Abstract
Multiple studies have reported synergized toxicity of PAH mixtures in developing fish larvae relative to the additive effect of the components. From a toxicological perspective, multiple mechanisms are known to contribute to synergism, such as altered toxicodynamics and kinetics, as well as increased oxidative stress. An understudied contributor to synergism is the accumulation of endogenous metabolites, for example: the aryl hydrocarbon receptor 2 (AhR2) agonist and tryptophan metabolite 6-Formylindolo(3,2-b)carbazole (FICZ). Fish larvae exposed to FICZ, alongside knock-down of cytochrome p450 (cyp1a), has been reported to induced symptoms of toxicity similar to those observed following exposure to PAHs or the dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin. Here, we explored if FICZ accumulates in newly hatched rainbow trout alevins (Oncorhynchus mykiss) exposed to two PAHs with different properties: retene (potent AhR2 agonist) and fluoranthene (weak AhR2 agonist and Cyp1a inhibitor), either alone or as a binary mixture for 3 and 7 days. We found that exposure to the mixture resulted in accumulation of endogenous FICZ, synergized the blue sac disease index (BSD), and altered the body burden profiles of the PAHs, when compared to the alevins exposed to the individual components. It is thus very plausible that accumulation of endogenously derived FICZ contributed to the synergized BSD index and toxicity in exposed alevins. Accumulation of endogenously derived FICZ is a novel finding that extends our general understanding on PAHs toxicity in developing fish larvae, while at the same time highlighting why environmental risk assessment of PAHs should not be based solely results from the assessment of individual compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a diverse and widespread group of environmental pollutants of either natural or anthropogenic origin (Wickström and Tolonen 1987; dos Santos et al. 2018) and are always present as complex mixtures (Tissot and Welte 1978). From an aquatic toxicological perspective, the exact mechanisms of toxicity are not fully understood, even after decades of research employing multiple species of fish and types of PAHs (Heintz et al. 2000; Brinkworth et al. 2003; Wassenberg and Di Giulio 2004; Van Tiem and Di Giulio 2011; Clark et al. 2013; Rigaud et al. 2020a). What is known is that different PAHs induce toxicity through different mechanisms and cause exposure and PAH specific toxicity profiles (Billiard et al. 2008; Incardona et al. 2011; Geier et al. 2018; Rigaud et al. 2020a, b; Eriksson et al. 2022a, b). Additionally, heart structure and function are especially sensitive to the influence of PAH(s) during early life development of fish (Incardona et al. 2011, 2009; Vehniäinen et al. 2016). The specificity of cardiotoxicity is hypothesized to be linked to interaction(s) between PAHs, as a part of crude oil, and lipoproteins bound to the cellular membrane (Incardona 2017). Furthermore, mixture composition has been observed and reported to modulate PAH toxicity outcome, relative to the components (Geier et al. 2018; Eriksson et al. 2022a, b). The best-known molecular mechanism related to PAH toxicity is that involving activation of the aryl-hydrocarbon receptor 2 (AhR2) (Massarsky et al. 2016; Doering et al. 2019). Activation of AhR2, by PAHs such as retene (Scott et al. 2011), benzo[a]pyrene (Incardona et al. 2011; Song et al. 2019), benzo[k]fluoranthene (Van Tiem and Di Giulio 2011), and pyrene (Incardona et al. 2011) induces the expression of, among many genes, cytochrome P450 (CYP1), particularly cyp1a, which encodes for an enzyme that initiates phase I metabolism of xenobiotics through hydroxylation (Billiard et al. 2008). By contrast, some PAHs, such as fluoranthene, can both induce the expression of cyp1a, and inhibit the function of the translated and functional enzyme by blocking its active site (Willett et al. 1998), which has previously been shown to synergize the toxicity of other PAHs (Wills et al. 2009; Van Tiem and Di Giulio 2011).
A commonly assessed biomarker of PAH toxicity in developing fish is the so called blue sac disease syndrome (BSD), which encompasses several symptoms of PAH induced toxicity: craniofacial deformities, hemorrhaging, yolk and pericardial edema and spinal curvatures (Billiard et al. 1999, 2006; Colavecchia et al. 2006). Although it is not fully known how BSD is induced (Scott et al. 2011; Clark et al. 2013), knockdown of ahr2 (but not cyp1a) is known to prevent the formation of BSD-related symptoms in developing zebrafish larvae (Danio rerio), which implies that downstream molecular events of AhR2, other than activation of Cyp1, are required for the manifestation of PAH toxicity (Van Tiem and Di Giulio 2011; Massarsky et al. 2016). Recently, the activation of cyclooxygenase-2 (cox2), which is linked to AhR2 activation, by PAH(s) has been reported to be involved in the induction of developmental toxicity in fish (Doering et al. 2019).
Some endogenous compounds, such as the tryptophan derivative FICZ (6-Formylindolo[3,2-b]carbazole), are also known to be able to activate AhR2 (Wincent et al. 2009). FICZ is a very potent AhR agonist, and during normal conditions, it is maintained at low levels by constant Cyp1-mediated metabolism (Wincent et al. 2009). Increased rate of formation of FICZ has been observed following three distinct processes: increased enzymatic activity, increased UV-irradiation, and increased levels of oxidative stress (Smirnova et al. 2016; Rannug and Rannug 2018). Newly hatched zebrafish larvae exposed to externally administrated FICZ, in combination with a Cyp1a-inhibitor (alpha-naphthoflavone) or cyp1a-knockdown, resulted in reduced metabolism of FICZ and increased occurrence of BSD-related symptoms, and other signs of toxicity that resemble those caused by exposure to PAHs (Wincent et al. 2016). It has therefore been hypothesized that the formation and accumulation of endogenous FICZ could contribute to AhR-mediated developmental toxicity in fish larvae (Wincent et al. 2012; Rannug and Rannug 2018).
Therefore, we investigated if exposure to two PAHs with different modes of action, retene (an AhR2 agonist) and fluoranthene (a weaker AhR2 agonist and Cyp1a inhibitor) (Barron et al. 2004), alone or as a binary mixture, could force the accumulation of endogenously derived FICZ in newly hatched and developing rainbow trout alevins (Oncorhynchus mykiss). By assessing the temporal development of the PAH and FICZ specific body burdens, in relation to the BSD index in developing rainbow trout, we aimed at uncovering how potential accumulation of FICZ could contribute to developmental toxicity.
Materials and methods
Experimental setup
Newly hatched 360 degree-days and healthy rainbow trout alevins (free from developmental deformities, edemas, and hemorrhages; provided by Hanka-Taimen Oy fish farm, Central Finland) were either exposed to dimethyl sulfoxide as control (DMSO; 20 µl L−1; Sigma–Aldrich, St-Louis, MO, USA) CAS-number 67-68-5), retene (nominally 32 µg L−1; MP Biomedicals, Illkirch, France) CAS-number 483-65-3), fluoranthene (nominally 50 µg L−1; Sigma Aldrich; CAS-number 206-44-0) or the binary mixture of the two PAHs (at aforementioned nominal concentrations), and sampled after 1, 3 and 7 days of exposure. Investigated nominal PAH concentrations were selected as to provoke toxicity, but not increase mortality rates, as per previously published studies on retene toxicity (Hodson et al. 2007; Scott and Hodson, 2008; Vehniäinen et al. 2016) and an unpublished assessment of fluoranthene toxicity (rainbow trout alevin exposed to 500 µg L−1 for 11 days, did not increase mortality). Additionally, the concentration of DMSO (20 µl L−1) can be considered as acceptable (<100 µl L−1; OECD 2013), and should not contribute to solvent-mediated toxicity (Maes et al. 2012). Exposures were performed in 1.5 litre Pyrex glass bowls, filled with 1 litre of filtered lake water obtained from Konnevesi Research station in Central Finland. Each treatment was performed in triplicates, and each replicate contained 15 alevins. Exposure water temperature was maintained at 10.8 ± 0.3 °C, and a 16:8 light to dark ratio employed. Relative oxygen saturation, pH and conductivity were measured at 103.23 ± 3.32%, 7.38 ± 0.09 and 17.53 ± 3.95 mS m−1, respectively and throughout the exposure duration. At sampling, 5 alevins per replicate were video recorded and photographed while the remaining 10 alevins (per replicate) were photographed and snap-frozen for later HPLC-analysis. Symptoms of blue sac disease (BSD) were assessed based upon the video recordings (pericardial edema) combined with photographs (yolk sac edema and hemorrhages) of the 5 individually sampled alevins in silico.
Blue sac disease index
BSD index was calculated through established convention for each exposure replicate (n = 3; Eq. 1) (Colavecchia et al. 2006; Scott et al. 2011). In order to compare the BSD indices reported by Eriksson et al. (2022a) with those obtained in this present study, the same symptoms of toxicity were assessed and scored in the same fashion: hemorrhages (HE; scored 0 or 1; not present or present), pericardial (PE; 0 or 1) and yolk sac edemas (YE: 0 or 1). The total score per replicate was then divided by the total maximum potential score per replicate: 15 (maximum score per individual alevin was 3, and 5 alevins per replicate were assessed).
Establishment of body burden through HPLC analysis and confirmation of FICZ
Preparation of alevins and HPLC analysis were performed as instructed by Rigaud et al. (2020a) and Eriksson et al. (2022a). In short, the 10 pooled alevins (per replicate) were homogenized by zirconium pellets (circumference of 1 and 2 mm; Next Advance, USA) in 70% acetonitrile (ACN; Fisher Scientific) using a standard model bullet blender (Next Advance). The homogenate was then centrifuged (Centrifuge 5415 R, Eppendorf, Germany) for 10 min at 14,000 rpm and 4 °C. The supernatant was collected and the pellet re-suspended in 70% ACN, centrifuged, the supernatant collected and pooled. The re-suspension step was performed twice. Body burden of the PAHs and FICZ was established through HPLC with fluorescence detector (Shimadzu UHPLC Nexera-system). Detection parameters and limits of the HPLC measurements are presented in Table 1. Subsequent area under the curve (AUC) for each respective compound was manually adjusted, background compensated (control AUC), and the body burden calculated based upon standard curves.
The presence of FICZ was confirmed using LC-MS/MS (Agilent 1290 ultra-high-pressure liquid chromatography system coupled to an Agilent 6460 triple quadrupole mass spectrometer). The mobile phase employed in LC-MS/MS analysis was created from double-distilled water and acetonitrile (70%), both fortified with 1.5 mM formic acid (Fischer Scientific). The ratio between the components of the mobile phase started at 1:1 which from minute 1 to 9 increased to 1:20 before returning to 1:1 from minute 10.5 to 11; finally, the post-time column stabilization lasted for 2.5 min. Retention time for FICZ was 3.54 min (LC-MS/MS).
qPCR preparation and analysis
Measurement of whole-body cyp1a expression was performed according to instructions reported by Rigaud et al. (2020a), which in turn was modified from Sivula et al. (2018). In short: the 5 individual alevin carcasses were treated using TRI reagent (Molecular Research Centre) in order to extract RNA. The concentration, purity (NanoDrop 1000, Thermo Fisher Scientific) and integrity of the RNA were assessed using a TapeStation and confirmed (eukaryote total RNA 6000 Nano-kit; Agilent) in accordance with the manufacturer’s instructions. RNA was DNase treated (DNase I, Fermentas) and using iScript cDNA Synthesis Kit (Bio-Rad, USA), 500 ng of RNA was reverse transcribed into cDNA and diluted 1:10 in nuclease-free water. Five µL of diluted cDNA were then mixed with 1.5 µL of forward and reverse primers (final concentration 300 nM; Table 2), 4.5 µL sterile H2O and 12.5 µL of iQ SYBR Green Supermix (Bio-Rad). The 25 µL qPCR reaction mixture was analyzed utilizing the CFX96 Real-Time PCR cycler (Bio-Rad) according to established protocol: 3 min at 95 °C; 40 cycles (10 s at 95 °C, 10 s at 58 °C, 30 s at 72 °C, 10 s at 95 °C) and melting curve increased from 55 °C to 95 °C in increments of 0.5 °C. The expression of cyp1a was calculated using Bio-Rad CFX Manager software (v.3.1), employing ndufa and rl17 as references genes (Table 2).
Data analysis
Statistical analyses were performed using R v.4.0.3 (The R Foundation for Statistical Computing, 2020) coupled with R-studio v.1.3.1093 (RStudio Team 2020). Significant differences were assessed using Kruskal–Wallis test combined with Dunn’s post-hoc test (KW + Dunn) and adjusted for multiple comparisons using Bonferroni’s method (Benjamini and Hochberg 1995). Exposure specific changes in the body burden of mixture exposed alevins, compared to those exposed to the components, were assessed using Mann–Whitney’s U-test.
In order to assess the effect of the mixture, relative to the additive effect of the components on the obtained BSD indices, background adjusted combination indices were calculated (CI; Eq. 2) (Foucquier and Guedj 2015) and compared with the results reported by Eriksson et al. (2022a):
where the average, and normalized effect (against control), exerted by retene and fluoranthene alone are represented by R and F, while the normalized effect exerted by the binary mixture is represented by M. A stronger effect by the mixture, relative to the components, is assumed if the CI is <1.
Results and discussion
The occurrence of blue sac disease related symptoms was dependent on both the type of exposure and duration (Table 3). Exposure to fluoranthene produced a weaker index after 3 days of exposure compared to Day 7. Exposure to retene produced, on average, similar BSD indices on both Day 3 and 7. By contrast, exposure to the binary mixture produced the strongest BSD index, irrespective of exposure duration. Yet, significance was only observed after 3 days of exposure (fluoranthene relative to mixture), while near-to-significance was obtained between control and mixture exposed alevins on Day 7 (p = 0.0532). Few replicates per treatment (n = 3) are plausibly obscuring some the statistical outcome, as the BSD indices reported by Eriksson et al. (2022a), who utilized a greater number of replicates (n = 6), were accompanied by statistical differences. Nevertheless, exposure to the mixture resulted in a greater than predicted BSD index (Table 3; irrespective of exposure duration), as the combination index was 0.39 after 3 days of exposure and 0.86 by Day 7. Hence, the results presented in Eriksson et al. (2022a) can therefore be considered as comparable, highlighting BSD indices as a functional proxy of nominal exposure concentrations in a standardized exposure study; at least when considering the effect of this binary mixture and combination indices.
Even though the actual PAH concentrations in water were not measured, we know from previous exposure studies that the concentrations of PAH in newly made solutions are very similar to the nominal concentration (Honkanen et al. 2020). Moreover, and as presented in Table 3, the same nominal concentrations caused very similar BSD indices as in the present study, relative to our previous work (Eriksson et al. 2022a), whereby strengthening the comparability.
The body burden of retene fluctuated non-significantly with time, irrespective of treatment (Fig. 1a). Hence, no significant difference in the body burden of retene was observed in mixture exposed alevins relative to those exposed to retene alone. By comparison, exposure to fluoranthene alone resulted in an increasing body burden with time, and a significantly greater body burden was observed by Day 7 compared to Day 1 (Fig. 1b). When co-exposed with retene, the body burden of fluoranthene diminished significantly compared to alevins exposed to fluoranthene alone for 7 days. Similar temporal patterns of accumulation were observed among alevins exposed to the mixture of retene and fluoranthene, as reported in our previous studies (Eriksson et al. 2022a, b). Hence, the reduction in the body burden of fluoranthene, when co-exposed with retene, reflects a broader and more potent activation of phase I and II metabolic processes, either transcriptomic (Eriksson et al. 2022a) or proteomic (Eriksson et al. 2022b), that can offset the inhibitory effect of fluoranthene upon Cyp1a.
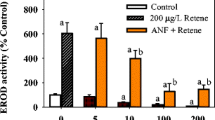
Boxplot representation of the exposure specific body burden profiles, per alevin, following exposure to retene (a; pmol), fluoranthene (b; pmol) and endogenously formed FICZ (c; fmol). Alevins were sampled after 1, 3 and 7 days of semi-static exposure to the PAHs alone (dark-grey-filled boxes) or as a binary mixture (white-filled boxes). Significant differences in body burden within each treatment, as per KW + Dunn, are denoted with upper- (fluoranthene) and lower-case letters (FICZ). Significant differences in body burden between larvae exposed to the mixture and the components are denoted with *. N per treatment = 3
Additionally, we were able to identify and quantify the accumulation of endogenously formed FICZ in alevins exposed to the mixture, but not in alevins exposed to fluoranthene or retene alone (Fig. 1c). However, it cannot be ruled out that exposure to retene and fluoranthene alone increased the rate of formation. Rather, it can only be stated that accumulation to detectable levels did not occur following exposure to the individual PAHs. Endogenously formed and accumulated FICZ can either be derived enzymatically from tryptamine or tryptophan, following UV-irradiation, or oxidation of tryptophan, as observed during increased oxidative stress (Smirnova et al. 2016; Rannug and Rannug 2018). UV-irradiation can be rejected as causative agent due to the architecture of the exposure facility (no windows), as the room was illuminated by yellow florescent light. That leaves enzymatic processes and oxidative stress as the most plausible causes; the latter being more likely due the known and established relationship between PAH toxicity and subsequently increased oxidative stress (Timme-Laragy et al. 2007; Song et al. 2019), altered iron metabolism (Rigaud et al. 2020b; Eriksson et al. 2022b) and activation of heat shock proteins (Räsänen et al. 2012) in PAH exposed fish larvae. The body burden of FICZ may also increase when subsequent metabolism by Cyp1a is inhibited (Wincent et al. 2012, 2016). In the present study, the body burden of FICZ peaked by Day 3 before decreasing significantly by Day 7. The dynamics of the body burden of FICZ, over time, thus suggests a link between actual Cyp1a inhibition and subsequent accumulation of FICZ in relation to development, and plausibly influenced by the maturation of the liver. However, Cyp1a inhibition by exposure to fluoranthene alone was not sufficient in causing accumulation of FICZ. Therefore, it can be postulated that alterations of multiple parallel molecular processes and events are required for FICZ accumulation in vivo.
Accumulation of PAHs and FICZ was reflected in the expression of cyp1a following 3 days of exposure. Exposure to fluoranthene resulted in a non-significantly increased expression relative to control, whereas exposure to retene increased the expression significantly. By contrast, exposure to the mixture resulted in a significantly stronger expression, relative to the other treatments and as a consequence, the measured expression was greater than the predicted additive effect exerted by the components (Table 4; combination index), results that were expected as per previous studies (Billiard et al. 2008; Eriksson et al. 2022a). As the experiment was designed to detect and quantify FICZ, it can only be assumed that accumulation of endogenously derived FICZ influences the expression of cyp1a. The underlying processes governing the toxicodynamic and kinetic processes are yet to be determined.
Combined, these findings highlight that the toxicity exerted by this mixture of PAHs could not have been predicted from the additive effect of the components in developing rainbow trout alevins, nor could the observed synergized BSD index in alevins exposed to the mixture be explained by the PAHs body burden alone. As FICZ is known to cause symptoms of BSD in fish (Wincent et al. 2016), it is plausible that accumulation of FICZ could contribute to the synergized BSD index among mixture exposed alevins. This is a novel mechanism of PAH mixture toxicity that could, at least, partly explain the synergism observed in organisms exposed to complex PAH mixtures such as crude oil (Billiard et al. 2008). However, it is unknown to what extent accumulated FICZ contributes to toxicity, nor if accumulation can occur in situ following environmental contamination.
Conclusions
Accumulation of endogenously derived FICZ is a novel discovery that will impact how PAH mixture toxicity is perceived. Accumulation of FICZ, which is a known AhR2 agonist that has been observed to induce developmental toxicity in zebrafish larvae, is likely to have influenced and aggravated developmental toxicity, as per the strong BSD indices. The underlying processes promoting accumulation are, in this case, unknown. Hypothetically, accumulation can be due to 1) decreased rate of xenobiotic metabolism due to increased substrate competition between the PAHs and FICZ for phase I and II metabolic enzymes; 2) increased rate of formation; or 3) a combination of decreased rate of phase I and II metabolism alongside increased rate of formation. Additionally, it is unknown if formation and accumulation of FICZ is tissue specific, evenly distributed throughout the developing organisms or produced in specific tissue(s) but distributed evenly. Moreover, it is unknown, although plausible, that exposure to other types of PAH mixtures (simple and complex), or crude oil, can result in the accumulation of FICZ. The same is true for species and life stage specificity, which must also be assessed. Therefore, more research on the nature of FICZ, in relation to developmental toxicity, toxicodynamics and kinetics are required to further the understanding of PAH toxicity in fish.
References
Barron MG, Heintz R, Rice SD (2004) Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res 58:95–100. https://doi.org/10.1016/j.marenvres.2004.03.001
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodolog) 57:289–300
Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT (2008) Nonadditive effects of PAHs on early vertebrate development: mechanisms and implications for risk assessment. Toxicolog Sci Official J Soc Toxicol 105:5–23. https://doi.org/10.1093/toxsci/kfm303
Billiard SM, Querbach K, Hodson PV (1999) Toxicity of retene to early life stages of two freshwater fish species. Environ Toxicol Chem 18:2070–2077. https://doi.org/10.1002/etc.5620180927
Billiard SM, Timme-Laragy A, Wassenberg DM, Cockman C, Di Giulio RT (2006) The role of the Aryl Hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to Zebrafish. Toxicolog Sci 92:526–536
Brinkworth LC, Hodson PV, Tabash S, Lee P (2003) CYP1A induction and blue sac disease in early developmental stages of rainbow trout (Oncorhynchus Mykiss) exposed to retene. J Toxicol Environ Health, Part A 66:627–646. https://doi.org/10.1080/15287390309353771
Clark B, Cooper E, Stapleton H, Di Giulio R (2013) Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River estuary (Virginia, USA). Environ Sci Technol 47. https://doi.org/10.1021/es401604b
Colavecchia M, Hodson P, Parrott J (2006) CYP1A induction and Blue Sac disease in early life stages of white suckers (Catostomus commersoni) exposed to oil sands. J Toxicol Environ Health Part A 69:967–994. https://doi.org/10.1080/15287390500362154
Doering J, Hecker M, Villeneuve D, Zhang X (2019) Adverse outcome pathway on aryl hydrocarbon receptor activation leading to early life stage mortality, via increased COX-2IS 12. https://doi.org/10.1787/bd46b538-en
dos Santos IF, Ferreira SLC, Domínguez C, Bayona JM (2018) Analytical strategies for determining the sources and ecotoxicological risk of PAHs in river sediment. Microchem J 137:90–97. https://doi.org/10.1016/j.microc.2017.09.025
Eriksson ANM, Rigaud C, Krasnov A, Wincent E, Vehniäinen E-R (2022a) Exposure to retene, fluoranthene, and their binary mixture causes distinct transcriptomic and apical outcomes in rainbow trout (Oncorhynchus mykiss) yolk sac alevins. Aqua Toxicol 106083. https://doi.org/10.1016/j.aquatox.2022.106083
Eriksson ANM, Rigaud C, Rokka A, Skaugen M, Lihavainen JH, Vehniäinen E-R (2022b) Changes in cardiac proteome and metabolome following exposure to the PAHs retene and fluoranthene and their mixture in developing rainbow trout alevins. Sci Total Environ 830:154846. https://doi.org/10.1016/j.scitotenv.2022.154846
Foucquier J, Guedj M (2015) Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3:e00149–e00149. https://doi.org/10.1002/prp2.149
Geier MC, James Minick D, Truong L, Tilton S, Pande P, Anderson KA, Teeguardan J, Tanguay RL (2018) Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol Appl Pharmacol 354:115–125. https://doi.org/10.1016/j.taap.2018.03.029
Heintz RA, Rice SD, Wertheimer AC, Bradshaw RF, Thrower FP, Joyce J, Short J (2000) Delayed effects on growth and marine survival of pink salmon Oncorhynchus gorbuscha after exposure to crude oil during embryonic development. Marine Ecology-progress Series. MAR ECOL-PROGR SER 208:205–216. https://doi.org/10.3354/meps208205
Hodson PV, Qureshi K, Noble CAJ, Akhtar P, Brown RS (2007) Inhibition of CYP1A enzymes by alpha-naphthoflavone causes both synergism and antagonism of retene toxicity to rainbow trout (Oncorhynchus mykiss). Aqua Toxicol 81:275–285. https://doi.org/10.1016/j.aquatox.2006.12.012
Honkanen JO, Rees CB, Kukkonen JVK, Hodson PV (2020) Temperature determines the rate at which retene affects trout embryos, not the concentration that is toxic. Aqua Toxicol 222:105471. https://doi.org/10.1016/j.aquatox.2020.105471
Incardona JP (2017) Molecular mechanisms of crude oil developmental toxicity in fish. Arch Environ Contam Toxicol 73:19–32. https://doi.org/10.1007/s00244-017-0381-1
Incardona JP, Carls MG, Day HL, Sloan CA, Bolton JL, Collier TK, Scholz NL (2009) Cardiac Arrhythmia is the primary response of Embryonic Pacific Herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol 43:201–207. https://doi.org/10.1021/es802270t
Incardona JP, Linbo TL, Scholz NL (2011) Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol 257:242–249. https://doi.org/10.1016/j.taap.2011.09.010
Maes J, Verlooy L, Buenafe OE, de Witte PAM, Esguerra CV, Crawford AD (2012) Evaluation of 14 organic solvents and carriers for screening applications in Zebrafish Embryos and Larvae. PLOS ONE 7:e43850
Massarsky A, Bone AJ, Dong W, Hinton DE, Prasad GL, Di Giulio RT (2016) AHR2 morpholino knockdown reduces the toxicity of total particulate matter to zebrafish embryos. Toxicol Appl Pharmacol 309:63–76. https://doi.org/10.1016/j.taap.2016.08.024
OECD, 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. https://doi.org/10.1787/9789264203709-en
Rannug A, Rannug U (2018) The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit Rev Toxicol 48:555–574. https://doi.org/10.1080/10408444.2018.1493086
Räsänen K, Arsiola T, Oikari A (2012) Fast genomic biomarker responses of retene and pyrene in liver of Juvenile Rainbow Trout, Oncorhynchus mykiss. Bull Environ Contam Toxicol 89:733–738. https://doi.org/10.1007/s00128-012-0770-0
Rigaud C, Eriksson A, Krasnov A, Wincent E, Pakkanen H, Lehtivuori H, Ihalainen J, Vehniäinen E-R (2020a) Retene, pyrene and phenanthrene cause distinct molecular-level changes in the cardiac tissue of rainbow trout (Oncorhynchus mykiss) larvae, part 1 – Transcriptomics. Sci Total Environ 745:141031. https://doi.org/10.1016/j.scitotenv.2020.141031
Rigaud C, Eriksson A, Rokka A, Skaugen M, Lihavainen J, Keinänen M, Lehtivuori H, Vehniäinen E-R (2020b) Retene, pyrene and phenanthrene cause distinct molecular-level changes in the cardiac tissue of rainbow trout (Oncorhynchus mykiss) larvae, part 2 – Proteomics and metabolomics. Sci Total Environ 746:141161. https://doi.org/10.1016/j.scitotenv.2020.141161
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Scott JA, Hodson PV (2008) Evidence for multiple mechanisms of toxicity in larval rainbow trout (Oncorhynchus mykiss) co-treated with retene and α-naphthoflavone. Aqua Toxicol 88:200–206. https://doi.org/10.1016/j.aquatox.2008.04.007
Scott JA, Incardona JP, Pelkki K, Shepardson S, Hodson PV (2011) AhR2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aqua Toxicol 101:165–174. https://doi.org/10.1016/j.aquatox.2010.09.016
Sivula L, Vehniäinen E-R, Vehniäinen E-R, Kukkonen JVK (2018) Toxicity of biomining effluents to Daphnia magna: acute toxicity and transcriptomic biomarkers. Chemosphere 210:304–311. https://doi.org/10.1016/j.chemosphere.2018.07.030
Smirnova A, Wincent E, Vikström Bergander L, Alsberg T, Bergman J, Rannug A, Rannug U (2016) Evidence for new light-independent pathways for generation of the endogenous Aryl Hydrocarbon receptor agonist FICZ. Chem Res Toxicol 29:75–86. https://doi.org/10.1021/acs.chemrestox.5b00416
Song Y, Nahrgang J, Tollefsen KE (2019) Transcriptomic analysis reveals dose-dependent modes of action of benzo(a)pyrene in polar cod (Boreogadus saida). Sci Total Environ 653:176–189. https://doi.org/10.1016/j.scitotenv.2018.10.261
Timme-Laragy A, Cockman CJ, Matson CW, Di Giulio RT (2007) Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aqua Toxicol 85:241–250. https://doi.org/10.1016/j.aquatox.2007.09.005
Tissot BP, Welte DH (1978) Composition of Crude Oils, In: Tissot BP, Welte DH (Eds.), Petroleum formation and occurrence: a new approach to oil and gas exploration. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 333–368. https://doi.org/10.1007/978-3-642-96446-6_19
Van Tiem LA, Di Giulio RT (2011) AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio). Toxicol Appl Pharmacol 254:280–287. https://doi.org/10.1016/j.taap.2011.05.002
Vehniäinen E-R, Bremer K, Scott JA, Junttila S, Laiho A, Gyenesei A, Hodson PV, Oikari AOJ (2016) Retene causes multifunctional transcriptomic changes in the heart of rainbow trout (Oncorhynchus mykiss) embryos. Environ Toxicol Pharmacol 41:95–102. https://doi.org/10.1016/j.etap.2015.11.015
Wassenberg D, Di Giulio R (2004) Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect 112:1658–1664. https://doi.org/10.1289/ehp.7168
Wickström K, Tolonen K (1987) The history of airborne polycyclic aromatic hydrocarbons (PAH) and perylene as recorded in dated lake sediments. Water Air Soil Pollut 32:155–175. https://doi.org/10.1007/BF00227691
Willett KL, Randerath K, Zhou G-D, Safe SH (1998) Inhibition of CYP1A1-dependent activity by the polynuclear aromatic hydrocarbon (PAH) fluoranthene. Biochem Pharmacol 55:831–839. https://doi.org/10.1016/S0006-2952(97)00561-3
Wills LP, Zhu S, Willett KL, Di Giulio RT (2009) Effect of CYP1A inhibition on the biotransformation of benzo[a]pyrene in two populations of Fundulus heteroclitus with different exposure histories. Aqua Toxicol 92:195–201. https://doi.org/10.1016/j.aquatox.2009.01.009
Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U (2009) The suggested physiologic Aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem 284:2690–2696. https://doi.org/10.1074/jbc.M808321200
Wincent E, Bengtsson J, Bardbori AM, Alsberg T, Luecke S, Rannug U, Rannug A (2012) Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 109:4479. https://doi.org/10.1073/pnas.1118467109
Wincent E, Kubota A, Timme-Laragy A, Jönsson ME, Hahn ME, Stegeman JJ (2016) Biological effects of 6-formylindolo[3,2-b]carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem Pharmacol 110–111:117–129. https://doi.org/10.1016/j.bcp.2016.04.012
Acknowledgements
We would like to acknowledge the contribution of laboratory technicians Mervi Koistinen, Emma Pajunen, and the laboratory personnel at Konnevesi research station for technical support. We also like to thank Hanka-Taimen OY fish farm for supplying us with rainbow trout alevins for scientific purposes and research.
Funding
Academy of Finland project number: 285296, 294066 and 319284 granted to Eeva-Riikka Vehniäinen. Open Access funding provided by University of Jyväskylä (JYU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eriksson, A.N.M., Rigaud, C., Wincent, E. et al. Endogenous AhR agonist FICZ accumulates in rainbow trout (Oncorhynchus mykiss) alevins exposed to a mixture of two PAHs, retene and fluoranthene. Ecotoxicology 31, 1382–1389 (2022). https://doi.org/10.1007/s10646-022-02593-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02593-9