd(II) adsorption and desorption on ferrihydrite: experiments and modeling. Environ Sci Process Impacts 20(6):934–942
Liu HJ, Yang F, Zheng YM, Kang J, Qu JH, Chen JP (2011) Improvement of metal adsorption onto chitosan/sargassum sp. composite sorbent by an innovative ion-imprint technology. Water Res 45(1):145–154
Mthembu PP, Elumalai V, Brindha K, Li PY (2020) Hydrogeochemical processes and trace metal contamination in groundwater: impact on human health in the Maputaland coastal aquifer. South Afr Exp Health 12(3):403–426
Ma J, Qin GT, Zhang YP, Sun JM, Jiang L (2018) Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J Clean Prod 182:776–782
Nascimento M, Paulo SMS, Souza VPD (2009) Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 88(9):1714–1719
Ou JH, Sheu YT, Tsang DCW, Sun YJ, Kao CM (2020) Application of iron/aluminum bimetallic nanoparticle system for chromium-contaminated groundwater remediation. Chemosphere 256:127158
Ohki A, Nakajima T, Sakaguchi Y, Iwashita A, Takanashi H (2005) Analysis of arsenic and some other elements in coal fly ash by x-ray photoelectron spectroscopy. J Hazard Mater 119(1–3):213–217
Qiu R, Cheng F, Huang H (2018) Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J Clean Prod 172(2):1918–1927
Rajamma R, Ball RJ, Tarelho LAC, Allen GC, Labrincha JA, Ferreira VM (2009) Characterisation and use of biomass fly ash in cement-based materials. J Hazard Mater 172(2–3):1049–1060
Seyed MHA, Ghadi A, Sharifzadeh BM, Javadian H, Maghsudi M, Kazemian H (2018) Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: a review. Fuel 217:320–342
Saber A, Tafazzoli M, Mortazavian S, James DE (2018) Investigation of kinetics and absorption isotherm models for hydroponic phytoremediation of waters contaminated with sulfate. J Environ Manage 207(1):276–291
Shaheen SM, Rinklebe J (2014) Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326
Shah B, Mistry C, Shah A (2013) Seizure modeling of Pb(II) and Cd(II) from aqueous solution by chemically modified sugarcane bagasse fly ash: isotherms, kinetics, and column study. Environ Sci Pollut Res 20(4):2193–2209
Sulaymon AH, Ebrahim SE, Mohammed-Ridha MJ (2013) Equilibrium, kinetic, and thermodynamic biosorption of Pb(II), Cr(III), and Cd(II) ions by dead anaerobic biomass from synthetic wastewater. Environ Sci Pollut Res 20(1):175–187
Shukla S, Seal S, Schwarz S, Zhou D (2001) Synthesis and characterization of nanocrystalline silver coating of fly ash cenosphere particles by electroless process. J Nanosci Nanotechnol 1(4):417–424
Visa M, Chelaru AM (2014) Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl Surf Sci 303(1):14–22
Wu YH, Pang HW, Liu Y, Wang XX, Yu SJ, Fu D, Chen JR, Wang XK (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut 246:608–620
Wang BD, Zhou YX, Li L, Xu H, Sun YL, Wang Y (2018) Novel synthesis of cyano-functionalized mesoporous silica nanospheres (MSN) from coal fly ash for removal of toxic metals from wastewater. J Hazard Mater 345:76–86
Wang J, Yu HY, Lin JF (2015) Treatment of Cr (VI) wastewater with modified coal fly ash. Appl Mech Mater 768(6):561–566
Wilińska I, Pacewska B (2019) Comparative investigation of reactivity of different kinds of fly ash in alkaline media. J Therm Anal Calorim 138(6):3857–3872
Wu FC, Tseng RL, Juang RS (2009) Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem Eng J 153(1–3):1–8
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78(15):446. https://doi.org/10.1007/s12665-019-8471-1
Wang S, Soudi M, Li L, Zhu ZH (2006) Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater. J Hazard Mater 133(1–3):243–251
Xiao J, Hu R, Chen GC (2020) Micro-nano-engineered nitrogenous bone biochar developed with a ballmilling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J Hazard Mater 387:121980
Yao ZT, Ji XS, Sarker PK, Tang JH, Ge LQ, Xia MS, Xi YQ (2015) A comprehensive review on the applications of coal fly ash. Earth-Sci Rev 141:105–121
Yang JL, Tang CL, Wang FW, Wu YH (2015) Co-contamination of Cu and Cd in paddy fields: using periphyton to entrap heavy metals. J Hazard Mater 304:150–158
Yao ZT, Xia MS, Sarker PK, Chen T (2014) A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 120:74–85
Zhao HL, Liu F, Liu HQ, Wang L, Zhang R, Hao Y (2020) Comparative life cycle assessment of two ceramsite production technologies for reusing municipal solid waste incinerator fly ash in China. Waste Manage 113:447–455
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Zhang P, O’Connor D, Wang YN, Jiang L, Xia TX, Wang LW, Tsang DCW, Ok YS, Hou DY (2019) A green biochar/iron oxide composite for methylene blue removal. J Hazard Mater 384:121286
Zhou H, Bhattarai R, Li Y, Li S, Fan Y (2019) Utilization of coal fly and bottom ash pellet for phosphorus adsorption: Sustainable management and evaluation. Resour Conserv Recycl 149:372–380
Zhao Q, Zhu X, Chen B (2017) Stable graphene oxide/poly (ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem Eng J 334:1119–1127
Zheng L, Wang W, Gao X (2016) Solidification and immobilization of mswi fly ash through aluminate geopolymerization: based on partial charge model analysis. Waste Manage 58:270–279
Zhou L, Chen YL, Zhang XH, Tian FM, Zu ZN (2014) Zeolites developed from mixed alkali modified coal fly ash for adsorption of volatile organic compounds. Mater Lett 119(15):140–142
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41761144059 and 42072286), the Fundamental Research Funds for the Central Universities of CHD (300102299301), the Fok Ying Tong Education Foundation (161098) and the Ten Thousand Talents Program (W03070125). The authors are grateful to the editor and anonymous reviewers for their useful comments, which helped to improve the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Song, F., Su, F. et al. Removal of Cadmium from Contaminated Groundwater Using a Novel Silicon/Aluminum Nanomaterial: An Experimental Study. Arch Environ Contam Toxicol 80, 234–247 (2021). https://doi.org/10.1007/s00244-020-00784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00784-1