Abstract
The conventional view of spawning in iteroparous bony fish, i.e., the “reproductive drain hypothesis,” is based on the observation that somatic growth (in length) slows down noticeably at approximately the time fish attain maturity, and hence the assumption is made that investment in gonadal development slows down growth. However, when this is translated as growth in weight, the weight at first maturity (or puberty) is usually smaller than the weight at which growth rate is highest, i.e., weight growth accelerates after first maturity. We solve this conundrum, with some emphasis on female cod (Gadus morhua), by proposing the hypothesis that the substantial loss of body mass experienced by fish as a result of spawning is quickly compensated for by increased somatic growth after the spawning period, notably because of the increase in food conversion efficiency resulting from a sudden loss of body weight, which necessarily leads to a large increase in relative oxygen supply via the gills. This is consistent with the argument developed elsewhere that declining relative oxygen supply by the gills, whose surface area cannot keep up with increasing body weight, is the reason for growth rate declining with weight in adult fish.




Similar content being viewed by others
Data availability
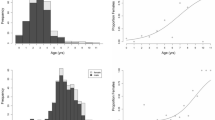
All the data used in this study are presented in its text and figures.
References
Ajiad A, Jakobsen T, Nakken O (1999) Sexual difference in maturation of Northeast Arctic cod. J Northw Atl Fish Sci 25:1–15
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4(2):147–190
Andrews AH, DeMartini EE, Eble JA, Taylor BM, Lou DC, Humphreys R (2016) Age and growth of bluespine unicornfish (Naso unicornis): a half-century life-span for a keystone browser, with a novel approach to bomb radiocarbon dating in the Hawaiian Islands. Can J Fish Aquat Sci 73:1575–1586
Audzijonyte A, Barneche DR, Baudron AR, Belmaker J, Clark TD, Marshall CT, Morrongiello JR, van Rijn I (2019) Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms? Glob Ecol Biogeogr 28(2):64–77
Caldwell LK, Pierce AL, Nagler JJ (2013) Metabolic endocrine factors involved in spawning recovery and rematuration of iteroparous female rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 194:124–132
Chabot D, Dutil J-D (1999) Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J Fish Biol 55:472–491
Charnov E (2008) Fish growth: Bertalanffy k is proportional to reproductive effort. Environ Biol Fish 83:185–187
Choat JH, Axe LM, Lou DC (1996) Growth and longevity in fishes of the family Scaridae. Mar Ecol Prog Ser 145:33–41
Daan N (1974) Growth of North Sea cod, Gadus morhua. Neth J Sea Res 8:27–48
Day T, Talyor PD (1997) Von Bertalanffy’s growth equation should not be used to model age and size at maturity. Am Nat 149(2):381–393
De Jager S, Dekkers WJ (1974) Relations between gill structure and activity in fish. Neth J Zool 25:276–308
Edwards RRC, Finlayson DM, Steele JH (1972) An experimental study of the oxygen consumption, growth, and metabolism of the cod (Gadus morhua L.). J Exp Mar Biol Ecol 8:299–309
Fenton GE, Short SA, Ritz DA (1991) Age determination of orange roughy, Hoplostethus atlanticus (Pisces: Trachichthyidae) using 210Pb:226Ra disequilibria. Mar Biol 109:197–202
Fordham SE, Trippel EA (1999) Feeding behaviour of cod (Gadus morhua) in relation to spawning. J Appl Ichthyol 15(1):1–9
Forster RP, Goldstein L (1969) Formation of excretory products. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 1. Academic Press, New York, pp 313–350
Froese R (2006) Cube law, condition factors and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Froese R, Flindt F, Meyer E, Meyer J, Egerland O (2020) Untersuchung zum Laichverhalten des Dorsches in der Kieler Bucht im Frühjahr 2020. Available at: http://www.fishbase.de/rfroese/LaichDorsch2020.pdf Accessed 1 Oct 2020
Froese R, Pauly D (2023) Comment on Metabolic scaling is the product of life-history optimization. Science 380(6643):eade6084
García-Fernández C, Domínguez-Petit R, Saborido-Rey F (2022) The use of daily growth to analyze individual spawning dynamics in an asynchronous population: the case of the European Hake from the southern stock. Fishes 7(4):208
Gerking SD (1952) The protein metabolism of sunfishes of different ages. Physiol Zool 25:358–372
Gerking SD (1971) Influence of rate of feeding and body weight on protein metabolism of bluegill sunfish. Physiol Zool 44:9–19
Goldberg AL, St John AC (1976) Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem 45:747–803
Greeley JR (1933) The growth rate of rainbow trout from some Michigan waters. Trans Am Fish Soc 63:361–378
Hawkins AJS (1991) Protein turnover: a functional appraisal. Funct Ecol 5:222–233
Heibo E, Magnhagen C, Vøllestad LA (2005) Latitudinal variation in life-history traits in Eurasian perch. Ecology 86(12):3377–3386
Heincke F (1908) Bericht über die Untersuchungen der Biologischen Anstalt auf Helgoland zur Naturgeschichte der Nutzfische. (1 April 1905 bis 1. Oktober 1907. In: Die Beteiligung Deutschlands an der Internationalen Meeresforschung, 4 & 5. Jahresbericht. Verlag von Otto Salle, Berlin, pp 67–150
Heinisch G, Corriero A, Medina A, Abascal FJ, de la Serna JM, Vassallo-Agius R, Ríos AB, García A, de la Gándara F, Fauvel C, Bridges CR, Mylonas CC, Karakulak SF, Oray I, De Metrio D, Rosenfeld H, Gordin H (2008) Spatial–temporal pattern of bluefin tuna (Thunnus thynnus L. 1758) gonad maturation across the Mediterranean Sea. Mar Biol 154(4):623–630
Hoar WS, Randall DJ, Donaldson EM (1983) Reproduction, part A: endocrine tissues and hormones. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology. Academic Press, New York, pp 229–231
Hubbs CL (1926) The structural consequence and modifications of the development rate in fishes, considered in reference to certain problems of evolution. Am Nat 60:57–81
Iles TD (1964) The duration of maturation stages in herring. ICES J Mar Sci 29(2):166–188
Iles TD (1974) The tactics and strategy of growth in fishes. In: Harden Jones ER (ed) Sea fisheries research. Elek Science, London, pp 331–345
Jardas I, Šantić M, Pallaoro A (2004) Diet composition and feeding intensity of horse mackerel, Trachurus trachurus (Osteichthyes: Carangidae) in the eastern Adriatic. Mar Biol 144(6):1051–1056
Jenkins LE, Pierce AL, Graham ND, Medeiros LR, Hatch DR, Nagler JJ (2019) Elevated plasma triglycerides and growth rate are early indicators of reproductive status in post-spawning female steelhead trout (Oncorhynchus mykiss). Conserv Physiol 7(1):coz038
Jobling M (1994) Fish Bioenergetics. Chapman and Hall, London
Jobling M, Meløy OH, Dos Santos J, Christiansen BJAI (1994) The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquac Int 2(2):75–90
Jones R (1976) Growth of fishes. In: Cushing DH, Walsh JJ (eds) The ecology of the seas. Blackwell Scientific Publications, London, pp 251–279
Kolding J, Haug L, Stefansson S (2008) Effect of ambient oxygen on growth and reproduction in Nile tilapia (Oreochromis niloticus). Can J Fish Aquat Sci 65:1413–1424. https://doi.org/10.1139/F08-059
Kooijman SALM (2000) Dynamic energy and mass budgets in biological systems. Cambridge University Press
Korsøen ØJ, Dempster T, Fosseidengen JE, Karlsen Ø, Oppedal F, Stien LH, Kristiansen TS (2013) Towards cod without spawning: artificial continuous light in submerged sea-cages maintains growth and delays sexual maturation for farmed Atlantic cod Gadus morhua. Aquac Environ Interact 3(3):245–255
Kozłowski J, Czarnołęski M, Dańko M (2004) Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr Comp Biol 44(6):480–493
Lagler KF, Bardach JE, Miller RR, Passino DRM (1977) Ichthyology, 2nd edn. John Wiley and Sons Inc, New York
Lavaud R, Filgueira R, Augustine S (2021) The role of dynamic energy budgets in conservation physiology. Conserv Physiol 9(1):coab083
Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20(2):201–219
Lester NP, Shuter BJ, Abrams PA (2004) Interpreting the von Bertalanffy model of somatic growth in fishes: the cost of reproduction. Proc Roy Soc London Ser B Biol Sci 271:1625–1631
Longhurst A, Pauly D (1987) Ecology of tropical oceans. Academic Press, San Diego
Mao X, Lan S, Xiao T, Huang Y, Jin H, Huan Z, Li J (2012) Research progress on culture biology of Japanese croaker Nibea japonica. Fish Sc 31(4):245–248 [in Chinese]
McKinley S, Van Der Kraak G, Power G (1998) Seasonal migrations and reproductive patterns in the lake sturgeon, Acipenser fulvescens, in the vicinity of hydroelectric stations in northern Ontario. Environ Biol Fish 51(3):245–256
Menzel DW (1958) Utilization of algae for growth by the angelfish Holocathus bermudensis. J Cons Int Explor Mer 24:308–313
Menzel DW (1960) Utilization of food by a Bermuda reef fish, Epinephelus guttatus. J Cons Int Explor Mer 25:216–222
Michalsen K, Johannesen E, Bogstad B (2008) Feeding of mature cod (Gadus morhua) on the spawning ground in Lofoten. ICES J Mar Sci 65:571–580
Mion M, Hilvarsson A, Hüssy K, Krumme U, Krüger-Johnsen M, McQueen K, Mohamed E, Motyka R, Orio A, Plikshs M, Radtke K (2020) Historical growth of Eastern Baltic cod (Gadus morhua): setting a baseline with international tagging data. Fish Res 223:105442
Moreira JM, Mendes AC, Maulvault AL, Marques A, Rosa R, Pousão-Ferreira P, Sousa T, Anacleto P, Marques GM (2022) Impacts of ocean warming and acidification on the energy budget of three commercially important fish species. Conserv Physiol 10(1):coac048
Navaluna NA, Pauly D (1988) Seasonality in the recruitment of Philippine fishes as related to monsoon wind patterns. In: Yañez-Arancibia A, Pauly D (eds) Proceedings of the IREP/OSLR workshop on the recruitment of coastal demersal communities, Campeche, Mexico, 21–25 April 1986. Supplement to IOC Workshop Rep. No 44, pp 167–179
Niimi AJ, Beamish FWH (1974) Bioenergetics and growth of largemouth bass (Micropterus salmoides) in relation to body weight and temperature. Can J Zool 52:447–456
Olson KR, Fromm PO (1971) Excretion of urea by two teleosts exposed to different concentrations of ambient ammonia. Comp Biochem Physiol 40:999–1007
Pandian TJ (1967) Intake, digestion, absorption and conversion of food in the fishes Megalops cyprinoides and Ophiocephalus striatus. Mar Biol 1:16–32
Pandian TJ (1970) Intake and conversion of food in the fish Limanda limanda exposed to different temperatures. Mar Biol 5:1–17
Pauly D (1986) A simple method for estimating the food consumption of fish populations from growth data and food conversion experiments. Fish Bull 4(4):827–842
Pauly D (2019) Gasping fish and panting squids: oxygen, temperature and the growth of water-breathing animals, 2nd edn. International Ecology Institute, Oldendorf/Luhe, Germany
Pauly D (2021) The Gill-Oxygen Limitation Theory (GOLT) and its critics. Science. Advances 7(2):eabc6050. https://doi.org/10.1126/sciadv.abc6050
Pauly D, Lam ME (2023) Too hot or too cold: the biochemical basis of temperature-size rules for fish and other ectotherms. Environ Biol Fish 106:1519–1527
Pauly D, Liang C (2022) A reconceptualization of the interactions between spawning and growth in bony fish. Sci Mar 84(4):e044. https://doi.org/10.3989/scimar.05280.044
Pedersen T, Jobling M (1989) Growth rates of large, sexually mature cod Gadus morhua, in relation to condition and temperature during an annual cycle. Aquaculture 81(2):161–168
Pütter A (1920) Studien über physiologische Ähnlichkeit. VI. Wachstumsähnlichkeiten. Pflüger's Archiv für die gesamte Physiologie des Menschen und der Tiere 180:293–340
Quince C, Abrams PA, Shuter BJ, Lester NP (2008) Biphasic growth in fish I: Theoretical foundations. J Theoret Biol 254(2):197–206
Reddin DG, Downton P, Fleming IA, Hansen LP, Mahon A (2011) Behavioural ecology at sea of Atlantic salmon (Salmo salar L.) kelts from a Newfoundland (Canada) river. Fish Oceanogr 20:174–191
Rösch R (2000) Gonadosomatic index (GSI) of female whitefish (Coregonus lavaretus) in Lake Constance. Limnologica 30:193–196
Roussow G (1957) Some considerations concerning sturgeon spawning periodicity. J Fish Board Can 14(4):553–572
Savitz J (1969) Effects of temperature and body weight on endogenous nitrogen excretion in the bluegill sunfish (Lepomis macrochirus). J Fish Board Can 26:1813–1821
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Shul’man GE (1974) Life cycles of fish: physiology and biochemistry. Israel Program of Scientific Translations & Wiley & Sons, New York
Silvert W, Pauly D (1987) On the compatibility of a new expression for gross conversion efficiency with the von Bertalanffy growth equation. Fish Bull 85(1):139–140
Sirotenko M, Istomin A (1978) Seasonal variations in the feeding of the Black Sea Trachurus mediterraneus ponticus Aleev. J Ichthyol 18:424–431
Solberg C, Willumsen L (2008) Differences in growth and chemical composition between male and female farmed cod (Gadus morhua) throughout a maturation cycle. Aquac Res 39(6):619–626
Stéquert B, Rodriguez JN, Cuisset B, Le Menn F (2001) Gonadosomatic index and seasonal variations of plasma sex steroids in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) from the western Indian Ocean. Aquat Living Resour 14:313–318
Temming A (1994a) Food conversion efficiency and the von Bertalanffy growth function I: a modification of Pauly’s model. Naga, The ICLARM Quarterly 17(1):38–39
Temming A (1994b) Food conversion efficiency and the von Bertalanffy growth function. Part II and conclusion: extension of the new model to the generalized von Bertalanffy growth function. Naga, The ICLARM Quarterly 17(4):41–45
Temming A, Herrmann J-P (2009) A generic model to estimate food consumption: linking von Bertalanffy’s growth model with Beverton and Holt’s and Ivlev’s concepts of net conversion efficiency. Can J Fish Aquat Sci 66:683–700
Trippel EA, Butts IA, Babin A, Neil SR, Feindel NJ, Benfey TJ (2014) Effects of reproduction on growth and survival in Atlantic cod, Gadus morhua, assessed by comparison to triploids. J Exp Mar Biol Ecol 451:35–43
Van der Have TM, De Jong G (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J Theoret Biol 183(3):329–340
von Bertalanffy L (1934) Untersuchungen über die Gesetzlichkeit des Wachstums I. Allgemeine Grundlagen der Theorie - Mathematisch-physiologische Gesetzlichkeiten des Wachstums bei Wassertieren. Roux’ Archiv für Entwicklungs-Mechanik 131:613–652
von Bertalanffy L (1951) Theoretische Biologie. Zweiter Band: Stoffwechsel, Wachstum. A Francke Verlag, Bern
Webb PW (1978) Partitioning of energy into metabolism and growth. In: Gerking SD (ed) Ecology of freshwater fish production. Blackwell Scientific Publications, Oxford, pp 184–214
Weeks SC (1996) The hidden cost of reproduction: reduced food intake caused by spatial constraints in the body cavity. Oikos 75(2):345–349
White CR, Alton LA, Bywater CL, Lombardi EJ, Marshall DJ (2022) Metabolic scaling is the product of life-history optimization. Science 377:834–839
Won ET, Borski RJ (2013) Endocrine regulation of compensatory growth in fish. Front Endocrinol 4:74. https://doi.org/10.3389/fendo.2013.00074
Wootton HF, Morrongiello JR, Schmitt T, Audzijonyte A (2022) Smaller adult fish size in warmer water is not explained by elevated metabolism. Ecol Lett 25(5):1177–1188
Funding
The data presented in Fig. 1 were provided by a project of the German Federal Agency for Nature Conservation (Bundesamt für Naturschutz, BfN), which provided RF funds from the Federal Ministry of the Environment, Nature Conservation and Nuclear Safety (BMU; grant agreement FKZ: 3521532201).
Author information
Authors and Affiliations
Contributions
Idea generation: DP, JM, RF, and PS; cases studies: DP, RF, JM, CJ, and PS; data analyses: DP and RF; first draft of manuscript: DP; editing of final manuscript: DP, RF, JM,CJ, and PS.
Corresponding author
Ethics declarations
Ethics approval
No approval of research ethics committees was required as this study analyzed only previously published data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pauly, D., Froese, R., Liang, C. et al. Post-spawning growth acceleration in fish as a result of reduced live weight and thus, increased food conversion efficiency. Environ Biol Fish 106, 2031–2043 (2023). https://doi.org/10.1007/s10641-023-01482-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01482-2