Abstract
Wild species living in captivity are subject to loss of genetic diversity, inbreeding depression, and differentiation among populations. Only very few species have been under human care for centuries but have not been selectively bred, have free-ranging movements most of the time, and retain porous barriers to gene flow between wild and captive populations. Such captive populations are expected to retain high levels of genetic diversity and anthropogenic factors should result in a limited genetic differentiation from wild populations. Asian elephants have been trained and used by humans for at least 4000 years as war animals, mounts of kings and draught animals. In Myanmar and Laos, elephants are still being used for hauling timber in the forest while retaining traditional management practices including seasonal release, free mating and movement. However, habitat fragmentation, isolation and reduced gene flows are threatening both semi-captive and wild pools. We genotyped 167 semi-captive elephants from Laos and Myanmar using a panel of 11 microsatellite loci to estimate the genetic diversity and population structure. We found that elephants of both countries presented high levels of genetic diversity and a low degree of inbreeding, if any. This agrees with the expected high level of genetic diversity in semi-captive populations. We found a weak differentiation along a geographical gradient from southern Laos to northern Myanmar but no differentiation between wild-caught and captive-born pools. The potential value for conservation of a large population of semi-captive elephants has been recognized but the conservation community has yet to fully explore the potential role semi-captive elephants could play in maintaining gene flows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic factors are important drivers of the evolutionary history of species and populations (Miraldo et al. 2016; Queirós et al. 2020). Among them landscape changes, such as habitat loss and fragmentation, connectivity impairment and population isolation have led to decrease in species abundance and limited gene flow (Kvie et al. 2019). These changes may further lead to depletion of genetic variation within populations as well as accelerating differentiation among populations (Frankham et al. 2004). Another major anthropogenic driver of changes in the genetic structure and diversity is the human exploitation of wild species through hunting, capture and breeding of wild populations (Barron et al. 2022). Genetic adaptation to captivity of wild species living under human care includes, among others, loss of genetic diversity and inbreeding depression that ultimately could further enhance differentiation between captive and wild populations (Frankham 2008). One would expect that the anthropogenic factors mentioned above might also shape the genetic structure and diversity of semi-captive populations. Only very few species have been under human care for centuries but have not been selectively bred, have free-ranging movements most of the time, and retain porous gene flow between wild and captive genetic pools through capture, release and/or reproduction. These free-ranging populations are usually managed under extensive herding systems that have undergone recent large-scale landscape changes, such as habitat loss and fragmentation, subdivide wild populations, reduce their size, and limit gene flow (Forbes et al. 2009; Kvie et al. 2019; Maurer et al. 2021). Among these semi-captive species, reindeer (Rangifer tarandus) has been managed under extensive husbandry practices, with capture and crosses breeding between wild and herded individuals. Both wild and semi-captive reindeer populations retain high levels of genetic diversity but the two groups show mild differentiation as a result of husbandry practices (Kvie et al. 2019; Røed et al. 2008).
Like the reindeer, the Asian elephant (Elephas maximus) is an iconic species in which captive herds are in close contact with wild individuals (Sukumar 2003; Vidya 2016). Asian elephant populations are in overall decline and are classified as Endangered by the International Union for Conservation of Nature (Williams et al. 2020). The total number of elephants has been reduced by at least 50% over the past three decades. Populations are affected by habitat fragmentation and loss, human–elephant conflicts, and poaching for ivory (Dublin et al. 2006; Hedges et al. 2005; Leimgruber et al. 2003; Sukumar 2006). The total population is estimated between 30,000 and 50,000 individuals, although these figures may be overestimates (Hedges et al. 2008). An estimated 14.500 to 16.000 elephants live in captivity or in semi-wild conditions, roaming freely at night or during idle periods in the forest (Leimgruber et al. 2008; Sukumar 2016). Elephants have been trained and used by humans for at least 4000 years as war animals, mounts of kings and draught animals for transport and forestry (Trautmann 2015). Nowadays elephants are employed as draught animals by the logging industry and by villagers, but also for tourism and representation in temples (Maurer 2018; Sukumar 2016). Over past centuries, large levels of gene flows have been retained between the captive and wild pools as most captive elephants were caught in the wild to maintain the tamed population (Lair 1997; Leimgruber et al. 2008; Sukumar 2016). The conservation value of semi-captive elephants is still disputed and could be seen, either as an opportunity or a threat for the survival of this endangered species (Hedges et al. 2018). For instance, the Myanmar Elephant Conservation Action Plan (MECAP) 2018–2027 (Hedges et al. 2018) recognizes that “the successful conservation of a species with captive relatives—including Asian Elephants—requires the captive population to be managed in such a manner that it does not constitute a threat to the wild population” (MECAP, p. 29). On the one hand, captive individuals could serve as a reservoir to support reintroduction among isolated wild populations (Dublin et al. 2006; Hedges et al. 2018). On the other hand, releases of captive individuals could favour the transmission of diseases to the wild pool (Lassausaie et al. 2015) or could increase crop raiding as habituated elephants could be fearless to humans (Hedges et al. 2018).
Myanmar and Laos are still using elephants for hauling timber in the forest while keeping traditional management practices of seasonal release, free mating and movement. Myanmar is home to around 2000 wild elephants living in large forest areas presenting low human densities (Leimgruber et al. 2011; Sampson et al. 2018; Songer et al. 2016; Thant et al. 2022). There are about 5000 captive elephants of which 3000 are owned by the government and 2000 are privately held (Asian Elephant Range States Meeting report 2017). These elephants are semi-captive and work for the timber industry (Crawley et al. 2020a, b). At night, captive elephants forage in the forest without supervision where they encounter tame and wild conspecifics (Lahdenperä et al. 2018). Laos hosts a population of 500–600 wild elephants living mostly in small and isolated herds, except for two populations of respectively 130 and 60–80 individuals living in Nakay and Nam Pouy protected areas (Menon and Tiwari 2019). Laos also shelters 450 captive elephants employed in the logging industry and more recently for tourism (Maurer et al. 2017). Most Lao captive elephants live in the northern province of Sayaboury.
In Laos and Myanmar, captive elephants share the same habitat as their wild conspecifics and interact regularly. In Laos, captive females are released part of the year and they can mate with wild males from the Nam Pouy protected area, thus contributing 80% of captive births in the country (Maurer et al. 2017, 2021). Gene flow from captive to wild elephants is also present, but to a lesser extent, through the release or accidental escape of captive elephants to the wild or through captive males mating wild living females (Lair 1997; Maurer et al. 2021; Thitaram et al. 2010). The capture of wild elephants was banned in Laos in 1989 (Suter et al. 2014). Routine capture has been banned in Myanmar for over two decades (Crawley et al. 2020a, b). As the elephant is a long-lived species, wild caught individuals are still found in the semi-captive population. Data from the Myanmar Timber Enterprise shows that the current population includes wild caught individuals and subsequent generations-F1, F2, F3, F4 and possibly later (Crawley et al. 2020a, b). In Laos the number of generations since capture is not known. Therefore, the traditional herding system in Laos and Myanmar provides an opportunity to investigate the impact of both recent and longstanding herding practices on the genetic structure and diversity of semi-captive populations.
Range wide genetic studies on elephants are mostly based on mtDNA (Fernando and Lande 2000; Vidya et al. 2009). Haplotypes of both α and β clades have been found in Laos and Myanmar—see detailed haplotypes and evolutionary distinctiveness by country in Budd et al. (2023a, b). Conversely, local population studies have mainly relied on nuclear microsatellites to evaluate nuclear genetic diversity and differentiation. Such local studies have been carried out in Laos (Ahlering et al. 2011; Budd et al. 2023a, b), Vietnam (Vidya et al. 2007), Cambodia (Gray et al. 2014), China (Zhang et al. 2015), Myanmar (Budd 2021; Kusza et al. 2018), Sumatra (Moßbrucker et al. 2015), Nepal (Flagstad et al. 2012), or at the country level such as in India (De et al. 2021), Thailand (Thitaram et al. 2010) or on Borneo (Goossens et al. 2016). Among these studies, the use of different microsatellite markers prevents any comprehensive analysis of population genetic parameters and geographic structure on wider geographical ranges. However, these studies agree that Southeast Asian populations of Thailand, Lao PDR, Cambodia and Myanmar harbored higher levels of allelic diversity and heterozygosity (Budd et al. 2023a, b) in accordance with Ahlering et al. (2011) showing high levels of diversity found in Nakai Protected Area, Lao PDR.
To investigate the genetic structure and diversity of semi-captive elephant populations, we conducted a large-scale population genetic study across Laos and Myanmar, two countries that maintain similar husbandry practices. We used blood samples taken by the veterinary medical services in the two countries to minimize genotyping errors associated with the use of environmental DNA. When known with certainty, we recorded the origin of the animal, either captured from the wild or captive born to study the structure of a large scale semi-captive population and investigate potential differentiation between wild and semi-captive pools.
Methods
Study sites
Myanmar and Laos represent a main part of the northern Indochina subtropical moist forests, a large ecoregion extending across the highlands of northern Myanmar, Laos, Vietnam and most of southern Yunnan Province in China. Monsoon forests are distributed over a mountainous landscape and form a broad range of habitats ranging from drought-deciduous savannah woodland to montane evergreen forests. This ecoregion has the second highest richness value for mammals in Asia and hosts large populations of Asian elephants (Olson and Dinerstein 1998).
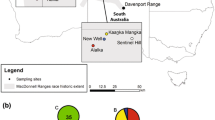
Wild elephant distribution and sampling sites in Laos and Myanmar. Number of samples used in the survey are given in brackets for the 5 populations. Nam Pouy and Nakay Protected areas are shown in green (wild elephant distribution). The map was drawn using QGIS version 2.14.0-Essen (www.qgis.org). Protected area shape files were downloaded from the World Database on Protected Areas (WDPA), IUCN and UNEP-WCMC (2017), available at: www.protectedplanet.net
Sample collection
Blood samples from 169 semi-captive elephants (Laos—99 and Myanmar—70) were collected across the two countries at the occasion of veterinary controls organized by respectively the Lao Elephant Care and Management Program (Department of Livestock) and Myanma Timber Entreprise veterinarians (Fig. 1). Among the 70 Myanmar samples, 48 originated from captive born and 19 from wild caught elephants. This information was not available for Lao samples. Approximatively 2 ml of blood was stored in 5 ml tubes filled with approximatively 2 ml of 95% ethanol or on FTA cards (Whatman). FTA cards were stored at room temperature and ethanol tubes at minus 20 °C for several months before export.
Export
Sample export was authorized by the Lao Department of Livestock and Fisheries (# 1782-30/09/2015) and import permit for the biological samples was issued by the French veterinary services, ministry of Forest, Agriculture and Environment (registration number 2015086, 06/10/2015). Export permit was issued by Forest Deparment, MoNREC, Myanmar (19 MM 000004/A/FD) and import permit by DREAL France (N° FR1903400028-I) under CITES. All samples were sent to the Center for Evolutionary and Functional Ecology (CEFE) laboratory, Montpellier, France, for DNA extraction and genotyping.
Laboratory
DNA was extracted from blood upon delivery at the CEFE. Negative controls were processed in parallel to control for potential contamination of reagents. DNA was extracted using DNeasy Blood & Tissue kit protocol. Purified DNA was eluted into 50 µl of elution buffer and stored at − 20 °C. We initially test a panel of 29 different microsatellites markers from the published literature on Asian elephants. We discarded loci with moderate amplification yields (less than 60%) and ambiguous scoring from blood samples. We finally selected 11 loci based on markers’ polymorphism.
PCR amplification of 11 microsatellite loci was carried out: EMU03, EMU07, EMU08, EMU11, EMU12, EMU14, FH60, FH94, FH103, LafMs05, LA2 (Comstock et al. 2000; Eggert et al. 2000; Kongrit et al. 2008; Nyakaana and Arctander 1998). Monoplex PCR were carried out for loci FH94, EMU08, EMU12 in 10 µl volumes, using 2 µl DNA, 0.2 µM of each primers, Qiagen Taq 1x and remaining of water. Multiplex PCRs were carried in 10 µl volume using DNA 2 µl, 0.2 µM of multiplex primers, Qiagen Taq 1x and remaining of water. The PCR consisted of an initial denaturation step at 95 °C for 15 min, followed by 30 cycles of three steps. These three steps included denaturation at 94 °C for 30 s, followed by annealing for 90 s, and extension at 72 °C for 1 min. A final extension was performed for 30 min at 60 °C. PCR products were electrophoresed, along with the internal size standard GS500LIZ, in ABI 3500XL 24-capillary-array Genetic Analyzer (Applied Biosystems) in the Genseq-CEMEB Facility in Montpellier. Genotypes were scored using the GeneMapper software version 4.0 (Applied Biosystems). The observations listed by Fernando et al. (2003) were followed as guidelines for scoring alleles from electropherograms.
Data analysis
We assessed null alleles, scoring errors and allelic dropout in the overall microsatellite data set using MICRO-CHECKER 2.2.3 (Van Oosterhout et al. 2004). The number of alleles per locus (Na), observed (Ho) and expected (He) heterozygosities, Fis, pairwise Fst values, analysis of molecular variance (AMOVA) and deviations from Hardy–Weinberg (HW) and linkage equilibrium (LE) between loci for each population were calculated using the R packages Adegenet, Pegas and Genepop (Jombart and Bateman 2008; Paradis 2010; R Core Team 2017). Evidence in favour of a recent population bottleneck was assessed using a test for heterozygosity excess (Cornuet and Luikart 1997) and a graphical test to detect mode shifts in allele frequency distributions (Luikart et al. 1998). Stepwise and two-phase models of mutation with 1000 randomizations were implemented in the program BOTTLENECK v.1.2.02 (Luikart and Cornuet 1999). Structuring into distinct gene pools across Laos and Myanmar and between wild and captive born individuals was investigated using Bayesian clustering as implemented in STRUCTURE v. 2.3.4 without spatial information (Falush et al. 2003). STRUCTURE was used under a model assuming admixture and allowing for correlation of allele frequencies between clusters. Models were run with and without population affiliation (Hubisz et al. 2009). We conducted ten runs for each value of K = 1–8 and each run consisted of a 10,000 burn-in followed by 100,000 iterations. The most likely value of K was assessed using Structure harvester (Earl and vonHoldt 2012). The use of the sampling location as default information has been shown to allow the correct inference of population clustering and ancestry when the sets of data used present weak structure signals that cannot be detected by basic models available for STRUCTURE (Hubisz et al. 2009). Population structure was also analysed using Discriminant Analysis of Principal Components and genetic isolation by distance (Jombart 2008; Jombart et al. 2010).
Results
Genetic diversity
We obtained 167 genotypes out of the 169 blood samples with 154 full genotypes, 12 genotypes with data missing for one locus and one genotype with data missing at 2 loci. Two samples with missing data at 8 loci were excluded from further analysis. Both the Lao and Myanmar samples presented high levels of genetic diversity with a mean number of alleles per locus of respectively 5.8 (SE 0.72) and 6.2 (SE 0.76) over the 11 loci (Table 1). The mean number of alleles is similar to previous results from a study on wild elephants in Cambodia (Gray et al. 2014) and captive elephants in Thailand (Thitaram et al. 2010), but lower than the 8.1 average allelic richness found in dung in a Lao wild population (Ahlering et al. 2011). We found moderate to high levels of observed heterozygosity depending on locus, ranging from 0.39 to 0.84 in Laos and 0.41 to 0.81 in Myanmar, consistent with studies using blood samples from Asian elephants in Thailand (Thitaram et al. 2010) and Myanmar (Kusza et al. 2018). There was no evidence for allele dropout. No significant deviation from Hardy Weinberg equilibrium was detected in sampling location. Null allele presence was only detected at locus FH60 in the Lao-north population. Null allele presence has been previously reported for loci FH94 and LA2 in dung samples (Gray et al. 2014).
No significant linkage disequilibrium (Fisher’s method) was observed between pairs of loci across all populations (see Supp mat. 1) with the exception of FH60/FH94 (Chi2 16.9, df 8, P-value 0.03).
The BOTTLENECK analysis (see Supp mat. 3) based on the stepwise mutation model did not evidence any significant signature of ongoing bottlenecks while the two phase mutation model supported a single case of ongoing bottleneck. However, that single case corresponded to the Lao-south sample, i.e. a location for which sample size was below the threshold for reliable results. The tests did not detect any deviation from the L-shaped distribution of allele frequencies predicted in the absence of population bottlenecks. The panmixia hypothesis and an apparent absence of selection are therefore supported by our analyses among and within populations.
Population structure
The Analysis of Molecular Variance (Table 2) indicated that most of the variance is found within individuals (95.9%) with limited differentiation between the two countries (2.4%). The highest but moderate pairwise Fst value of 0.06 is observed between the southern Lao population and the northern Myanmar one (Fig. 2). No statistically significant Fis value was found within population (See Supp mat. 2).
Pairwise Fst for the 5 sampling locations
Bayesian clustering analysis of microsatellite genotypes using STRUCTURE with no prior information did not evidence any marked structure (Fig. 3a). Ln P (X|K) and Delta K values both suggest K = 2 (Supp mat. 4), a result which indicates that there is a single or two genetic clusters. However, when using the 5 sampling locations as priors, STRUCTURE segregated the three Myanmar locations from the two Lao ones, confirming the presence of some genetic differentiation into a Lao and a Myanmar gene pool as suggested by pairwise Fst analysis (Fig. 3b). Furthermore, we tested if Myanmar elephants born in captivity and wild born ones belonged to a same or different gene pools. Wild born individuals (MWC in Fig. 3c) were assigned to the same cluster as captive born ones within Myanmar (MCB in Fig. 3c), while retaining together a weak differentiation comparatively to the Lao populations.
Individual assignment probabilities of Asian elephants to genetic clusters using the model-based program STRUCTURE. A Model-based structuring of Lao-Myanmar populations without prior; B with location prior; C Captive born and wild caught populations in Myanmar with priors. Red and green colours show the assignment probability for each individual to one of the two putative ancestor population. Individuals are grouped by populations: Laos south (LS), Laos north (LN), Myanmar east (ME), Myanmar west (MW), Myanmar north (MN). C: Myanmar captive born (MCB), Myanmar wild caught (MWC)
Discriminant Analysis of Principal Components (DACP) and Genetic isolation by distance analyses further evidenced some limited differentiation between populations along a geographic gradient (Fig. 4a and b). These results support the absence of a marked population structure but a weak differentiation among sampling locations found in Bayesian clustering. DACP analysis also confirmed that wild caught elephants are not differentiated from captive born elephants (Fig. 4c) as shown in STRUCTURE (Fig. 3b).
Discussion
We genotyped 167 semi-captive elephants from Laos and Myanmar using a panel of 11 microsatellite loci. Elephants of both countries presented high levels of genetic diversity with an observed heterozygosity per locus ranging from 0.48 to 0.84 and a low degree of inbreeding, if any. This is in agreement with the expected high level of genetic diversity in semi-captive populations of wild species.
Our mean observed and expected heterozygosity (0.65) are similar to the mean observed heterozygosity found in wild elephants in central Laos (0.67; Ahlering et al. 2011) using non-invasive samples but slightly lower than their estimate of expected heterozygosity under Hardy Weinberg equilibrium (0.74). Fernando et al. (2003) found a much lower expected heterozygosity across Laos (0.43) using different microsatellite markers. The 5 loci shared between our study and the Ahlering study (EMU03, EMU07, EMU12, EMU14, FH94) presented higher numbers of alleles in dung samples than in our blood samples from the semi-captive population in central Laos based on similar numbers of individuals. Locus EMU07 presented the largest deviation with Ahlering scoring 15 different alleles, while we found only 11 alleles throughout Laos and 12 when considering the whole dataset from both countries. This was the locus presenting the highest number of alleles in our data set and was hence the most difficult to score. The lower allelic richness found in the semi-captive population may be the result of a decrease in diversity compared to the wild pool. Alternatively, it may be the intrinsic result of the legitimate selection of the most polymorphic loci for the investigation of social structure despite of the occurrence of some genotyping errors due to degraded DNA. The expected lack of differentiation between wild and semi-captive pool in Laos suggests the possibility that genotyping errors explain the very high level of alleles and heterozygosity found in Nakay wild elephants.
We carefully selected 11 loci after testing an initial panel of 29 different markers based on their polymorphism, amplification yields and unambiguous reading from blood samples. Based on these observations, we may add some recommendations to the detailed protocols previously defined. Amplification success rates should be systematically mentioned in any study, as a prior indicative of the genotyping quality of the samples. Markers should be tested beforehand on a large panel of blood/dung samples, taking advantage of the large and genetically similar captive population across the region. Any marker showing null alleles, possible slippage, allelic dropouts or somewhat ambiguously scoring markers in blood samples should be avoided in non-invasive genotyping studies. While some highly polymorphic markers could be useful within the framework of CMR protocols, this high polymorphism potentially biases genetic diversity estimators. Further, genetic diversity inferred from these studies should be assessed using a restricted panel of markers chosen for their reliability and representativeness of Asian elephant genetic diversity across the region.
Semi-captive reindeers share management similarities with the Asian elephant. A study based on 12 microsatellites shown a mild differentiation between wild and semi-captive populations of Norwegian reindeers using Fst and Bayesian hierarchical clustering (Kvie et al. 2019). We also analyzed the population structure of semi-captive elephants from Laos and Myanmar but did not find mild differentiation between the wild and semi-captive pools. Pairwise Fst values and Bayesian clustering showed a weak differentiation between a Lao and a Myanmar gene pool but not between populations within each country. Our study confirmed that population differentiation was weak using nuclear DNA over 1500 km from south Laos to northern Myanmar. Wild caught and captive born elephants from Myanmar did not differ significantly, suggesting that the captive and wild populations in Myanmar are homogenous, as is probably also the case in Laos. Similarly Thitaram et al. (2010) concluded that the captive pool is genetically representative of the wild one in Thailand. Male and/or anthropic-mediated gene flow allow such high levels of diversity and heterozygosity within the wild and the captive pools. Like Myanmar and Thailand, Laos has always based its traditional elephant rearing system on capture and cross-breeding between wild males and captive females (Maurer et al. 2021; Thitaram et al. 2010). This continuous gene flow induced a lack of genetic differentiation between wild and semi-captive populations.
As the elephant is a long-lived species, with a generation time of two decades, the population is slow to show genetic changes. However, the anthropogenic factors that have allowed the preservation of the genetic diversity and population structure of the wild and semi-captive populations in these two countries are shifting rapidly. Over the past decades, habitat loss and fragmentation have broken many of the larger habitat patches into smaller patches that now support small isolated wild elephant populations (Dublin et al. 2006) especially in Thailand and Indochina (Hedges et al. 2008). Wild elephant populations are affected by population size reduction, fragmentation and gene flows disruption leading to high risks of diversity loss and inbreeding. Similarly, the semi-captive population shares similar threats. The traditional extensive form of elephant management is disappearing. Seasonal release of captive elephants that has allowed cross-breeding between captive and wild mates is restricted because of agricultural land expansion and access ban in protected areas (Maurer et al. 2021). Captive elephants are increasingly kept in suburban areas isolated from their wild conspecifics. Fecundity is also impeded by the limited availability of captive breeders (Maurer et al. 2017).
We did not observe any evidence of local genetic drift, but the small population sizes at local level and habitat fragmentation (Budd et al. 2023a, b; Songer et al. 2016) will mechanically lead to loss of genetic diversity as current levels of heterozygosity could not be upheld on the long term. Unlike the Bornean population that viably sustains high level of inbreeding (Goossens et al. 2016), wild and captive populations from the Indochina-Myanmar complex have never been selected on their resistance to inbreeding. Inbreeding has been revealed in a small and declining population in Vietnam showing low mitochondrial and microsatellite diversity and the signature of a recent population bottleneck (Vidya et al. 2007). Small populations, such as those in Southeast Asia, which harbour the greatest diversity, are also among those with the highest rates of habitat loss and fragmentation (Williams et al. 2020). They are therefore exposed to a high risk of extinction at local level. Consequently, the maintenance of gene flows is of paramount importance for the conservation of these isolated populations. Further research is needed to confirm whether the weak genetic differentiation, if any, and the similarity in alleles frequencies at nuclear loci found in our study, could justify considering the Lao and Myanmar semi-captive and wild pools as a homogenous Management Unit (MU) (Moritz 1994). The geographical demarcation of this MU should be further investigated by adding samples originating from neighbouring countries to check whether or not these populations show substantial divergence in allele frequencies with a potential maximum area ranging from peninsular Malaysia, Vietnam up to northern India.
The potential value for conservation of a large population of captive elephants has been recognized (Dublin et al. 2006). If captive populations are to form reservoirs or insurance populations to support the survival of isolated wild populations, then it is important that their genetic diversity is representative of extant diversity in the wild (Sato et al. 2017). There will likely be more translocation of elephants in the future as moving elephants, especially small pocketed herds, will become a necessity as part of meta-population management programs (Asian Elephant Range States Meeting report 2017). However, translocating wild elephants has proved to be risky as it often creates Human Elephant Conflicts (HEC). This technique, while solving the inbreeding problem, will increase HEC (Asian Elephant Range States Meeting report 2017). Semi-captive elephants that are used to forage in their natural habitat can ultimately play the role of ‘temporary’ migrants into inbred wild populations avoiding the risks associated with translocating wild individuals. In this context, the captive elephant diversity should be actively managed over the long term. However, current conservation strategies in Laos tend to restrain the interactions between the wild and captive pools (Maurer et al. 2021). In Myanmar, the 2018–2027 Conservation and Management plan recommendations regarding the captive population rather focus on population management and monitoring (Dublin et al. 2006; Hedges et al. 2018). Elephant conservationists cannot afford to ignore semi-captive populations in conservation planning for the long-term genetic sustainability of the species.
Conclusion
Our study showed that the population of semi-captive Asian elephants from Laos and Myanmar has high levels of genetic diversity and cannot be differentiated from the wild pool. Wild and semi-captive populations of Asian elephants have been closely interconnected for millennia. Today both pools are exposed to similar threats such as poaching, habitat destruction, isolation and potential loss of diversity. Governments, managers and conservationists should favour cooperation and a global approach considering the potential of a unique MU extending from Laos to Myanmar and probably even on a larger scale. Semi-captive and wild pools should also be managed with an inclusive and global approach to maintain the species’ abundance and genetic diversity. Finally, researchers should further investigate the structure and range of MU using methodologies that will allow better cooperation and exchange of data between them. Within this scope, the use of SNPs and genetic markers associated with next-generation sequencing should be developed as they can facilitate the comparison of results between studies and laboratories and as they are less sensitive to DNA degradation (Prado et al. 2023).
References
Ahlering MA, Hedges S, Johnson A, Tyson M, Schuttler SG, Eggert LS (2011) Genetic diversity, social structure, and conservation value of the elephants of the Nakai Plateau, Lao PDR, based on non-invasive sampling. Conserv Genet 12(2):413–422. https://doi.org/10.1007/s10592-010-0148-y
Asian Elephant Range States Meeting Report (2017) In: Asian Elephant Range States Meeting. Jakarta
Barron ES, Chaudhary RP, Carvalho Ribeiro S, Gilman E, Hess J, Hilborn R, Danner MC (2022) Status of and trends in the use of wild species and its implications for wild species, the environment and people. In: J. M. Fromentin, M. R. Emery, J. Donaldson, M. C. Danner, A. Hallosserie, & D. Kieling (Eds.), Thematic assessment report on the sustainable use of wild species of the intergovernmental science-policy platform on biodiversity and ecosystem services. Bonn, Germany
Budd K (2021) Conservation genetics of conflict in the Asian elephant. Elephas maximus. University of Missouri, Columbia
Budd K, Gunn JC, Sullivan LL, Eggert LS (2023a) Identification of conservation priority units in the Asian elephant, Elephas maximus. Conserv Genet. https://doi.org/10.1007/s10592-023-01542-1
Budd K, Suddychan D, Tyson M, Coudrat CNZ, Mcwilliam A, Hallam CD, Eggert LS (2023b) Effects of a hydropower project on a high-value Asian elephant population. Ecol Evol 13(April):1–14. https://doi.org/10.1002/ece3.10353
Comstock KE, Wasser SK, Ostrander EA (2000) Polymorphic microsatellite DNA loci identified in the African elephant (Loxodonta). Mol Ecol 9:993–1011. https://doi.org/10.1046/j.1365-294X.2000.00939.x
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, Vol. 0, p. {ISBN} 3-900051-07-0. https://doi.org/http://www.R-project.org/
Cornuet JM, Luikart G (1997) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Crawley JAH, Lahdenperä M, Min Oo Z, Htut W, Nandar H, Lummaa V (2020a) Taming age mortality in semi-captive Asian elephants. Sci Rep 10(1):1–8. https://doi.org/10.1038/s41598-020-58590-7
Crawley JAH, Lahdenperä M, Seltmann MW, Htut W, Aung HH, Nyein K, Lummaa V (2020b) Investigating changes within the handling system of the largest semi-captive population of Asian elephants. PLoS ONE. https://doi.org/10.1371/journal.pone.0209701
De R, Sharma R, Davidar P, Arumugam N, Sedhupathy A, Puyravaud JP, Goyal SP (2021) Pan-india population genetics signifies the importance of habitat connectivity for wild Asian elephant conservation. Global Ecol Conserv 32:e01888. https://doi.org/10.1016/J.GECCO.2021.E01888
Dublin H, Desai A, Hedges S, Vié JC, Bambaradeniya C, Lopez A (2006) Elephant Range States Meeting. Kuala Lumpur
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res 4(2):359–361. https://doi.org/10.1007/s12686-011-9548-7
Eggert LS, Ramakrishnan U, Mundy NI, Woodruff SD (2000) Polymorphic microsatellite DNA markers in the African elephant (Loxondonta Africana) and their use in the Asian elephant (Elephas maximus). Mol Ecol 9:2155–2157. https://doi.org/10.1046/j.1365-294X.2000.01053.x
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164(4):1567–1587. https://doi.org/10.1111/j.1471-8286.2007.01758.x
Fernando P, Lande R (2000) Molecular genetic and behavioral analysis of social organization in the Asian elephant (Elephas maximus). Behav Ecol Sociobiol 48:84–91. https://doi.org/10.1007/s002650000218
Fernando P, Vidya TNC, Rajapakse C, Dangolla A, Melnick DJ (2003) Reliable noninvasive genotyping: fantasy or reality? J Hered 94(2):115–123. https://doi.org/10.1093/jhered/esg022
Flagstad Ø, Pradhan NMB, Kvernstuen LG, Wegg P (2012) Conserving small and fragmented populations of large mammals: non-invasive genetic sampling in an isolated population of Asian elephants in Nepal. J Nat Conserv 20(3):181–190
Forbes BC, Stammler F, Kumpula T, Meschtyb N, Pajunen A, Kaarlejärvi E (2009) High resilience in the Yamal-Nenets social–ecological system, west Siberian Arctic, Russia. Proc Natl Acad Sci 106(52):22041–22048. https://doi.org/10.1073/pnas.0908286106
Frankham R (2008) Genetic adaptation to captivity in species conservation programs. Mol Ecol 17:325–333. https://doi.org/10.1111/j.1365-294X.2007.03399.x
Frankham R, Ballou JD, Briscoe DA (2004) A Primer of Conservation Genetics. In: Oryx (Vol. 39). https://doi.org/10.1017/S0030605305000487
Goossens B, Sharma R, Othman N, Kun-rodrigues C, Sakong R, Ancrenaz M, Chikhi L (2016) Habitat fragmentation and genetic diversity in natural populations of the Bornean elephant: implications for conservation. Biol Conserv 196:80–92. https://doi.org/10.1016/j.biocon.2016.02.008
Gray TNE, Vidya TNC, Potdar S, Bharti DK, Sovanna P (2014) Population size estimation of an Asian elephant population in eastern Cambodia through non-invasive mark-recapture sampling. Conserv Genet 15(4):803–810. https://doi.org/10.1007/s10592-014-0579-y
Hedges S, Tyson MJ, Sitompul AF, Kinnaird MF, Gunaryadi D (2005) Distribution, status, and conservation needs of Asian elephants (Elephas maximus) in Lampung Province. Sumatra Indonesia Biol Conserv 124(1):35–48. https://doi.org/10.1016/j.biocon.2005.01.004
Hedges S, Fisher K, Rose R (2008) Range-wide mapping workshop for Asian elephants (Elephas maximus), Cambodia, October 2008. Phnom Penh
Hedges S, Leimgruber P, Lynam A, Mar KU, Riddle H, Thaw UWN, Soe P (2018) Myanmar elephant conservation action plan (MECAP): 2018–2027. Nay Pyi Taw.
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Res, 9(5), 1322–1332. Accessed from http://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/j.1755-0998.2009.02591.x/full
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11):1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Jombart T, Bateman A (2008) Genetics and population analysis adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11):1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. https://doi.org/10.1371/journal.pcbi.1000455
Kongrit C, Siripunkaw C, Brockelman WY, Akkarapatumwong V, Wright TF, Eggert LS (2008) Isolation and characterization of dinucleotide microsatellite loci in the Asian elephant (Elephas maximus). Mol Ecol Resour 8(1):175–177. https://doi.org/10.1111/j.1471-8286.2007.01916.x
Kusza S, Suchentrunk F, Pucher H, Mar KU, Zachos FE (2018) High levels of mitochondrial genetic diversity in Asian elephants (Elephas maximus) from Myanmar. Hystrix
Kvie KS, Heggenes J, Bårdsen BJ, Røed KH (2019) Recent large-scale landscape changes, genetic drift and reintroductions characterize the genetic structure of Norwegian wild reindeer. Conserv Genet 20:1405–1419. https://doi.org/10.1007/s10592-019-01225-w
Lahdenperä M, Mar KU, Courtiol A, Lummaa V (2018) Differences in age-specific mortality between wild-caught and captive-born Asian elephants. Nat Commun. https://doi.org/10.1038/s41467-018-05515-8
Lair R (1997) Gone astray: the care and management of the Asian elephant in domesticity. Food and Agriculture Organization of the United Nations, Bangkok
Lassausaie J, Bret A, Bouapao X, Chanthavong V, Castonguay-Vanier J, Quet F, Bouchard B (2015) Tuberculosis in Laos, who is at risk: the mahouts or their elephants? Epidemiol Infect 143(5):922–931. https://doi.org/10.1017/S0950268814002180
Leimgruber P, Gagnon JB, Wemmer C, Kelly DS, Songer MA, Selig ER (2003) Fragmentation of Asia’s remaining wildlands: implications for Asian elephant conservation. Anim Conserv 6(4):347–359. https://doi.org/10.1017/S1367943003003421
Leimgruber P, Senior B, Aung M, Songer MA, Mueller T, Wemmer C, Ballou JD (2008) Modeling population viability of captive elephants in Myanmar (Burma): implications for wild populations. Anim Conserv 11(3):198–205. https://doi.org/10.1111/j.1469-1795.2008.00172.x
Leimgruber P, Min Oo Z, Aung M, Kelly D, Wemmer C, Senior B, Songer M (2011) Current status of Asian elephants in Myanmar. Gajah 35:35, 76–86
Luikart PS, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reduction in the effective population size using allele frequency data. J Hered 90(4):502–503
Luikart G, Allendorf FW, Sherwin WB (1998) Distortion of allele frequency distributions bottlenecks. J Hered 89:238–247
Maurer G (2018) Conservation de l’éléphant d’Asie (Elephas maximus) par l’étude des interactions entre humains, populations sauvages et semi-captives d’éléphants: une approche intégrée des dimensions démographiques, génétiques, économiques et socioculturelles (Université Montpellier). Accessed from https://tel.archives-ouvertes.fr/tel-01834575/file/2018_MAURER_archivage.pdf
Maurer G, Rashford BS, Chanthavong V, Mulot B, Gimenez O (2017) Wild-captive interactions and economics drive dynamics of Asian elephants in Laos. Sci Rep 7(1):14800. https://doi.org/10.1038/s41598-017-13907-x
Maurer G, Gimenez O, Mulot B, Lescureux N (2021) Under pressure: How human-wild‐captive elephant social‐ecological system in Laos is teetering due to global forces and sociocultural changes. People Nat. https://doi.org/10.1002/pan3.10247
Menon V, Tiwari SK (2019) Population status of Asian elephants Elephas maximus and key threats. Int Zoo Yearbook 53:17–30. https://doi.org/10.1111/izy.12247
Miraldo A, Li S, Borregaard MK, Flórez-Rodríguez A, Gopalakrishnan S, Rizvanovic M, Nogués-Bravo D (2016) An anthropocene map of genetic diversity. Science, 353(6307). Accessed from http://science.sciencemag.org/
Moritz C (1994) Defining evolutionarily significant units for conservation. Trends Ecol Evol 9(10):373–375. https://doi.org/10.1016/0169-5347(94)90057-4
Moßbrucker AM, Apriyana I, Fickel J, Imron MA, Pudyatmoko S (2015) Non-invasive genotyping of Sumatran elephants: implications for conservation the Sumatran elephant (Elephas maximus sumatranus) is one of three currently recognized subspecies. Trop Conserv Sci 8(3):745–759
Nyakaana S, Arctander P (1998) Isolation and characterization of microsatellite loci in the African elephant, Loxodonta africana. Mol Ecol 7(10):1436
Olson DM, Dinerstein E (1998) The global 200: a representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv Biol 12(3):502–515. https://doi.org/10.1046/j.1523-1739.1998.012003502.x
Paradis E (2010) Pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics. https://doi.org/10.1093/bioinformatics/btp696
Prado NA, Armstrong EE, Brown JL, Goldenberg SZ, Leimgruber P, Pearson VR, Campana MG (2023) Genomic resources for Asian (Elephas maximus) and African savannah elephant (Loxodonta africana) conservation and health research. J Hered 114(5):529–538. https://doi.org/10.1093/JHERED/ESAD034
Queirós J, Gortázar C, Alves PC (2020) Deciphering anthropogenic effects on the genetic background of the red deer in the Iberian Peninsula. Front Ecol Evol 8:515401. https://doi.org/10.3389/FEVO.2020.00147/BIBTEX
Røed KH, Flagstad Ø, Nieminen M, Holand Ø, Dwyer MJ, Røv N, Vilà C (2008) Genetic analyses reveal independent domestication origins of eurasian reindeer. Proc Royal Soc B: Biol Sci 275(1645):1849–1855. https://doi.org/10.1098/rspb.2008.0332
Sampson C, McEvoy J, Oo ZM, Chit AM, Chan AN, Tonkyn D, Leimgruber P (2018) New elephant crisis in Asia—early warning signs from Myanmar. PLoS ONE 13(3):1–13. https://doi.org/10.1371/journal.pone.0194113
Sato Y, Ogden R, Komatsu M, Maeda T, Inoue-Murayama M (2017) Integration of wild and captive genetic management approaches to support conservation of the endangered Japanese golden eagle. Biol Conserv 213(April):175–184. https://doi.org/10.1016/j.biocon.2017.07.008
Songer M, Aung M, Allendorf TD, Calabrese JM, Leimgruber P (2016) Drivers of change in Myanmar’s wild elephant distribution. Trop Conserv Sci 9(4):1940082916673749. https://doi.org/10.1177/1940082916673749
Sukumar R (2003) The living elephants: evolutionary ecology, behavior, and conservation. Oxford University Press, New York
Sukumar R (2006) A brief review of the status, distribution and biology of wild Asian elephants Elephas maximus. Int Zoo Yearbook 40(1):1–8. https://doi.org/10.1111/j.1748-1090.2006.00001.x
Sukumar R (2016) The Human–Elephant Relationship through the ages: a brief Macro-scale History. In: Locke P, Buckingham J (eds) Conflict, negotiation, and coexistence: Rethinking Human Elephant relations in South Asia. Oxford University Press, Delhi, pp 31–46
Suter IC, Maurer G, Baxter G (2014) Population viability of captive Asian elephants in the Lao PDR. Endanger Species Res 24(1):1–7. https://doi.org/10.3354/esr00578
Thant ZM, May R, Røskaft E (2022) Human-elephant coexistence challenges in Myanmar: an analysis of fatal elephant attacks on humans and elephant mortality human-elephant conflict (HEC) attack mortality poaching. J Nat Conserv 69:126260. https://doi.org/10.1016/j.jnc.2022.126260
Thitaram C, Somgird C, Mahasawangkul S, Angkavanich T, Roongsri R, Thongtip N, Lenstra JA (2010) Genetic assessment of captive elephant (Elephas maximus) populations in Thailand. Conserv Genet 11(1):325–330. https://doi.org/10.1007/s10592-009-0018-7
Trautmann TR (2015) Elephants and kings: an environmental history. University of Chicago Press, Chicago
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4(3):535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Vidya TNC (2016) Evolutionary history and population genetic structure of Asian elephants in India. Indian J History Sci 51(2B):391–405. https://doi.org/10.16943/ijhs/2016/v51i2.2/48453
Vidya TNC, Varma S, Dang NX, Thanh T, Sukumar R (2007) Minimum population size, genetic diversity, and social structure of the Asian elephant in Cat Tien National Park and its adjoining areas, Vietnam, based on molecular genetic analyses. Conserv Genet 8(6):1471–1478. https://doi.org/10.1007/s10592-007-9301-7
Vidya TNC, Sukumar R, Melnick DJ (2009) Range-wide mtDNA phylogeography yields insights into the origins of Asian elephants. Proc Royal Soc B: Biol Sci 276(1658):893–902. https://doi.org/10.1098/rspb.2008.1494
Williams C, Tiwari SK, Goswami VR, de Silva S, Kumar A, Baskaran N, Menon V (2020) Elephas maximus (Asian Elephant). Accessed 27 Dec, 2023, from The IUCN Red List of Threatened Species 2020 website: https://www.iucnredlist.org/species/7140/45818198
Zhang L, Dong L, Lin L, Feng L, Yan F, Wang L, Luo A (2015) Asian elephants in China: estimating population size and evaluating habitat suitability. PLoS ONE 10(5):e0124834. https://doi.org/10.1371/journal.pone.0124834
Acknowledgements
We thank the Ministry of Natural Resources and Environmental Conservation, the Government of the Union of Myanmar for giving permission to work with Myanma Timber Enterprise (MTE). We thank MTE managing director and all the vets and officers involved in data collection. We thank the Ministry of Agriculture and Forestry of the Lao People’s Democratic Republic, the veterinarians of the Department of Livestock and Fisheries, the ElefantAsia project and Dr Jerome Lassausaie, for collecting samples in Laos.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
GM, OG and FK designed the study. VC and ZM collected the data. GM and MD performed lab work. GM analysed the data with FK. GM wrote the paper with contributions from FK. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maurer, G., Dubois, MP., Oo, Z.M. et al. Genetic structure and diversity of semi-captive populations: the anomalous case of the Asian elephant. Conserv Genet (2024). https://doi.org/10.1007/s10592-024-01617-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10592-024-01617-7