Abstract
An efficient and environmentally benign protocol is accomplished for the rapid production of amide and carboxylic ester via aminocarbonylation and alkoxycarbonylation of aryl halides with various amines and alcohols, respectively. The protocol is advantageous compared to those reported in the literature including using a recyclable heterogeneous palladium catalyst comprising palladium nanoparticles immobilized on magnetic covalent triazine-based framework (CTF) support (Pd/Fe3O4@SiO2-CTF), safe Mo(CO)6 as the solid CO source, being environmentally more benign due to using a readily-available and biodegradable room-temperature ionic liquid, choline hydroxide (ChOH), serves a dual role as solvent as well as the base without addition of auxiliary base. More significantly, Pd/Fe3O4@SiO2-CTF showed good recyclability, recycled six times with negligible change in catalytic performance which is an important characteristic from the viewpoint of sustainable chemistry.
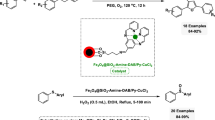
Graphical Abstract
An efficient and clean protocol has been developed for the rapid production of amide and carboxylic ester via aminocarbonylation and alkoxycarbonylation of aryl halides with various amines and alcohols, respectively. The protocol is advantageous due to using a recyclable heterogeneous palladium catalyst (Pd/Fe3O4@SiO2@CTF), safe Mo(CO)6 as the solid CO source, and a readily available and biodegradable room-temperature ionic liquid, choline hydroxide (ChOH), dually as solvent and the base to scavenge the acid of hydrogen halide (HX) without addition of auxiliary base. More significantly, Pd/Fe3O4@SiO2@CTF showed good recyclability, recycled six times with negligible change in catalytic performance which is an important characteristic from the viewpoint of sustainable chemistry.






Similar content being viewed by others
References
Schoenberg A, Bartoletti I, Heck R (1974) Palladium-catalyzed carboalkoxylation of aryl, benzyl, and vinylic halides. J Org Chem 39(23):3318–3326
Schoenberg A, Heck R (1974) Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J Org Chem 39(23):3327–3331
Hu Q, Wang L, Wang C, Wu Y, Ding Z, Yuan R (2017) Ligand-free Pd (0)/SiO 2-catalyzed aminocarbonylation of aryl iodides to amides under atmospheric CO pressure. RSC Adv 7(59):37200–37207
Dang TT, Zhu Y, Ghosh SC, Chen A, Chai CL, Seayad AM (2012) Atmospheric pressure aminocarbonylation of aryl iodides using palladium nanoparticles supported on MOF-5. Chem Commun 48(12):1805–1807
Lei Y, Xiao S, Li G, Gu Y, Wu H, Shi K (2017) Mild and efficient Pd (PtBu3) 2-catalyzed aminocarbonylation of aryl halides to aryl amides with high selectivity. Appl Organomet Chem 31(7):e3637
Zhang J, Hou Y, Ma Y, Szostak M (2018) Synthesis of amides by mild palladium-catalyzed aminocarbonylation of arylsilanes with amines enabled by copper (II) fluoride. J Org Chem 84(1):338–345
Fang W, Deng Q, Xu M, Tu T (2013) Highly efficient aminocarbonylation of iodoarenes at atmospheric pressure catalyzed by a robust acenaphthoimidazolyidene allylic palladium complex. Org Lett 15(14):3678–3681
Munday RH, Martinelli JR, Buchwald SL (2008) Palladium-catalyzed carbonylation of aryl tosylates and mesylates. J Am Chem Soc 130(9):2754–2755
Khedkar MV, Sasaki T, Bhanage BM (2013) Immobilized palladium metal-containing ionic liquid-catalyzed alkoxycarbonylation, phenoxycarbonylation, and aminocarbonylation reactions. ACS Catal 3(3):287–293
Bal Reddy C, Ram S, Kumar A, Bharti R, Das P (2019) Supported palladium nanoparticles that catalyze aminocarbonylation of aryl halides with amines using oxalic acid as a sustainable CO source. Chemistry 25(16):4067–4071
Iranpoor N, Firouzabadi H, Etemadi-Davan E, Nematollahi A, Firouzi HR (2015) A novel nickel-catalyzed synthesis of thioesters, esters and amides from aryl iodides in the presence of chromium hexacarbonyl. New J Chem 39(8):6445–6452
Wan Y, Alterman M, Larhed M, Hallberg A (2002) Dimethylformamide as a carbon monoxide source in fast palladium-catalyzed aminocarbonylations of aryl bromides. J Org Chem 67(17):6232–6235
Khedkar MV, Khan SR, Lambat TL, Chaudhary RG, Abdala AA (2020) CO Surrogates: a green alternative in palladium-catalyzed co gas free carbonylation reactions. Curr Org Chem 24(22):2588–2600
Peng J-B, Qi X, Wu X-F (2017) Recent achievements in carbonylation reactions: a personal account. Synlett 28(02):175–194
Cao J, Zheng Z-J, Xu Z, Xu L-W (2017) Transition-metal-catalyzed transfer carbonylation with HCOOH or HCHO as non-gaseous C1 source. Coord Chem Rev 336:43–53
Jiang J, Yoon S (2018) A metalated porous porphyrin polymer with [Co (CO) 4]− anion as an efficient heterogeneous catalyst for ring expanding carbonylation. Sci Rep 8(1):1–6
Wu X-F (2018) Palladium-catalyzed synthesis of aldehydes from aryl iodides and formic acid with propylphosphonic anhydride as the activator. Sci Rep 8(1):1–4
De La Cruz LK, Bauer N, Cachuela A, Tam WS, Tripathi R, Yang X et al (2022) Light-activated CO donor as a universal CO surrogate for Pd-catalyzed and light-mediated carbonylation. Org Lett 24(27):4902–4907
Åkerbladh L, Odell LR, Larhed M (2019) Palladium-catalyzed molybdenum hexacarbonyl-mediated gas-free carbonylative reactions. Synlett 30(02):141–155
Odell LR, Russo F, Larhed M (2012) Molybdenum hexacarbonyl mediated CO gas-free carbonylative reactions. Synlett 23(05):685–698
Brennführer A, Neumann H, Beller M (2009) Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew Chem Int Ed 48(23):4114–4133
Barnard CF (2008) Palladium-catalyzed carbonylation—a reaction come of age. Organometallics 27(21):5402–5422
Zhang J, Ma Y, Ma Y (2018) Synthesis of secondary amides through the palladium (II)-catalyzed aminocarbonylation of arylboronic acids with amines or hydrazines and carbon monoxide. Eur J Org Chem 2018(14):1720–1725
Urbán B, Papp M, Skoda-Földes R (2019) Carbonylation of aryl halides in the presence of heterogeneous catalysts. Curr Green Chem 6(2):78–95
Vavasori A, Calgaro L, Quartarone G, Ronchin L, Tortato C (2016) New magnetically recoverable palladium-based catalysts active in the alkoxycarbonylation of iodobenzene. Pure Appl Chem 88(5):445–455
Lei Y, Wan Y, Li G, Zhou X-Y, Gu Y, Feng J et al (2017) Palladium supported on an amphiphilic porous organic polymer: a highly efficient catalyst for aminocarbonylation reactions in water. Mater Chem Front 1(8):1541–1549
Zhu Y, Chuanzhao L, Biying AO, Sudarmadji M, Chen A, Tuan DT et al (2011) Stabilized well-dispersed Pd (0) nanoparticles for aminocarbonylation of aryl halides. Dalton Trans 40(36):9320–9325
Hajipour A-R, Tavangar-Rizi Z, Iranpoor N (2016) Palladium-catalyzed carbonylation of aryl halides: an efficient, heterogeneous and phosphine-free catalytic system for aminocarbonylation and alkoxycarbonylation employing Mo (CO) 6 as a solid carbon monoxide source. RSC Adv 6(82):78468–78476
Dang TT, Zhu Y, Ngiam JS, Ghosh SC, Chen A, Seayad AM (2013) Palladium nanoparticles supported on ZIF-8 as an efficient heterogeneous catalyst for aminocarbonylation. ACS Catal 3(6):1406–1410
Papp M, Szabó P, Srankó D, Sáfrán G, Kollár L, Skoda-Földes R (2017) Mono-and double carbonylation of aryl iodides with amine nucleophiles in the presence of recyclable palladium catalysts immobilised on a supported dicationic ionic liquid phase. RSC Adv 7(70):44587–44597
Layek S, Agrahari B, Ganguly R, Das P, Pathak DD (2020) Carbonylative Suzuki coupling reactions catalyzed by ONO pincer–type Pd (II) complexes using chloroform as a carbon monoxide surrogate. Appl Organomet Chem 34(3):e5414
Liu M, Guo L, Jin S, Tan B (2019) Covalent triazine frameworks: synthesis and applications. J Mater Chem A 7(10):5153–5172
Wang K, Huang H, Liu D, Wang C, Li J, Zhong C (2016) Covalent triazine-based frameworks with ultramicropores and high nitrogen contents for highly selective CO2 capture. Environ Sci Technol 50(9):4869–4876
Deng Q-W, Ren G-Q, Li Y-J, Yang L, Zhai S-L, Yu T et al (2020) Hydrogen and CO2 storage in high surface area covalent triazine–based frameworks. Mater Today Energy 18:100506
Lee G-Y, Lee J, Vo HT, Kim S, Lee H, Park T (2017) Amine-functionalized covalent organic framework for efficient SO 2 capture with high reversibility. Sci Rep 7(1):1–10
Gu C, Liu D, Huang W, Liu J, Yang R (2015) Synthesis of covalent triazine-based frameworks with high CO 2 adsorption and selectivity. Polym Chem 6(42):7410–7417
Artz J (2018) Covalent triazine-based frameworks—tailor-made catalysts and catalyst supports for molecular and nanoparticulate species. ChemCatChem 10(8):1753–1771
Mullangi D, Nandi S, Shalini S, Sreedhala S, Vinod CP, Vaidhyanathan R (2015) Pd loaded amphiphilic COF as catalyst for multi-fold Heck reactions, CC couplings and CO oxidation. Sci Rep 5(1):1–12
Siebels M, Schlüsener C, Thomas J, Xiao Y-X, Yang X-Y, Janiak C (2019) Rhodium nanoparticles supported on covalent triazine-based frameworks as re-usable catalyst for benzene hydrogenation and hydrogen evolution reaction. J Mater Chem A 7(19):11934–11943
Bi J, Fang W, Li L, Wang J, Liang S, He Y et al (2015) Covalent triazine-based frameworks as visible light photocatalysts for the splitting of water. Macromol Rapid Commun 36(20):1799–1805
Li J, Zhang L, Liu X, Shang N, Gao S, Feng C et al (2018) Pd nanoparticles supported on a covalent triazine-based framework material: an efficient and highly chemoselective catalyst for the reduction of nitroarenes. New J Chem 42(12):9684–9689
Cheng HY, Wang T (2021) Covalent organic frameworks in catalytic organic synthesis. Adv Synth Catal 363(1):144–193
Wang Z, Liu C, Huang Y, Hu Y, Zhang B (2016) Covalent triazine framework-supported palladium as a ligand-free catalyst for the selective double carbonylation of aryl iodides under ambient pressure of CO. Chem Commun 52(14):2960–2963
Ismael A, Gevorgyan A, Skrydstrup T, Bayer A (2020) Renewable solvents for palladium-catalyzed carbonylation reactions. Org Process Res Dev 24(11):2665–2675
de Albuquerque DY, de Moraes JR, Schwab RS (2019) Palladium-catalyzed aminocarbonylation reaction to access 1, 2, 3-triazole-5-carboxamides using dimethyl carbonate as sustainable solvent. Eur J Org Chem 2019(39):6673–6681
Gadilohar BL, Shankarling GS (2017) Choline based ionic liquids and their applications in organic transformation. J Mol Liq 227:234–261
Abelló S, Medina F, Rodríguez X, Cesteros Y, Salagre P, Sueiras JE et al (2004) Supported choline hydroxide (ionic liquid) as heterogeneous catalyst for aldol condensation reactions. Chem Commun 9:1096–1097
Kwon GT, Joo SR, Park SY, Kim SH (2020) Choline hydroxide as a versatile medium for catalyst-free O-functionalization of phenols. Bull Korean Chem Soc 41(12):1200–1205
Joo SR, Kwon GT, Kim SH (2020) A combination of biocompatible room temperature ionic liquid and palladium catalyst for base-and ligand-free suzuki coupling reactions. Asian J Org Chem 9(4):584–587
Wang DL, Liu H, Yang D, Wang P, Lu Y, Liu Y (2017) Aminocarbonylation of aryl halides to produce primary amides by using NH4HCO3 dually as ammonia surrogate and base. ChemCatChem 9(22):4206–4211
Salamatmanesh A, Kazemi Miraki M, Yazdani E, Heydari A (2018) Copper (I)–caffeine complex immobilized on silica-coated magnetite nanoparticles: a recyclable and eco-friendly catalyst for click chemistry from organic halides and epoxides. Catal Lett 148(10):3257–3268
Mart M, Tylus W, Trzeciak A (2019) Pd/DNA as a highly active and recyclable catalyst for aminocarbonylation and hydroxycarbonylation in water: the effect of Mo (CO) 6 on the reaction course. Mol Catal 462:28–36
Gockel SN, Hull KL (2015) Chloroform as a carbon monoxide precursor: in or ex situ generation of CO for Pd-catalyzed aminocarbonylations. Org Lett 17(13):3236–3239
Wang Z, Yin Z, Wu XF (2017) Pd/C-catalyzed aminocarbonylation of aryl Iodides with anthranils in water using Mo (CO) 6 as the CO source. Chemistry 23(60):15026–15029
Acknowledgements
We are grateful to Tarbiat Modares University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jadidi Nejad, M., Shariatipour, M. & Heydari, A. A Combination of Biocompatible Room Temperature Ionic Liquid and Supported Palladium Nanoparticles Catalyst for Aminocarbonylation and Alkoxycarbonylation. Catal Lett 153, 1957–1973 (2023). https://doi.org/10.1007/s10562-022-04141-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04141-w