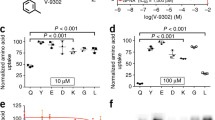
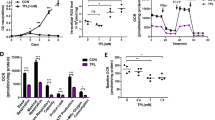
Summary
Targeting tumor metabolic vulnerabilities such as “glutamine addiction” has become an attractive approach for the discovery of novel antitumor agents. Among various mechanisms explored, SLC1A5, a membrane transporter that plays an important role in glutamine cellular uptake, represents a viable target to interfere with tumor’s ability to acquire critical nutrients during proliferation. In the present study, a stably transfected HEK293 cell line with human SLC1A5 (HEK293-SLC1A5) was established for the screening and identification of small molecule SLC1A5 inhibitors. This in vitro system, in conjunction with direct measurement of SLC1A5-mediated L-glutamine-2,3,3,4,4-D5 (substrate) uptake, was practical and efficient in ensuring the specificity of SLC1A5 inhibition. Among a group of diverse compounds tested, mianserin (a tetracyclic antidepressant) demonstrated a marked inhibition of SLC1A5-mediated glutamine uptake. Subsequent investigations using SW480 cells demonstrated that mianserin was capable of inhibiting SW480 tumor growth both in vitro and in vivo, and the in vivo antitumor efficacy was correlated to the reduction of glutamine concentrations in tumor tissues. Computational analysis revealed that hydrophobic interactions between SLC1A5 and its inhibitors could be a critical factor in drug design. Taken together, the current findings confirmed the feasibility of targeting SLC1A5-mediated glutamine uptake as a novel approach for antitumor intervention. It is anticipated that structural insights obtained based on homology modeling would lead to the discovery of more potent and specific SLC1A5 inhibitors for clinical development.






Similar content being viewed by others
Data Availability
The data presented in this study are available in the paper and Supplementary Information.
References
Schumann T, König J, Henke C, Willmes DM, Bornstein SR, Jordan J, Fromm MF, Birkenfeld AL (2020) Solute Carrier Transporters as Potential Targets for the Treatment of Metabolic Disease. Pharmacol Rev 72(1):343–379. https://doi.org/10.1124/pr.118.015735
Yahyaoui R, Pérez-Frías J (2019) Amino Acid Transport Defects in Human Inherited Metabolic Disorders. Int J Mol Sci 21(1). https://doi.org/10.3390/ijms21010119
Hanahan D (2022) Hallmarks of Cancer: New Dimensions. Cancer Discov 12(1):31–46. https://doi.org/10.1158/2159-8290.Cd-21-1059
Pizzagalli MD, Bensimon A, Superti-Furga G (2021) A guide to plasma membrane solute carrier proteins. FEBS J 288(9):2784–2835. https://doi.org/10.1111/febs.15531
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35(8):427–433. https://doi.org/10.1016/j.tibs.2010.05.003
Scalise M, Pochini L, Console L, Losso MA, Indiveri C (2018) The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front Cell Dev Biol 6:96. https://doi.org/10.3389/fcell.2018.00096
Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, Schmitt A, Poulain L, Green AS, Uzunov M et al (2013) Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122(20):3521–3532. https://doi.org/10.1182/blood-2013-03-493163
Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JC et al (2018) Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med 24(2):194–202. https://doi.org/10.1038/nm.4464
Feng Y, Pathria G, Heynen-Genel S, Jackson M, James B, Yin J, Scott DA, Ronai ZA (2021) Identification and Characterization of IMD-0354 as a Glutamine Carrier Protein Inhibitor in Melanoma. Mol Cancer Ther 20(5):816–832. https://doi.org/10.1158/1535-7163.Mct-20-0354
Toda K, Nishikawa G, Iwamoto M, Itatani Y, Takahashi R, Sakai Y, Kawada K (2017) Clinical Role of ASCT2 (SLC1A5) in KRAS-Mutated Colorectal Cancer. Int J Mol Sci 18(8). https://doi.org/10.3390/ijms18081632
Ma H, Wu Z, Peng J, Li Y, Huang H, Liao Y, Zhou M, Sun L, Huang N, Shi M et al (2018) Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int J Cancer 142(12):2578–2588. https://doi.org/10.1002/ijc.31274
Suzuki M, Toki H, Furuya A, Ando H (2017) Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem Biophys Res Commun 482(4):651–657. https://doi.org/10.1016/j.bbrc.2016.11.089
Wang W, Zou W (2020) Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy. Mol Cell 80(3):384–395. https://doi.org/10.1016/j.molcel.2020.09.006
Garibsingh RA, Ndaru E, Garaeva AA, Shi Y, Zielewicz L, Zakrepine P, Bonomi M, Slotboom DJ, Paulino C, Grewer C et al (2021) Rational design of ASCT2 inhibitors using an integrated experimental-computational approach. Proc Natl Acad Sci U S A 118(37). https://doi.org/10.1073/pnas.2104093118
Osanai-Sasakawa A, Hosomi K, Sumitomo Y, Takizawa T, Tomura-Suruki S, Imaizumi M, Kasai N, Poh TW, Yamano K, Yong WP et al (2018) An anti-ASCT2 monoclonal antibody suppresses gastric cancer growth by inducing oxidative stress and antibody dependent cellular toxicity in preclinical models. Am J Cancer Res 8(8):1499–1513
Mazzei M, Vascellari M, Zanardello C, Melchiotti E, Vannini S, Forzan M, Marchetti V, Albanese F, Abramo F (2019) Quantitative real time polymerase chain reaction (qRT-PCR) and RNAscope in situ hybridization (RNA-ISH) as effective tools to diagnose feline herpesvirus-1-associated dermatitis. Vet Dermatol 30(6):491-e147. https://doi.org/10.1111/vde.12787
Bai X, Moraes TF, Reithmeier RAF (2017) Structural biology of solute carrier (SLC) membrane transport proteins. Mol Membr Biol 34(1–2):1–32. https://doi.org/10.1080/09687688.2018.1448123
Altamura AC, De Novellis F, Mauri MC, Gomeni R (1987) Plasma and brain pharmacokinetics of mianserin after single and multiple dosing in mice. Prog Neuropsychopharmacol Biol Psychiatry 11(1):23–33. https://doi.org/10.1016/0278-5846(87)90028-5
Canul-Tec JC, Assal R, Cirri E, Legrand P, Brier S, Chamot-Rooke J, Reyes N (2017) Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature 544(7651):446–451. https://doi.org/10.1038/nature22064
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L et al (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(Web Server issue):W252–258. https://doi.org/10.1093/nar/gku340
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27(3):343–350. https://doi.org/10.1093/bioinformatics/btq662
Sybyl-X 2.0 (2012) Tripos International, St, USA, Louis, MO
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron 36(22):3219–3228
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791. https://doi.org/10.1002/jcc.21256
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8(2):127–134
Colas C, Grewer C, Otte NJ, Gameiro A, Albers T, Singh K, Shere H, Bonomi M, Holst J, Schlessinger A (2015) Ligand Discovery for the Alanine-Serine-Cysteine Transporter (ASCT2, SLC1A5) from Homology Modeling and Virtual Screening. PLoS Comput Biol 11(10):e1004477. https://doi.org/10.1371/journal.pcbi.1004477
Garibsingh RA, Otte NJ, Ndaru E, Colas C, Grewer C, Holst J, Schlessinger A (2018) Homology Modeling Informs Ligand Discovery for the Glutamine Transporter ASCT2. Front Chem 6:279. https://doi.org/10.3389/fchem.2018.00279
Ni Y, Duan Z, Zhou D, Liu S, Wan H, Gui C, Zhang H (2020) Identification of Structural Features for the Inhibition of OAT3-Mediated Uptake of Enalaprilat by Selected Drugs and Flavonoids. Front Pharmacol 11:802. https://doi.org/10.3389/fphar.2020.00802
Xiang Y, Liu S, Yang J, Wang Z, Zhang H, Gui C (2020) Investigation of the interactions between flavonoids and human organic anion transporting polypeptide 1B1 using fluorescent substrate and 3D-QSAR analysis. Biochim Biophys Acta Biomembr 5:183210. https://doi.org/10.1016/j.bbamem.2020.183210
Garaeva AA, Oostergetel GT, Gati C, Guskov A, Paulino C, Slotboom DJ (2018) Cryo-EM structure of the human neutral amino acid transporter ASCT2. Nat Struct Mol Biol 25(6):515–521. https://doi.org/10.1038/s41594-018-0076-y
Yu X, Plotnikova O, Bonin PD, Subashi TA, McLellan TJ, Dumlao D, Che Y, Dong YY, Carpenter EP, West GM et al (2019) Cryo-EM structures of the human glutamine transporter SLC1A5 (ASCT2) in the outward-facing conformation. eLife 8. https://doi.org/10.7554/eLife.48120
Albers T, Marsiglia W, Thomas T, Gameiro A, Grewer C (2012) Defining substrate and blocker activity of alanine-serine-cysteine transporter 2 (ASCT2) Ligands with Novel Serine Analogs. Mol Pharmacol 81(3):356–365. https://doi.org/10.1124/mol.111.075648
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534. https://doi.org/10.1016/j.cell.2008.11.044
Scalise M, Pochini L, Pingitore P, Hedfalk K, Indiveri C (2015) Cysteine is not a substrate but a specific modulator of human ASCT2 (SLC1A5) transporter. FEBS Lett 589(23):3617–3623. https://doi.org/10.1016/j.febslet.2015.10.011
Zhang H, Cui K, Yao S, Yin Y, Liu D, Huang Z (2021) Comprehensive molecular and clinical characterization of SLC1A5 in human cancers. Pathol Res Pract 224:153525. https://doi.org/10.1016/j.prp.2021.153525
Brogden RN, Heel RC, Speight TM, Avery GS (1978) Mianserin: a review of its pharmacological properties and therapeutic efficacy in depressive illness. Drugs 16(4):273–301. https://doi.org/10.2165/00003495-197816040-00001
Kelder J, Funke C, De Boer T, Delbressine L, Leysen D, Nickolson V (1997) A comparison of the physicochemical and biological properties of mirtazapine and mianserin. J Pharm Pharmacol 49(4):403–411. https://doi.org/10.1111/j.2042-7158.1997.tb06814.x
Raiteri M, Angelini F, Bertollini A (1976) Comparative study of the effects of mianserin, a tetracyclic antidepressant, and of imipramine on uptake and release of neurotransmitters in synaptosomes. J Pharm Pharmacol 28(6):483–488. https://doi.org/10.1111/j.2042-7158.1976.tb02770.x
Olivier B, Soudijn W, van Wijngaarden I (2000) Serotonin, dopamine and norepinephrine transporters in the central nervous system and their inhibitors. Prog Drug Res 54:59–119. https://doi.org/10.1007/978-3-0348-8391-7_3
Valentini V, Frau R, Di Chiara G (2004) Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem 88(4):917–927. https://doi.org/10.1046/j.1471-4159.2003.02238.x
Bhutia YD (1863) Ganapathy V (2016) Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta 10:2531–2539. https://doi.org/10.1016/j.bbamcr.2015.12.017
Scalise M, Pochini L, Galluccio M, Console L, Indiveri C (2020) Glutamine transporters as pharmacological targets: From function to drug design. Asian J Pharm Sci 15(2):207–219. https://doi.org/10.1016/j.ajps.2020.02.005
Acknowledgements
The authors were grateful for the technical assistance provided by Dr. Fanyi Meng during the establishment of the SW480 tumor xenograft model as well as database search and analysis.
Funding
This research was supported by the National Natural Science Foundation of China (No. 82173880).
Author information
Authors and Affiliations
Contributions
Z.D. and Z.Z. participated in the design and conduct of in vitro and in vivo experiments in addition to data analysis and manuscript drafting and editing. F.L., Y.Z. and X.G. contributed to the establishment of in vitro cell models as well as in vivo xenograft models. C.G. was instrumental in computational analysis and manuscript drafting. H.Z. was involved in conceptualization of the studies and manuscript drafting, editing and finalization.
Corresponding author
Ethics declarations
Ethics approval
All experimental procedures were performed in accordance with the NIH guidelines for Care and Use of Laboratory Animals; all procedures and protocols were approved by the Institutional Animal Care and Use Committee at Soochow University (#202107A0121). Patents A patent application on a method for screening of SLC1A5 inhibitors and mianserin’s use as antitumor agent has been filed with the China National Intellectual Property Administration (Application number: CN202111328929.3).
Competing interests
The authors declare no potential conflicts of interest during the study and preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, Z., Zhou, Z., Lu, F. et al. Antitumor activity of mianserin (a tetracyclic antidepressant) primarily driven by the inhibition of SLC1A5-mediated glutamine transport. Invest New Drugs 40, 977–989 (2022). https://doi.org/10.1007/s10637-022-01284-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01284-w