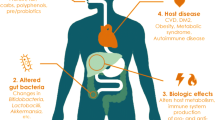
Abstract
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer mortality worldwide. Colitis-associated colorectal cancer (CAC) is a subtype of CRC associated with inflammatory bowel disease (IBD). It is well known that individuals with IBD have a 2–3 times higher risk of developing CRC than those who do not, rendering CAC a major cause of death in this group. Although the etiology and pathogenesis of CAC are incompletely understood, animal models of chronic inflammation and human cohort data indicate that changes in the intestinal environment, including host response dysregulation and gut microbiota perturbations, may contribute to the development of CAC. Genomic alterations are a hallmark of CAC, with patterns that are distinct from those in sporadic CRC. The discovery of the biological changes that underlie the development of CAC is ongoing; however, current data suggest that chronic inflammation in IBD increases the risk of developing CAC. Therefore, a deeper understanding of the precise mechanisms by which inflammation triggers genetic alterations and disrupts intestinal homeostasis may provide insight into novel therapeutic strategies for the prevention of CAC.

Similar content being viewed by others
Abbreviations
- APC:
-
Adenomatous polyposis coli
- CD:
-
Crohn’s disease
- CAC:
-
Colitis-associated colorectal cancer
- CRC:
-
Colorectal cancer
- CSCs:
-
Cancer stem cells
- DCC:
-
Deleted in colon cancer
- DSS:
-
Dextran sulfate sodium
- EMT:
-
Epithelial–mesenchymal transition
- ETBF:
-
Enterotoxigenic Bacteroides fragilis
- FAP:
-
Familial adenomatous polyposis
- FMT:
-
Fecal microbiota transplantation
- GI:
-
Gastrointestinal
- GSK3β:
-
Glycogen synthase kinase-3β
- HDC:
-
Histidine decarboxylase
- H2S:
-
Hydrogen sulfide
- IL-22BP:
-
IL-22 binding protein
- IBD:
-
Inflammatory bowel disease
- IEC:
-
Intestinal epithelial cell
- KRAS:
-
Kirsten rat sarcoma virus
- Lcn2:
-
Lipocalin-2
- LGR5:
-
Leucine-rich repeat-containing G-protein-coupled receptor 5
- MSI:
-
Microsatellite instability
- Myd88:
-
Myeloid differentiation factor 88
- NF-κB:
-
Nuclear factor kappa-light-chain enhancer
- NLRP3:
-
NOD-like receptor protein 3
- NOS:
-
Nitric oxide synthase
- pks:
-
Polyketide synthase
- PRR:
-
Pattern recognition receptor
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- STAT3:
-
Signal transducer and activator of transcription 3
- Th:
-
T helper
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- Tregs:
-
Regulatory T cells
- UC:
-
Ulcerative colitis
- 5-ASA:
-
5-Aminosalicylic acid
References
Kaplan, G. G. (2015). The global burden of IBD: From 2015 to 2025. Nature reviews Gastroenterology & hepatology, 12(12), 720–727.
Eaden, J. A., Abrams, K. R., & Mayberry, J. F. (2001). The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut, 48(4), 526–535. https://doi.org/10.1136/gut.48.4.526
Jess, T., Loftus, E. V., Jr., Velayos, F. S., Harmsen, W. S., Zinsmeister, A. R., Smyrk, T. C., et al. (2006). Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: A population-based study from Olmsted County. Minnesota. Inflamm Bowel Dis, 12(8), 669–676. https://doi.org/10.1097/00054725-200608000-00001
Andersen, N. N., & Jess, T. (2013). Has the risk of colorectal cancer in inflammatory bowel disease decreased? World Journal of Gastroenterology, 19(43), 7561–7568. https://doi.org/10.3748/wjg.v19.i43.7561
Lewis, J. D., Deren, J. J., & Lichtenstein, G. R. (1999). Cancer risk in patients with inflammatory bowel disease. Gastroenterology Clinics of North America, 28(2), 459–477, x, https://doi.org/10.1016/s0889-8553(05)70065-0.
Wanders, L. K., Dekker, E., Pullens, B., Bassett, P., Travis, S. P., & East, J. E. (2014). Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: A meta-analysis. Clinical Gastroenterology and Hepatology, 12(5), 756–764. https://doi.org/10.1016/j.cgh.2013.07.024
Fantini, M. C., & Guadagni, I. (2021). From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Digestive and Liver Disease, 53(5), 558–565. https://doi.org/10.1016/j.dld.2021.01.012
Waldner, M. J., & Neurath, M. F. (2008). Cytokines in colitis associated cancer: Potential drug targets? Inflammation & Allergy: Drug Targets, 7(3), 187–194. https://doi.org/10.2174/187152808785748137
Bezzio, C., Festa, S., Saibeni, S., & Papi, C. (2017). Chemoprevention of colorectal cancer in ulcerative colitis: Digging deep in current evidence. Expert Review of Gastroenterology & Hepatology, 11(4), 339–347. https://doi.org/10.1080/17474124.2017.1292129
Lopez, A., Pouillon, L., Beaugerie, L., Danese, S., & Peyrin-Biroulet, L. (2018). Colorectal cancer prevention in patients with ulcerative colitis. Best Practice & Research Clinical Gastroenterology, 32–33, 103–109. https://doi.org/10.1016/j.bpg.2018.05.010
Yaeger, R., Shah, M. A., Miller, V. A., Kelsen, J. R., Wang, K., Heins, Z. J., et al. (2016). Genomic alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology, 151(2), 278-287.e276. https://doi.org/10.1053/j.gastro.2016.04.001
Kameyama, H., Nagahashi, M., Shimada, Y., Tajima, Y., Ichikawa, H., Nakano, M., et al. (2018). Genomic characterization of colitis-associated colorectal cancer. World Journal of Surgical Oncology, 16(1), 121. https://doi.org/10.1186/s12957-018-1428-0
RJ Porter MJ Arends AMD Churchhouse S Din 2021 Inflammatory bowel disease-associated colorectal cancer: Translational risks from mechanisms to medicines Journal of Crohn's and Colitis https://doi.org/10.1093/ecco-jcc/jjab102
Ullman, T. A., & Itzkowitz, S. H. (2011). Intestinal inflammation and cancer. Gastroenterology, 140(6), 1807–1816. https://doi.org/10.1053/j.gastro.2011.01.057
Itzkowitz, S. H., & Yio, X. (2004). Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology, 287(1), G7–17, https://doi.org/10.1152/ajpgi.00079.2004.
Cooks, T., Pateras, I. S., Tarcic, O., Solomon, H., Schetter, A. J., Wilder, S., et al. (2013). Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell, 23(5), 634–646. https://doi.org/10.1016/j.ccr.2013.03.022
Khor, B., Gardet, A., & Xavier, R. J. (2011). Genetics and pathogenesis of inflammatory bowel disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Nature, 474(7351), 307–317, https://doi.org/10.1038/nature10209.
Chandrasinghe, P., Cereser, B., Moorghen, M., Al Bakir, I., Tabassum, N., Hart, A., et al. (2018). Role of SMAD proteins in colitis-associated cancer: From known to the unknown. Oncogene, 37(1), 1–7. https://doi.org/10.1038/onc.2017.300
Cooper, H. S., Everley, L., Chang, W. C., Pfeiffer, G., Lee, B., Murthy, S., et al. (2001). The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology, 121(6), 1407–1416. https://doi.org/10.1053/gast.2001.29609
Flaeisher, A. S., Esteller, M., Harpaz, N., Leytin, A., Rashid, A., Xu, Y., et al. (2000). Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Research, 60(17), 4864–4868.
Caooks, T., Pateras, I. S., Tarcic, O., Solomon, H., Schetter, A. J., Wilder, S., et al. (2013). Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell, 23(5), 634–646. https://doi.org/10.1016/j.ccr.2013.03.022
Burmer, G. C., Rabinovitch, P. S., Haggitt, R. C., Crispin, D. A., Brentnall, T. A., Kolli, V. R., et al. (1992). Neoplastic progression in ulcerative colitis: Histology, DNA content, and loss of a p53 allele. Gastroenterology, 103(5), 1602–1610. https://doi.org/10.1016/0016-5085(92)91184-6
Hussain, S. P., Amstad, P., Raja, K., Ambs, S., Nagashima, M., Bennett, W. P., et al. (2000). Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Research, 60(13), 3333–3337.
Hsieh, C. J., Klump, B., Holzmann, K., Borchard, F., Gregor, M., & Porschen, R. (1998). Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Research, 58(17), 3942–3945.
Moriyama, T., Matsumoto, T., Nakamura, S., Jo, Y., Mibu, R., Yao, T., et al. (2007). Hypermethylation of p14 (ARF) may be predictive of colitic cancer in patients with ulcerative colitis. Diseases of the Colon and Rectum, 50(9), 1384–1392. https://doi.org/10.1007/10350-007-0302-x
Lang, S. M., Stratakis, D. F., Heinzlmann, M., Heldwein, W., Wiebecke, B., & Loeschke, K. (1999). Molecular screening of patients with long standing extensive ulcerative colitis: Detection of p53 and Ki-ras mutations by single strand conformation polymorphism analysis and differential hybridisation in colonic lavage fluid. Gut, 44(6), 822–825. https://doi.org/10.1136/gut.44.6.822
Bläker, H., von Herbay, A., Penzel, R., Groß, S., & Otto, H. F. (2002). Genetics of adenocarcinomas of the small intestine: Frequent deletions at chromosome 18q and mutations of the SMAD4 gene. Oncogene, 21(1), 158–164. https://doi.org/10.1038/sj.onc.1205041
Maru, D., Wu, T. T., Canada, A., Houlihan, P. S., Hamilton, S. R., & Rashid, A. (2004). Loss of chromosome 18q and DPC4 (Smad4) mutations in appendiceal adenocarcinomas. Oncogene, 23(3), 859–864. https://doi.org/10.1038/sj.onc.1207194
Perreault, N. (2018). Ulcerative colitis-associated carcinoma: Epithelial SMAD4-mediated signaling is a key guardian. Cellular and molecular gastroenterology and hepatology, 6(3), 350–351. https://doi.org/10.1016/j.jcmgh.2018.06.004
Tarafa, G., Villanueva, A., Farré, L., Rodríguez, J., Musulén, E., Reyes, G., et al. (2000). DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene, 19(4), 546–555. https://doi.org/10.1038/sj.onc.1203353
Itzkowitz, S. H. (2006). Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterology Clinics of North America, 35(3), 553–571. https://doi.org/10.1016/j.gtc.2006.07.002
Foersch, S., & Neurath, M. F. (2014). Colitis-associated neoplasia: Molecular basis and clinical translation. Cellular and Molecular Life Sciences, 71(18), 3523–3535. https://doi.org/10.1007/s00018-014-1636-x
Bayik, D., & Lathia, J. D. (2021). Cancer stem cell–immune cell crosstalk in tumour progression. Nature Reviews Cancer, 21(8), 526–536. https://doi.org/10.1038/s41568-021-00366-w
Barker, N., Ridgway, R. A., van Es, J. H., van de Wetering, M., Begthel, H., van den Born, M., et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457(7229), 608–611. https://doi.org/10.1038/nature07602
Iwaya, M., Ota, H., Nakajima, T., Uehara, T., Riddell, R., & Conner, J. (2021). Most colitis associated carcinomas lack expression of LGR5: A preliminary study with implications for unique pathways of carcinogenesis compared to sporadic colorectal carcinoma. BMC Cancer, 21(1), 119. https://doi.org/10.1186/s12885-021-07835-3
Yasuda, H., Tanaka, K., Okita, Y., Araki, T., Saigusa, S., Toiyama, Y., et al. (2011). CD133, OCT4, and NANOG in ulcerative colitis-associated colorectal cancer. Oncology Letters, 2(6), 1065–1071. https://doi.org/10.3892/ol.2011.415
Grivennikov, S. I. (2013). Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin Immunopathol, 35(2), 229–244. https://doi.org/10.1007/s00281-012-0352-6
Hofseth, L. J., Saito, S., Hussain, S. P., Espey, M. G., Miranda, K. M., Araki, Y., et al. (2003). Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A, 100(1), 143–148. https://doi.org/10.1073/pnas.0237083100
Hussain, S. P., Hofseth, L. J., & Harris, C. C. (2003). Radical causes of cancer. Nature Reviews Cancer, 3(4), 276–285. https://doi.org/10.1038/nrc1046
Bartsch, H., & Nair, J. (2005). Accumulation of lipid peroxidation-derived DNA lesions: Potential lead markers for chemoprevention of inflammation-driven malignancies. Mutation Research, 591(1–2), 34–44. https://doi.org/10.1016/j.mrfmmm.2005.04.013
Rachmilewitz, D., Stamler, J. S., Bachwich, D., Karmeli, F., Ackerman, Z., & Podolsky, D. K. (1995). Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut, 36(5), 718–723. https://doi.org/10.1136/gut.36.5.718
Kimura, H., Hokari, R., Miura, S., Shigematsu, T., Hirokawa, M., Akiba, Y., et al. (1998). Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut, 42(2), 180–187. https://doi.org/10.1136/gut.42.2.180
O’Sullivan, J. N., Bronner, M. P., Brentnall, T. A., Finley, J. C., Shen, W. T., Emerson, S., et al. (2002). Chromosomal instability in ulcerative colitis is related to telomere shortening. Nature Genetics, 32(2), 280–284. https://doi.org/10.1038/ng989
Osburn, W. O., Karim, B., Dolan, P. M., Liu, G., Yamamoto, M., Huso, D. L., et al. (2007). Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. International Journal of Cancer, 121(9), 1883–1891. https://doi.org/10.1002/ijc.22943
Kusaba, T., Nakayama, T., Yamazumi, K., Yakata, Y., Yoshizaki, A., Inoue, K., et al. (2006). Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncology Reports, 15(6), 1445–1451.
Grivennikov, S. I., & Karin, M. (2010). Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine & Growth Factor Reviews, 21(1), 11–19. https://doi.org/10.1016/j.cytogfr.2009.11.005
Kusaba, T., Nakayama, T., Yamazumi, K., Yakata, Y., Yoshizaki, A., Nagayasu, T., et al. (2005). Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. Journal of Clinical Pathology, 58(8), 833–838. https://doi.org/10.1136/jcp.2004.023416
Corvinus, F. M., Orth, C., Moriggl, R., Tsareva, S. A., Wagner, S., Pfitzner, E. B., et al. (2005). Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia, 7(6), 545–555. https://doi.org/10.1593/neo.04571
Lassmann, S., Schuster, I., Walch, A., Göbel, H., Jütting, U., Makowiec, F., et al. (2007). STAT3 mRNA and protein expression in colorectal cancer: Effects on STAT3-inducible targets linked to cell survival and proliferation. Journal of Clinical Pathology, 60(2), 173–179. https://doi.org/10.1136/jcp.2005.035113
Yu, H., Pardoll, D., & Jove, R. (2009). STATs in cancer inflammation and immunity: A leading role for STAT3. Nature Reviews Cancer, 9(11), 798–809. https://doi.org/10.1038/nrc2734
Atreya, R., & Neurath, M. F. (2008). Signaling molecules: The pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Current Drug Targets, 9(5), 369–374. https://doi.org/10.2174/138945008784221116
Han, J., & Theiss, A. L. (2014). Stat3: Friend or foe in colitis and colitis-associated cancer? Inflammatory bowel diseases, 20(12), 2405–2411. https://doi.org/10.1097/MIB.0000000000000180
Grivennikov, S., Karin, E., Terzic, J., Mucida, D., Yu, G. Y., Vallabhapurapu, S., et al. (2009). IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell, 15(2), 103–113. https://doi.org/10.1016/j.ccr.2009.01.001
Yu, H., Kortylewski, M., & Pardoll, D. (2007). Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nature Reviews Immunology, 7(1), 41–51. https://doi.org/10.1038/nri1995
Jin, W. (2020). Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells, 9(1), 217. https://doi.org/10.3390/cells9010217
Nenci, A., Becker, C., Wullaert, A., Gareus, R., van Loo, G., Danese, S., et al. (2007). Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature, 446(7135), 557–561. https://doi.org/10.1038/nature05698
Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z. W., Egan, L. J., et al. (2004). IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118(3), 285–296. https://doi.org/10.1016/j.cell.2004.07.013
Greten, F. R., & Karin, M. (2004). The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Letters, 206(2), 193–199. https://doi.org/10.1016/j.canlet.2003.08.029
Schottelius, A. J., & Dinter, H. (2006). Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treatment and Research, 130, 67–87. https://doi.org/10.1007/0-387-26283-0_3
Rogler, G., Brand, K., Vogl, D., Page, S., Hofmeister, R., Andus, T., et al. (1998). Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology, 115(2), 357–369. https://doi.org/10.1016/s0016-5085(98)70202-1
Lee, G., Goretsky, T., Managlia, E., Dirisina, R., Singh, A. P., Brown, J. B., et al. (2010). Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology, 139(3), 869–881, 881.e861–869, https://doi.org/10.1053/j.gastro.2010.05.037.
Eaden, J., Abrams, K., Ekbom, A., Jackson, E., & Mayberry, J. (2000). Colorectal cancer prevention in ulcerative colitis: A case-control study. Alimentary Pharmacology & Therapeutics, 14(2), 145–153. https://doi.org/10.1046/j.1365-2036.2000.00698.x
Bernstein, C. N., Eaden, J., Steinhart, A. H., Munkholm, P., & Gordon, P. H. (2002). Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflammatory Bowel Diseases, 8(5), 356–361. https://doi.org/10.1097/00054725-200209000-00007
Cooper, H. S., Murthy, S., Kido, K., Yoshitake, H., & Flanigan, A. (2000). Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis, 21(4), 757–768, https://doi.org/10.1093/carcin/21.4.757.
Chang, W. C., Coudry, R. A., Clapper, M. L., Zhang, X., Williams, K. L., Spittle, C. S., et al. (2007). Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis, 28(11), 2375–2381. https://doi.org/10.1093/carcin/bgm134
Claessen, M. M., Schipper, M. E., Oldenburg, B., Siersema, P. D., Offerhaus, G. J., & Vleggaar, F. P. (2010). WNT-pathway activation in IBD-associated colorectal carcinogenesis: Potential biomarkers for colonic surveillance. Cellular Oncology, 32(4), 303–310. https://doi.org/10.3233/clo-2009-0503
Liu, Z. G. (2005). Molecular mechanism of TNF signaling and beyond. Cell Research, 15(1), 24–27. https://doi.org/10.1038/sj.cr.7290259
Kruglov, A. A., Kuchmiy, A., Grivennikov, S. I., Tumanov, A. V., Kuprash, D. V., & Nedospasov, S. A. (2008). Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: Mouse models. Cytokine & Growth Factor Reviews, 19(3–4), 231–244. https://doi.org/10.1016/j.cytogfr.2008.04.010
Feldmann, M. (2009). Translating molecular insights in autoimmunity into effective therapy. Annual Review of Immunology, 27, 1–27. https://doi.org/10.1146/annurev-immunol-082708-100732
Kollias, G. (2004). Modeling the function of tumor necrosis factor in immune pathophysiology. Autoimmunity Reviews, 3(Suppl 1), S24-25.
Ford, A. C., Sandborn, W. J., Khan, K. J., Hanauer, S. B., Talley, N. J., & Moayyedi, P. (2011). Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. American Journal of Gastroenterology, 106(4), 644–659, quiz 660, https://doi.org/10.1038/ajg.2011.73.
Popivanova, B. K., Kitamura, K., Wu, Y., Kondo, T., Kagaya, T., Kaneko, S., et al. (2008). Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. The Journal of Clinical Investigation, 118(2), 560–570. https://doi.org/10.1172/jci32453
Voronov, E., & Apte, R. N. (2015). IL-1 in colon inflammation, colon carcinogenesis and invasiveness of colon cancer. Cancer microenvironment : Official journal of the International Cancer Microenvironment Society, 8(3), 187–200. https://doi.org/10.1007/s12307-015-0177-7
Wang, Y., Wang, K., Han, G. C., Wang, R. X., Xiao, H., Hou, C. M., et al. (2014). Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunology, 7(5), 1106–1115. https://doi.org/10.1038/mi.2013.126
Garlanda, C., Riva, F., Veliz, T., Polentarutti, N., Pasqualini, F., Radaelli, E., et al. (2007). Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Research, 67(13), 6017–6021. https://doi.org/10.1158/0008-5472.can-07-0560
Mao, L., Kitani, A., Strober, W., & Fuss, I. J. (2018). The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. [Review]. Frontiers in Immunology, 9(2566), https://doi.org/10.3389/fimmu.2018.02566.
Guo, W., Sun, Y., Liu, W., Wu, X., Guo, L., Cai, P., et al. (2014). Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy, 10(6), 972–985. https://doi.org/10.4161/auto.28374
Bettelli, E., Carrier, Y., Gao, W., Korn, T., Strom, T. B., Oukka, M., et al. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature, 441(7090), 235–238. https://doi.org/10.1038/nature04753
Dominitzki, S., Fantini, M. C., Neufert, C., Nikolaev, A., Galle, P. R., Scheller, J., et al. (2007). Cutting edge: Trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. The Journal of Immunology, 179(4), 2041–2045. https://doi.org/10.4049/jimmunol.179.4.2041
Li, Y., de Haar, C., Chen, M., Deuring, J., Gerrits, M. M., Smits, R., et al. (2010). Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut, 59(2), 227–235. https://doi.org/10.1136/gut.2009.184176
Reinisch, W., Gasché, C., Tillinger, W., Wyatt, J., Lichtenberger, C., Willheim, M., et al. (1999). Clinical relevance of serum interleukin-6 in Crohn’s disease: Single point measurements, therapy monitoring, and prediction of clinical relapse. American Journal of Gastroenterology, 94(8), 2156–2164. https://doi.org/10.1111/j.1572-0241.1999.01288.x
Atreya, R., Mudter, J., Finotto, S., Müllberg, J., Jostock, T., Wirtz, S., et al. (2000). Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nature Medicine, 6(5), 583–588. https://doi.org/10.1038/75068
Ito, H., Takazoe, M., Fukuda, Y., Hibi, T., Kusugami, K., Andoh, A., et al. (2004). A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology, 126(4), 989–996; discussion 947, https://doi.org/10.1053/j.gastro.2004.01.012.
De Simone, V., Pallone, F., Monteleone, G., & Stolfi, C. (2013). Role of T(H)17 cytokines in the control of colorectal cancer. Oncoimmunology, 2(12), e26617. https://doi.org/10.4161/onci.26617
Ernst, M., & Putoczki, T. (2014). IL-17 cuts to the chase in colon cancer. Immunity, 41(6), 880–882. https://doi.org/10.1016/j.immuni.2014.12.004
Wu, S., Rhee, K. J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Medicine, 15(9), 1016–1022. https://doi.org/10.1038/nm.2015
Hyun, Y. S., Han, D. S., Lee, A. R., Eun, C. S., Youn, J., & Kim, H.-Y. (2012). Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis, 33(4), 931–936. https://doi.org/10.1093/carcin/bgs106
Leppkes, M., Becker, C., Ivanov, I. I., Hirth, S., Wirtz, S., Neufert, C., et al. (2009). RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology, 136(1), 257–267. https://doi.org/10.1053/j.gastro.2008.10.018
Chae, W. J., & Bothwell, A. L. (2011). IL-17F deficiency inhibits small intestinal tumorigenesis in ApcMin/+ mice. Biochemical and Biophysical Research Communications, 414(1), 31–36. https://doi.org/10.1016/j.bbrc.2011.09.016
Song, X., Gao, H., Lin, Y., Yao, Y., Zhu, S., Wang, J., et al. (2014). Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity, 40(1), 140–152. https://doi.org/10.1016/j.immuni.2013.11.018
Yang, X. O., Chang, S. H., Park, H., Nurieva, R., Shah, B., Acero, L., et al. (2008). Regulation of inflammatory responses by IL-17F. Journal of Experimental Medicine, 205(5), 1063–1075. https://doi.org/10.1084/jem.20071978
Grivennikov, S. I., Wang, K., Mucida, D., Stewart, C. A., Schnabl, B., Jauch, D., et al. (2012). Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature, 491(7423), 254–258. https://doi.org/10.1038/nature11465
Chan, I. H., Jain, R., Tessmer, M. S., Gorman, D., Mangadu, R., Sathe, M., et al. (2014). Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunology, 7(4), 842–856. https://doi.org/10.1038/mi.2013.101
Keir, M., Yi, Y., Lu, T., & Ghilardi, N. (2020). The role of IL-22 in intestinal health and disease. Journal of Experimental Medicine, 217(3), e20192195. https://doi.org/10.1084/jem.20192195
Sabihi, M., Böttcher, M., Pelczar, P., & Huber, S. (2020). Microbiota-dependent effects of IL-22. Cells, 9(10), https://doi.org/10.3390/cells9102205.
Doulabi, H., Masoumi, E., Rastin, M., Foolady Azarnaminy, A., Esmaeili, S. A., & Mahmoudi, M. (2022). The role of Th22 cells, from tissue repair to cancer progression. Cytokine, 149, 155749. https://doi.org/10.1016/j.cyto.2021.155749
Wang, C., Gong, G., Sheh, A., Muthupalani, S., Bryant, E. M., Puglisi, D. A., et al. (2017). Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer. Mucosal Immunology, 10(6), 1504–1517. https://doi.org/10.1038/mi.2017.9
Huber, S., Gagliani, N., Zenewicz, L. A., Huber, F. J., Bosurgi, L., Hu, B., et al. (2012). IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature, 491(7423), 259–263. https://doi.org/10.1038/nature11535
Jiang, R., Wang, H., Deng, L., Hou, J., Shi, R., Yao, M., et al. (2013). IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer, 13, 59. https://doi.org/10.1186/1471-2407-13-59
Wei, H.-X., Wang, B., & Li, B. (2020). IL-10 and IL-22 in mucosal immunity: driving protection and pathology. [Review]. Frontiers in Immunology, 11(1315), https://doi.org/10.3389/fimmu.2020.01315.
Lüthi, A. U., Cullen, S. P., McNeela, E. A., Duriez, P. J., Afonina, I. S., Sheridan, C., et al. (2009). Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity, 31(1), 84–98. https://doi.org/10.1016/j.immuni.2009.05.007
O’Donnell, C., Mahmoud, A., Keane, J., Murphy, C., White, D., Carey, S., et al. (2016). An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. British Journal of Cancer, 114(1), 37–43. https://doi.org/10.1038/bjc.2015.433
Chen, J., He, Y., Tu, L., & Duan, L. (2020). Dual immune functions of IL-33 in inflammatory bowel disease. Histology and Histopathology, 35(2), 137–146, https://doi.org/10.14670/hh-18-149.
Maywald, R. L., Doerner, S. K., Pastorelli, L., De Salvo, C., Benton, S. M., Dawson, E. P., et al. (2015). IL-33 activates tumor stroma to promote intestinal polyposis. Proceedings of the National Academy of Sciences of the United States of America, 112(19), E2487-2496. https://doi.org/10.1073/pnas.1422445112
Mertz, K. D., Mager, L. F., Wasmer, M. H., Thiesler, T., Koelzer, V. H., Ruzzante, G., et al. (2016). The IL-33/ST2 pathway contributes to intestinal tumorigenesis in humans and mice. Oncoimmunology, 5(1), e1062966. https://doi.org/10.1080/2162402x.2015.1062966
Malik, A., Sharma, D., Zhu, Q., Karki, R., Guy, C. S., Vogel, P., et al. (2016). IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. The Journal of Clinical Investigation, 126(12), 4469–4481. https://doi.org/10.1172/JCI88625
Kamada, N., & Núñez, G. (2013). Role of the gut microbiota in the development and function of lymphoid cells. The Journal of Immunology, 190(4), 1389–1395. https://doi.org/10.4049/jimmunol.1203100
Kamada, N., Seo, S. U., Chen, G. Y., & Núñez, G. (2013). Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology, 13(5), 321–335. https://doi.org/10.1038/nri3430
Nagao-Kitamoto, H., & Kamada, N. (2017). Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune network, 17(1), 1–12. https://doi.org/10.4110/in.2017.17.1.1
Richard, M. L., Liguori, G., Lamas, B., Brandi, G., da Costa, G., Hoffmann, T. W., et al. (2018). Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes, 9(2), 131–142. https://doi.org/10.1080/19490976.2017.1379637
Priyamvada, P. (2021). Dysbiosis in microbiome leading to colitis-associated cancer: gut microbiome correlation with CAC. In P. Ashok Kumar (Ed.), Diagnostic and Treatment Methods for Ulcerative Colitis and Colitis-Associated Cancer (pp. 142–169). Hershey, PA, USA: IGI Global.
Dove, W. F., Clipson, L., Gould, K. A., Luongo, C., Marshall, D. J., Moser, A. R., et al. (1997). Intestinal neoplasia in the ApcMin mouse: Independence from the microbial and natural killer (beige locus) status. Cancer Research, 57(5), 812–814.
Zackular, J. P., Baxter, N. T., Iverson, K. D., Sadler, W. D., Petrosino, J. F., Chen, G. Y., et al. (2013). The gut microbiome modulates colon tumorigenesis. mBio, 4(6), e00692–00613, https://doi.org/10.1128/mBio.00692-13.
Tanaka, Y., Ito, S., & Isobe, K.-I. (2016). Vancomycin-sensitive bacteria trigger development of colitis-associated colon cancer by attracting neutrophils. Scientific Reports, 6(1), 23920. https://doi.org/10.1038/srep23920
Poutahidis, T., Haigis, K. M., Rao, V. P., Nambiar, P. R., Taylor, C. L., Ge, Z., et al. (2007). Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis, 28(12), 2614–2623. https://doi.org/10.1093/carcin/bgm180
Arthur, J. C., & Jobin, C. (2013). The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes, 4(3), 253–258. https://doi.org/10.4161/gmic.24220
Arthur, J. C., Perez-Chanona, E., Muhlbauer, M., Tomkovich, S., Uronis, J. M., Fan, T. J., et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Science, 338(6103), 120–123, https://doi.org/10.1126/science.1224820.
Tomkovich, S., Yang, Y., Winglee, K., Gauthier, J., Mühlbauer, M., Sun, X., et al. (2017). Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Research, 77(10), 2620–2632. https://doi.org/10.1158/0008-5472.CAN-16-3472
Yang, Y., Gharaibeh, R. Z., Newsome, R. C., & Jobin, C. (2020). Amending microbiota by targeting intestinal inflammation with TNF blockade attenuates development of colorectal cancer. Nat Cancer, 1(7), 723–734. https://doi.org/10.1038/s43018-020-0078-7
Zhu, W., Miyata, N., Winter, M. G., Arenales, A., Hughes, E. R., Spiga, L., et al. (2019). Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. Journal of Experimental Medicine, 216(10), 2378–2393. https://doi.org/10.1084/jem.20181939
Chung, L., Thiele Orberg, E., Geis, A. L., Chan, J. L., Fu, K., DeStefano Shields, C. E., et al. (2018). Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host & Microbe, 23(2), 203-214.e205. https://doi.org/10.1016/j.chom.2018.01.007
Hwang, S., Lee, C. G., Jo, M., Park, C. O., Gwon, S. Y., Hwang, S., et al. (2020). Enterotoxigenic Bacteroides fragilis infection exacerbates tumorigenesis in AOM/DSS mouse model. International Journal of Medical Sciences, 17(2), 145–152. https://doi.org/10.7150/ijms.38371
Li, S., Peppelenbosch, M. P., & Smits, R. (2019). Bacterial biofilms as a potential contributor to mucinous colorectal cancer formation. Biochimica et Biophysica Acta - Reviews on Cancer, 1872(1), 74–79. https://doi.org/10.1016/j.bbcan.2019.05.009
Chew, S. S., Tan, L. T., Law, J. W., Pusparajah, P., Goh, B. H., Ab Mutalib, N. S., et al. (2020). Targeting gut microbial biofilms-a key to hinder colon carcinogenesis? Cancers (Basel), 12(8), https://doi.org/10.3390/cancers12082272.
Dejea, C. M., Fathi, P., Craig, J. M., Boleij, A., Taddese, R., Geis, A. L., et al. (2018). Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science, 359(6375), 592–597. https://doi.org/10.1126/science.aah3648
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host & Microbe, 14(2), 207–215. https://doi.org/10.1016/j.chom.2013.07.007
Yu, M. R., Kim, H. J., & Park, H. R. (2020). Fusobacterium nucleatum accelerates the progression of colitis-associated colorectal cancer by promoting EMT. Cancers (Basel), 12(10), https://doi.org/10.3390/cancers12102728.
Flynn, K. J., Baxter, N. T., & Schloss, P. D. (2016). Metabolic and community synergy of oral bacteria in colorectal cancer. mSphere, 1(3), https://doi.org/10.1128/mSphere.00102-16.
Li, X., Zhu, S., Zhang, T., & Chen, X. (2021). Association between oral microflora and gastrointestinal tumors (Review). Oncology Reports, 46(2), https://doi.org/10.3892/or.2021.8111.
Wang, X., Jia, Y., Wen, L., Mu, W., Wu, X., Liu, T., et al. (2021). Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Research, 81(10), 2745–2759. https://doi.org/10.1158/0008-5472.can-20-3827
Kitamoto, S., Nagao-Kitamoto, H., Hein, R., Schmidt, T. M., & Kamada, N. (2020). The bacterial connection between the oral cavity and the gut diseases. Journal of Dental Research, 99(9), 1021–1029. https://doi.org/10.1177/0022034520924633
Zhang, Y., Weng, Y., Gan, H., Zhao, X., & Zhi, F. (2018). Streptococcus gallolyticus conspires myeloid cells to promote tumorigenesis of inflammatory bowel disease. Biochemical and Biophysical Research Communications, 506(4), 907–911. https://doi.org/10.1016/j.bbrc.2018.10.136
Chichlowski, M., Sharp, J. M., Vanderford, D. A., Myles, M. H., & Hale, L. P. (2008). Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comparative Medicine, 58(6), 534–541.
Erdman, S. E., Rao, V. P., Poutahidis, T., Rogers, A. B., Taylor, C. L., Jackson, E. A., et al. (2009). Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1027–1032. https://doi.org/10.1073/pnas.0812347106
Li, L. N., Liu, Y., Zhang, H. C., Wu, T., Dai, Y., & Wang, W. H. (2020). Helicobacter pylori infection reduces TAMs infiltration in a mouse model of AOM/DSS induced colitis-associated cancer. PLoS ONE, 15(11), e0241840. https://doi.org/10.1371/journal.pone.0241840
Karamzin, A. M., Ropot, A. V., Sergeyev, O. V., & Khalturina, E. O. (2021). Akkermansia muciniphila and host interaction within the intestinal tract. Anaerobe, 72, 102472. https://doi.org/10.1016/j.anaerobe.2021.102472
Zhang, T., Ji, X., Lu, G., & Zhang, F. (2021). The potential of Akkermansia muciniphila in inflammatory bowel disease. Applied Microbiology and Biotechnology, 105(14–15), 5785–5794. https://doi.org/10.1007/s00253-021-11453-1
Wang, L., Tang, L., Feng, Y., Zhao, S., Han, M., Zhang, C., et al. (2020). A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut, 69(11), 1988–1997. https://doi.org/10.1136/gutjnl-2019-320105
Wang, F., Cai, K., Xiao, Q., He, L., Xie, L., & Liu, Z. (2022). <i>Akkermansia muciniphila</i> administration exacerbated the development of colitis-associated colorectal cancer in mice. [Research Paper]. Journal of Cancer, 13(1), 124–133, https://doi.org/10.7150/jca.63578.
Moschen, A. R., Gerner, R. R., Wang, J., Klepsch, V., Adolph, T. E., Reider, S. J., et al. (2016). Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host & Microbe, 19(4), 455–469. https://doi.org/10.1016/j.chom.2016.03.007
Wallace, J. L. (2010). Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxidants & Redox Signaling, 12(9), 1125–1133. https://doi.org/10.1089/ars.2009.2900
Mottawea, W., Chiang, C. K., Mühlbauer, M., Starr, A. E., Butcher, J., Abujamel, T., et al. (2016). Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nature Communications, 7, 13419. https://doi.org/10.1038/ncomms13419
Kim, S. W., Kim, H. M., Yang, K. M., Kim, S. A., Kim, S. K., An, M. J., et al. (2010). Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflammatory Bowel Diseases, 16(9), 1514–1525. https://doi.org/10.1002/ibd.21262
Wang, Q., Wang, K., Wu, W., Lv, L., Bian, X., Yang, L., et al. (2020). Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice. Applied Microbiology and Biotechnology, 104(13), 5915–5928. https://doi.org/10.1007/s00253-020-10621-z
Silveira, D. S. C., Veronez, L. C., Lopes-Júnior, L. C., Anatriello, E., Brunaldi, M. O., & Pereira-da-Silva, G. (2020). Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World Journal of Gastroenterology, 26(43), 6782–6794. https://doi.org/10.3748/wjg.v26.i43.6782
Matsumoto, S., Hara, T., Nagaoka, M., Mike, A., Mitsuyama, K., Sako, T., et al. (2009). A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology, 128(1 Suppl), e170–e180. https://doi.org/10.1111/j.1365-2567.2008.02942.x
Gao, C., Ganesh, B. P., Shi, Z., Shah, R. R., Fultz, R., Major, A., et al. (2017). Gut microbe–mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. The American Journal of Pathology, 187(10), 2323–2336. https://doi.org/10.1016/j.ajpath.2017.06.011
Kahouli, I., Malhotra, M., Westfall, S., Alaoui-Jamali, M. A., & Prakash, S. (2017). Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc (Min/+) mouse model. Applied Microbiology and Biotechnology, 101(5), 1999–2019, https://doi.org/10.1007/s00253-016-7885-x.
Wang, Z., Hua, W., Li, C., Chang, H., Liu, R., Ni, Y., et al. (2019). Protective role of fecal microbiota transplantation on colitis and colitis-associated colon cancer in mice is associated with Treg cells. [Original Research]. Frontiers in Microbiology, 10(2498), https://doi.org/10.3389/fmicb.2019.02498.
Borody, T. J., & Khoruts, A. (2011). Fecal microbiota transplantation and emerging applications. [Research Support, Non-U.S. Gov't Review]. Nature Reviews Gastroenterology & Hepatology, 9(2), 88–96, https://doi.org/10.1038/nrgastro.2011.244.
van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E. G., de Vos, W. M., et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine, 368(5), 407–415. https://doi.org/10.1056/NEJMoa1205037
Mann, E. R., You, J., Horneffer-van der Sluis, V., Bernardo, D., Omar Al-Hassi, H., Landy, J., et al. (2013). Dysregulated circulating dendritic cell function in ulcerative colitis is partially restored by probiotic strain <i>Lactobacillus casei</i> Shirota. Mediators of Inflammation, 2013, 573576. https://doi.org/10.1155/2013/573576
Yang, X. D., Ai, W., Asfaha, S., Bhagat, G., Friedman, R. A., Jin, G., et al. (2011). Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nature Medicine, 17(1), 87–95. https://doi.org/10.1038/nm.2278
Ghanavati, R., Asadollahi, P., Shapourabadi, M. B., Razavi, S., Talebi, M., & Rohani, M. (2020). Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microbial Pathogenesis, 139, 103829. https://doi.org/10.1016/j.micpath.2019.103829
Tian, Y., Xu, Q., Sun, L., Ye, Y., & Ji, G. (2018). Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. Journal of Nutritional Biochemistry, 57, 103–109. https://doi.org/10.1016/j.jnutbio.2018.03.007
Lowe, E. L., Crother, T. R., Rabizadeh, S., Hu, B., Wang, H., Chen, S., et al. (2010). Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE, 5(9), e13027. https://doi.org/10.1371/journal.pone.0013027
Pastille, E., Faßnacht, T., Adamczyk, A., Ngo Thi Phuong, N., Buer, J., & Westendorf, A. M. (2021). Inhibition of TLR4 signaling impedes tumor growth in colitis-associated colon cancer. [Original Research]. Frontiers in Immunology, 12(1608), https://doi.org/10.3389/fimmu.2021.669747.
Fukata, M., Shang, L., Santaolalla, R., Sotolongo, J., Pastorini, C., España, C., et al. (2011). Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflammatory Bowel Diseases, 17(7), 1464–1473. https://doi.org/10.1002/ibd.21527
Uronis, J. M., Mühlbauer, M., Herfarth, H. H., Rubinas, T. C., Jones, G. S., & Jobin, C. (2009). Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE, 4(6), e6026. https://doi.org/10.1371/journal.pone.0006026
Lopez, A., & Peyrin-Biroulet, L. (2013). 5-Aminosalicylic acid and chemoprevention: Does it work? Digestive Diseases, 31(2), 248–253. https://doi.org/10.1159/000353806
Koelink, P. J., Robanus-Maandag, E. C., Devilee, P., Hommes, D. W., Lamers, C. B. H. W., & Verspaget, H. W. (2009). 5-Aminosalicylic acid inhibits colitis-associated but not sporadic colorectal neoplasia in a novel conditional Apc mouse model. Carcinogenesis, 30(7), 1217–1224. https://doi.org/10.1093/carcin/bgp113
Velayos, F. S., Terdiman, J. P., & Walsh, J. M. (2005). Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: A systematic review and metaanalysis of observational studies. American Journal of Gastroenterology, 100(6), 1345–1353. https://doi.org/10.1111/j.1572-0241.2005.41442.x
Zhao, L. N., Li, J. Y., Yu, T., Chen, G. C., Yuan, Y. H., & Chen, Q. K. (2014). 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: An updated meta-analysis. PLoS ONE, 9(4), e94208. https://doi.org/10.1371/journal.pone.0094208
Nguyen, G. C., Gulamhusein, A., & Bernstein, C. N. (2012). 5-Aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: A meta-analysis of non-referral populations. Official journal of the American College of Gastroenterology | ACG, 107(9).
Lopetuso, L. R., Petito, V., Zinicola, T., Graziani, C., Gerardi, V., Arena, V., et al. (2016). Infliximab does not increase colonic cancer risk associated to murine chronic colitis. World Journal of Gastroenterology, 22(44), 9727–9733. https://doi.org/10.3748/wjg.v22.i44.9727
Biancone, L., Petruzziello, C., Calabrese, E., Zorzi, F., Naccarato, P., Onali, S., et al. (2009). Long-term safety of Infliximab for the treatment of inflammatory bowel disease: Does blocking TNFalpha reduce colitis-associated colorectal carcinogenesis? Gut, 58(12), 1703. https://doi.org/10.1136/gut.2008.176461
Baars, J. E., Looman, C. W., Steyerberg, E. W., Beukers, R., Tan, A. C., Weusten, B. L., et al. (2011). The risk of inflammatory bowel disease-related colorectal carcinoma is limited: Results from a nationwide nested case-control study. American Journal of Gastroenterology, 106(2), 319–328. https://doi.org/10.1038/ajg.2010.428
Lu, M. J., Qiu, X. Y., Mao, X. Q., Li, X. T., & Zhang, H. J. (2018). Systematic review with meta-analysis: Thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics, 47(3), 318–331. https://doi.org/10.1111/apt.14436
Zhang, Q., Pi, J., Woods, C. G., & Andersen, M. E. (2010). A systems biology perspective on Nrf2-mediated antioxidant response. Toxicology and Applied Pharmacology, 244(1), 84–97. https://doi.org/10.1016/j.taap.2009.08.018
Zhao, Y., Guo, Q., Zhao, K., Zhou, Y., Li, W., Pan, C., et al. (2017). Small molecule GL-V9 protects against colitis-associated colorectal cancer by limiting NLRP3 inflammasome through autophagy. Oncoimmunology, 7(1), e1375640–e1375640. https://doi.org/10.1080/2162402X.2017.1375640
Irrazabal, T., Thakur, B. K., Kang, M., Malaise, Y., Streutker, C., Wong, E. O. Y., et al. (2020). Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nature Communications, 11(1), 1802. https://doi.org/10.1038/s41467-020-15549-6
Acknowledgements
This work was supported by the Crohn’s and Colitis Foundation grant 632826 (to H.N.-K.), the Office of the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the Peer-Reviewed Cancer Research Program under Award No. W81XWH2010547 and the Prevent Cancer Foundation (to S.K.), and the National Institutes of Health grants DK108901, DK119219, AI142047, and DK125087 (to N.K.).
Author information
Authors and Affiliations
Contributions
H.N.-K., S.K., and N.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagao-Kitamoto, H., Kitamoto, S. & Kamada, N. Inflammatory bowel disease and carcinogenesis. Cancer Metastasis Rev 41, 301–316 (2022). https://doi.org/10.1007/s10555-022-10028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10028-4