Abstract
Little is known about the distribution and ecology of juvenile green sturgeon throughout their range and specifically within California’s Central Valley and the San Francisco Bay Estuary. The objective of our study was to identify rearing movement patterns of juvenile green sturgeon and their interaction with Delta and Bay habitats. Acoustic biotelemetry was used to record juvenile movements and residence within different regions of this highly modified watershed. We document a diversity of movements exhibited by 31 acoustically tagged juvenile green sturgeon monitored over a 9-month period. The juvenile sturgeon either (1) remained in the Delta or moved (2) into the Carquinez Straits, (3) into San Pablo Bay, (4) into San Pablo Bay but returned to Delta, (5) through the estuary and likely left through the mouth of the bay, (6) or left the estuary only to return later. Spatiotemporal habitat quality varies within and between Delta and Bay habitats. Variation in water year and hydrologic connectivity are likely to influence habitat quality and quantity, and behaviors may be differentially optimized for fitness in response to environmental variation. Our results provide initial evidence supporting multiple behavioral movement patterns and a broad use of regional habitats for green sturgeon in the California Central Valley. This apparent behavioral diversity observed through movement patterns may ensure long-term success of all population segments over time in an extremely variable environment, ultimately supporting overall population stability (i.e., the portfolio effect). Enhanced conservation efforts may be needed to protect juvenile green sturgeon and the habitats they rely upon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The green sturgeon (Acipenser medirostris) is a megafauna fish which has existed in a prehistoric state for more than 200 million years. Their diverse behavioral patterns expose green sturgeon to a range of anthropogenic threats as individuals utilize the Central Valley Corridor, CA, USA. Habitat degradation, water extraction, fragmentation, and flow alteration dominate the functional ecology of the world’s large rivers, including the Sacramento and San Joaquin Rivers and estuary (Nilsson et al. 2005; Grill et al. 2019). As a result, freshwater ecosystems and diversity are imperiled at all scales (Strayer and Dudgeon 2010; Reid et al. 2019). Indeed, > 83% of California’s native fish fauna, most of which is concentrated in the Central Valley, is considered to be in some form of strong decline (Moyle et al. 2011; Katz et al. 2013).
Yet surprisingly little is known about the basic ecology of green sturgeon, even though the species is experiencing a protracted population decline. Currently, the southern Distinct Population Segment (sDPS) is listed as threatened under the US Endangered Species Act, while the northern Distinct Population Segment (nDPS) is listed as a National Marine Fisheries Service “Species of Special Concern” (NOAA 2008; Moyle et al. 2015).
In the Central Valley, water diversions and subsequent alterations of river flows and temperatures, contaminants, an altered prey-base, and competition with non-native species for habitat likely deleteriously impact the population of juvenile green sturgeon (NOAA 2008). For example, juveniles that spend a significant amount of time in the southern Delta may be impacted by water exports at the State and Federal pumping facilities. Estuarine-oriented juveniles residing near Carquinez Strait may be more impacted by point-source contaminants (Stewart et al. 2020) or to a lesser known extent mortality through shipping commerce and prop strikes (Demetras et al. 2020). Severity of the individual threats can be quite variable across the watershed; thus, conservation management strategies should be wide-reaching but nimble depending on the region and threat.
For sDPS green sturgeon, the last decade has been profitable in terms of knowledge gained from directed research (e.g., Verhille et al. 2014; Klimley et al. 2015; Rodgers et al. 2019). However, there remains a major gap in understanding of juvenile life stages, leading to high levels of uncertainty in science-based conservation management decisions. Advancements with respect to early life stages are challenging because biologists are faced with (1) working in one of the more complicated ecosystems west of the Continental Divide; and (2) encounter rates of individuals in the wild are low, a function of behavior and population size.
Adult spawning is known to occur seasonally. This is based on the collection of eggs on artificial substrate samplers, in a 94 km reach from 426 to 332 river kilometers (rkm) upriver from April to July, with the peak generally occurring in May to June (Poytress et al. 2015). Historically, the putative spawning grounds were divided by an impassable dam located near the town of Red Bluff, California (rkm 479). Operation of the dam gates occurred from May 15 to September 14 each year until 2009 when removal plans began to facilitate adult passage. Rotary screw traps operated by the US Fish and Wildlife Service are utilized primarily for estimating salmon smolt production. While these traps are by no means designed to capture sturgeon, periodic captures of post-exogenous feeding larvae do occur, particularly in above average recruitment years, and provide a rare collection opportunity.
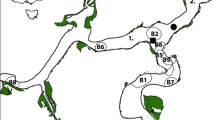
The putative nursery grounds for sDPS green sturgeon likely includes the Sacramento-San Joaquin Delta, Suisun Bay, San Pablo Bay “North Bay”, and San Francisco Bay “Central Bay” (Fig. 1). Descriptions of residency and regional habitat use have largely been informed by laboratory-based physiological studies (Allen and Cech 2007; Sardella et al. 2008; Allen et al. 2011; Poletto et al. 2013, 2018). Distribution and temporal habitat usage descriptions have been largely based on incidental captures within ongoing monitoring efforts, research focused on non-sturgeon species, and from both the California State Water Project (SWP) and federal Central Valley Project (CVP) fish salvage data. Prior to this work, the most comprehensive body of literature regarding juvenile green sturgeon utilization of the putative nursery grounds consisted of several paragraphs in a multi-species survey from > 50 years ago (Radtke 1966). This study reported 138 green sturgeon captured by gill net in the Central Delta between June and August of 1964 with individuals ranging from 200 to 580 mm. Length frequencies from the survey were characterized as two different age classes (Radtke 1966). Combined, the SWP and CVP entrainment monitoring program in the southern region of the Sacramento-San Joaquin Delta accounted for 180 individuals salvaged between 1980 and 2016, of which 62% were measured (J.C. Heublein, NOAA, pers. comm.). Similar to results from Radtke (1966), entrainment monitoring resulted in length frequencies ranging from 125 to 774 mm and were bimodally distributed, again suggesting multiple year classes.
Controlled physiological experimental studies have provided insight into the potential timing of the movement from freshwater to estuarine environments, albeit inferred. Juvenile green sturgeon increase their tolerance of salinity as they grow. nDPS green sturgeon with a total length (TL) of ~ 50 mm TL are tolerant of short-term exposure to salinity of 15‰ at 42 days post-hatch (dph), with increasing tolerance to salinities of 35‰ once they reach a TL of 250 mm at an average of 134 dph (Allen et al. 2011). Additionally, hatchery reared nDPS green sturgeon, 140 dph with lengths ranging from 380 to 525 mm TL that were acclimated to both salt and freshwater, spent significantly more time in the highest salt concentrations during salinity preference experiments (Poletto et al. 2013). Finally, isotope analysis of nDPS adult green sturgeon from the Klamath basin was shown to have entered the estuarine environment between 0.5 and 1.5 years of age (Allen et al. 2009). From this body of physiological literature, it is hypothesized that ocean entry is a linear process where individuals progress nearer to the ocean as they grow in size.
Limited information does exist on the behavior of juvenile green sturgeon in the San Francisco Bay estuary. Six yearling green sturgeon, released in the Central Delta, were continually tracked over a period of 5 days while recording GPS coordinates, depths, and water temperatures (Thomas et al. 2019). These individuals swam at or near the bottom at depths ranging from > 3 to 20 m. Directed movement was largely confined to the main channel of the San Joaquin River, with only one individual straying from the main channel. Juveniles exhibited positive and negative rheotaxis during sustained directional movements during ebb tidal cycles indicating non-passive transport in response to tidal process.
The Central Valley physiographic province is a hotspot of fish diversification in California (Moyle 2002). Native fishes often present multiple intraspecific movement patterns, and many of these phenotypes and genotypes vary specifically with respect to timing of migration. Classically, there are four runs of Chinook salmon in the Central Valley that vary in the timing of adult migration from the ocean as well as juvenile outmigration from freshwater. Population variability within each run was likely historically high—a product of the variable Mediterranean climate of the Central Valley. However, the existence of four runs (and multiple populations of some runs) ultimately promotes long-term stability of the Chinook salmon population overall. This phenomenon is referred to as “the portfolio effect” and is of increasing interest to conservation scientists (Schindler et al. 2010, 2015; Carlson and Satterthwaite 2011; Satterthwaite and Carlson 2015; Yamane et al. 2017). Adult green sturgeon exhibits behavioral plasticity in their movements and habitat used during their spawning migration (Thomas et al. 2014; Steel et al. 2018) and thus may benefit from the portfolio effect; yet little is known regarding green sturgeon plasticity across the various life stages, especially at the juvenile stage. Kessel et al. (2018) have found different migration routes with an unrestricted spatial corridor that indicates behavioral plasticity in the movement patterns of adult lake sturgeon (Acipenser fulvescens).
The primary objective of our study was to provide the first description of the spatiotemporal distribution and regional rearing habitat utilization of juvenile green sturgeon. Coded ultrasonic transmitters were placed on 31 juveniles, and their movements and periods of residence were recorded by autonomous acoustic receivers in different regions of the Sacramento-San Joaquin Delta and San Francisco Estuary. We predicted that the juveniles would exhibit behavioral plasticity to their out-migratory movements as adults had been shown to display. Also, we suspected that the juveniles might enter the open ocean to increase their rate of growth due to greater feeding opportunities in the coastal waters.
Methods
Juvenile capture and rearing
Acquiring individuals can be a challenge for completing tagging studies on juveniles of rare species like green sturgeon. In this study, juveniles were sourced from multiple rotary screw traps operated by the US Fish and Wildlife Service (USFWS) immediately downstream of the Red Bluff Diversion Dam (RBDD) June through September, 2012. Eggs of green sturgeon have been collected on substrate samplers in a reach of the Sacramento River 94 rkm long at rkm 426–332, encompassing the reaches at which the post-larvae were collected (Poytress et al. 2015). Individuals captured in screw traps were held in live wells. Each day, the juveniles were transported by truck to the University of California, Davis (UC Davis) in a 114-L aerated transport tank. Transport densities were low, with a typical daily transport consisting of fewer than five post-exogenous feeding individuals (~ 0–40 mm). The post-larval juveniles were separated into two groups (each comprised of 16 juveniles) and then held at the Center for Aquatic Biology and Aquaculture (CABA) at the university in two 1.5 m diameter, 600-L aerated fiberglass circular tanks. These circular rearing tanks were configured as flow-through systems with water sourced from the onsite well. Rearing tanks were covered with shade cloth in order to limit light exposure. The juveniles were fed a ration of commercial diet ad libitum (SilverCup, Nelson & Sons, Inc.; www.silvercup.com), and water temperatures were held at a near optimal growth of 18 °C until juveniles reached comparable sizes to those described by Radtke (1966).
Tag specifications, implantation, and juvenile release
Movements of juvenile green sturgeon were determined by surgically implanting coded ultrasonic transmitters (Vemco Ltd., V-9L) in juvenile green sturgeon (see Thomas et al. 2019 for additional surgery details). Each transmitter was cylindrical (9 mm diameter and 29 mm length), weighed 4.7 g in air, and had a battery life of 270 days. They had an average transmission rate of 60 s with coded pulse trains occurring pseudo-randomly in time with intervals varying from 30 to 90 s. Juveniles were anesthetized prior to surgery in a water bath consisting of 350 mg/l of MS-222 buffered with 6 g/l of NaCl and 420 mg/l of NaHCO3. Individuals were inverted in a surgical cradle and irrigated with a maintenance dose of MS-222 (15 mg/l MS-222, buffered with 6 g/l of NaCl and 420 mg/l of NaHCO3). A 10-mm longitudinal incision was made using a scalpel (Sharpoint microsurgical knife) between the midline and between the third and fourth ventral scute. After implanting the tag in the peritoneum, the incision was closed using PDSII monofilament sutures (size 4/0 cutting needle) with a single far-near-far-near cross stitch completed with three surgical square knots. Upon completion of the surgery, individuals were measured, moved back to the rearing tank, and monitored for recovery which we defined as the time to regain equilibrium and exhibit volitional swimming. The mean fork length at time of tagging was 41.3 cm (SD = 4.5), while mean weight at time of tagging was 453.7 g (SD = 184.0 g) (Table 1). Total surgery time averaged 4 min. All fish recovered quickly, and no mortalities occurred in response to the tagging process. Tag burden across all individuals was < 2% of body weight. An evaluation of tag effects was performed prior to this study as reported in Miller et al. (2014). Tag effect study results showed no differences in growth or swimming performance for comparative groups of tagged, sham, and control fish using the same tag specifications and surgical technique across all groups (Miller et al. 2014).
All tagged fish were transported and released together at Santa Clara Shoals (N 38.09439, W 121.65280) in the Central Delta on 28 February 2013, 14 days post-surgery (Fig. 1). This location at rkm 105 was chosen for release because juvenile green sturgeon had been captured here in the past (see Radtke 1966). Individuals released during this study would have been young of the year and approximately 8–10 months old at the time of release on.
Array of tag-detecting receivers
The detection range of autonomous receivers varies by location based upon degree of spherical spreading and absorption, which are functions of the depth, bottom type, and local speed of water flow. Ammann et al. (2007) determined that range of detection of a V-09 at 200 m was > 80% in open water of depth ~ 11 m and > 70% detection efficiency up to 500 m. Similarly, in river tests characterized by slow moving water of depth ~ 6 m showed > 80% detection efficiency up to a distance 475 m and > 70% at a distance of 500 m in a shallow slough such as exist throughout the Delta (Ammann et al. 2007). There was little difference between performances of three independent transmitters in each of the two range tests.
Movements of sturgeon carrying coded transmitters were determined with an array of ~ 250 autonomous receivers distributed within the Sacramento-San Joaquin watershed. The Delta region was instrumented with 53 autonomous receivers which had previously been used in Delta salmonid survival studies (Chapman et al. 2013, 2014; Singer et al. 2013; Hearn et al. 2014). As apparent on delta and estuary maps (Figs. SI-1, SI-2), monitors were placed at critical junctions such that receivers within the represented reach were acoustically independent of fish transporting along adjacent channels. Redundant receivers in lines and gates were pooled into single locations. Detections were summed for monitors in specific regions as defined in down-river order from those farthest from the Golden Gate Bridge to that at the mouth of San Francisco Bay, as follows: (1) East Delta, (2) between East and Central Delta, (3) North Delta, (4) Central Delta, (5) Suisun Bay, (6) Carquinez Straight, (7) San Pablo Bay, (8) Richmond Bridge, (9) San Francisco Bay, and (10) Golden Gate Bridge.
Analytical techniques
Regional use by juvenile green sturgeon was estimated from the total number of days within a given region divided by the total number of days in system. Individual tracks across the 270-d tag life were plotted using R (R Core Team 2017). We first did a coarse examination for behavioral patterns in transitions between regions. Different movement patterns were characterized ad hoc based upon a visual inspection of individual waterfall plots.
We used hierarchical clustering to identify groups of statistically distinct behavioral patterns. Also, the R package, factoextra (V 1.0.6) (Kassambara and Mundt 2020) was utilized to implement Partitioning Around Medoids (PAM) (see Kaufman and Rousseeuw (1990)) algorithm, a more robust hierarchical clustering method than a typical K-means. We generated a spatiotemporal matrix of normalized presence/absence derived from the detection histories of all individuals across the study time frame, where for a given day across the study timeline, a binary presence or absence was assigned to the associated rkm (regional indicator) of the receiver location. We assumed that individuals remained within the region of last detection until either a new first detection occurred within the original region or elsewhere in the system. If a new detection occurred in a new region, we considered these individuals transitioned to that new region. All river kilometer estimates were measured from beneath the Golden Gate Bridge (rkm 0) located at the mouth of the San Francisco Estuary. The number of clusters selected (k) was determined using the within-cluster sum of squares (WSS) method, where k is varied from 1 to n clusters and the optimal k selection occurs at the point of inflection along the curve.
Results
Thirty-one juvenile green sturgeon with coded transmitters were released at Santa Clara Shoals (rkm 104) in the Central Delta on 28 February 2013. Individual sturgeon were detected for up to 290 days—longer than the estimated battery life of the tags (Table 1) because no kill time was requested on the transmitters. The variable life of the tags may have influenced our results although the longest period of detection slightly exceeded the standard kill time used by Vemco Ltd.
Six movement patterns were exhibited by juvenile green sturgeon. Our first analysis is qualitative, based on summarizing telemetry records in the various study regions (Fig. 2). We ultimately pooled the three sub-regions of the Delta into one Delta category. Tagged juvenile green sturgeon either (1) remained in the Delta; (2) moved into the Carquinez Straits; (3) migrated into San Pablo Bay; (4) moved into San Pablo Bay but returned to Delta; (5) migrated through the estuary, left the bay, and did not return; or (6) left the estuary only to later return during the 9-month period. Twelve (39%) of the 31 green sturgeon remained in the Delta for the period within which they were detected, including the individual noted as GS-23. This fish was first detected after released immediately upriver of Santa Clara Shoals in early March where it appeared to reside for 4 months until the end of July, after which point, it swam upriver to the Medford Channel and downriver to Franks Tract, then continued downstream to the Antioch Bridge before returning to Santa Clara Shoals (Fig. 2a). Juvenile GS-03 moved below the Delta boundary and Antioch Bridge and remained in the Suisun Bay/Carquinez Strait region through most of November (Fig. 2b). Individual GS-32 migrated downstream where it spent 6 months from mid-June to mid-December near the Richmond Bridge (Fig. 2c). Juvenile green sturgeon GS-06 stayed at Santa Clara Shoals for 4 months before it migrated downstream to the Richmond Bridge (Fig. 2d). It then reversed direction and returned to the Delta where it exhibited an oscillatory pattern moving up and downstream but generally remaining in the Delta. In contrast, juvenile green sturgeon GS-18 and GS-12 migrated downstream throughout the entire length of the estuary and exited the bay during the month of June into coastal marine waters (Fig. 2e, f). The former left and never returned, but the latter remained in either coastal or oceanic waters for 3 months before returning through the Golden Gate at the beginning of September. GS-12 appeared to migrate towards the South Bay before being again detected by the two cross-bay arrays at the mouth of San Francisco Bay. Movement type exhibited by each tagged sturgeon is identified in Table 1, and proportions of sturgeon exhibiting each movement pattern are shown in Fig. 3.
Percentages of tagged green sturgeon exhibiting six movement patterns in the Sacramento-San Joaquin watershed defined as the following: (a) remains in Delta (D), (b) moves into Carquinez Straits (D-CS), (c) moves into San Pablo Bay, (d) moves into Pablo Bay but returns to Delta (D-SP-D), (e) leaves estuary (D-O), and (f) leaves estuary for ocean and returns to estuary (D-O-E)
Tagged juvenile green sturgeon resided mostly within the Delta (Fig. 3). All 31 tagged individuals were detected in the Central Delta near the release site (see Table 1), and individuals remained within this region an average of 87.7 days (SD = 91.8) over the 9-month tag life. A total of fifteen individuals (48%) spent on average 26.7 days (SD = 39.8) in the Eastern Delta, while a total of 16 (52%) juveniles spent 29.0 days (SD = 45.4) between the Eastern and Central Delta regions. Only six individuals (19%) were detected in the North Delta and averaged 9.8 days (SD = 13.3) within the region. Moving towards the western regions, a total of 19 (61%) and 18 (58%) individuals averaged 11 days (SD = 13.3) and 25 days (SD = 33.9), respectively, within the Suisun Bay and Carquinez Strait regions. The next-most inhabited regions were San Pablo Bay and in the vicinity of the Richmond Bridge where 15 (48%) and 12 (39%) individuals spent an average of 26.0 days (SD = 34.8) and 39.7 (SD = 58.2) days, respectively. It is notable that the San Francisco Bay and Golden Gate Bridge regions had the fewest number of individuals utilize these regions. Nine juveniles (29%) resided on average 28 days (SD = 30.3) within San Francisco Bay. We detected seven juveniles (23%) at the two cross-bay arrays near the Golden Gate Bridge averaging 27.1 days (SD = 37.7) at the closest array to the ocean. We assume individuals which were detected at the Golden Gate line made an ocean entry. Two individuals (GS 18 and 31) (7%) appeared to leave the system near the beginning of July. Four individuals (GS 07, 12, 25, and 27) (13%) likely made an ocean entry but either returned to the Golden Gate region or moved further upstream for the remainder of the study.
Total unique detections by individual green sturgeon within regions varied seasonally (Fig. 4). During spring (March to May 2013), most of the juvenile green sturgeon were detected within the Delta. During March, six individuals were detected by the automated receivers in the Antioch Bridge region and San Joaquin/Middle River reach (Central Delta), two near Frank’s Tract, three at Black’s Slough, with the westernmost sturgeon detected at the Petaluma River. One individual was detected at the Golden Gate array in April and two in May 2013. By summer (June and July), juvenile green sturgeon had dispersed widely throughout the watershed with six fish detected by the array west of Richmond Bridge. Only four fish were detected within the watershed during August, leading to speculation that they may have left the system to inhabit coastal waters. During fall, individuals detected within the watershed dispersed with equal numbers of fish detected below and above Antioch Bridge. During winter, we detected green sturgeon frequently in the Delta. It is possible that juveniles favor the Delta during winter and spring when waters are coolest and the lower bays during the summer and fall when waters are overall warmer throughout the upper regions of the system.
The number of juvenile green sturgeon detected at locations with receivers during the four seasons. The seasons are defined as between the vernal and autumn equinoxes and summer and winter solstices. The sizes of the solid squares are proportional to the number of detections of juvenile green sturgeon within that region
The optimal number of clusters (k) selected using the WSS elbow method showed a clear inflection point at k = 3. With an optimal clustering of k = 3, partitioning results indicated three centroids to which individuals could be assigned, with individual sturgeon represented by points within each of the clusters across the two dimensions (Fig. 5). Out of 31 fish, eight were assigned to cluster 1. Individuals partitioned to this cluster represented juvenile sturgeon that either had few detections within the Delta, but were not detected elsewhere, or individuals who exhibited extended Delta residency (red cluster) (Fig. 6). The second cluster consisted of 13 individuals and was characterized by transient individuals which moved between the lower Delta and middle estuary (green cluster). The third cluster consisted of the remaining nine individuals and was perhaps the most varied cluster across both dimensions. Individuals in cluster three either entered the ocean or utilized those regions nearest to San Francisco Bay across the study duration (blue cluster). Dimension 1 of the cluster analysis explained 78.1% of variation between clusters, while dimension 2 explained 11.4% of the variation.
Partitioning Around Medoids clustering of normalized presence versus absence matrix by river kilometer and date of year for each individual green sturgeon using the optimal k = 3 selection. Clustering results shown here can be thought of as the clustering of individuals for similarities in use across two dimensions (space and time), based upon the individual’s distribution of presence/absence when compared against all other individuals
Distance within the Sacramento-San Joaquin watershed in river kilometers (rkm) over the 9-month period that the ultrasonic tags were active for 31 juvenile green sturgeon. Each plot is identified by its VEMCO Ltd. Code, but can be cross-referenced to the plots in Fig. 3 and occupation percentages in Table 1 by starting with one in the upper right and proceeding consecutively from left to right and down row by row (5 missing lacking detection by a monitor) until 32 at the bottom right. The curves are color coded according to the PAM clustering results, with red referring to cluster 1, green cluster 2, and blue cluster 3
Discussion
We documented several distinct movement patterns by juvenile green sturgeon using differences in regional habitat use of passively tracked fish. Over a 9-month period, we found through inspection of plots that individuals exhibited six varied but distinct spatial movement patterns. These movements, however, were not consistent with a strict definition of long-distance, annual migrations in response to changes in temperature, food supply, or the amount of daylight for the purpose of feeding or mating. Some of the movements were within outmigration pathways within the Sacramento-San Joaquin watershed; others were temporary or permanent visits to the coastal waters. We performed a PAM clustering analysis, on the normalized presence absence of individuals within a region for a given time, to quantify statistical typologies of movements among individuals. The PAM clustering using k = 3 resulted in the most biologically reasonable solution without data overfitting. The analysis indicated that (1) some individuals moved very little, staying within the Delta, (2) others moved down in the watershed into San Pablo and San Francisco Bays, (3) while a few fish exited the Golden Gate, those that did either stayed in the ocean or returned to the bay.
Flexible movement patterns are likely adaptive for green sturgeon, especially in California’s Central Valley at the southern end of their distribution. Sturgeon species are classically defined by “periodic” life histories characterized by high fecundity, low juvenile survivorship, and delayed age-at-sexual maturity (Winemiller and Rose 1992). It is believed that recruitment of juvenile sturgeon in the Central Valley often occurs under specific environmental conditions, e.g., during wet climatic periods in California. Long-term population abundance data does not currently exist for green sturgeon in the Central Valley to test this assertion. However, reporting of cohort recruitment into the white sturgeon sport fishery does provide some evidence of episodic recruitment which aligns well with past high-water years (CDFW 2017).
Despite a small population size, currently and likely historically, the green sturgeon’s genetics may provide for added heterogeneity and phenotypic plasticity through their 8 N polyploidy (Schulte 2004). Israel et al. (2004) provide evidence for maintained genetic diversity within the sDPS of green sturgeon despite a rather small population size, perhaps implying a need for rapidly adaptive life-history characteristics and behaviors. Given the capability of green sturgeon to navigate essentially the entire corridor of the Sacramento River, Delta, Bay, and adjacent marine habitats, it is perhaps not surprising that individual behaviors of juveniles vary widely. Yet prevalence of distinct and diagnosable behaviors is intriguing and implies an evolutionary basis for wide tolerances to environmental niches based upon their broad distribution across ecoregions. Green sturgeon are already known to possess two genetically distinct population segments at the macro-ecological scale, termed a Northern Distinct Population Segment (nDPS) and a Southern Distinct Population Segment (sDPS) (Israel et al. 2004). The two DPS spawning populations are separated geographically with the former present in the Klamath River in northern California, Rogue River in Oregon, and the latter in the Sacramento River in central California State. Therefore, additional diversification within the green sturgeon complex is feasible and should be considered in future green sturgeon research and management plans.
Many other native California fishes exhibit diversification of life-history strategies. Chinook Salmon Oncorhynchus tshawytscha lives in the same regions of the Sacramento-San Joaquin watershed as the green sturgeon and also possesses key variations in its behavioral chronology of movement patterns. Biologists have identified four different evolutionarily significant units (ESUs) of Chinook salmon in the Central Valley, distinguished by variations in spawning run timing, spawning time, former spawning habitat, and times of emergence, freshwater residency, and ocean entry of juveniles (Fisher 1994). Thus, timing of immigration and emigration differ from each other due to drastically different environmental conditions throughout the year (Yoshiyama et al. 1998). There is strong evidence based on microsatellite studies that each run is genetically distinct (Prince et al. 2017; Banks et al. 2000).
Results from this study point to an emerging need to explore the genotypic and phenotypic basis for spatial differences in habitat use within the juvenile life history in sDPS green sturgeon. For example, while this study focused on juvenile green sturgeon, it is possible that distinct adult green sturgeon life histories also exist. Adult sDPS green sturgeon appear to have different outmigration strategies. A proportion of the population leaves the upper Sacramento River immediately after spawning in early summer, while others “over-summer,” migrating later in winter (Steel et al. 2018). Similarly, juveniles appear to exhibit divergent movement patterns while maturing in the lower watershed.
Unraveling the genotype-to-phenotype pathways will take time and additional research. Yet in the interlude, protective conservation management practices remain necessary, in part because of the federal listing status of green sturgeon, but also because the species is indeed in decline and in jeopardy of extirpation (Adams et al. 2007). Consideration of habitat quantity and quality is essential for successful fish conservation management (Sass et al. 2017); thus, some prioritization of habitat management actions will ultimately be required to sufficiently protect juvenile green sturgeon in all their habitats. Results from this study indicate that juvenile green sturgeon have an almost ubiquitous approach to utilizing habitats within the three described regions. Given green sturgeon appear to be ubiquitous throughout the region, special consideration must be given to adaptive management strategies that are specific to sturgeon that do not rely upon conventions typically used in salmonid management (i.e., work windows).
The existence of multiple movement patterns by juvenile green sturgeon provides an early indication of the potential importance of these patterns of movement to the stability of the population overall. For example, multiple runs of salmon statistically compress population variance across all runs of salmon over time (Tilman et al. 1998). Understanding and acknowledging the simple existence of this effect and managing for an intraspecific life-history diversity is essential to sustaining myriad species and ecosystems (Chang et al. 2020). Because of limited tag life, unexplored genomic differences, limited population data, and low ability to capture wild individuals, it remains difficult to discern a portfolio effect for green sturgeon in the region. Future studies should focus on several priority science questions: (1) Do the observed patterns of movement by juvenile green sturgeon scale to adults; (2) Are different movement patterns based in genetic differences or are they strictly plasticity operating among individuals; (3) If patterns of movement are genetically wired, how can juvenile green sturgeon populations be best protected and managed; and (4) Is a portfolio effect a fundamental aspect to the ecology of green sturgeon populations in California and throughout Western North America?
Telemetry techniques such as those used in this study will continue to provide actionable science towards enhanced conservation and management of green sturgeon and a diversity of other species (e.g., Heublein et al. 2009; Thomas et al. 2014; Mora et al. 2018; Steel et al. 2018). Through long-term tagging of juveniles and adults, habitat utilization can continually be mapped to assist in conservation prioritization of habitats used by juvenile green sturgeon in the Sacramento River ecosystem. Furthermore, future consideration as to the effect of flow conveyance projects within the Delta and ultimately the estuary should appropriately weigh against the likelihood of disturbing the broad range of habitats used by this highly rare species.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request or may be found through our open source database (BARD; http://cftc.metro.ucdavis.edu/biotelemetry-autonomous-real-time-database/landingmap).
References
Adams PB, Grimes C, Hightower JE, Lindley ST, Moser ML, Parsley MJ (2007) Population status of North American Green Sturgeon, Acipenser medirostris. Environ Biol Fish 79:339–356
Allen PJ, Cech JJ (2007) Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79:211–229
Allen PJ, Hobbs JA, Cech JJ Jr, Van Eenennaam JP, Doroshov SI (2009) Using trace elements in pectoral fin rays to assess life history movements in sturgeon: estimating age at initial seawater entry in Klamath River green sturgeon. Trans Am Fish Soc 138:240–250
Allen PJ, McEnroe M, Forostyan T, Cole S, Nicholl M, Hodge B, Cech JJ Jr (2011) Ontogeny of salinity tolerance and evidence for seawater-entry preparation in juvenile green sturgeon, Acipenser medirostris. J Comp Physiol B 181:903–920
Ammann AJ, Sandstrom P, Chapman E, Michel C, Klimley P, MacFarlane R, Lindley S (2007) The performance of Vemco V7, V9, and V16 transmitters and receivers under varying conditions. Poster, 8th Biennial State of the Estuary Conference, Oakland
Banks MA, Rashbrook VK, Calavetta MJ, Dean CA, Hedgecock D (2000) Analysis of microsatellite DNA resolves genetic structure and diversity of Chinook salmon (Oncorhynchus tshawytscha) in California’s Central Valley. Can J Fish Aquat Sci 57:915–927
[CDFW] California Department of Fish and Wildlife (2017) Predicting 2015 and 2016 White Sturgeon Year Class Index. https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentId=139781&inline
Carlson SM, Satterthwaite WH (2011) Weakened portfolio effect in a collapsed salmon population complex. Can J Fish Aquat Sci 68:1579–1589
Chang C-W, Ye H, Miki T, Deyle ER, Souissi S, Anneville O, Adrian R, Chiang Y-R, Ichise S, Kumagai M, S-iS M, Shiah F-K, Wu J-T, Hsieh C-h, Sugihara G (2020) Long-term warming destabilizes aquatic ecosystems through weakening biodiversity-mediated causal networks. Glob Chang Biol 26:6413–6423
Chapman ED, Hearn AR, Michel CJ, Ammann AJ, Lindley ST, Thomas MJ, Sandstrom PT, Singer GP, Peterson ML, MacFarlane RB, Klimley AP (2013) Diel movements of out-migrating Chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (Oncorhynchus mykiss) smolts in the Sacramento/San Joaquin watershed. Environ Biol Fish 96:273–286
Chapman ED, Hearn AR, Singer GP, Brostoff WN, LaCivita PE, Klimley AP (2014) Movements of out-migrating steelhead smolts through the San Francisco Bay Estuary. Environ Biol Fish 98:1069–1080
Demetras N, Helwig B, McHuron A (2020) Reported vessel strike as a source of mortality of White Sturgeon in San Francisco Bay. Calif Fish Game 106:59–65
Fisher FW (1994) Past and present status of Central Valley chinook salmon. Conserv Biol 8:870–873
Grill G, Lehner B, Thieme M, Geenen B, Tickner D, Antonelli F, Babu S, Borrelli P, Cheng L, Crochetiere H, Ehalt Macedo H, Filgueiras R, Goichot M, Higgins J, Hogan Z, Lip B, McClain ME, Meng J, Mulligan M, Nilsson C, Olden JD, Opperman JJ, Petry P, Reidy Liermann C, Sáenz L, Salinas-Rodríguez S, Schelle P, Schmitt RJP, Snider J, Tan F, Tockner K, Valdujo PH, van Soesbergen A, Zarfl C (2019) Mapping the world’s free-flowing rivers. Nature 569:215–221
Hearn AR, Chapman ED, Singer GP, Brostoff WN, LaCivita PE, Klimley AP (2014) Movements of out-migrating late-fall run Chinook salmon (Oncorhynchus tshawytscha) smolts through the San Francisco Bay Estuary. Environ Biol Fish 97:851–863
Heublein JC, Kelly JT, Crocker CE, Klimley AP, Lindley ST (2009) Migration of green sturgeon, Acipenser medirostris, in the Sacramento River. Environ Biol Fish 84:245–258
Israel JA, Cordes JF, Blumberg MA, May B (2004) Geographic patterns of genetic differentiation among collections of green sturgeon. N Am J Fish Manag 24:922–931
Kassambara A, Mundt F (2020) Factoextra: extract and visualize results of multivariate data analyses. R package version 1.0.7
Katz J, Moyle PB, Quiñones RM, Israel J, Purdy S (2013) Impending extinction of salmon, steelhead, and trout (Salmonidae) in California. Environ Biol Fish 96:1169–1186
Kaufman L, Rousseeuw PJ (1990) Finding groups in data: an introduction to cluster analysis. Wiley, New York
Kessel ST, Hondorp DW, Holbrook CM, Boase JC, Chiotti JA, Thomas MV, Krueger CC (2018) Divergent migration within lake sturgeon (Acipenser fulvescens) populations: multiple distinct patterns exist across an unrestricted migration corridor. J Anim Ecol 87:259–273
Klimley AP, Chapman ED, Cech JJ, Cocherell DE, Fangue NA, Gingras M, Jackson Z, Miller EA, Mora EA, Poletto JB, Schreier AM, Seesholtz A, Sulak KJ, Thomas MJ, Woodbury D, Wyman MT (2015) Sturgeon in the Sacramento-San Joaquin watershed: new insights to support conservation and management. San Fran Est Watershed Sci 13:1–19
Miller EA, Froehlich HE, Cocherell DE, Thomas MJ, Cech JJ Jr, Klimley AP, Fangue NA (2014) Effects of acoustic tagging on juvenile green sturgeon incision healing, swimming performance, and growth. Environ Biol Fish 97:647–658
Mora EA, Battleson RD, Lindley ST, Thomas MJ, Bellmer R, Zarri LJ, Klimley AP (2018) Estimating the annual spawning run size and population size of the southern distinct population segment of green sturgeon. Trans Am Fish Soc 147:195–203
Moyle PB (2002) Inland fishes of California. University of California Press, Berkeley
Moyle PB, Katz JVE, Quiñones RM (2011) Rapid decline of California’s native inland fishes: a status assessment. Biol Conserv 144:2414–2423
Moyle PB, Quiñones RM, Katz JV, Weaver J (2015) Fish species of special concern in California. Sacramento: California Department of Fish and Wildlife. www.wildlife.ca.gov
Nilsson C, Reidy CA, Dynesius M, Revenga C (2005) Fragmentation and flow regulation of the world’s large river systems. Science 308:405–408
[NOAA] National Oceanic and Atmosphere Administration (2008) Endangered and threatened wildlife and plants; proposed rulemaking to designate critical habitat for the threatened Southern distinct population segment of North American green sturgeon Federal Register 73: 52084–52110
Poletto JB, Cocherell DE, Klimley AP, Cech JJ Jr, Fangue NA (2013) Behavioral salinity preferences of juvenile green sturgeon Acipenser medirostris acclimated to fresh water and full-strength salt water. J Fish Biol 82:671–685
Poletto JB, Martin B, Danner E, Baird SE, Cocherell DE, Hamda N, Cech JJ Jr, Fangue NA (2018) Assessment of multiple stressors on the growth of larval green sturgeon Acipenser medirostris: implications for recruitment of early life-history stages. J Fish Biol 93:952–960
Poytress WR, Gruber JJ, Van Eenennaam JP, Gard M (2015) Spatial and temporal distribution of spawning events and habitat characteristics of Sacramento River Green Sturgeon. Trans Am Fish Soc 144:1129–1142
Prince DJ, O’Rourke SM, Thompson TQ, Ali OA, Lyman HS, Saglam IK, Hotaling TJ, Spidle AP, Miller MR (2017) The evolutionary basis of premature migration in Pacific salmon highlights the utility of genomics for informing conservation. Sci Adv 3(8):e1603198
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Radtke LD (1966) Distribution of smelt, juvenile sturgeon and starry flounder in the Sacramento – San Joaquin Delta. Fish Bull Cal Dept Fish Game 136:115–119
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873
Rodgers EM, Poletto JB, Gomez Isaza DF, Van Eenennaam JP, Connon RE, Todgham AE, Seesholtz A, Heublein JC, Cech JJ Jr, Kelly JT, Fangue NA (2019) Integrating physiological data with the conservation and management of fishes: a meta-analytical review using the threatened green sturgeon (Acipenser medirostris). Conserv Physiol 7:1–22
Sardella BA, Sanmarti E, Kültz D (2008) The acute temperature tolerance of green sturgeon (Acipenser medirostris) and the effect of environmental salinity. J Exp Zool A Ecol Gen Physiol 309:477–483
Sass GG, Rypel AL, Stafford JD (2017) Inland fisheries habitat management: lessons learned from wildlife ecology and a proposal for change. Fisheries 42:197–209
Satterthwaite W, Carlson S (2015) Weakening portfolio effect strength in a hatchery-supplemented Chinook salmon population complex. Can J Fish Aquat Sci 72:1860–1875
Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS (2010) Population diversity and the portfolio effect in an exploited species. Nature 465:609–612
Schindler DE, Armstrong JB, Reed TE (2015) The portfolio concept in ecology and evolution. Front Ecol Environ 13:257–263
Schulte PM (2004) Changes in gene expression as biochemical adaptations to environmental change: a tribute to Peter Hochachka. Comp Biochem Physiol B Biochem Mol Biol 139:519–529
Singer G, Hearn A, Chapman E, Peterson M, LaCivita P, Brostoff W, Bremner A, Klimley AP (2013) Interannual variation of reach specific migratory success for Sacramento River hatchery yearling late-fall run Chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (Oncorhynchus mykiss). Environ Biol Fish 96:363–379
Steel AE, Thomas MJ, Klimley AP (2018) Reach specific use of spawning habitat by adult green sturgeon (Acipenser medirostris) under different operation schedules at Red Bluff Diversion Dam. J Appl Ichthyol 35:1–8
Stewart AR, Feyrer F, Johnson RC (2020) Resolving selenium exposure risk: spatial, temporal, and tissue-specific variability of an endemic fish in a large, dynamic estuary. Sci Total Environ 707:135919
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Thomas MJ, Peterson ML, Chapman ED, Hearn AR, Singer GP, Battleson RD, Klimley AP (2014) Behavior, movements, and habitat use of adult green sturgeon, Acipenser medirostris, in the upper Sacramento River. Environ Biol Fish 97:133–146
Thomas MJ, Peterson ML, Chapman ED, Fangue NA, Klimley AP (2019) Individual habitat usage patterns and behavior of acoustically-tagged juvenile green sturgeon in the Sacramento-San Joaquin Delta. Environ Biol Fish 102:1025–1037
Tilman D, Lehman CL, Bristow CE (1998) Diversity-stability relationships: statistical inevitability or ecological consequence? Am Nat 151:277–282
Verhille C, Poletto J, Cocherell D, DeCourten B, Baird SC Jr, Fangue N (2014) Larval green and white sturgeon swimming performance in relation to water-diversion flows. Conserv Physiol 2:1–14
Winemiller KO, Rose KA (1992) Patterns of life-history diversification in North American fishes: implications for population regulation. Can J Fish Aquat Sci 49:2196–2218
Yamane L, Botsford LW, Kilduff DP (2017) Tracking restoration of population diversity via the portfolio effect. J Appl Ecol 55:472–481
Yoshiyama RM, Fisher FW, Moyle PB (1998) Historical abundances and decline of Chinook Salmon in the Central Valley Region of California. N Am J Fish Manag 18:487–552
Acknowledgements
We thank biologists of the FWS under the supervision of William Poytress and Josh Gruber for capturing post-larval green sturgeon and providing the opportunity to have these rare samples. We would also like to acknowledge the efforts of junior staff and students from UC Davis, who participated in various aspects of field work along the way, and their energy and efforts should be recognized. We appreciate the feedback and comments provided by the two anonymous reviewers which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and conflict of interest statement
We acknowledge funding provided by the USBOR (United States Bureau of Reclamation) for the research program, entitled “Sacramento River Green Sturgeon Migration and Population Assessment” (Cooperative Agreement: R10AC20012) (A. Peter Klimley, Principal Investigator). NAF and ALR were supported by the Agricultural Experiment Station of the University of California, Project CA-D-WFB-2098-H and CA-D-WFB-2467-H, respectively. ALR is also supported by the California Trout and Peter B. Moyle Endowment for Coldwater Fish Conservation. This work was approved through the University of California, Davis Institutional Animal Care and Use Committee. The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

ESM 1
Fig. SI-1. The distribution of autonomous monitors within the Delta region. Note the yellow star marker denotes the Santa Clara Shoal release site (PNG 2353 kb)

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, M.J., Rypel, A.L., Singer, G.P. et al. Movement patterns of juvenile green sturgeon (Acipenser medirostris) in the San Francisco Bay Estuary. Environ Biol Fish 105, 1749–1763 (2022). https://doi.org/10.1007/s10641-022-01245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01245-5