Abstract
Supported metal nanoparticles, M-NPs, are of great scientific and economic interest as they encompass application in chemical manufacturing, oil refining and environmental catalysis. Oxidation and hydrogenation reactions are among the major reactions catalyzed by supported M-NPs. Although supported M-NPs are preferable due to their easy recovery and reuse, there are still some practical issues regarding their catalytic activity and deactivation. This review highlights the general features of supported M-NPs as catalysts with particular attention to copper, gold, platinum, palladium, ruthenium, silver, cobalt and nickel and their catalytic evaluation in various reactions. The catalytic performance of noble M-NPs has been explored extensively in various selective oxidation and hydrogenation reactions. In general, noble metals are expensive and sensitive to poisons. Despite their significant merits and potential (easily available, comparatively inexpensive and less sensitive to poisons), catalysis by base M-NPs is relatively less explored. Therefore, activity of base M-NPs can be improved, and still, there is potential for such catalysts.

Adapted with permission from [28]




Reprinted with permission from [216]

Reprinted with permission from [241]




Reprinted with permission from [295]

Adapted with permission from [10]


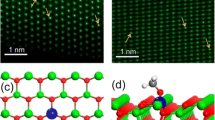
Adapted with permission from [341]

Reprinted with permission from [379]

Reprinted with permission from [380]
Similar content being viewed by others
References
Cuenya BR, Behafarid F (2015) Nanocatalysis: size-and shape-dependent chemisorption and catalytic reactivity. Surf Sci Rep 70:135–187
Schlögl R, Abd Hamid SB (2004) Nanocatalysis: mature science revisited or something really new? Angew Chem Int Ed 43:1628–1637
Monfared A, Mohammadi R, Ahmadi S, Nikpassand M, Hosseinian A (2019) Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions. RSC Adv 9:3185–3202
Navalón S, García H (2016) Nanoparticles for catalysis. Nanomaterials 6:123
Dimitratos N, Lopez-Sanchez JA, Hutchings GJ (2012) Selective liquid phase oxidation with supported metal nanoparticles. Chem Sci 3:20–44
Astruc D (2008) Nanoparticles and catalysis. Wiley, New York
Shang L, Bian T, Zhang B, Zhang D, Wu L, Tung C, Yin Y, Zhang T (2014) Graphene-supported ultrafine metal nanoparticles encapsulated by mesoporous silica: robust catalysts for oxidation and reduction reactions. Angew Chem 126:254–258
Xia Y, Yang H, Campbell CT (2013) Nanoparticles for catalysis. Acc Chem Res 46:1671–1672
Philippot K, Serp P (2013) Nanomaterials in catalysis. Wiley-VCH, Weinheim
Albonetti S, Mazzoni R, Cavani F (2014) Homogeneous, heterogeneous and nanocatalysis. In: Cardona F, Parmeggiani C (eds) Transition metal catalysis in aerobic alcohol oxidation. Royal Society of Chemistry, Cambridge, pp 1–39
Choudhary VR, Dumbre DK, Bhargava SK (2009) Oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over nanogold supported on TiO2 and other transition and rare-earth metal oxides. Ind Eng Chem Res 48:9471–9478
Albonetti S, Pasini T, Lolli A, Blosi M, Piccinini M, Dimitratos N, Lopez-Sanchez JA, Morgan DJ, Carley AF, Hutchings GJ (2012) Selective oxidation of 5-hydroxymethyl-2-furfural over TiO2-supported gold–copper catalysts prepared from preformed nanoparticles: effect of Au/Cu ratio. Catal Today 195:120–126
Della Pina C, Falletta E, Rossi M (2008) Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold–copper catalyst. J Catal 260:384–386
Pritchard J, Kesavan L, Piccinini M, He Q, Tiruvalam R, Dimitratos N, Lopez-Sanchez JA, Carley AF, Edwards JK, Kiely CJ (2010) Direct synthesis of hydrogen peroxide and benzyl alcohol oxidation using Au–Pd catalysts prepared by sol immobilization. Langmuir 26:16568–16577
Lippits MJ, Nieuwenhuys BE (2010) Direct conversion of ethanol into ethylene oxide on copper and silver nanoparticles: effect of addition of CeOx and Li2O. Catal Today 154:127–132
Wang D, Astruc D (2015) The golden age of transfer hydrogenation. Chem Rev 115:6621–6686
Simakova OA, Davis RJ, Murzin DY (2013) Selective hydrogenation reactions. In: Biomass process over gold catalysts. Springer Briefs in Molecular Science. Springer, Heidelberg, pp 39–40
Liao F, Huang Y, Ge J, Zheng W, Tedsree K, Collier P, Hong X, Tsang SC (2011) Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH. Angew Chem Int Ed 50:2162–2165
Hou R (2017) Selective hydrogenation of 1,3-butadiene on Pd–Ni bimetallic catalyst: from model surfaces to supported catalysts. In: Catalytic and process study of the selective hydrogenation of acetylene and 1,3-butadiene. Springer theses. Springer, Singapore, pp 45–72
Smith AM, Whyman R (2014) Review of methods for the catalytic hydrogenation of carboxamides. Chem Rev 114:5477–5510
Pritchard J, Filonenko GA, van Putten R, Hensen EJM, Pidko EA (2015) Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: history, advances and future directions. Chem Soc Rev 44:3808–3833
Ouyang G, Wang CX, Yang GW (2009) Surface energy of nanostructural materials with negative curvature and related size effects. Chem Rev 109:4221–4247
Schmid G (2004) Nanoparticles: from theory to application. Wiley-VCH, Weinheim
Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 44:7852–7872
Thielbeer F, Donaldson K, Bradley M (2011) Zeta potential mediated reaction monitoring on nano and microparticles. Bioconjug Chem 22:144–150
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12:743–754
Aiken JD III, Lin Y, Finke RG (1996) A perspective on nanocluster catalysis: polyoxoanion and (n-C4H9)4N + stabilized Ir(0) ~ 300 nanocluster ‘soluble heterogeneous catalysts’. J Mol Catal A Chem 114:29–51
Antonels NC (2014) The evaluation of dendrimer encapsulated ruthenium nanoparticles, immobilised on silica, as catalysts in various catalytic reactions and the effect of ionic liquids on the catalytic activity. University of Johannesburg, Johannesburg
Belyakova OA, Slovokhotov YL (2003) Structures of large transition metal clusters. Russ Chem Bull 52:2299–2327
Kidwai M (2010) Nanoparticles in green catalysis. In: Handbook of green chemistry. Online, Wiley Online Library, pp 81–92
Toshima N (2003) In: Liz-Marzan LM, Kamat PV (eds) Nanoscale materials, vol 444. Kluwer Acad. Pub., London, pp 79–96
Thathagar MB, Beckers J, Rothenberg G (2002) Copper-catalyzed Suzuki cross-coupling using mixed nanocluster catalysts. J Am Chem Soc 124:11858–11859
Calo V, Nacci A, Monopoli A, Ieva E, Cioffi N (2005) Copper bronze catalyzed heck reaction in ionic liquids. Org Lett 7:617–620
Lipshutz BH, Taft BR (2006) Heterogeneous copper-in-charcoal-catalyzed click chemistry. Angew Chem Int Ed 45:8235–8238
Jammi S, Sakthivel S, Rout L, Mukherjee T, Mandal S, Mitra R, Saha P, Punniyamurthy T (2009) CuO nanoparticles catalyzed C–N, C–O, and C–S cross-coupling reactions: scope and mechanism. J Org Chem 74:1971–1976
Ranu BC, Dey R, Chatterjee T, Ahammed S (2012) Copper nanoparticle-catalyzed carbon–carbon and carbon–heteroatom bond formation with a greener perspective. Chemsuschem 5:22–44
Mitsudome T, Mikami Y, Ebata K, Mizugaki T, Jitsukawa K, Kaneda K (2008) Copper nanoparticles on hydrotalcite as a heterogeneous catalyst for oxidant-free dehydrogenation of alcohols. Chem Commun 39:4804–4806
Ndolomingo MJ, Meijboom R (2015) Kinetic analysis of catalytic oxidation of methylene blue over γ-Al2O3 supported copper nanoparticles. Appl Catal A Gen 506:33–43
Hutchings GJ, Haruta M (2005) A golden age of catalysis: a perspective. Appl Catal A Gen 291:2–5
Haruta M (2005) Catalysis: gold rush. Nature 437:1098–1099
Sermon PA, Bond GC, Wells PB (1979) Hydrogenation of alkenes over supported gold. J Chem Soc Faraday 1 Trans Phys Chem Condens Phases 75:385–394
Bond GC (1972) The catalytic properties of gold. Gold Bull 5:11–13
Schwank J (1985) Gold in bimetallic catalysts. Gold Bull 18:2–10
Hashmi ASK, Hutchings GJ (2006) Gold catalysis. Angew Chem Int Ed 45:7896–7936
Hutchings GJ, Brust M, Schmidbaur H (2008) Gold—an introductory perspective. Chem Soc Rev 37:1759–1765
Corma A, Serna P (2006) Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313:332–334
Hutchings GJ (1996) Catalysis: a golden future. Gold Bull 29:123–130
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 C. Chem Lett 16:405–408
Abad A, Concepción P, Corma A, García H (2005) A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew Chem Int Ed 44:4066–4069
Malta G, Kondrat SA, Freakley SJ, Davies CJ, Lu L, Dawson S, Thetford A, Gibson EK, Morgan DJ, Jones W (2017) Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355:1399–1403
Hashmi ASK, Rudolph M (2008) Gold catalysis in total synthesis. Chem Soc Rev 37:1766–1775
Marion N, Nolan SP (2008) N-Heterocyclic carbenes in gold catalysis. Chem Soc Rev 37:1776–1782
Ndolomingo MJ, Meijboom R (2017) Selective liquid phase oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over γ-Al2O3 supported copper and gold nanoparticles. Appl Surf Sci 398:19–32
Wang C, Daimon H, Onodera T, Koda T, Sun S (2008) A general approach to the size-and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew Chem Int. Ed 47:3588–3591
Tan TL, Wang L-L, Zhang J, Johnson DD, Bai K (2015) Platinum nanoparticle during electrochemical hydrogen evolution: adsorbate distribution, active reaction species, and size effect. ACS Catal 5:2376–2383
Ovejero G, Rodríguez A, Vallet A, García J (2011) Studies in catalytic wet air oxidation as a process to destroy CI Basic Yellow 11 in aqueous stream over platinum catalyst. Color Technol 127:10–17
Gomes HT, Samant PV, Serp P, Kalck P, Figueiredo JL, Faria JL (2004) Carbon nanotubes and xerogels as supports of well-dispersed Pt catalysts for environmental applications. Appl Catal B Environ 54:175–182
Daas BM, Ghosh S (2016) Fuel cell applications of chemically synthesized zeolite modified electrode (ZME) as catalyst for alcohol electro-oxidation—a review. J Electroanal Chem 783:308–315
Lange J, Price R, Ayoub PM, Louis J, Petrus L, Clarke L, Gosselink H (2010) Valeric biofuels: a platform of cellulosic transportation fuels. Angew Chem Int Ed 49:4479–4483
Liu X, Guo Y, Xu W, Wang Y, Gong X, Guo Y, Guo Y, Lu G (2011) Catalytic properties of Pt/Al2O3 catalysts in the aqueous-phase reforming of ethylene glycol: effect of the alumina support. Kinet Catal 52:817–822
Stanislaus A, Marafi A, Rana MS (2010) Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal Today 153:1–68
Bingwa N, Ndolomingo MJ, Noh J-H, Antonels N, Haumann M, Meijboom R (n.d) Synergistic effect of mesoporous transition metal oxides and Pt nanoparticles in aerobic oxidation of ethanol and ionic liquid induced selectivity. Manuscript under review
Smidt J, Hafner W, Jira R, Sedlmeier J, Sieber R, Rüttinger R, Kojer H (1959) Katalytische Umsetzungen von Olefinen an Platinmetall-Verbindungen Das Consortium-Verfahren zur Herstellung von Acetaldehyd. Angew Chem 71:176–182
Reddy CB, Bharti R, Kumar S, Das P (2016) Supported palladium nanoparticles-catalyzed decarboxylative coupling approaches to aryl alkynes, indoles and pyrrolines synthesis. RSC Adv. 6:71117–71121
Kumar S, Chaudhary A, Bhattacherjee D, Thakur V, Das P (2017) Supported palladium nanoparticles as switchable catalyst for aldehyde conjugate/s and acetate ester syntheses from alcohols. New J Chem 41:3242–3245
Shiju NR, Guliants VV (2009) Recent developments in catalysis using nanostructured materials. Appl Catal A Gen 356:1–17
Shah D, Kaur H (2016) Supported palladium nanoparticles: a general sustainable catalyst for microwave enhanced carbon-carbon coupling reactions. J Mol Catal A Chem 424:171–180
Ncube P, Hlabathe T, Meijboom R (2015) Palladium nanoparticles supported on mesoporous silica as efficient and recyclable heterogenous nanocatalysts for the Suzuki C–C coupling reaction. J Clust Sci 26:1873–1888
Iqbal S, Kondrat SA, Jones DR, Schoenmakers DC, Edwards JK, Lu L, Yeo BR, Wells PP, Gibson EK, Morgan DJ (2015) Ruthenium nanoparticles supported on carbon: an active catalyst for the hydrogenation of lactic acid to 1, 2-propanediol. ACS Catal 5:5047–5059
Tan J, Cui J, Cui X, Deng T, Li X, Zhu Y, Li Y (2015) Graphene-modified Ru nanocatalyst for low-temperature hydrogenation of carbonyl groups. ACS Catal 5:7379–7384
Sun J, Li X, Taguchi A, Abe T, Niu W, Lu P, Yoneyama Y, Tsubaki N (2013) Highly-dispersed metallic Ru nanoparticles sputtered on H-beta zeolite for directly converting syngas to middle isoparaffins. ACS Catal 4:1–8
Manzer LE (2004) Catalytic synthesis of α-methylene-γ-valerolactone: a biomass-derived acrylic monomer. Appl Catal A Gen 272:249–256
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554
Protsenko II, Nikoshvili LZ, Matveeva VG, Sulman EM, Rebrov E (2016) Selective hydrogenation of levulinic acid to gamma-valerolactone using polymer-based ru-containing catalysts. Chem Eng Trans 52:679–684
Frohning CD, Kölbel H, Ralek M, Rottig W, Schnur F, Schulz H (1977) Fischer–Tropsch synthese. In: Falbe J (ed) Chemierohstoffe Aus Kohle, pp 218–219
Vannice MA (1975) The catalytic synthesis of hydrocarbons from H2CO mixtures over the group VIII metals: III. Metal–support effects with Pt and Pd catalysts. J Catal 40:129–134
Kitano M, Kanbara S, Inoue Y, Kuganathan N, Sushko PV, Yokoyama T, Hara M, Hosono H (2015) Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat Commun 6:6731
Ndolomingo MJ, Meijboom R (2019) Noble and base-metal nanoparticles supported on mesoporous metal oxides: efficient catalysts for the selective hydrogenation of levulinic acid to γ-valerolactone. Catal Lett 149:2807–2822
Sun Y (2013) Controlled synthesis of colloidal silver nanoparticles in organic solutions: empirical rules for nucleation engineering. Chem Soc Rev 42:2497–2511
Rizzello L, Pompa PP (2014) Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev 43:1501–1518
Dong XY, Gao ZW, Yang KF, Zhang WQ, Xu LW (2015) Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal Sci Technol 5:2554–2574
Gawande MB, Guo H, Rathi AK, Branco PS, Chen Y, Varma RS, Peng D-L (2013) First application of core-shell Ag@ Ni magnetic nanocatalyst for transfer hydrogenation reactions of aromatic nitro and carbonyl compounds. RSC Adv 3:1050–1054
Bingwa N, Meijboom R (2015) Evaluation of catalytic activity of Ag and Au dendrimer-encapsulated nanoparticles in the reduction of 4-nitrophenol. J Mol Catal A Chem 396:1–7
Chaki NK, Tsunoyama H, Negishi Y, Sakurai H, Tsukuda T (2007) Effect of Ag-doping on the catalytic activity of polymer-stabilized Au clusters in aerobic oxidation of alcohol. J Phys Chem C 111:4885–4888
Nemanashi-Maumela M, Nongwe I, Motene RC, Davids BL, Meijboom R (2017) Au and Ag nanoparticles encapsulated within silica nanospheres using dendrimers as dual templating agent and their catalytic activity. Mol Catal 438:184–196
Guo JZ, Cui H, Zhou W, Wang W (2008) Ag nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. J Photochem Photobiol A Chem 193:89–96
Khodakov AY, Chu W, Fongarland P (2007) Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem Rev 107:1692–1744
van Deelen TW, Nijhuis JJ, Krans NA, Zečević J, de Jong KP (2018) Preparation of Cobalt nanocrystals supported on metal oxides to study particle growth in Fischer–Tropsch catalysts. ACS Catal 8:10581–10589
Sadek R, Chalupka KA, Mierczynski P, Rynkowski J, Gurgul J, Dzwigaj S (2019) Cobalt based catalysts supported on two kinds of beta zeolite for application in Fischer–Tropsch synthesis. Catalysts 9:497
Hertrich MF, Scharnagl FK, Pews-Davtyan A, Kreyenschulte CR, Lund H, Bartling S, Jackstell R, Beller M (2019) Supported cobalt nanoparticles for hydroformylation reactions. Chem Eur J 25:5534–5538
Bingwa N, Ndolomingo MJ, Mabate T, Dube S, Meijboom R (2019) Surface property–activity relations of Co/Sn oxide nanocatalysts evaluated using a model reaction: surface characterization study. Catal Lett 149:2940–2949
Seo S, Perez GA, Tewari K, Comas X, Kim M (2018) Catalytic activity of nickel nanoparticles stabilized by adsorbing polymers for enhanced carbon sequestration. Sci Rep 8(11786):1–11
Seo S, Nguyen M, Mastiani M, Navarrete G, Kim M (2017) Microbubbles loaded with nickel nanoparticles: a perspective for carbon sequestration. Anal Chem 89:10827–10833
Bhaduri GA, Šiller L (2013) Nickel nanoparticles catalyse reversible hydration of carbon dioxide for mineralization carbon capture and storage. Catal Sci Technol 3:1234–1239
Khurana JM, Vij K (2012) Nickel nanoparticles: a highly efficient catalyst for one pot synthesis of tetraketones and biscoumarins. J Chem Sci 124:907–912
Kamata H, Tian ZQ, Izumi Y, Choong CKS, Chang J, Schreyer M, Chen L, Borgna A (2018) Dispersed and high loading Ni catalyst stabilized in porous SiO2 matrix for substituted natural gas production. Catal Today 299:193–200
Li S, Tang H, Gong D, Ma Z, Liu Y (2017) Loading Ni/La2O3 on SiO2 for CO methanation from syngas. Catal Today 297:298–307
Naito M, Yokoyama T, Hosokawa K, Nogi K (2018) Nanoparticle technology handbook. Elsevier, Amsterdam
Merkel TJ, Herlihy KP, Nunes J, Orgel RM, Rolland JP, DeSimone JM (2009) Scalable, shape-specific, top-down fabrication methods for the synthesis of engineered colloidal particles. Langmuir 26:13086–13096
Wang X, Zhuang J, Peng Q, Li Y (2005) A general strategy for nanocrystal synthesis. Nature 437:121–124
Peng Z, Yang H (2009) Designer platinum nanoparticles: control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 4:143–164
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Dupont J, Scholten JD (2010) On the structural and surface properties of transition-metal nanoparticles in ionic liquids. Chem Soc Rev 39:1780–1804
Gurav JL, Jung I-K, Park H-H, Kang ES, Nadargi DY (2010) Silica aerogel: synthesis and applications. J Nanomater 2010:1–11
Jiang Z-J, Liu C-Y, Sun L-W (2005) Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B 109:1730–1735
Hori K, Matsune H, Takenaka S, Kishida M (2006) Preparation of silica-coated Pt metal nanoparticles using microemulsion and their catalytic performance. Sci Technol Adv Mater 7:678–684
He L, Lin Q, Liu Y, Huang Y (2014) Unique catalysis of Ni–Al hydrotalcite derived catalyst in CO2 methanation: cooperative effect between Ni nanoparticles and a basic support. J Energy Chem 23:587–592
Peng X, Cheng K, Kang J, Gu B, Yu X, Zhang Q, Wang Y (2015) Impact of hydrogenolysis on the selectivity of the Fischer–Tropsch synthesis: diesel fuel production over mesoporous Zeolite-Y-supported cobalt nanoparticles. Angew Chem Int Ed 54:4553–4556
Cheng D, Jin W, Zhan X, Chen F (2016) Alumina membrane coated activated carbon: a novel strategy to enhance the mechanical properties of a solid catalyst. RSC Adv. 6:10229–10232
Raybaud P, Chizallet C, Mager-Maury C, Digne M, Toulhoat H, Sautet P (2013) From γ-alumina to supported platinum nanoclusters in reforming conditions: 10 years of DFT modeling and beyond. J Catal 308:328–340
Bagheri S, Muhd Julkapli N, Bee Abd Hamid S (2014) Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci World J 2014:1–21. https://doi.org/10.1155/2014/727496
Ntais S, Isaifan RJ, Baranova EA (2014) An X-ray photoelectron spectroscopy study of platinum nanoparticles on yttria-stabilized zirconia ionic support: insight into metal support interaction. Mater Chem Phys 148:673–679
Mori K, Hara T, Mizugaki T, Ebitani K, Kaneda K (2004) Hydroxyapatite-supported palladium nanoclusters: a highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J Am Chem Soc 126:10657–10666
Kantam ML, Chakravarti R, Pal U, Sreedhar B, Bhargava S (2008) nanocrystalline magnesium oxide-stabilized palladium (0): an efficient and reusable catalyst for selective reduction of nitro compounds. Adv Synth Catal 350:822–827
Wang LC, Liu Y-M, Chen M, Cao Y, He H-Y, Fan K-N (2008) MnO2 nanorod supported gold nanoparticles with enhanced activity for solvent-free aerobic alcohol oxidation. J Phys Chem C 112:6981–6987
Wu H, Wang L, Zhang J, Shen Z, Zhao J (2011) Catalytic oxidation of benzene, toluene and p-xylene over colloidal gold supported on zinc oxide catalyst. Catal Commun 12:859–865
Yamada Y, Yano K, Xu Q, Fukuzumi S (2010) Cu/Co3O4 nanoparticles as catalysts for hydrogen evolution from ammonia borane by hydrolysis. J Phys Chem C 114:16456–16462
Pérez-Mayoral E, Calvino-Casilda V, Soriano E (2016) Metal-supported carbon-based materials: opportunities and challenges in the synthesis of valuable products. Catal Sci Technol 6:1265–1291
Haber J, Block JH, Delmon B (1995) Manual of methods and procedures for catalyst characterization (Technical Report). Pure Appl Chem 67(1995):1257–1306
Poncelet G, Martens J, Delmon B, Grange P, Jacobs PA (1995) Preparation of catalysts VI: scientific bases for the preparation of heterogeneous catalysts. Elsevier, Amsterdam
Ertl G, Knà H, Weitkamp J (2008) Preparation of solid catalysts. Wiley, New York
Qiu S, Zhang X, Liu Q, Wang T, Zhang Q, Ma L (2013) A simple method to prepare highly active and dispersed Ni/MCM-41 catalysts by co-impregnation. Catal Commun 42:73–78
Del Angel G, Bonilla A, Pena Y, Navarrete J, Fierro JLG, Acosta DR (2003) Effect of lanthanum on the catalytic properties of PtSn/γ-Al2O3 bimetallic catalysts prepared by successive impregnation and controlled surface reaction. J Catal 219:63–73
Agnihotry SA, Sharma N, Deepa M (2002) Ion exchange derived precursor materials for deposition of WO3 electrochromic films: spectroscopic investigations. J Sol-Gel Sci Technol 24:265–270
Jones AC, Hitchman ML (2009) Chemical vapour deposition: precursors, processes and applications. Royal Society of Chemistry, Cambridge
Schüth F, Hesse M, Unger KK (2008) In: Rostrup-Nielsen JR, Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis, pp 100–109
Rochas C, Rinaudo M (1984) Mechanism of gel formation in κ-carrageenan. Biopolym Orig Res Biomol 23:735–745
Murzin D (2013) Engineering catalysis. Walter de Gruyter, Berlin
Taghvaei H, Heravi M, Rahimpour MR (2017) Synthesis of supported nanocatalysts via novel non-thermal plasma methods and its application in catalytic processes. Plasma Process Polym 14(1600204):1–20
Di L, Zhang J, Zhang X (2018) A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process Polym 15(1700234):1–19
Wang W, Wang Z, Wang J, Zhong C, Liu C (2017) Highly active and stable Pt–Pd alloy catalysts synthesized by room-temperature electron reduction for oxygen reduction reaction. Adv Sci 4:1600486-1–1600486-9
Wang X, Xu W, Liu N, Yu Z, Li Y, Qiu J (2015) Synthesis of metallic Ni–Co/graphene catalysts with enhanced hydrodesulfurization activity via a low-temperature plasma approach. Catal Today 256:203–208
Kozhevin VM, Yavsin DA, Kouznetsov VM, Busov VM, Mikushkin VM, Nikonov SY, Gurevich SA, Kolobov A (2000) Granulated metal nanostructure deposited by laser ablation accompanied by cascade drop fission. J Vac Sci Technol B Microelectron Nanom Struct Process Meas Phenom 18:1402–1405
Lokteva ES, Golubina EV (2019) Metal–support interactions in the design of heterogeneous catalysts for redox processes. Pure Appl Chem 91:609–631
Rostovshchikova TN, Lokteva ES, Kavalerskaya NE, Gurevich SA, Kozhevin VM, Yavsin DA (2013) Surface density of particles in the design of nanostructured catalysts. Theor Exp Chem 49:40–45
Zhu Q-L, Xu Q (2016) Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chemistry 1:220–245
Zhu Q-L, Xu Q (2014) Metal–organic framework composites. Chem Soc Rev 43:5468–5512
Campelo JM, Luna D, Luque R, Marinas JM, Romero AA (2009) Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem Chem Sustain Energy Mater 2:18–45
Zhong H, Liu C, Wang Y, Wang R, Hong M (2016) Tailor-made porosities of fluorene-based porous organic frameworks for the pre-designable fabrication of palladium nanoparticles with size, location and distribution control. Chem Sci 7:2188–2194
Aijaz A, Karkamkar A, Choi YJ, Tsumori N, Rönnebro E, Autrey T, Shioyama H, Xu Q (2012) Immobilizing highly catalytically active Pt nanoparticles inside the pores of metal–organic framework: a double solvents approach. J Am Chem Soc 134:13926–13929
Chen Y, Zhu Q-L, Tsumori N, Xu Q (2015) Immobilizing highly catalytically active noble metal nanoparticles on reduced graphene oxide: a non-noble metal sacrificial approach. J Am Chem Soc 137:106–109
Zhu Q-L, Tsumori N, Xu Q (2015) Immobilizing extremely catalytically active palladium nanoparticles to carbon nanospheres: a weakly-capping growth approach. J Am Chem Soc 137:11743–11748
Maciver DS, Tobin HH, Barth RT (1963) Catalytic aluminas I. Surface chemistry of eta and gamma alumina. J Catal 2:485–497
Rozita Y, Brydson R, Scott AJ (2010) An investigation of commercial gamma-Al2O3 nanoparticles. J Phys Conf Ser 241:12096-1–12096-4
Krokidis X, Raybaud P, Gobichon AE, Rebours B, Euzen P, Toulhoat H (2001) Theoretical study of the dehydration process of boehmite to γ-alumina. J Phys Chem B 105:5121–5130
Lippens BC, De Boer JH (1964) Study of phase transformations during calcination of aluminum hydroxides by selected area electron diffraction. Acta Crystallogr 17:1312–1321
Vaezifar S, Faghihian H, Kamali M (2008) The influence of alumina used as a support on the catalytic properties of Pt/Sn/Al2O3 systems in the dehydrogenation of isobutane. J Iran Chem Res 1:19–24
Levin I, Bendersky LA, Brandon DG, Rühle M (1997) Cubic to monoclinic phase transformations in alumina. Acta Mater 45:3659–3669
Halvarsson M (2016) CVD alumina. http://fy.chalmers.se/~f10mh/Halvarsson/CVD_alumina.html. Accessed 22 June 2016
Ndolomingo MJ, Meijboom R (2016) Kinetics of the catalytic oxidation of morin on γ-Al2O3 supported gold nanoparticles and determination of gold nanoparticles surface area and sizes by quantitative ligand adsorption. Appl Catal B Environ 199:142–154
Lippits MJ, Nieuwenhuys BE (2010) Direct conversion of ethanol into ethylene oxide on gold-based catalysts: effect of CeOx and Li2O addition on the selectivity. J Catal 274:142–149
Trewyn BG, Nieweg JA, Zhao Y, Lin VS-Y (2008) Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem Eng J 137:23–29
Nandiyanto ABD, Kim S-G, Iskandar F, Okuyama K (2009) Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater 120:447–453
Vunain E, Ncube P, Jalama K, Meijboom R (2018) Confinement effect of rhodium(I) complex species on mesoporous MCM-41 and SBA-15: effect of pore size on the hydroformylation of 1-octene. J Porous Mater 25:303–320
Xu J, Li K, Shi W, Li R, Peng T (2014) Rice-like brookite titania as an efficient scattering layer for nanosized anatase titania film-based dye-sensitized solar cells. J Power Sources 260:233–242
Ruppert AM, Grams J, Jędrzejczyk M, Matras-Michalska J, Keller N, Ostojska K, Sautet P (2015) Titania-supported catalysts for levulinic acid hydrogenation: influence of support and its impact on γ-valerolactone yield. Chemsuschem 8:1538–1547
Dai H (2001) Carbon nanotubes: synthesis, structure, properties, and applications. In: Dresselhaus MS, Dresselhaus G, Avouris P (eds) Topics in applied physics, vol 80. Springer, pp 29–54
Ravat V, Nongwe I, Meijboom R, Bepete G, Coville NJ (2013) Pd on boron-doped hollow carbon spheres–PdO particle size and support effects. J Catal 305:36–45
Lam E, Luong JHT (2014) Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal 4:3393–3410
Gluhoi AC, Bogdanchikova N, Nieuwenhuys BE (2005) The effect of different types of additives on the catalytic activity of Au/Al2O3 in propene total oxidation: transition metal oxides and ceria. J Catal 229:154–162
Lippits MJ (201) Catalytic behavior of Cu, Ag and Au nanoparticles. A comparison
Lee J-K, Kung MC, Kung HH (2008) Cooperative catalysis: a new development in heterogeneous catalysis. Top Catal 49:136–144
Lippits MJ, Gluhoi AC, Nieuwenhuys BE (2007) A comparative study of the effect of addition of CeOx and Li2O on γ-Al2O3 supported copper, silver and gold catalysts in the preferential oxidation of CO. Top Catal 44:159–165
Gluhoi AC, Lin SD, Nieuwenhuys BE (2004) The beneficial effect of the addition of base metal oxides to gold catalysts on reactions relevant to air pollution abatement. Catal Today 90:175–181
Gluhoi AC, Bogdanchikova N, Nieuwenhuys BE (2005) Alkali (earth)-doped Au/Al2O3 catalysts for the total oxidation of propene. J Catal 232:96–101
Gluhoi AC, Nieuwenhuys BE (2007) Structural and chemical promoter effects of alkali (earth) and cerium oxides in CO oxidation on supported gold. Catal Today 122:226–232
Gluhoi AC (2005) Fundamental studies focused on understanding of gold catalysis. Faculty of Mathematics & Natural Sciences, Institute of Chemistry, Leiden University, Leiden
Gluhoi AC, Tang X, Marginean P, Nieuwenhuys BE (2006) Characterization and catalytic activity of unpromoted and alkali (earth)-promoted Au/Al2O3 catalysts for low-temperature CO oxidation. Top Catal 39:101–110
de Miguel SR, Caballero Martinez A, Castro AA, Scelza OA (1996) Effect of lithium addition upon γ-Al2O3 for isopropanol dehydration. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 65:131–136
Lippits MJ, Iwema RRHB, Nieuwenhuys BE (2009) A comparative study of oxidation of methanol on γ-Al2O3 supported group IB metal catalysts. Catal Today 145:27–33
Grisel RJH, Weststrate CJ, Goossens A, Crajé MWJ, Van der Kraan AM, Nieuwenhuys BE (2002) Oxidation of CO over Au/MOx/Al2O3 multi-component catalysts in a hydrogen-rich environment. Catal Today 72:123–132
Yang Q, Liu G, Liu Y (2017) Perovskite-type oxides as the catalyst precursors for preparing supported metallic nanocatalysts: a review. Ind Eng Chem Res 57:1–17
Grabowska E (2016) Selected perovskite oxides: characterization, preparation and photocatalytic properties—a review. Appl. Catal. B Environ. 186:97–126
Lam SM, Sin JC, Mohamed AR (2017) A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review. Mater Res Bull 90:15–30
Chen J, He Z, Li G, An T, Shi H, Li Y (2017) Visible-light-enhanced photothermocatalytic activity of ABO3-type perovskites for the decontamination of gaseous styrene. Appl Catal B Environ 209:146–154
Pacchioni G, Freund HJ (2018) Controlling the charge state of supported nanoparticles in catalysis: lessons from model systems. Chem Soc Rev 47:8474–8502
Tosoni S, Pacchioni G (2017) Trends in adhesion energies of gold on MgO (100), Rutile TiO2 (110), and CeO2 (111) surfaces: a comparative DFT study. J Phys Chem C 121:28328–28338
Corma A, García H, Llabrés i Xamena FX (2010) Engineering metal organic frameworks for heterogeneous catalysis. Chem Rev 110:4606–4655
Chen L, Xu Q (2019) Metal-organic framework composites for catalysis. Matter 1:57–89
Cui Y, Li B, He H, Zhou W, Chen B, Qian G (2016) Metal–organic frameworks as platforms for functional materials. Acc Chem Res 49:483–493
Zhu QL, Li J, Xu Q (2013) Immobilizing metal nanoparticles to metal–organic frameworks with size and location control for optimizing catalytic performance. J Am Chem Soc 135:10210–10213
Hawxwell SM, Espallargas GM, Bradshaw D, Rosseinsky MJ, Prior TJ, Florence AJ, van de Streek J, Brammer L (2007) Ligand flexibility and framework rearrangement in a new family of porous metal–organic frameworks. Chem Commun 15:1532–1534
Jiao L, Wang Y, Jiang H, Xu Q (2018) Metal–organic frameworks as platforms for catalytic applications. Adv Mater 30(1703663):1–23
Xu C, Fang R, Luque R, Chen L, Li Y (2019) Functional metal–organic frameworks for catalytic applications. Coord Chem Rev 388:268–292
Tu W, Xu Y, Yin S, Xu R (2018) Rational design of catalytic centers in crystalline frameworks. Adv Mater 30(1707582):1–29
Kang Y-S, Lu Y, Chen K, Zhao Y, Wang P, Sun W-Y (2019) Metal–organic frameworks with catalytic centers: from synthesis to catalytic application. Coord Chem Rev 378:262–280
Dang S, Zhu Q-L, Xu Q (2018) Nanomaterials derived from metal–organic frameworks. Nat Rev Mater 3(17075):1–14
Chen L, Luque R, Li Y (2017) Controllable design of tunable nanostructures inside metal–organic frameworks. Chem Soc Rev 46:4614–4630
O’Neill LD, Zhang H, Bradshaw D (2010) Macro-/microporous MOF composite beads. J Mater Chem 20:5720–5726
Li G, Zhao S, Zhang Y, Tang Z (2018) Metal-organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: recent progress and perspectives. Adv Mater 30(1800702):1–43
Sun J-L, Chen Y-Z, Ge B-D, Li J-H, Wang G-M (2018) Three-shell Cu@Co@Ni nanoparticles stabilized with a metal–organic framework for enhanced tandem catalysis. ACS Appl Mater Interfaces 11:940–947
Lu G, Li S, Guo Z, Farha OK, Hauser BG, Qi X, Wang Y, Wang X, Han S, Liu X (2012) Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat Chem 4:310–316
Yang Q, Xu Q, Yu S, Jiang H (2016) Pd nanocubes@ ZIF-8: integration of plasmon-driven photothermal conversion with a metal-organic framework for efficient and selective catalysis. Angew Chem Int Ed 55:3685–3689
Chen Y-Z, Gu B, Uchida T, Liu J, Liu X, Ye B-J, Xu Q, Jiang H-L (2019) Location determination of metal nanoparticles relative to a metal–organic framework. Nat Commun 10(3462):1–10
Gadipelli S, Travis W, Zhou W, Guo Z (2014) A thermally derived and optimized structure from ZIF-8 with giant enhancement in CO2 uptake. Energy Environ Sci 7:2232–2238
Liu B, Shioyama H, Akita T, Xu Q (2008) Metal-organic framework as a template for porous carbon synthesis. J Am Chem Soc 130:5390–5391
Liu B, Han S, Tanaka K, Shioyama H, Xu Q (2009) Metal–organic framework (MOF) as a precursor for synthesis of platinum supporting zinc oxide nanoparticles. Bull Chem Soc Jpn 82:1052–1054
Ruppert AM, Weckhuysen BM (2008) Metal–support interactions. In: Handbook of heterogeneous catalysis, pp 1178–1188
Bingwa N, Patala R, Noh J-H, Ndolomingo MJ, Tetyana S, Bewana S, Meijboom R (2017) Synergistic effects of gold–palladium nanoalloys and reducible supports on the catalytic reduction of 4-nitrophenol. Langmuir 33:7086–7095
Munoz M, de Pedro ZM, Casas JA, Rodriguez JJ (2014) Improved γ-alumina-supported Pd and Rh catalysts for hydrodechlorination of chlorophenols. Appl Catal A Gen 488:78–85
Baeza JA, Calvo L, Gilarranz MA, Rodriguez JJ (2014) Effect of size and oxidation state of size-controlled rhodium nanoparticles on the aqueous-phase hydrodechlorination of 4-chlorophenol. Chem Eng J 240:271–280
Gómez-Sainero LM, Seoane XL, Fierro JLG, Arcoya A (2002) Liquid-phase hydrodechlorination of CCl4 to CHCl3 on Pd/carbon catalysts: nature and role of Pd active species. J Catal 209:279–288
Ordóñez S, Díaz E, Bueres RF, Asedegbega-Nieto E, Sastre H (2010) Carbon nanofibre-supported palladium catalysts as model hydrodechlorination catalysts. J Catal 272:158–168
Baeza JA, Calvo L, Gilarranz MA, Mohedano AF, Casas JA, Rodriguez JJ (2012) Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase. J Catal 293:85–93
Ewbank JL, Kovarik L, Diallo FZ, Sievers C (2015) Effect of metal–support interactions in Ni/Al2O3 catalysts with low metal loading for methane dry reforming. Appl Catal A Gen 494:57–67
Molina CB, Calvo L, Gilarranz MA, Casas JA, Rodriguez JJ (2009) Pd–Al pillared clays as catalysts for the hydrodechlorination of 4-chlorophenol in aqueous phase. J Hazard Mater 172:214–223
Lokteva ES, Peristyy AA, Kavalerskaya NE, Golubina EV, Yashina LV, Rostovshchikova TN, Gurevich SA, Kozhevin VM, Yavsin DA, Lunin VV (2012) Laser electrodispersion as a new chlorine-free method for the production of highly effective metal-containing supported catalysts. Pure Appl Chem 84:495–508
Cao S, Tao FF, Tang Y, Li Y, Yu J (2016) Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem Soc Rev 45:4747–4765
Bingwa N, Meijboom R (2014) Kinetic evaluation of dendrimer-encapsulated palladium nanoparticles in the 4-nitrophenol reduction reaction. J Phys Chem C 118:19849–19858
Prestianni A, Ferrante F, Sulman EM, Duca D (2014) Density functional theory investigation on the nucleation and growth of small palladium clusters on a hyper-cross-linked polystyrene matrix. J Phys Chem C 118:21006–21013
Ncube P, Bingwa N, Baloyi H, Meijboom R (2015) Catalytic activity of palladium and gold dendrimer-encapsulated nanoparticles for methylene blue reduction: a kinetic analysis. Appl Catal A Gen 495:63–71
Cortese R, Schimmenti R, Prestianni A, Duca D (2018) DFT calculations on subnanometric metal catalysts: a short review on new supported materials. Theor Chem Acc 137(59):2–8
Zhang C, Michaelides A, King DA, Jenkins SJ (2010) Positive charge states and possible polymorphism of gold nanoclusters on reduced ceria. J Am Chem Soc 132:2175–2182
Tosoni S, Pacchioni G (2019) Oxide-supported gold clusters and nanoparticles in catalysis: a computational chemistry perspective. ChemCatChem 11:73–89
Häkkinen H, Landman U (2000) Gold clusters (AuN, 2 < ~ N < ~ 10) and their anions. Phys Rev B 62:R2287–R2290
Janiak C (2013) Ionic liquids for the synthesis and stabilization of metal nanoparticles. Zeitschrift Für Naturforsch B 68:1059–1089
An K, Alayoglu S, Ewers T, Somorjai GA (2012) Colloid chemistry of nanocatalysts: a molecular view. J Colloid Interface Sci 373:1–13
Lou XW, Yuan C, Rhoades E, Zhang Q, Archer LA (2006) Encapsulation and Ostwald ripening of Au and Au–Cl complex nanostructures in silica shells. Adv Funct Mater 16:1679–1684
Ostwald WZ (1901) Blocking of Ostwald ripening allowing long-term stabilization. Phys Chem 37:385
Kim M, Phan VN, Lee K (2012) Exploiting nanoparticles as precursors for novel nanostructure designs and properties. CrystEngComm 14:7535–7548
Ma Z, Yu J, Dai S (2010) Preparation of inorganic materials using ionic liquids. Adv Mater 22:261–285
Roucoux A, Schulz J, Patin H (2002) Reduced transition metal colloids: a novel family of reusable catalysts? Chem Rev 102:3757–3778
Scholten JD, Leal BC, Dupont J (2011) Transition metal nanoparticle catalysis in ionic liquids. ACS Catal 2:184–200
Hardacre C, Parvulescu V (2014) Catalysis in ionic liquirds: from catalyst synthesis to application. Royal Society of Chemistry, Cambridge
Bönnemann H, Richards RM (2001) Nanoscopic metal particles–synthetic methods and potential applications. Eur J Inorg Chem 2001:2455–2480
Pan C, Pelzer K, Philippot K, Chaudret B, Dassenoy F, Lecante P, Casanove M-J (2001) Ligand-stabilized ruthenium nanoparticles: synthesis, organization, and dynamics. J Am Chem Soc 123:7584–7593
Neouze M-A (2010) About the interactions between nanoparticles and imidazolium moieties: emergence of original hybrid materials. J Mater Chem 20:9593–9607
Vollmer C, Janiak C (2011) Naked metal nanoparticles from metal carbonyls in ionic liquids: easy synthesis and stabilization. Coord Chem Rev 255:2039–2057
Weingärtner H (2008) Understanding ionic liquids at the molecular level: facts, problems, and controversies. Angew Chem Int Ed 47:654–670
Xiao D, Rajian JR, Cady A, Li S, Bartsch RA, Quitevis EL (2007) Nanostructural organization and anion effects on the temperature dependence of the optical Kerr effect spectra of ionic liquids. J Phys Chem B 111:4669–4677
Kuwabata S, Tsuda T, Torimoto T (2010) Room-temperature ionic liquid. A new medium for material production and analyses under vacuum conditions. J Phys Chem Lett 1:3177–3188
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150
Ndolomingo MJ, Meijboom R (2016) Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption–chemisorption. Appl Surf Sci 390:224–235
Qian W, Texter J, Yan F (2017) Frontiers in poly (ionic liquid) s: syntheses and applications. Chem Soc Rev 46:1124–1159
Vijayakrishna K, Charan KTP, Manojkumar K, Venkatesh S, Pothanagandhi N, Sivaramakrishna A, Mayuri P, Kumar AS, Sreedhar B (2016) Ni nanoparticles stabilized by poly (ionic liquids) as chemoselective and magnetically recoverable catalysts for transfer hydrogenation reactions of carbonyl compounds. ChemCatChem 8:1139–1145
Charan KTP, Pothanagandhi N, Vijayakrishna K, Sivaramakrishna A, Mecerreyes D, Sreedhar B (2014) Poly (ionic liquids) as “smart” stabilizers for metal nanoparticles. Eur Polym J 60:114–122
Zimmerman SC (1999) Dendritic macromolecules: concepts, syntheses, perspectives. J Chem Educ 76:31
Scott RWJ, Wilson OM, Crooks RM (2005) Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J Phys Chem B 109:692–704
Kim Y-G, Oh S-K, Crooks RM (2004) Preparation and characterization of 1–2 nm dendrimer-encapsulated gold nanoparticles having very narrow size distributions. Chem Mater 16:167–172
Deraedt C, Ye R, Ralston WT, Toste FD, Somorjai GA (2017) Dendrimer-stabilized metal nanoparticles as efficient catalysts for reversible dehydrogenation/hydrogenation of N-heterocycles. J Am Chem Soc 139:18084–18092
Noh J-H, Meijboom R (2014) Catalytic evaluation of dendrimer-templated Pd nanoparticles in the reduction of 4-nitrophenol using Langmuir-Hinshelwood kinetics. Appl Surf Sci 320:400–413
Krüger C, Agarwal S, Greiner A (2008) Stoichiometric functionalization of gold nanoparticles in solution through a free radical polymerization approach. J Am Chem Soc 130:2710–2711
Bronger R, Le TD, Bastin S, García-Antón J, Citadelle C, Chaudret B, Lecante P, Igau A, Philippot K (2011) Multi-site coordination N-phosphanylamidine ligands as stabilizers for the synthesis of ruthenium nanoparticles. New J Chem 35:2653–2660
Jadhav SA, Brunella V, Scalarone D (2015) Polymerizable ligands as stabilizers for nanoparticles. Part Part Syst Charact 32:417–428
Khlebtsov BN, Khlebtsov NG (2011) On the measurement of gold nanoparticle sizes by the dynamic light scattering method. Colloid J 73:118–127
Bienert R, Emmerling F, Thünemann AF (2009) The size distribution of’gold standard’nanoparticles. Anal Bioanal Chem 395:1651–1660
Pyrz WD, Buttrey DJ (2008) Particle size determination using TEM: a discussion of image acquisition and analysis for the novice microscopist. Langmuir 24:11350–11360
Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC (2017) Scanning electron microscopy and X-ray microanalysis. Springer, Berlin
Willets KA, Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58:267–297
Guha S, Li M, Tarlov MJ, Zachariah MR (2012) Electrospray–differential mobility analysis of bionanoparticles. Trends Biotechnol 30:291–300
Couteau O, Roebben G (2011) Measurement of the size of spherical nanoparticles by means of atomic force microscopy. Meas Sci Technol 22(065101):1–8
Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV–Vis spectra. Anal Chem 79:4215–4221
Janz A, Köckritz A, Yao L, Martin A (2010) Fundamental calculations on the surface area determination of supported gold nanoparticles by alkanethiol adsorption. Langmuir 26:6783–6789
Perumal S, Hofmann A, Scholz N, Rühl E, Graf C (2011) Kinetics study of the binding of multivalent ligands on size-selected gold nanoparticles. Langmuir 27:4456–4464
Gadogbe M, Ansar SM, He G, Collier WE, Rodriguez J, Liu D, Chu I-W, Zhang D (2013) Determination of colloidal gold nanoparticle surface areas, concentrations, and sizes through quantitative ligand adsorption. Anal Bioanal Chem 405:413–422
Sing KSW (1998) Adsorption methods for the characterization of porous materials. Adv Colloid Interface Sci 76:3–11
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Anderson JA (2012) Supported metals in catalysis. World Scientific, Singapore
Berndt H, Pitsch I, Evert S, Struve K, Pohl M-M, Radnik J, Martin A (2003) Oxygen adsorption on Au/Al2O3 catalysts and relation to the catalytic oxidation of ethylene glycol to glycolic acid. Appl Catal A Gen 244:169–179
Chiorino A, Manzoli M, Menegazzo F, Signoretto M, Vindigni F, Pinna F, Boccuzzi F (2009) New insight on the nature of catalytically active gold sites: quantitative CO chemisorption data and analysis of FTIR spectra of adsorbed CO and of isotopic mixtures. J Catal 262:169–176
Clément M, Ménard H, Rowntree PA (2008) Determination of the surface area of Pd and nanometric Au aggregates supported on a micrometric solid support by thiol adsorption and GC–MS. Langmuir 24:8045–8049
Van Vegten N, Haider P, Maciejewski M, Krumeich F, Baiker A (2009) Chemisorption of methyl mercaptane on titania-supported Au nanoparticles: viability of Au surface area determination. J Colloid Interface Sci 339:310–316
Ulman A (1996) Formation and structure of self-assembled monolayers. Chem Rev 96:1533–1554
Dubois LH, Nuzzo RG (1992) Synthesis, structure, and properties of model organic surfaces. Annu Rev Phys Chem 43:437–463
Hinterwirth H, Kappel S, Waitz T, Prohaska T, Lindner W, Lämmerhofer M (2013) Quantifying thiol ligand density of self-assembled monolayers on gold nanoparticles by inductively coupled plasma–mass spectrometry. ACS Nano 7:1129–1136
Mahmoud MA, Garlyyev B, El-Sayed MA (2013) Determining the mechanism of solution metallic nanocatalysis with solid and hollow nanoparticles: homogeneous or heterogeneous. J Phys Chem C 117:21886–21893
Folkers JP, Gorman CB, Laibinis PE, Buchholz S, Whitesides GM, Nuzzo RG (1995) Self-assembled monolayers of long-chain hydroxamic acids on the native oxide of metals. Langmuir 11:813–824
Haider P, Baiker A (2007) Gold supported on Cu–Mg–Al-mixed oxides: strong enhancement of activity in aerobic alcohol oxidation by concerted effect of copper and magnesium. J Catal 248:175–187
Punniyamurthy T, Velusamy S, Iqbal J (2005) Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem Rev 105:2329–2364
Herves P, Perez-Lorenzo M, Liz-Marzan LM, Dzubiella J, Lu Y, Ballauff M (2012) Catalysis by metallic nanoparticles in aqueous solution: model reactions. Chem Soc Rev 41:5577–5587
Zhang W, Yang Z, Wang X, Zhang Y, Wen X, Yang S (2006) Large-scale synthesis of β-MnO2 nanorods and their rapid and efficient catalytic oxidation of methylene blue dye. Catal Commun 7:408–412
Grabowski LR, Van Veldhuizen EM, Pemen AJM, Rutgers WR (2007) Breakdown of methylene blue and methyl orange by pulsed corona discharge. Plasma Sources Sci Technol 16:226–232
Zhang L, Nie Y, Hu C, Hu X (2011) Decolorization of methylene blue in layered manganese oxide suspension with H2O2. J Hazard Mater 190:780–785
Salem IA (2000) Kinetics of the oxidative color removal and degradation of bromophenol blue with hydrogen peroxide catalyzed by copper(II)-supported alumina and zirconia. Appl Catal B Environ 28:153–162
El-Dein AM, Libra JA, Wiesmann U (2003) Mechanism and kinetic model for the decolorization of the azo dye Reactive Black 5 by hydrogen peroxide and UV radiation. Chemosphere 52:1069–1077
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Zhou L, Song W, Chen Z, Yin G (2013) Degradation of organic pollutants in wastewater by bicarbonate-activated hydrogen peroxide with a supported cobalt catalyst. Environ Sci Technol 47:3833–3839
Sopajaree K (1997) Photocatalytic oxidation of methylene blue by titanium dioxide in an integrated photoreactor-membrane filtration unit. Doctoral dissertation, University of Texas at Arlington
Mills A, Davies RH, Worsley D (1993) Water purification by semiconductor photocatalysis. Chem Soc Rev 22:417–425
Salem IA, El-Maazawi MS (2000) Kinetics and mechanism of color removal of methylene blue with hydrogen peroxide catalyzed by some supported alumina surfaces. Chemosphere 41:1173–1180
Ilunga AK, Meijboom R (2016) Catalytic oxidation of methylene blue by dendrimer encapsulated silver and gold nanoparticles. J Mol Catal A Chem 411:48–60
Elango G, Roopan SM (2016) Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J Photochem Photobiol B Biol 155:34–38
Liu Y, Jin W, Zhao Y, Zhang G, Zhang W (2017) Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl Catal B Environ 206:642–652
Atchudan R, Edison TNJI, Perumal S, Karthikeyan D, Lee YR (2016) Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J Photochem Photobiol B Biol 162:500–510
Edison TNJI, Atchudan R, Lee YR (2016) Optical sensor for dissolved ammonia through the green synthesis of silver nanoparticles by fruit extract of Terminalia chebula. J Clust Sci 27:683–690
Sriskandakumar T, Opembe N, Chen C-H, Morey A, King’ondu C, Suib SL (2009) Green decomposition of organic dyes using octahedral molecular sieve manganese oxide catalysts. J Phys Chem A 113:1523–1530
Nuengmatcha P, Chanthai S, Mahachai R, Oh WC (2016) Sonocatalytic performance of ZnO/graphene/TiO2 nanocomposite for degradation of dye pollutants (methylene blue, texbrite BAC-L, texbrite BBU-L and texbrite NFW-L) under ultrasonic irradiation. Dye Pigment 134:487–497
Wieprecht T, Heinz U, Xia J, Schlingloff G, Dannacher J (2004) Terpyridine-manganese complexes: a new class of bleach catalysts for detergent applications. J Surfactants Deterg 7:59–66
Dannacher JJ (2006) Catalytic bleach: most valuable applications for smart oxidation chemistry. J Mol Catal A Chem 251:159–176
Colombini MP, Andreotti A, Baraldi C, Degano I, Łucejko JJ (2007) Colour fading in textiles: a model study on the decomposition of natural dyes. Microchem J 85:174–182
Osman A, Makris DP (2011) Oxidation of morin (2′,3,4′,5,7-pentahydroxylavone) with a peroxidase homogenate from onion. Int Food Res J 18:1085–1089
Topalovic T (2007) Catalytic bleaching of cotton: molecular and macroscopic aspects. University of Twente, Twente
Polzer F, Wunder S, Lu Y, Ballauff M (2012) Oxidation of an organic dye catalyzed by MnOx nanoparticles. J Catal 289:80–87
Nemanashi M, Meijboom R (2015) Catalytic behavior of different sizes of dendrimer-encapsulated Aun nanoparticles in the oxidative degradation of morin with H2O2. Langmuir 31:9041–9053
Bingwa N, Bewana S, Haumann M, Meijboom R (2017) Revisiting kinetics of morin oxidation: surface kinetics analysis. Appl Surf Sci 426:497–503
Ilunga AK, Legodi IR, Gumbi S, Meijboom R (2018) Isothermic adsorption of morin onto the reducible mesoporous manganese oxide materials surface. Appl Catal B Environ 224:928–939
Bingwa N, Bewana S, Ndolomingo MJ, Mawila N, Mogudi B, Ncube P, Carleschi E, Doyle BP, Haumann M, Meijboom R (2018) Effect of alkali and alkaline earth metal dopants on catalytic activity of mesoporous cobalt oxide evaluated using a model reaction. Appl Catal A Gen 555:189–195
Yamaguchi K, Mori K, Mizugaki T, Ebitani K, Kaneda K (2000) Creation of a monomeric Ru species on the surface of hydroxyapatite as an efficient heterogeneous catalyst for aerobic alcohol oxidation. J Am Chem Soc 122:7144–7145
Mallat T, Baiker A (1994) Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal Today 19:247–283
Besson M, Gallezot P (2000) Selective oxidation of alcohols and aldehydes on metal catalysts. Catal Today 57:127–141
Crozon A-B, Besson M, Gallezot P (1998) Oxidation of 9-decen-1-ol (rosalva) by air in aqueous media on platinum catalysts. New J Chem 22:269–273
Della Pina C, Falletta E, Prati L, Rossi M (2008) Selective oxidation using gold. Chem Soc Rev 37:2077–2095
Pagliaro M, Campestrini S, Ciriminna R (2005) Ru-based oxidation catalysis. Chem Soc Rev 34:837–845
Schultz MJ, Adler RS, Zierkiewicz W, Privalov T, Sigman MS (2005) Using mechanistic and computational studies to explain ligand effects in the palladium-catalyzed aerobic oxidation of alcohols. J Am Chem Soc 127:8499–8507
ten Brink G-J, Arends IWCE, Sheldon RA (2000) Green, catalytic oxidation of alcohols in water. Science 287:1636–1639
Vazylyev M, Sloboda-Rozner D, Haimov A, Maayan G, Neumann R (2005) Strategies for oxidation catalyzed by polyoxometalates at the interface of homogeneous and heterogeneous catalysis. Top Catal 34:93–99
Bolm C, Beller M (2004) Transition metals for organic synthesis. Wiley-VCH, Weinheim
Xiao S, Zhang C, Chen R, Chen F (2015) Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe3O4 microspheres. New J Chem 39:4924–4932
Choudhary VR, Dumbre DK, Uphade BS, Narkhede VS (2004) Solvent-free oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide using transition metal containing layered double hydroxides and/or mixed hydroxides. J Mol Catal A Chem 215:129–135
Miedziak PJ, He Q, Edwards JK, Taylor SH, Knight DW, Tarbit B, Kiely CJ, Hutchings GJ (2011) Oxidation of benzyl alcohol using supported gold–palladium nanoparticles. Catal Today 163:47–54
Enache DI, Edwards JK, Landon P, Solsona-Espriu B, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Knight DW, Hutchings GJ (2006) Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 311:362–365
Meenakshisundaram S, Nowicka E, Miedziak PJ, Brett GL, Jenkins RL, Dimitratos N, Taylor SH, Knight DW, Bethell D, Hutchings GJ (2010) Oxidation of alcohols using supported gold and gold–palladium nanoparticles. Faraday Discuss 145:341–356
Kumar A, Kumar VP, Srikanth A, Vishwanathan V, Chary KVR (2016) Vapor phase oxidation of benzyl alcohol over nano Au/SBA-15 catalysts: effect of preparation methods. Catal Lett 146:35–46
Wang C, Wu X, Xiao J (2008) Broader, greener, and more efficient: recent advances in asymmetric transfer hydrogenation. Chem Asian J 3:1750–1770
Wu X, Xiao J (2007) Aqueous-phase asymmetric transfer hydrogenation of ketones—a greener approach to chiral alcohols. Chem Commun 24:2449–2466
Clapham SE, Hadzovic A, Morris RH (2004) Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord Chem Rev 248:2201–2237
Vincent T, Guibal E (2003) Chitosan-supported palladium catalyst. 3. Influence of experimental parameters on nitrophenol degradation. Langmuir 19:8475–8483
Feng ZV, Lyon JL, Croley JS, Crooks RM, Vanden Bout DA, Stevenson KJ (2009) Synthesis and catalytic evaluation of dendrimer-encapsulated Cu nanoparticles. An undergraduate experiment exploring catalytic nanomaterials. J Chem Educ 86:368–372
Lee J, Park JC, Song H (2008) A nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction of p-nitrophenol. Adv Mater 20:1523–1528
Evangelista V, Acosta B, Miridonov S, Smolentseva E, Fuentes S, Simakov A (2015) Highly active Au-CeO2@ ZrO2 yolk–shell nanoreactors for the reduction of 4-nitrophenol to 4-aminophenol. Appl Catal B Environ 166:518–528
Nemanashi M, Meijboom R (2013) Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J Colloid Interface Sci 389:260–267
Antonels NC, Meijboom R (2013) Preparation of well-defined dendrimer Encapsulated ruthenium nanoparticles and their evaluation in the reduction of 4-nitrophenol according to the Langmuir-Hinshelwood approach. Langmuir 29:13433–13442
An K, Alayoglu S, Musselwhite N, Plamthottam S, Melaet G, Lindeman AE, Somorjai GA (2013) Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J Am Chem Soc 135:16689–16696
Karim W, Spreafico C, Kleibert A, Gobrecht J, VandeVondele J, Ekinci Y, van Bokhoven JA (2017) Catalyst support effects on hydrogen spillover. Nature 541:68–71
Wang Q, Zhang Y, Zhou Y, Zhang Z, Xu Y, Zhang C, Sheng X (2015) Synthesis of dendrimer-templated Pt nanoparticles immobilized on mesoporous alumina for p-nitrophenol reduction. New J Chem 39:9942–9950
El-Bahy ZM (2013) Preparation and characterization of Pt-promoted NiY and CoY catalysts employed for 4-nitrophenol reduction. Appl Catal A Gen 468:175–183
Lin F, Doong R (2011) Bifunctional Au–Fe3O4 heterostructures for magnetically recyclable catalysis of nitrophenol reduction. J Phys Chem C 115:6591–6598
Zhou Z, Kooi S, Flytzani-Stephanopoulos M, Saltsburg H (2008) The role of the interface in CO oxidation on Au/CeO2 multilayer nanotowers. Adv Funct Mater 18:2801–2807
Rath PC, Saikia D, Mishra M, Kao H-M (2018) Exceptional catalytic performance of ultrafine Cu2O nanoparticles confined in cubic mesoporous carbon for 4-nitrophenol reduction. Appl Surf Sci 427:1217–1226
Morère J, Tenorio MJ, Torralvo MJ, Pando C, Renuncio JAR, Cabanas A (2011) Deposition of Pd into mesoporous silica SBA-15 using supercritical carbon dioxide. J Supercrit Fluids 56:213–222
Liu X, Zhao X, Zhu L, Liu N, Tian T (2018) Palladium nanoparticles covered on amine-functionalized mesoporous hollow SiO2 spheres for the reduction of 4-nitrophenol. Catal Lett 148:173–180
Chow LCH (2011) China’s energy future: a framing comment. Eurasian Geogr Econ 52:523–528
Hassan H, Lim JK, Hameed BH (2016) Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour Technol 221:645–655
Gallezot P (2012) Conversion of biomass to selected chemical products. Chem Soc Rev 41:1538–1558
Bond JQ, Alonso DM, Wang D, West RM, Dumesic JA (2010) Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 327:1110–1114
Serrano-Ruiz JC, West RM, Dumesic JA (2010) Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu Rev Chem Biomol Eng 1:79–100
Biddy MJ, Davis R, Humbird D, Tao L, Dowe N, Guarnieri MT, Linger JG, Karp EM, Salvachúa D, Vardon DR (2016) The techno-economic basis for coproduct manufacturing to enable hydrocarbon fuel production from lignocellulosic biomass. ACS Sustain Chem Eng 4:3196–3211
Luo W, Sankar M, Beale AM, He Q, Kiely CJ, Bruijnincx PCA, Weckhuysen BM (2015) High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone. Nat Commun 6(6540):1–10
Li W, Xie J-H, Lin H, Zhou Q-L (2012) Highly efficient hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by iridium pincer complexes. Green Chem 14:2388–2390
Climent MJ, Corma A, Iborra S (2014) Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem 16:516–547
Geilen F, Engendahl B, Harwardt A, Marquardt W, Klankermayer J, Leitner W (2010) Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system. Angew Chem 122:5642–5646
Yan Z, Lin L, Liu S (2009) Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst. Energy Fuels 23:3853–3858
Amenuvor G, Makhubela BCE, Darkwa J (2016) Efficient solvent-free hydrogenation of levulinic acid to γ-valerolactone by pyrazolylphosphite and pyrazolylphosphinite ruthenium(II) complexes. ACS Sustain Chem Eng 4:6010–6018
Hu Q, Yang L, Fan G, Li F (2016) Hydrogenation of biomass-derived compounds containing a carbonyl group over a copper-based nanocatalyst: insight into the origin and influence of surface oxygen vacancies. J Catal 340:184–195
Kong X, Wu S, Jin Y, Liu L, Liu J (2017) Continuous hydrogenation of ethyl levulinate to γ-valerolactone over Cu-Zn/ZrO2 catalyst with alumina binder. Energy Fuels 31:12232–12237
Stevens JG, Bourne RA, Twigg MV, Poliakoff M (2010) Real-time product switching using a twin catalyst system for the hydrogenation of furfural in supercritical CO2. Angew Chem 122:9040–9043
Yan K, Liao J, Wu X, Xie X (2013) A noble-metal free Cu-catalyst derived from hydrotalcite for highly efficient hydrogenation of biomass-derived furfural and levulinic acid. RSC Adv 3:3853–3856
Li Z, Zuo M, Jiang Y, Tang X, Zeng X, Sun Y, Lei T, Lin L (2016) Stable and efficient CuCr catalyst for the solvent-free hydrogenation of biomass derived ethyl levulinate to γ-valerolactone as potential biofuel candidate. Fuel 175:232–239
Shimizu K, Kanno S, Kon K (2014) Hydrogenation of levulinic acid to γ-valerolactone by Ni and MoOx co-loaded carbon catalysts. Green Chem 16:3899–3903
Nemanashi M, Noh J-H, Meijboom R (2018) Hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by mesoporous supported dendrimer-derived Ru and Pt catalysts: an alternative method for the production of renewable biofuels. Appl Catal A Gen 550:77–89
Piskun AS, de Haan JE, Wilbers E, van de Bovenkamp HH, Tang Z, Heeres HJ (2016) Hydrogenation of levulinic acid to γ-valerolactone in water using millimeter sized supported Ru catalysts in a packed bed reactor. Acs Sustain Chem Eng 4:2939–2950
Dutta S (2012) Catalytic materials that improve selectivity of biomass conversions. RSC Adv 2:12575–12593
Omoruyi U, Page S, Hallett J, Miller PW (2016) Homogeneous catalyzed reactions of levulinic acid: to γ-valerolactone and beyond. Chemsuschem 9:2037–2047
Xie C, Song J, Zhou B, Hu J, Zhang Z, Zhang P, Jiang Z, Han B (2016) Porous hafnium phosphonate: novel heterogeneous catalyst for conversion of levulinic acid and esters into γ-valerolactone. ACS Sustain Chem Eng 4:6231–6236
Wright WRH, Palkovits R (2012) Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. Chemsuschem 5:1657–1667
Baijun L, Lianhai L, Bingchun W, Tianxi C, Iwatani K (1998) Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl Catal A Gen 171:117–122
Sitthisa S, Resasco DE (2011) Hydrodeoxygenation of furfural over supported metal catalysts: a comparative study of Cu, Pd and Ni. Catal Lett 141:784–791
Zhong H, Li Q, Liu J, Yao G, Wang J, Zeng X, Huo Z, Jin F (2017) New method for highly efficient conversion of biomass-derived levulinic acid to γ-valerolactone in water without precious metal catalysts. ACS Sustain Chem Eng 5:6517–6523
Wang S, Huang H, Dorcet V, Roisnel T, Bruneau C, Fischmeister C (2017) Efficient Iridium catalysts for base-free hydrogenation of levulinic acid. Organometallics 36:3152–3162
Pileidis FD, Titirici M (2016) Levulinic acid biorefineries: new challenges for efficient utilization of biomass. Chemsuschem 9:562–582
Zhang J, Chen J, Guo Y, Chen L (2015) Effective upgrade of levulinic acid into γ-valerolactone over an inexpensive and magnetic catalyst derived from hydrotalcite precursor. ACS Sustain Chem Eng 3:1708–1714
Horváth IT, Mehdi H, Fábos V, Boda L, Mika LT (2008) γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem 10:238–242
Alonso DM, Wettstein SG, Dumesic JA (2013) Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem 15:584–595
Tang X, Zeng X, Li Z, Hu L, Sun Y, Liu S, Lei T, Lin L (2014) Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew Sustain Energy Rev 40:608–620
Hengne AM, Rode CV (2012) Cu–ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone. Green Chem 14:1064–1072
Yang Y, Gao G, Zhang X, Li F (2014) Facile fabrication of composition-tuned Ru–Ni bimetallics in ordered mesoporous carbon for levulinic acid hydrogenation. ACS Catal 4:1419–1425
Delhomme C, Schaper LA, Zhang-Preße M, Raudaschl-Sieber G, Weuster-Botz D, Kühn FE (2013) Catalytic hydrogenation of levulinic acid in aqueous phase. J Organomet Chem 724:297–299
Tukacs JM, Király D, Strádi A, Novodarszki G, Eke Z, Dibó G, Kégl T, Mika LT (2012) Efficient catalytic hydrogenation of levulinic acid: a key step in biomass conversion. Green Chem 14:2057–2065
Fábos V, Mika LT, Horváth IT (2014) Selective conversion of levulinic and formic acids to γ-valerolactone with the shvo catalyst. Organometallics 33:181–187
Joo F, Tóth Z, Beck MT (1977) Homogeneous hydrogenations in aqueous solutions catalyzed by transition metal phosphine complexes. Inorg Chim Acta 25:L61–L62
Deng L, Zhao Y, Li J, Fu Y, Liao B, Guo Q (2010) Conversion of levulinic acid and formic acid into γ-valerolactone over heterogeneous catalysts. Chemsuschem 3:1172–1175
Wang J, Jaenicke S, Chuah G-K (2014) Zirconium-Beta zeolite as a robust catalyst for the transformation of levulinic acid to γ-valerolactone via Meerwein–Ponndorf–Verley reduction. RSC Adv 4:13481–13489
Al-Shaal MG, Wright WRH, Palkovits R (2012) Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem 14:1260–1263
Galletti AMR, Antonetti C, De Luise V, Martinelli M (2012) A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem 14:688–694
Selva M, Gottardo M, Perosa A (2012) Upgrade of biomass-derived levulinic acid via Ru/C-catalyzed hydrogenation to γ-valerolactone in aqueous–organic–ionic liquids multiphase systems. ACS Sustain Chem Eng 1:180–189
Tan J, Cui J, Deng T, Cui X, Ding G, Zhu Y, Li Y (2015) Water-promoted hydrogenation of levulinic acid to γ-valerolactone on supported ruthenium catalyst. ChemCatChem 7:508–512
Braden DJ, Henao CA, Heltzel J, Maravelias CC, Dumesic JA (2011) Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid. Green Chem 13:1755–1765
Wettstein SG, Bond JQ, Alonso DM, Pham HN, Datye AK, Dumesic JA (2012) RuSn bimetallic catalysts for selective hydrogenation of levulinic acid to γ-valerolactone. Appl Catal B Environ 117:321–329
Zhu S, Zhu Y, Hao S, Zheng H, Mo T, Li Y (2012) One-step hydrogenolysis of glycerol to biopropanols over Pt–H4SiW12O40/ZrO2 catalysts. Green Chem 14:2607–2616
Wang Y, Zhou J, Guo X (2015) Catalytic hydrogenolysis of glycerol to propanediols: a review. RSC Adv 5:74611–74628
Vasiliadou ES, Eggenhuisen TM, Munnik P, De Jongh PE, De Jong KP, Lemonidou AA (2014) Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl Catal B Environ 145:108–119
Xiao Z, Jin S, Wang X, Li W, Wang J, Liang C (2012) Preparation, structure and catalytic properties of magnetically separable Cu–Fe catalysts for glycerol hydrogenolysis. J Mater Chem 22:16598–16605
van Ryneveld E, Mahomed AS, van Heerden PS, Green MJ, Friedrich HB (2011) A catalytic route to lower alcohols from glycerol using Ni-supported catalysts. Green Chem 13:1819–1827
Marinoiu A, Ionita G, Gáspár CL, Cobzaru C, Marinescu D, Teodorescu C, Oprea S (2009) Selective hydrogenolysis of glycerol to propylene glycol using heterogeneous catalysts. React Kinet Mech Catal 99:111–118
Marinoiu A, Ionita G, Gáspár C-L, Cobzaru C, Oprea S (2009) Glycerol hydrogenolysis to propylene glycol. React Kinet Catal Lett 97:315–320
Amada Y, Shinmi Y, Koso S, Kubota T, Nakagawa Y, Tomishige K (2011) Reaction mechanism of the glycerol hydrogenolysis to 1,3-propanediol over Ir–ReOx/SiO2 catalyst. Appl Catal B Environ 105:117–127
Vasiliadou ES, Lemonidou AA (2011) Parameters affecting the formation of 1,2-propanediol from glycerol over Ru/SiO2 catalyst. Org Process Res Dev 15:925–931
Zhang JS, Delgass WN, Fisher TS, Gore JP (2007) Kinetics of Ru-catalyzed sodium borohydride hydrolysis. J Power Sources 164:772–781
Wunder S, Polzer F, Lu Y, Mei Y, Ballauff M (2010) Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J Phys Chem C 114:8814–8820
Rill C, Kolar ZI, Kickelbick G, Wolterbeek HT, Peters JA (2009) Kinetics and thermodynamics of adsorption on hydroxyapatite of the [160Tb] terbium complexes of the bone-targeting ligands DOTP and BPPED. Langmuir 25:2294–2301
Wunder S, Lu Y, Albrecht M, Ballauff M (2011) Catalytic activity of faceted gold nanoparticles studied by a model reaction: evidence for substrate-induced surface restructuring. ACS Catal 1:908–916
Vannice MA, Joyce WH (2005) Kinetics of catalytic reactions. Springer, Berlin
Acknowledgements
This work is financially supported by the University of Johannesburg and in the part by the National Research Foundation of South Africa (Grant specific unique reference numbers (UID) 85386, 117997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ndolomingo, M.J., Bingwa, N. & Meijboom, R. Review of supported metal nanoparticles: synthesis methodologies, advantages and application as catalysts. J Mater Sci 55, 6195–6241 (2020). https://doi.org/10.1007/s10853-020-04415-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04415-x